Abstract
Premature ventricular contractions (PVCs) and slow coronary flow phenomenon (SCFP) are primarily separate entities. Each one of them has different characteristics and a diverse spectrum of presentation. However, and despite many suggested theories, a comprehensive understanding of the etiology of both of them is still a matter of debate. PVCs, which can be triggered by consuming cannabis (marijuana), and through decreasing the diastolic time (DT), can affect the slow blood flow found in SCFP even more and worsen the clinical picture in patients who have PVCs and SCFP. In this paper, we present a patient who uses marijuana and has PVCs and SCFP, try to address different aspects of PVCs and SCFP, pinpoint any suspected interaction between both of them and the role of marijuana in this context.
<Learning objective: (i) PVCs are extra abnormal heartbeats arising in one of the ventricles and disrupting the normal rhythm of the heart. (ii) PVCs are very common and occur in a broad spectrum of the population including those with and without underlying heart disease. (iii) SCFP is an angiographic finding characterized by delayed progression of the contrast injected inside large coronary arteries without any significant CAD. (iv) It has an incidence of 1% among patients who undergo coronary angiography, especially those presenting with acute coronary syndrome. (v) PVCs, which can be triggered by consuming cannabis (marijuana), and throughout decreasing the DT, can affect the slow blood flow found in SCFP even more and worsen the clinical picture in patients who have PVCs and SCFP.>
Keywords: Premature ventricular contractions, Coronary blood flow, Marijuana
Introduction
Premature ventricular contractions (PVCs) are extra abnormal heartbeats arising in one of the ventricles and disrupting the normal rhythm of the heart. PVCs are very common and occur in a broad spectrum of the population including those with and without underlying heart disease. They are more common in men than women, in African Americans than whites, and in those with organic heart disease. The ability to detect recorded PVCs is mainly depending on the period of monitoring, especially in asymptomatic individuals.
Slow coronary flow phenomenon (SCFP) is an angiographic finding characterized by delayed progression of the contrast injected inside large coronary arteries without any significant coronary artery disease (CAD). It was first recognized in 1972. It is more common in male smokers [1], with an overall incidence of 1% among patients who undergo coronary angiography, especially those presenting with acute coronary syndrome [2].
Case report
A 37-year-old female with a history of mitral regurgitation (MR), hypertension, and syncope, presented to the emergency department with chest pain, dizziness, headache, and shortness of breath for the past two weeks. She also reported a history of palpitations for many years, which have increased over the past two weeks. Laboratory evaluation showed potassium of 3.6 mEq/L (N = 3.5–5 mEq/L), magnesium of 1.9 mg/dL (N = 1.5–2.3 mg/dL), and negative troponin times two. Urine was found to be positive for opiates and marijuana. The patient admitted to using marijuana for 10 years. Electrocardiography (ECG) at rest revealed normal sinus rhythm with PVCs in a bigeminal pattern (Fig. 1). On exercise stress test, the patient developed non-sustained run of ventricular tachycardia at almost 300 beats per minute. Chest X-ray and computed tomography of the chest came back normal. Nuclear medicine stress test was also normal with no evidence of reversible ischemia or scar. Transthoracic echocardiogram showed abnormal left ventricle diastolic filling pattern, PVCs, trace MR, and an ejection fraction of 50–55%. To rule out any significant CAD, cardiac catheterization was done and revealed slow coronary blood flow with a thrombolysis in myocardial infarction (TIMI) score of II in left anterior descending (LAD) artery (Video 1) and mildly slow coronary blood flow with a TIMI score of II–III in right coronary artery (RCA) without any overt CAD. Occasional couplets without ectopy and mild MR were also noted. The patient was put on the maximal dose of metoprolol and counseled to abstain from marijuana with a possibility to consider catheter ablation therapy in case she continues to be symptomatic with documented PVCs and ventricular tachycardia after that.
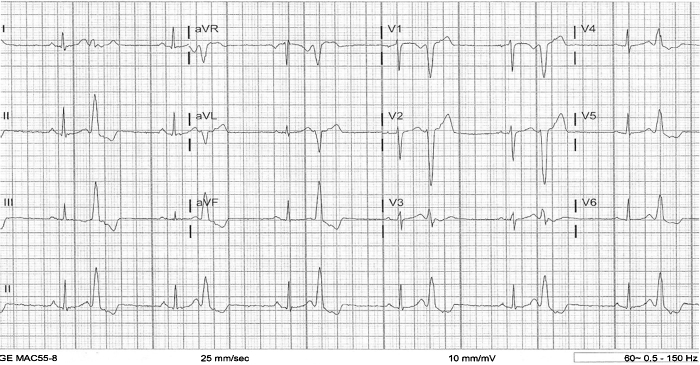
Fig. 1.
