Abstract
We report a case of torsades de pointes (TdP) induced by donepezil without QT prolongation. An 86-year-old woman was admitted to our hospital because of a syncopal attack. She had been treated for Alzheimer's disease with donepezil. Initial 12-lead electrocardiogram showed atrial fibrillation and normal corrected QT interval. After admission, atrial fibrillation spontaneously recovered to normal sinus rhythm on electrocardiographic monitoring. On the second day, electrocardiographic monitoring documented TdP. We discontinued donepezil immediately. After washout of donepezil, TdP was not observed again. Corrected QT interval was normal throughout hospitalization. This case suggests that donepezil may cause life-threatening ventricular arrhythmias without QT prolongation. Even if corrected QT interval is normal in patients taking donepezil and experiencing symptoms associated with TdP, electrocardiographic monitoring is recommended.
<Learning objective: Donepezil may cause life-threatening ventricular arrhythmias without QT prolongation. Even if corrected QT interval is normal in patients taking donepezil and experiencing symptoms associated with torsades de pointes, electrocardiographic monitoring is recommended.>
Keywords: Arrhythmias, QT interval, Ventricular arrhythmia
Introduction
“Proarrhythmia” or “arrhythmogenicity” indicates the capacity of a cardiac or noncardiac drug to aggravate an existing arrhythmia or provoke a new arrhythmia [1].
Donepezil is an acetylcholinesterase inhibitor used widely for the treatment of Alzheimer's disease. Side effects of donepezil are basically cholinergic dependent. The most common adverse events are nausea, diarrhea, malaise, dizziness, and insomnia. Donepezil is considered a safe agent with few adverse effects on the cardiovascular system. However, since the heart is rich in cholinesterase, its cholinergic effects could generate serious cardiac disorders such as bradycardia, atrioventricular block, and QT prolongation [2]. QT prolongation may lead to torsades de pointes (TdP), a polymorphic ventricular arrhythmia that can progress to ventricular fibrillation and sudden death. The present report describes a rare case of TdP induced by donepezil without QT prolongation.
Case report
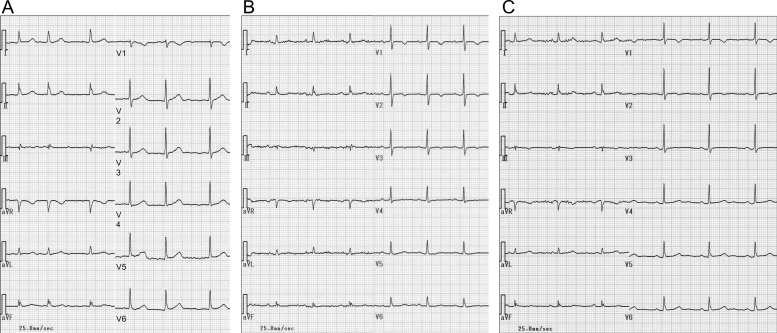
An 86-year-old woman was admitted to our hospital because of a syncopal attack. On admission, she was alert and asymptomatic. She had no history of cardiovascular disease or previous syncope, and no family history of cardiovascular disease or sudden death. She had been treated for Alzheimer's disease with donepezil 5 mg/day for three years by her home physician. She also had hypertension, which had been treated with amlodipine 5 mg/day. Because she had lived in a nursing home, these drugs were administered to her by nurses without fail. She had no symptoms of acetylcholine poisoning, such as miosis or dysuria. Her blood pressure was 124/60 mmHg. She had neither cardiac murmur nor symptoms of heart failure. Neurological findings, chest radiograph, and transthoracic echocardiogram were unremarkable. Initial 12-lead electrocardiogram showed atrial fibrillation (heart rate, 75 beats/min) and normal corrected QT (QTc) interval (QT 390 ms; QTc 436 ms) (Fig. 1A). The interval between the peak and the end of the T wave (Tp–Te) was 100 ms; and corrected Tp–Te interval was 112 ms. She had not undergone 12-lead electrocardiogram before donepezil medication. Blood tests were within normal limits (potassium 3.69 mEq/l, magnesium 2.0 mg/dl, calcium 8.7 mg/dl, aspartate aminotransferase 20 IU/l, alanine aminotransferase 10 IU/l, blood urea nitrogen 14.6 mg/dl, creatinine 0.85 mg/dl).
Fig. 1.
Changes in 12-lead electrocardiogram. (A) Admission, (B) just after the documentation of torsades de pointes on the second day, and (C) the day before discharge.
