Abstract
An adolescent male trauma patient developed new asymptomatic ST segment elevations that mimicked a myocardial infarction on infero-lateral telemetry leads on hospital day #8, following burn excision and skin grafting. This was confirmed on 12 lead electrocardiogram. Laboratory test results indicated normal potassium. Troponins ×3 were negative. X-rays indicated marked gaseous gastric distention. A nasogastric tube was placed with evacuation of 400 mL of fluid and resolution of gastric distention. After gastric decompression, the ST segment elevations resolved. This case illustrates the need to consider acute gastric distention in the differential of acute ST segment elevation.
<Learning objective: Electrocardiographic ST segment elevation generally indicates myocardial infarction. However, in the asymptomatic patient with normal troponins, alternative explanations, such as gastric distention should be considered.>
Keywords: Gastric distention, ST elevation, Electrocardiogram
Introduction
ST segment elevation is generally associated with significant coronary pathology. We describe the case of a teenage victim of blunt trauma, who had acute onset of ST segment elevation, mimicking a myocardial infarction, from acute gastric distention. It resolved promptly with nasogastric decompression. The concept of QRS axis changes with gastric distention has been described [1]; however, there is minimal literature about ST-T wave changes secondary to gastric distention [2].
Case history
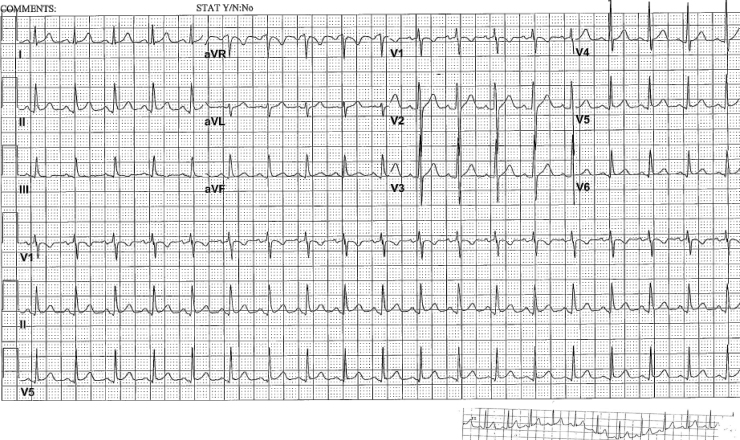
An obese 15-year-old male was brought to our facility following a pedestrian vs. car accident. He was hit by a car which probably drove over a part of him. Past medical history was notable for febrile seizures in childhood. Family history was notable for hypertension and diabetes. He was not taking any medications. His injuries and their initial management included: pelvic fracture managed with a femoral traction pin; partial urethral disruption managed via Foley catheterization; small left pneumothorax and bilateral pulmonary contusions managed conservatively; clavicle fracture and right acromion fracture managed conservatively; and 11% body surface area burns for which wound care was performed. A toxicology screen was negative. Admission electrocardiogram (ECG) was normal. On hospital day (HD) #4, he underwent burn scrub debridement and orthopedic surgery to repair the pelvis and remove the femoral traction pin. On HD #8, he underwent burn wound excision and skin grafting, during which he received 2 units of packed red blood cells. That evening he had a fever to 38.7 °C and new-onset ST segment elevation on telemetry leads (Fig. 1). He denied chest pain, shortness of breath, or any other complaints. His pulse was 117, respiratory rate was 16, blood pressure was 137/66 mm Hg, and SpO2 was 100% on 1 L oxygen by nasal cannula. He was not in distress, was tachycardic, with clear lung fields. His abdomen was soft, not tender, and not distended. Extremities were without edema. A 12-lead ECG demonstrated sinus tachycardia and confirmed new ST segment elevation in infero-lateral leads (Fig. 2A). Troponin I was negative ×3; each level was drawn eight hours apart. Potassium was 4.3 mmol/L, magnesium was 1.8 mg/dl, calcium was 7.5 mg/dl, and creatinine was 0.42 mg/dl. Hemoglobin was 9.8 g/dl. Radiographic imaging indicated new acute marked gaseous gastric distention that was not present pre-operatively (Fig. 3). A nasogastric (NGT) tube was placed and 400 mL of fluid were evacuated. On imaging shortly after NGT insertion, decreased gastric distention was noted; it resolved completely on subsequent imaging several hours later. Following gastric decompression, ST segment changes resolved on telemetry and subsequent ECGs confirmed complete resolution