Abstract
We report a 66-year-old male with a history of Roux-en-Y gastric bypass surgery who began dabigatran for new onset atrial fibrillation. After 5 weeks of therapy, his transesophageal echocardiogram prior to electrocardioversion showed severe spontaneous echo contrast. Cardioversion was postponed and anticoagulant therapy was continued. The following day, he suffered a thromboembolic stroke. Concern arose that postoperative malabsorption could have resulted in subtherapeutic anticoagulation. This notion was strengthened by a second patient who had subtherapeutic serum levels despite maximal dosing. To the best of our knowledge, we are the first to report impaired absorption of dabigatran following Roux-en-Y gastric bypass surgery.
<Learning objective: Dabigatran has a predictable pharmacokinetic profile, allowing for a fixed-dose regimen that does not require frequent monitoring or dietary modifications. However, its absorption in patients who have undergone Roux-en-Y gastric bypass surgery has not been studied. Postoperative malabsoprtion, a major complication following Roux-en-Y gastric bypass surgery, can result in inadequate anticoagulation. As a result of unpredictable absorption, strategies allowing for routine monitoring may be best in this population.>
Keywords: Atrial fibrillation, Morbid obesity, Roux-en-Y gastric bypass surgery, Dabigatran
Introduction
Roux-en-Y gastric bypass (RYGB) is the preferred surgical approach to morbid obesity because of its high level of effectiveness and durability. However, the procedure is associated with postoperative malabsorptive complications. Morbid obesity carries a significant risk for atrial fibrillation, with rates as high as 52% in males and 46% in females [1]. Dabigatran, an oral direct thrombin inhibitor, is one of several novel anticoagulants approved for non-valvular atrial fibrillation. The use of dabigatran in patients who have undergone RYGB is not well studied.
Case report
A 66-year-old Caucasian male with diabetes mellitus, hypertension, and chronic systolic congestive heart failure (ejection fraction 20%) secondary to nonischemic cardiomyopathy had previously undergone RYBG in 2001. On routine examination, he was noted to have an irregular rhythm, confirmed by electrocardiogram to be atrial fibrillation. His CHADS2 score was 3. Given his normal renal function, anticoagulation was initiated with dabigatran (150 mg every 12 h). Transesophageal echocardiogram (TEE) at that time showed severe left ventricular systolic dysfunction with global hypokinesis. The left atrium and left ventricle were markedly dilated, but no evidence of valvular disease or left atrial thrombus was seen. Therapy was continued for 5 weeks with plans for electrical cardioversion. However, his repeat TEE showed severe spontaneous echo contrast throughout the left atrium and left atrial appendage, as well as a low left atrial appendage emptying velocity (20 cm/s) (Fig. 1, Video 1). He was compliant with anticoagulant therapy. Plans were made to continue therapy and reassess for possible cardioversion in 6 weeks. He presented to the emergency department the following day with left-sided weakness, facial droop, and slurred speech. Magnetic resonance imaging (MRI) of the brain revealed a small focus of restricted diffusion in the posterior right insular cortex, consistent with acute infarction of the right middle cerebral artery (Fig. 2). This event was thought to be thromboembolic in nature. Coagulation studies showed partial prothrombin time (PTT) 26 s (reference range: 22–34 s), prothrombin (PT) 13.7 s (reference range: 11.4–14 s), and an international normalized ratio (INR) 1.1 (reference range: 0.9–1.1). He was started on intravenous heparin and bridged to warfarin. Fortunately, his neurologic symptoms resolved. He has not experienced further thromboembolic events.
Fig. 1.
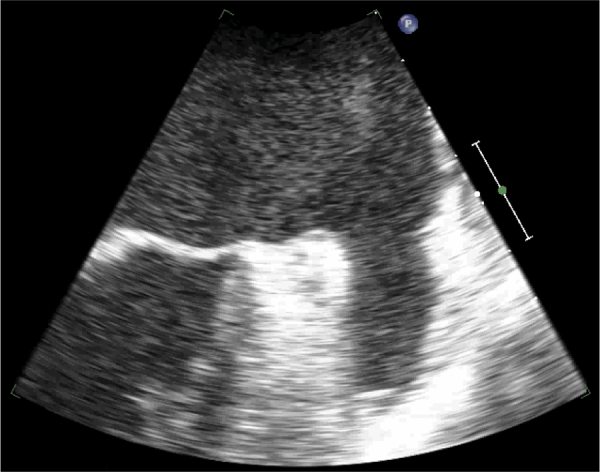
Transesophageal echocardiogram showing severe spontaneous echo contrast with thrombus formation in the left atrial appendage.
Fig. 2.