Electrocardiogram of the patient showing normal sinus rhythm associated with a bigeminal pattern of premature ventricular contractions.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jccase.2013.06.006.
Coronary angiography of the patient showing thrombolysis in myocardial infarction grade II slow coronary blood flow in left anterior descending artery.
Discussion
The prevelance of PVCs is increased in patients diagnosed with hypertension (HTN) [3] especially when it is associated with left ventricular hypertrophy, dilated cardiomyopathy and heart failure (HF), post acute myocardial infarction (MI), and congenital heart disease.
PVCs often cause no symptoms. In many patients, the presence of PVCs could result in the sensation of fluttering, pounding, skipped beats, palpitations, dizziness, and/or near syncope.
The etiology of PVCs is not well known. Many mechanisms may explain the origin of PVCs, including enhanced normal or abnormal automaticity inside the heart, triggered activity in Purkinje cells of the ventricular myocardium, or reentry. Anxiety, alcohol, caffeine, tobacco, exercise, illicit drugs, hypokalemia, HTN, ischemia, infarction, excessive calcium, drug toxicity (such as digoxin), or an underlying heart disease could result in PVCs through previously mentioned mechanisms.
ECG is the mainstay of diagnosis of PVCs. This includes standard ECG, exercise stress ECG, holter monitor, and event recorder depending primarily on the frequency of PVCs which helps to decide the best way to detect them.
Only in symptomatic patients do PVCs need to be diagnosed and treated. Beside eliminating previously mentioned possible triggers, beta blockers and calcium channel blockers are recommended as first-line therapy for symptomatic PVCs, especially with outflow tract morphology in a structurally normal heart. Antiarrhythmic medications, such as amiodarone can sometimes be tried but with caution because of its side effects.
Frequent PVCs may be associated with worsening of systolic heart failure in patients with a dilated cardiomyopathy. Small studies have suggested that in selected patients, radiofrequency ablation of ectopic ventricular foci is associated with an improvement in left ventricular function and clinical improvement in symptoms 4, 5, 6, 7.
The 2006 American College of Cardiology/American Heart Association/European Society of Cardiology guidelines for the management of ventricular arrhythmias included suggestions regarding ablation therapy for PVCs [8]. They note that ablation therapy of PVCs may be useful if they are frequent, symptomatic, and monomorphic, if they are refractory to medical therapy, if the patient chooses to avoid long-term medical therapy, or if they consistently provoke ventricular arrhythmia storm of a similar morphology [9].
SCFP, as a separate entity, has a widely diverse presentation including chest discomfort, unstable angina, non ST elevation MI, ST elevation MI or nonsustained ventricular tachycardia. It usually presents with recurrent rest pain requiring urgent admission.
The etiology of SCFP is not completely understood. It is speculated that it is caused by acute but recurrent perturbations of microvascular function. Histopathological examination (light and electron microscope) of left and right ventricular endomyocardial biopsies taken from some patients showed fibromuscular hyperplasia, myofibrilar hypertrophy, endothelial degeneration with swollen endothelial cells encroaching on the lumen, luminal size reduction, mitochondrial abnormalities, lipofuscin deposition, and glycogen content reduction, which can cause the elevation in resting coronary artery resistances, especially toward microvasculature beds, found in SCFP. Normal and pathological zones often coexisted in the same specimen (patchy appearance). Thus, in some patients with slow coronary flow and patent coronary arteries, functional obstruction of microvessels seems to be implicated, as it is relieved by dipyridamole infusion. This shows also that small-vessel CAD can cause classic angina pectoris.
The diagnosis can be suspected when the coronary angiogram shows large patent arteries with slow flow of the angiographic contrast medium and it can be confirmed by endomyocardial biopsy.
Another study suggested that elevation in plasma homocysteine, even if it is mild, may play a role in the pathogenesis of SCFP by severely disturbing vascular endothelial function and subsequently impairing coronary blood flow [10], and showed that patients with SCFP have statistically significant raised level of plasma homocysteine compared to control subjects with normal coronary flow.