of the infero-lateral ST segment elevation, with persistent sinus tachycardia. The QRS axis remained within normal limits throughout and there was no Q wave development (Fig. 2B and C). The patient remained asymptomatic. Given the patient's youth, we felt that he would be at very low risk for myocardial ischemia or pericarditis, especially with negative cardiac enzymes, without symptoms of myocardial ischemia, and with the resolution of the ECG findings after NG decompression. As such, we felt that further work-up via echocardiogram, angiography, or cardiac stress study was not indicated. The patient was discharged to an acute rehabilitation facility on HD #23, following further burn and orthopedic procedures.
Fig. 1.
Multi-lead telemetry from the evening of hospital day #8, following surgery, demonstrating significant ST segment elevations in leads II, III, aVF. Abnormalities in lead aVR are also noted. Some respiratory variation in the electrocardiogram is also noted.
Fig. 2.
(A) Initial 12 lead electrocardiogram (ECG) demonstrating ST-segment elevation in infero-lateral leads. Corresponding telemetry lead II from same time frame is also shown. (B) 12 lead ECG performed approximately 4 h after initial ECG, and following commencement of nasogastric decompression, demonstrating resolution of ST-segment elevation and no Q waves. (C) 12 lead ECG performed approximately 13 h after initial ECG, with ongoing nasogastric decompression, demonstrating complete resolution of ST-segment elevations and no Q waves. Corresponding telemetry lead II from same time frame is also shown.
Fig. 3.

Chest X-ray demonstrating marked gaseous distention of the stomach. No pneumothorax is seen.
Discussion
To our knowledge, there are no previous English-language case reports with ST segment elevation secondary to gastric distention in trauma patients. The mechanism of this phenomenon is unclear. We hypothesize several possible mechanisms: (1) a change in the heart's position secondary to gastric distention; (2) changes in the patient's body position; (3) Brugada syndrome which was unmasked by the fever; (4) elevated vagal tone in absence of Brugada syndrome; (5) stress-related/catecholamine-associated cardiomyopathy; (6) variant angina; (7) pericarditis; (8) blunt cardiac injury; or (9) perhaps an irritative/direct compression effect on the heart from gastric distention.
The concept of changes in the ECG secondary to variation in diaphragmatic position has been described. Specifically, the concept of gastric dilation causing a leftward shift of the QRS axis was described by Duke in 1965, in 12 healthy volunteers [1]. He found that gastric distention via rapid instillation of air, and thus a shift in the cardiac position in the chest, had greater effects on upright ECGs (leftward axis shift in 75% of volunteers) than supine ECGs (leftward axis shift in 25% of volunteers). Changes in the P axis have also been described in restrictive and obstructive lung disease [3]. It was suggested that changes in the diaphragm level anatomically distort the right atrium, resulting in an alteration of the depolarization course [3]. Furthermore, electrical alternans has been described secondary to severe diaphragmatic eventration with resultant cardiac displacement [4]. However, our patient did not demonstrate a significant change in P axis, QRS axis, or electrical alternans.
Another consideration is whether changes in body position resulted in these ST segment changes. A study of 18 patients with ischemic heart disease and 22 healthy student volunteers found that 22% of ischemic heart disease patients and 9% of healthy subjects, respectively, developed greater than 1-mm ST segment changes with position changes [5]. However, our patient remained supine with head of bed elevated throughout.