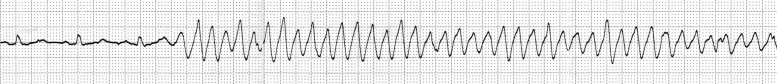
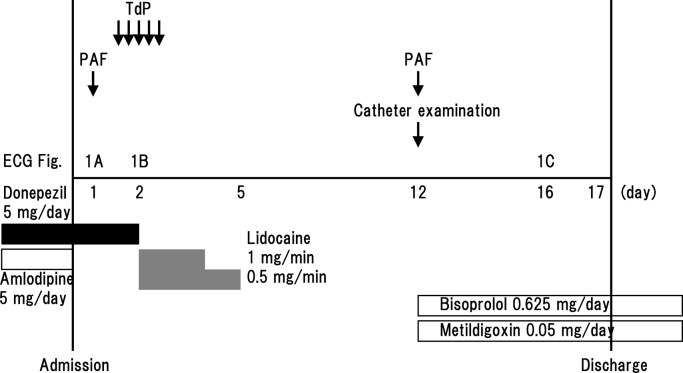
After admission, she was prescribed bed rest. Because her blood pressure dropped to 98/58 mmHg, amlodipine was halted. At midnight, atrial fibrillation spontaneously recovered to normal sinus rhythm on electrocardiographic monitoring and we only detected a short pause (1.4 s). Several hours later, in the daytime on the second day, electrocardiographic monitoring documented TdP when she lay on the bed (Fig. 2, Fig. 3). This arrhythmia was sustained for 10 s and terminated spontaneously without medication or direct current cardioversion. Repeat 12-lead electrocardiogram revealed normal sinus rhythm (heart rate, 78 beats/min) and normal QTc interval (QT 380 ms; QTc 433 ms) (Fig. 1B). We started an intravenous infusion of lidocaine at the rate of 1 mg/min. Although we could not measure donepezil concentration in her blood, we discontinued donepezil immediately. We detected TdP total five times for 2 h. Every TdP terminated spontaneously within 30 s, without other drugs or direct current cardioversion. She experienced no syncope, but she was assumed to have fallen because of Adams–Stokes syndrome due to TdP. After washout of donepezil, we found neither TdP nor worsening of dementia. We performed catheter examination 10 days after the withdrawal of donepezil and 7 days after the withdrawal of lidocaine. Coronary arteriogram demonstrated no significant stenosis of coronary arteries. Programmed ventricular stimulation could not induce ventricular fibrillation. As we confirmed paroxysmal atrial fibrillation again, we selected medical follow-up with bisoprolol and metildigoxin for paroxysmal atrial fibrillation. In the serial electrocardiogram follow up, QTc interval was normal. Twelve-lead electrocardiogram on the day before discharge noted normal sinus rhythm (heart rate, 64 beats/min) (Fig. 1C). Tp–Te interval was 80 ms; and corrected Tp–Te interval was 83 ms. She was discharged in a stable condition without administration of donepezil.
Fig. 2.
Electrocardiographic monitoring on the second day documented torsades de pointes.
Fig. 3.
Clinical course of the case. ECG, electrocardiogram; PAF, paroxysmal atrial fibrillation; TdP, torsades de pointes.
Discussion
Cardiac and noncardiac drugs have been known to facilitate proarrhythmia [1]. Drugs that are generally identified to confer a risk of TdP include antiarrhythmic agents, inotropic agents, antibiotic agents, antipsychotic agents, and antihistamines. Most of the drugs produce either QT prolongation or an increase in adrenergic tone.
Donepezil works at the level of the central nervous system and is highly specific for neural acetylcholinesterases. This specificity minimizes peripheral adverse effects in therapeutic doses. A previous study found that donepezil therapy for each dose has no significant effect on electrocardiographic parameters, including heart rate, PR, QT and QTc interval, and QRS duration, in elderly patients with Alzheimer's disease compared to baseline [3]. It was demonstrated that donepezil has neither negative chronotropic and arrhythmogenic effects nor hypotensive effects. However, it has rarely been reported that bradycardia, atrioventricular block, and TdP were seen in patients receiving donepezil [2].
In the current case, plasma donepezil level was not measured. However, we confirmed no TdP after washout of donepezil. Therefore, we concluded that TdP might be induced by donepezil. Donepezil has a terminal elimination half-life of 50–70 h in young volunteers; in elderly volunteers, the half-life of donepezil is extended to over 100 h. High concentration of donepezil could result in TdP.
Cholinesterase is distributed abundantly in the heart and its inhibition may affect cardiac function, especially in elderly patients. Donepezil inhibits acetylcholinesterase and increases the concentration of acetylcholine by preventing degradation. High levels of acetylcholine may lead to the development of ventricular arrhythmias via vagal tone stimulation [4].
Activation of acetylcholine receptors opens voltage-dependent calcium ion channels and increases intracellular calcium ion concentration. The rise in intracellular calcium ion concentration can contribute to enhanced automaticity or early afterdepolarization following prolongation of action potential duration and trigger ventricular tachyarrhythmias. Elevated calcium ion concentration can also depress conduction and elicit reentry. Reentry depends on prolongation of repolarization and is manifested either as ventricular tachycardia or ventricular fibrillation.