As a treatment, dipyridamole, which has dilatator properties on coronary microvessels, proved to be useful in most patients with SCFP. It abolishes functional obstruction in coronary arteries with diameters less than 200 μm and is considered far superior in treating SCFP as compared to nitroglycerine. Other therapies proved to be effective also include simvastatin [11], atorvastatin [12], nebivolol [13], and mibefradil [14] although the use of the latter is limited because of drug interactions caused by its inhibition of the cytochrome P450 3A4 pathway [15].
Beside mean arterial pressure, blood flow in coronary arteries depends on heart contractility. During systole, and because of the big muscular mass of the left ventricle (LV), extravascular compression prevents any antegrade blood flow in left coronary artery (LCA) particularly at the end of isovolumetric contraction. Conversely, and during diastole and especially early diastole which represents isovolumetric relaxation, coronary blood flow in LCA becomes maximal (Fig. 2).
Fig. 2.
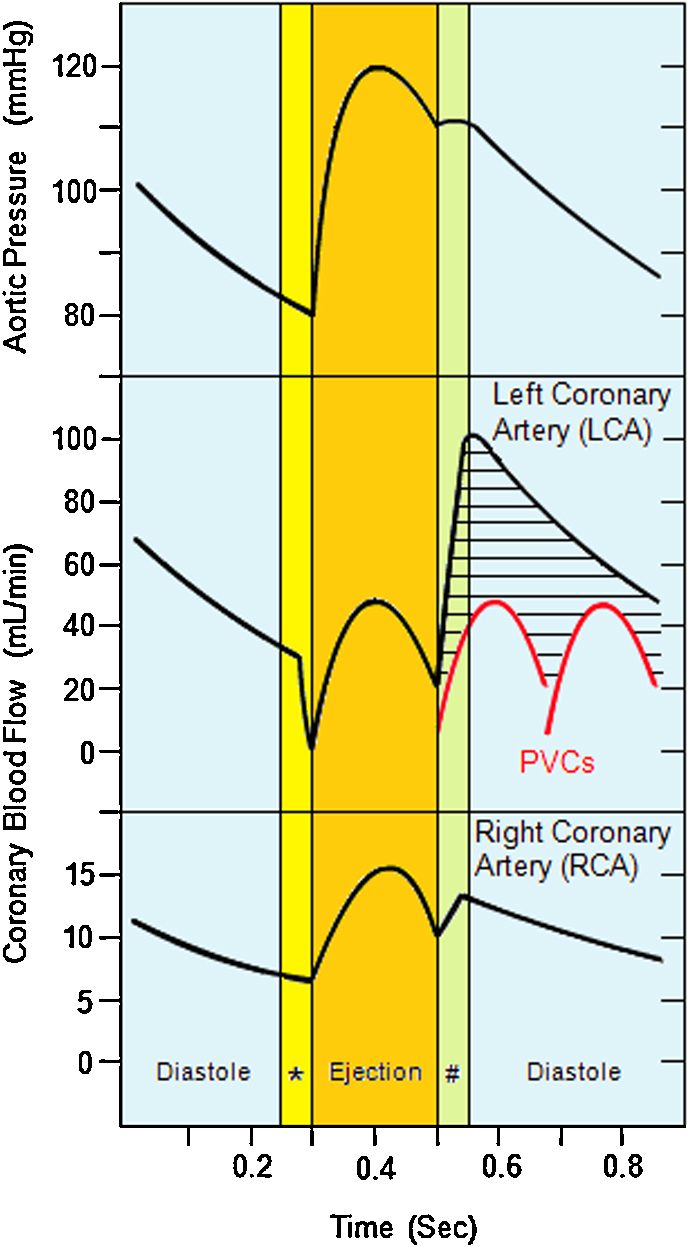
Illustrating the changes in aortic pressure, blood flow in left coronary artery (LCA) and blood flow in right coronary artery throughout normal cardiac cycle of systole and diastole. Two premature ventricular contractions (PVCs) (red curves) and their effect on blood flow in LCA (black shaded area) are shown for comparison.*, Isovolumetric contraction. #, Isovolumetric relaxation. (For interpretation of references to color in this figure legend, the reader is referred to the web version of this article,)
In right coronary artery (RCA), blood flow is maximal during peak systole, because extravascular compression within the right ventricle is less than its counterpart in the LV preserving antegrade blood flow in RCA in both systole and diastole.