Yet another intriguing possibility is that the patient's fever of 38.7 °C unmasked Brugada syndrome [6]. Ikeda et al. described the full stomach test as a novel way of identifying patients at risk for Brugada syndrome, i.e. ST elevation in leads V1–V3 [7]. Specifically, patients are at risk of Brugada syndrome when an ECG done shortly after ingestion of a large meal demonstrates greater than 1-mm augmentation of ST elevation at the J point. Elevated vagal tone has been associated with ST elevation, and thus enhanced vagal tone following a large meal facilitates ST elevation [7]. It is thought that enhanced vagal tone or acetylcholine increase calcium currents and can increase ST elevation in patients with Brugada syndrome [8]. Brugada syndrome is also thought to be mediated by transient outward potassium current, Ito [9]. It has also been hypothesized that the syndrome is mediated by a functional reduction in the myocardial sodium current because of an increased proportion of sodium channels that enter the intermediate inactivation (Im) state [10]. Interestingly, no pathognomonic findings were noted on histopathologic analyses on myocardial biopsy specimens in 21 patients with Brugada syndrome [11]. Since the ECG “location” of Brugada syndrome is the anterior right ventricular outflow tract (leads V1 and V2), it is hard to ascribe the infero-lateral location of the ECG changes in this patient as Brugada changes. The prompt resolution of ECG changes with gastric decompression would also argue against Brugada syndrome.
Another consideration is a visceral-cardiac reflex, secondary to gastric distention, resulting in increased vagal tone. To this end, the concept of vagally mediated symmetrical T-wave inversion has been reported in patients with biliary pathology 12, 13. The increased vagal tone may cause transient coronary vasospasm [12]. It is possible that our patient had increased vagal tone which adversely affected coronary perfusion. However, he was asymptomatic from a cardiac and abdominal standpoint and was tachycardic.
An additional differential diagnosis is stress-related cardiomyopathy, including Takotsubo syndrome. While he did not have an echocardiogram or coronary angiogram, his presentation would argue against this phenomenon. He was asymptomatic, i.e. he did not have any chest pain or shortness of breath. There was also no pulmonary edema on chest X-ray [14]. He did not experience any hypotension; his blood pressure and heart rate remained fairly stable during this time. While infero-lateral ST segment elevation may be seen with Takotsubo syndrome, it is more common for it to be in the precordial leads. The ECG change also resolved within several hours as opposed to a few days or longer with Takotsubo syndrome [14]. Furthermore, Takotsubo cardiomyopathy occurs primarily in postmenopausal women [15]. With regards to other etiologies for stress-related cardiomyopathy, he did not have a cerebral subarachnoid hemorrhage [16]. While there was no evaluation done for pheochromocytoma, there was nothing in history or subsequent hospital course to suggest this [16].
Another alternative etiology would be variant (Prinzmetal) angina/coronary artery spasm. While there are case reports in the literature of this phenomenon in adolescents, our patient did not have any chest pain, nor was he diaphoretic 17, 18, 19. However, this possibility underscores the importance of performing an ECG in pediatric patients with chest pain, dyspnea, and diaphoresis because of the rare instance that they may have variant angina [18]. Pericarditis is also unlikely because of the localized nature of the ECG changes and their short duration, i.e. resolution with gastric decompression, as well as the absence of a pericardial friction rub.
Perhaps he sustained blunt cardiac injury from the accident, which resulted in myocardial susceptibility to acute gastric distention. However, his admitting ECG was normal and other than the above event, he only had sinus tachycardia on telemetry throughout his hospitalization. As per practice management guidelines of the Eastern Association for the Surgery of Trauma in 1998, with a normal admission ECG, the risk of a blunt cardiac injury that requires treatment is considered insignificant and no further work-up is recommended [20]. These were recently revised to indicate that a normal troponin value along with a normal ECG rules out blunt cardiac injury [21]. Hence, blunt cardiac injury seems an unlikely etiology in our patient.