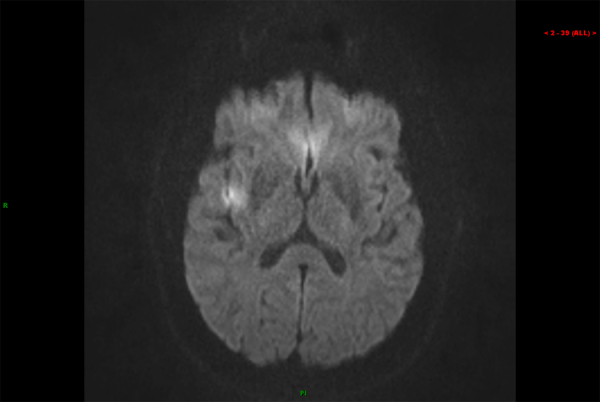
Diffusion-weighted magnetic resonance image showing acute infarction in the right insular cortex.
A 67-year-old Caucasian female with diabetes mellitus, congestive heart failure, and gastroesophageal reflux disease presented with new onset atrial fibrillation, which failed electrical cardioversion. She underwent RYGB in 2007. She took pantoprazole daily. Her CHADS2 score was 2 and she was started on dabigatran (150 mg every 12 h). She continued therapy for 9 months. Given concerns regarding the absorption of dabigatran after RYGB raised by the first case, her serum trough level was measured and discovered to be subtherapeutic (21 ng/ml, reference range: 31–225 ng/ml) [2]. She was transitioned to warfarin to avoid potential thromboembolic complications.
Discussion
Dabigatran is an increasingly popular anticoagulant being used in the treatment of non-valvular atrial fibrillation. In the RE-LY trial, dabigatran at 150 mg twice daily was associated with lower rates of stroke and systemic embolism when compared to adjusted-dose warfarin [3]. Dabigatran has a predictable pharmacokinetic profile, allowing for a fixed-dose regimen that does not require routine monitoring or dietary modifications. While direct thrombin inhibitors cannot be reliably monitored through traditional coagulation assays, time-dependent effects can be observed. The anticoagulant activity of dabigatran can be reflected in a prolonged PTT, although this test cannot quantify its precise anticoagulant effect [2]. Little impact is seen on PT/INR. Most importantly, all changes on coagulation assays are time-dependent on the most recent dose. Peak changes are observed two hours after administration [2].
RYGB is considered a combination restriction-malabsorption procedure, as it involves creation of a small gastric pouch and exclusion of the proximal small intestine from the digestive tract [4]. Postoperative malabsorption occurs for several reasons. The decreased intestinal length reduces time and surface area available for absorption. Additionally, changes in gastric pH after RYGB alter medication solubility, which can further be compounded by the use of proton pump inhibitors and H2 blockers. Dabigatran etexilate, the prodrug of dabigatran, is rapidly absorbed in the stomach and small intestine. It is coated with tartaric acid, creating an acidic microenvironment, in an attempt to maximize absorption independent of gastric pH [5]. Proton pump inhibitors have been shown to decrease serum concentrations of dabigatran, although their concurrent use has not proven to be clinically significant [6]. The extent to which these factors affect the absorption of dabigatran following RYGB is unclear. Nonetheless, these cases demonstrate subtherapeutic anticoagulation, both clinically and by laboratory testing, despite maximal dosing. As a result of unpredictable absorption, anticoagulation strategies allowing for routine monitoring may be best in this population.
Conclusion
The use of dabigatran following RYGB requires further investigation due to the potentially grave consequences of impaired absorption.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Supplementary material related to this article found, in the online version, at http://dx.doi.org/10.1016/j.jccase.2013.03.013.
Appendix A. Supplementary data
The following are the supplementary data to this article:
Transesophageal echocardiogram showing severe spontaneous echo contrast with thrombus formation in the left atrial appendage.
References
- 1.Wang T.J., Parise H., Levy D., D’Agostino R.B., Sr., Wolf P.A., Vasan R.S., Benjamin E.J. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 2.van Ryn J., Stangier J., Haertter S., Liesenfeld K.H., Wienen W., Feuring M., Clemens A. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116–1127. doi: 10.1160/TH09-11-0758. [DOI] [PubMed] [Google Scholar]
- 3.Connolly S.J., Ezekowitz M.D., Yusuf S., Eikelbloom J., Oldgren J., Parekh A., Pogue J., Reilly P.A., Themeles E., Varrone J., Wang S., Alings M., Xavier D., Zhu J., Diaz R. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 4.DeMaria E.J. Bariatric surgery for morbid obesity. N Engl J Med. 2007;356:2176–2183. doi: 10.1056/NEJMct067019. [DOI] [PubMed] [Google Scholar]
- 5.Douketis J.D. Pharmacologic properties of the new oral anticoagulants: a clinician-oriented review with a focus on perioperative management. Curr Pharm Des. 2010;16:3436–3441. doi: 10.2174/138161210793563338. [DOI] [PubMed] [Google Scholar]
- 6.Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet. 2008;47:285–295. doi: 10.2165/00003088-200847050-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transesophageal echocardiogram showing severe spontaneous echo contrast with thrombus formation in the left atrial appendage.