The fact that the highest blood flow in LCA occurs throughout different stages of diastole, which is not the case in RCA, assumes that any PVC happening through diastole will have a bigger impact on blood flow in LCA compared to RCA. This, in turn, will be reflected as a slow blood flow (low TIMI score) in LCA more than in RCA.
In patients with SCFP, resting coronary artery resistances, as mentioned above, are abnormally elevated, however, these resistances respond normally to vasodilator stimuli such as papaverine and adenosine and during exercise [16].
Marijuana affects the heart in different ways. The acute cardiovascular effects of marijuana, which are palpitations, tachycardia, elevated blood pressure, and a greater myocardial oxygen demand, are mainly caused by increased release of catecholamines. Chronic use of marijuana can worsen any underlying disease through prolonged vasoconstriction and causes digital clubbing. Very high doses of marijuana may conversely cause bradycardia and hypotension [17].
After a thorough review of the literature, it was noted that there was a lack of description of any specific timeframe, after which marijuana consumption will have effects on the heart, resulting in myocardial ischemia or heart failure. Patient's age and the presence of underlying CAD are key players in determining how fast the results of smoking marijuana will take effect on the heart, especially when it comes to chronic use.
The mechanisms by which marijuana affects the heart are diverse including increased cardiac work through elevated catecholamines and carboxyhemoglobin levels, as well as possible episodes of intense postural hypotension [18]. Moreover, smoking cannabis was rarely found to trigger MI. Among cannabis users who sustained an acute MI, the risk was nearly five times higher within the first hour after smoking compared to periods of nonuse (relative risk 4.8, 95% CI 2.9–9.5) [19]. The elevated risk rapidly declined thereafter.
Increased release of catecholamines caused by marijuana can enhance normal or abnormal automaticity inside the heart and subsequently cause PVCs, which in turn, will reduce diastolic time (DT). The reduction in DT in combination with the direct vasoconstrictive effect of marijuana, will potentially lead to “physiologic” slow blood flow in coronary arteries or exacerbate any baseline SCFP.
Conclusion
PVCs documented through ECG, SCFP reflected in low TIMI score during coronary angiography, and marijuana use in symptomatic patients, could or could not be etiologically related to each other, but can exacerbate each other.
In this population of patients, breaking this vicious circle of exacerbation must be given the priority. Counseling these patients about abstaining from marijuana and starting them on a gradually increasing dose of beta blockers to reach the maximal dose must be at the top of the list of treatment options of PVCs and should be tried before any further invasive procedures are considered.
References
- 1.Goel P.K., Gupta S.K., Agarwal A., Kapoor A. Slow coronary flow: a distinct angiographic subgroup in syndrome X. Angiology. 2001;52:507–514. doi: 10.1177/000331970105200801. [DOI] [PubMed] [Google Scholar]
- 2.Simpson R.J., Jr., Cascio W.E., Schreiner P.J., Crow R.S., Rautaharju P.M., Heiss G. Prevalence of premature ventricular contractions in a population of African American and white men and women: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2002;143:535–540. doi: 10.1067/mhj.2002.120298. [DOI] [PubMed] [Google Scholar]
- 3.Singh S., Kothari S.S., Bahl V.K. Coronary slow flow phenomenon: an angiographic curiosity. Indian Heart J. 2004;56:613–617. [PubMed] [Google Scholar]
- 4.Satish O.S., Yeh K.H., Wen M.S., Wang C.C. Premature ventricular contraction-induced concealed mechanical bradycardia and dilated cardiomyopathy. J Cardiovasc Electrophysiol. 2005;16:88–91. doi: 10.1046/j.1540-8167.2005.04473.x. [DOI] [PubMed] [Google Scholar]