In considering these differential diagnoses, Brugada syndrome, stress-related cardiomyopathy, variant angina, pericarditis, and blunt cardiac injury appear unlikely. As such, we hypothesize that the infero-lateral ECG changes were largely mediated via a direct effect of gastric distention on the heart. This may represent a type of irritative phenomenon and/or a direct pressure-type effect on the heart. The absence of a significant change in QRS axis in our patient would argue against major cardiac displacement. Furthermore, there was also no significant change in P axis. In support of this hypothesis is one English-language case report of a middle-aged female with T wave changes secondary to gastric distention [2]. She presented with acute sharp retrosternal chest pain while working at her desk. She had no significant history. ECG revealed T wave inversion and slight ST segment elevations in leads I, aVL, and V5–V6. There was no shift in the QRS axis. Chest X-ray indicated marked gastric distention. Her symptoms resolved immediately with NGT insertion. A subsequent ECG demonstrated resolution of the anterior T wave inversion although T wave flattening remained. A treadmill exercise test and stress thallium perfusion scintigraphy test were also normal. The mechanism was unclear.
Conclusion
To our knowledge, via a Pubmed search, this is the first case in the English-language literature of gastric distention leading to acute ST segment elevation in a trauma patient. This case illustrates the need to consider acute gastric distention in the differential of acute ST segment elevation that is concerning for a myocardial infarction. The mechanism is yet to be elucidated. In difficult situations, an echocardiogram may be of benefit to help differentiate this pathology from ST segment elevation myocardial infarction.
Footnotes
This paper was presented in part at the 42nd annual congress of the Society for Critical Care Medicine in San Juan, Puerto Rico in January 2013.
References
- 1.Duke M. Positional effects of gastric distention upon the mean electrical axis of the QRS complex of the electrocardiogram. Vasc Dis. 1965;2:161–167. [PubMed] [Google Scholar]
- 2.Frais M., Rodgers K. Dramatic electrocardiographic T wave changes associated with gastric dilatation. Chest. 1990;98:489–490. doi: 10.1378/chest.98.2.489. [DOI] [PubMed] [Google Scholar]
- 3.Shah N.S., Koller S.M., Janower M.L., Spodick D.H. Diaphragm levels as determinants of P axis in restrictive vs obstructive pulmonary disease. Chest. 1995;107:697–700. doi: 10.1378/chest.107.3.697. [DOI] [PubMed] [Google Scholar]
- 4.Gul E.E., Can I., Ozbek O. Displacement of the heart by diaphragm: is this heart alternating? J Electrocardiol. 2011;44:465–466. doi: 10.1016/j.jelectrocard.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Adams M.G., Drew B.J. Body position effects on the ECG: implication for ischemia monitoring. J Electrocardiol. 1997;30:285–291. doi: 10.1016/s0022-0736(97)80040-4. [DOI] [PubMed] [Google Scholar]
- 6.Keller D.I., Rougier J.S., Kucera J.P., Benammar N., Fressart V., Guicheney P., Madle A., Fromer M., Schläpfer J., Abriel H. Brugada syndrome and fever: genetic and molecular characterization of patients carrying SCN5A mutations. Cardiovasc Res. 2005;67:510–519. doi: 10.1016/j.cardiores.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda T., Abe A., Yusu S., Nakamura K., Ishiguro H., Mera H., Yotsukura M., Yoshino H. The full stomach test as a novel diagnostic technique for identifying patients at risk of Brugada syndrome. J Cardiovasc Electrophysiol. 2006;17:602–607. doi: 10.1111/j.1540-8167.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- 8.Miyazaki T., Mitamura H., Miyoshi S., Soejima K., Aizawa Y., Ogawa S. Autonomic and antiarrhythmic drug modulation of ST segment elevation in patients with Brugada syndrome. J Am Coll Cardiol. 1996;27:1061–1070. doi: 10.1016/0735-1097(95)00613-3. [DOI] [PubMed] [Google Scholar]