The beat-to-beat alterations in duration and amplitude of action potential are a consistent precursor to ventricular fibrillation in optical mapping studies using detailed measurements of conduction and refractoriness. Tp–Te is associated with the transmural dispersion of repolarization, which is related to the vulnerability to ventricular arrhythmias. A prolonged Tp–Te interval caused by an increased transmural dispersion of repolarization has potential arrhythmogenic effects. In this case, Tp–Te interval on admission, during administration of donepezil, was prolonged, compared to that after washout of donepezil. Thus, donepezil might induce TdP via reentrant excitation caused by heterogeneity in action potential duration and effective refractory period.
Risk factors promoting proarrhythmia are old age, female sex, electrolyte imbalance (hypokalemia, hypomagnesemia, or hypocalcemia), bradycardia, atrial arrhythmias (atrial fibrillation and flutter), ischemic heart disease, congestive heart failure, overdose of a drug, rapid rate of intravenous infusion with a drug, use of other drugs that prolong QT interval directly or indirectly, a family history of sudden death, congenital long QT syndrome, and polymorphisms of genes coding ion channels or enzymes involved in drug metabolism [1].
Our patient had multiple risk factors for TdP: increasing age, female sex, and paroxysmal atrial fibrillation. Coronary arteriogram demonstrated no ischemic heart disease and we thought that ischemia was not involved in this arrhythmic event. Because QTc interval was normal throughout hospitalization, we did not investigate the genetic analysis of congenital long QT syndrome.
Some factors inducing specific gene mutations and selective action potential prolongation may lead to a proarrhythmic state precipitating the onset of QT prolongation and TdP 5, 6. This indicates that there are individual specificities and differences in sensitivity to the drug, and gene abnormalities are assumed behind these differences.
Unlike in previous reports [2], QT prolongation, hypokalemia, and bradycardia were absent in our case. QT prolongation is a predictor of fatal ventricular arrhythmia and sudden death. The risk of TdP increases with increasing length of QTc interval, but the relationship is not linear. It is important to emphasize that QT prolongation is only a surrogate marker or signal of arrhythmic risk and not the risk itself.
An implantable cardioverter defibrillator (ICD) terminates ventricular tachycardia and ventricular fibrillation, and reduces the rate of sudden cardiac death 7, 8. There was no indication for ICD implantation in the present case.
In conclusion, this case suggests that donepezil may cause life-threatening ventricular arrhythmias without QT prolongation. Caution is needed for symptoms, such as dizziness, palpitation, or syncope, which could indicate cardiac arrhythmias in any patient prescribed donepezil. Even if QTc interval is normal in patients taking donepezil and experiencing symptoms associated with TdP, electrocardiographic monitoring is recommended.
Conflict of interest
Authors declare no conflict of interest.
References
- 1.Kerin N.Z., Somberg J. Proarrhythmia definition, risk factors, causes, treatment, and controversies. Am Heart J. 1994;128:575–585. doi: 10.1016/0002-8703(94)90634-3. [DOI] [PubMed] [Google Scholar]
- 2.Takaya T., Okamoto M., Yodoi K., Hata K., Kijima Y., Nakajima H., Nishikawa Y., Kita T., Ito M., Seo T., Kawashima S. Torsades de pointes with QT prolongation related to donepezil use. J Cardiol. 2009;54:507–511. doi: 10.1016/j.jjcc.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Isik A.T., Yildiz G.B., Bozoglu E., Yay A., Aydemir E. Cardiac safety of donepezil in elderly patients with Alzheimer disease. Intern Med. 2012;51:575–578. doi: 10.2169/internalmedicine.51.6671. [DOI] [PubMed] [Google Scholar]
- 4.Omiya T., Shimizu A., Ueyama T., Yoshiga Y., Doi M., Hiratsuka A., Fukuda M., Yoshida M., Matsuzaki M. Effects of isoproterenol and propranolol on the inducibility and frequency of ventricular fibrillation in patients with Brugada syndrome. J Cardiol. 2012;60:47–54. doi: 10.1016/j.jjcc.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Ito S., Taketani T., Sugamori T., Okada T., Sato H., Adachi T., Takeda M., Kodani N., Takahashi N., Endo A., Yoshitomi H., Tanabe K., Shimizu W. A case of long QT syndrome having compound mutations of KCNH2 and SCN5A. J Cardiol Case. 2012;6:e166–e169. doi: 10.1016/j.jccase.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morita H. The compound mutation, a model for acquire long QT syndrome. J Cardiol Case. 2012;6:e187. doi: 10.1016/j.jccase.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hadano Y., Ogawa H., Wakeyama T., Takaki A., Iwami T., Kimura M., Miyazaki Y., Okada H., Shimizu A., Matsuzaki M. Defibrillation efficacy of a subcutaneous array lead: a case report. J Cardiol Case. 2010;1:e21–e24. doi: 10.1016/j.jccase.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsico F., Savarese G., Sardu C., D’Ascia C., Ruggiero D., Casaretti L., Parisi V., Musella F., Pirozzi E., Formisano R., Losco T., Filardi P.P. Implantable cardioverter defibrillator to prevent sudden cardiac death in a patient with systemic sclerosis: a clinical case. J Cardiol Case. 2012;5:e166–e170. doi: 10.1016/j.jccase.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]