- 5.Chugh S.S., Shen W.K., Luria D.M., Smith H.C. First evidence of premature ventricular complex-induced cardiomyopathy: a potentially reversible cause of heart failure. J Cardiovasc Electrophysiol. 2000;11:328–329. doi: 10.1111/j.1540-8167.2000.tb01802.x. [DOI] [PubMed] [Google Scholar]
- 6.Takemoto M., Yoshimura H., Ohba Y., Matsumoto Y., Yamamoto U., Mohri M., Yamamoto H., Origuchi H. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J Am Coll Cardiol. 2005;45:1259–1265. doi: 10.1016/j.jacc.2004.12.073. [DOI] [PubMed] [Google Scholar]
- 7.Efremidis M., Letsas K.P., Sideris A., Kardaras F. Reversal of premature ventricular complex-induced cardiomyopathy following successful radiofrequency catheter ablation. Europace. 2008;10:769–770. doi: 10.1093/europace/eun060. [DOI] [PubMed] [Google Scholar]
- 8.Zipes D.P., Camm A.J., Borggrefe M., Buxton A.E., Chaitman B., Fromer M., Gregoratos G., Klein G., Moss A.J., Myerburg R.J., Priori S.G., Quinones M.A., Roden D.M., Silka M.J., Tracy C. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death–executive summary: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2006;27:2099–2140. doi: 10.1093/eurheartj/ehl199. [DOI] [PubMed] [Google Scholar]
- 9.Haïssaguerre M., Shoda M., Jaïs P., Nogami A., Shah D.C., Kautzner J., Arentz T., Kalushe D., Lamaison D., Griffith M., Cruz F., de Paola A., Gaïta F., Hocini M., Garrigue S. Mapping and ablation of idiopathic ventricular fibrillation. Circulation. 2002;106:962–967. doi: 10.1161/01.cir.0000027564.55739.b1. [DOI] [PubMed] [Google Scholar]
- 10.Riza Erbay A., Turhan H., Yasar A.S., Ayaz S., Sahin O., Senen K., Sasmaz H., Yetkin E. Elevated level of plasma homocysteine in patients with slow coronary flow. Int J Cardiol. 2005;102:419–423. doi: 10.1016/j.ijcard.2004.05.064. [DOI] [PubMed] [Google Scholar]
- 11.Cakmak M., Tanriverdi H., Cakmak N., Evrengul H., Cetemen S., Kuru O. Simvastatin may improve myocardial perfusion abnormality in slow coronary flow. Cardiology. 2008;110:39–44. doi: 10.1159/000109405. [DOI] [PubMed] [Google Scholar]
- 12.Fan Y., Lin J.H., Dong G., Zhu J., Yin F., Yang S.S. The effect of carvedilol on coronary flow reserve in patients with dilated cardiomyopathy. Zhonghua Nei Ke Za Zhi. 2010;49:217–219. [PubMed] [Google Scholar]
- 13.Tiryakioglu S., Tiryakioglu O., Ari H., Basel M.C., Bozat T. Effects of nebivolol on endothelial function and exercise parameters in patients with slow coronary flow. Clin Med Cardiol. 2009;3:115–119. doi: 10.4137/cmc.s3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beltrame J.F., Turner S.P., Leslie S.L., Solomon P., Freedman S.B., Horowitz J.D. The angiographic and clinical benefits of mibefradil in the coronary slow flow phenomenon. J Am Coll Cardiol. 2004;44:57–62. doi: 10.1016/j.jacc.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 15.Becquemont L., Funck-Brentano C., Mibefradil Jaillon P. a potent CYP3A inhibitor, does not alter pravastatin pharmacokinetics. Fundam Clin Pharmacol. 1999;13:232–236. doi: 10.1111/j.1472-8206.1999.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 16.Beltrame J.F., Limaye S.B., Wuttke R.D., Horowitz J.D. Coronary hemodynamic and metabolic studies of the coronary slow flow phenomenon. Am Heart J. 2003;146:84–90. doi: 10.1016/S0002-8703(03)00124-8. [DOI] [PubMed] [Google Scholar]
- 17.Gruber A.J., Pope H.G., Jr. Marijuana use among adolescents. Pediatr Clin North Am. 2002;49:389–413. doi: 10.1016/s0031-3955(01)00011-6. [DOI] [PubMed] [Google Scholar]
- 18.Jones R.T. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42(11 Suppl.):58S–63S. doi: 10.1002/j.1552-4604.2002.tb06004.x. [DOI] [PubMed] [Google Scholar]
- 19.Mittleman M.A., Lewis R.A., Maclure M., Sherwood J.B., Muller J.E. Triggering myocardial infarction by marijuana. Circulation. 2001;103:2805–2809. doi: 10.1161/01.cir.103.23.2805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Coronary angiography of the patient showing thrombolysis in myocardial infarction grade II slow coronary blood flow in left anterior descending artery.