- 9.Calloe K., Cordeiro J.M., Di Diego J.M., Hansen R.S., Grunnet M., Olesen S.P., Antzelevitch C. A transient outward potassium current activator recapitulates the electrocardiographic manifestations of Brugada syndrome. Cardiovasc Res. 2009;81:686–694. doi: 10.1093/cvr/cvn339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D.W., Makita N., Kitabatake A., Balser J.R., George A.L., Jr. Enhanced Na+ channel intermediate inactivation in Brugada syndrome. Circ Res. 2000;87:E37–E43. doi: 10.1161/01.res.87.8.e37. [DOI] [PubMed] [Google Scholar]
- 11.Zumhagen S., Spieker T., Rolinck J., Baba H.A., Breithardt G., Böcker W., Eckardt L., Paul M., Wichter T., Schulze-Bahr E. Absence of pathognomonic or inflammatory patterns in cardiac biopsies from patients with Brugada syndrome. Circ Arrhythm Electrophysiol. 2009;2:16–23. doi: 10.1161/CIRCEP.107.737882. [DOI] [PubMed] [Google Scholar]
- 12.Aksay E., Ersel M., Kiyan S., Musalar E., Gungor H. Acute coronary syndrome mimicked by acute cholecystitis. Emerg Med Australas. 2010;22:343–346. doi: 10.1111/j.1742-6723.2010.01291.x. [DOI] [PubMed] [Google Scholar]
- 13.Krasna M.J., Flancbaum L. Electrocardiographic changes in cardiac patients with acute gallbladder disease. Am Surg. 1986;52:541–543. [PubMed] [Google Scholar]
- 14.Prasad A., Lerman A., Rihal C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408–417. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Nef H.M., Möllmann H., Akashi Y.J., Hamm C.W. Mechanisms of stress (Takotsubo) cardiomyopathy. Nat Rev Cardiol. 2010;7:187–193. doi: 10.1038/nrcardio.2010.16. [DOI] [PubMed] [Google Scholar]
- 16.Richard C. Stress-related cardiomyopathies. Ann Intensive Care. 2011;1:39. doi: 10.1186/2110-5820-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holt D.B., Singh G.K., Rhee E.K., Billadello J., Ludomirsky A. Images in cardiovascular medicine. Prinzmetal angina in an adolescent: adjunctive role of tissue synchronization imaging. Circulation. 2005;112:e91–e92. doi: 10.1161/CIRCULATIONAHA.104.498006. [DOI] [PubMed] [Google Scholar]
- 18.Wang A.C., Chen S.J., Lee P.C., Hwang B.T., Tsai M.C. Variant angina in an adolescent coexisting with intermittent Wolff–Parkinson–White syndrome. Am J Emerg Med. 2008;26:968.e5–968.e7. doi: 10.1016/j.ajem.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Karaaslan S., Baysal T., Başpinar O., Oran B. Adolescent with variant angina. Pediatr Int. 2003;45:478–480. doi: 10.1046/j.1442-200x.2003.01760.x. [DOI] [PubMed] [Google Scholar]
- 20.Eastern Association for the Surgery of Trauma. http://www.east.org/resources/treatment-guidelines/blunt-cardiac-blunt-injury-screening-for [last accessed 15.09.12].
- 21.Clancy K., Velopulos C., Bilaniuk J.W., Collier B., Crowley W., Kurek S., Lui F., Nayduch D., Sangosanya A., Tucker B., Haut E.R., Eastern Association for the Surgery of Trauma Screening for blunt cardiac injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012;73(5, Suppl. 4):S301–S306. doi: 10.1097/TA.0b013e318270193a. [DOI] [PubMed] [Google Scholar]