Abstract
Stem cell differentiation into a variety of lineages is known to involve signaling from the extracellular niche, including from the physical properties of that environment. What regulates stem cell responses to these cues is there ability to activate different mechanotransductive pathways. Here, we will review the structures and pathways that regulate stem cell commitment to a cardiomyocyte lineage, specifically examining proteins within muscle sarcomeres, costameres, and intercalated discs. Proteins within these structures stretch, inducing a change in their phosphorylated state or in their localization to initiate different signals. We will also put these changes in the context of stem cell differentiation into cardiomyocytes, their subsequent formation of the chambered heart, and explore negative signaling that occurs during disease.
1. INTRODUCTION
Mechanics play an essential role in developing or maintaining function at every stage of the heart’s lifespan, from differentiation and maturation to regulation of cardiac structure with advancing age. Cells not only respond to external stresses but are also capable of affecting them through internal and external restructuring. This remarkable ability is due to the abundance of mechanosensitive molecules and mechanisms populating the cardiac tissue, which form a closed feedback loop in which mechanics regulate mechanics.
Mechanotransduction, the process by which cells sense external forces and translate them into biochemical signals that can change cell function, is regulated in the heart by a diverse array of factors operating at different length scales. Externally, arterial blood pressure, valve compliance, passive stiffness and adhesivity of the cellular niche, and ventricular wall stress have all been shown to impact form and function of the heart. Through intracellular mechanosensitive pathways, cardiac cells can sense these changes and remodel themselves and their surroundings in order to achieve and maintain a level of function that meets physiological demand.1 Evidence also suggests that “inside-out” mechanical signaling is crucial for tissue morphogenesis, maintenance of homeostasis, and prolonging function over decades of life.2–4
In this chapter, we will first describe the establishment of cardiac fate from stem cells and subsequent morphogenesis of the heart. Then, we will highlight several major mechanosensitive subcompartments of the heart, noting the way in which they engage in mechanical and biochemical cross talk. Throughout the chapter, we will also discuss how mechanical signaling helps establish cardiac fate, construct the contractile apparatus, shape cardiac morphogenesis, regulate force transmission between myocytes and their niche, and underline multiscale remodeling during aging and altered mechanical loads. We will also argue that establishment and long-term heart maintenance are highly dependent upon the cardiomyocyte’s ability to remodel its intracellular structure in order to adapt to changing mechanical loads and physiological demand. By dissecting the effector and affected pathways of cardiac mechanotransduction, we hope that the reader will appreciate how mechanics regulates cardiac differentiation and how physical parameters help engineer the function of adult cardiac myocytes in addition to developing a better understanding of the pathophysiology of genetic and age-related cardiomyopathies.
2. CARDIAC MORPHOGENESIS DURING THE LIFESPAN OF THE HEART
2.1. Specification, differentiation, and heart morphogenesis
The cells that eventually become the myocardium are derived from the mesoderm within the primitive streak.5,6 Early cardiogenesis is driven by time-dependent biochemical signaling, such as bone morphogenic protein (BMP) and suppression of wingless-related integration site (WNT) signaling.7,8 At this stage, cardiac progenitors begin to migrate and form two populations of cells, one of which will eventually become the early, beating heart tube and the other the outflow tract and portions of the right heart.5,9 It is shortly after formation of the heart tube that contractions begin and underline further growth and remodeling to loop and subdivide into a primitive four-chambered heart. Morphogenesis can continue in embryonic mice hearts ex vivo10 as it is guided by an internal mechanism: the forces created by interactions of myosin and actin.11
2.2. Cell maturation and maintenance
For the remainder of embryogenesis, growth is caused by hyperplasia or proliferation of the early cardiomyocyte population.12,13 Postnatal cardiomyocytes continue remodeling in a process dubbed maturation, which includes both hyperplasia and hypertrophy that are considered adaptive as the growth contributes to improved function.13 Postmaturation myocardial remodeling, either through concentric or through eccentric hypertrophy, is underlined by the addition of sarcomeres, remodeling of cortical ultrastructure, protein expression, and altered cell morphology, and is associated with age-related dysfunction such as impaired fractional shortening.1,14,15 While primarily composed of terminally differentiated adult cardiomyocytes, cardiac stem cells have also been recently identified16–18; despite the presence of these progenitor cells, however, the adult heart is still thought to have limited regenerative potential compared to other tissue systems given that the heart does not repair itself like other muscles. Therefore, adult cardiomyocytes must be extremely responsive to these changing mechanical environments (e.g., elevated arterial pressure, fibrosis) to maintain function over several decades, and mechanosensitive molecules provide a convenient feedback mechanism to maintain cardiac function.
3. MECHANOSENSITIVE COMPARTMENTS IN CARDIOMYOCYTES
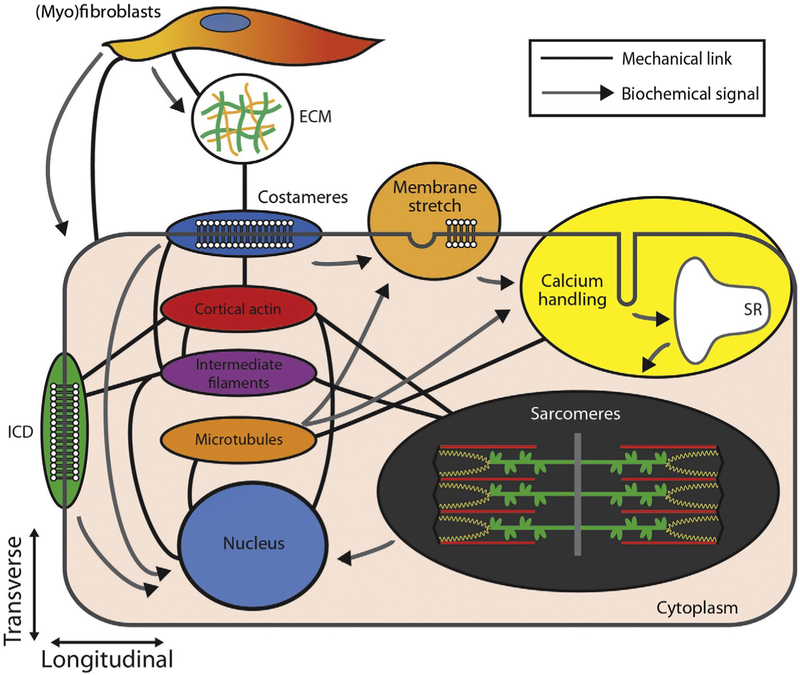
Cardiomyocytes are composed of several subcompartments involved in mechanotransduction, including the contractile sarcomeres, the cytoskeletal filament networks, transmembrane cell–cell and cell–matrix junctions, stretch-sensitive membrane structures, and calcium-handling machinery as shown in Fig. 9.1. Each of these is involved in providing structural integrity as well as generation, sensation, transmission, and/or modulation of forces in the heart. In the following sections, we will summarize the basic structure and highlight known and hypothesized functions of each of these subcompartments.
Figure 9.1.
Mechanosignaling between subcellular compartments. Illustration highlights the mechanical and chemical interplay of indicated intracellular, extracellular, and membrane components. Black lines indicate mechanic and/or structural interaction between elements, whereas arrows indicate the direction of signaling pathways. Note the following abbreviations: intercalated disc, ICD; sarcoplasmic reticulum, SR; extracellular matrix, ECM.
4. THE SARCOMERE
The primary function of the heart is to pump blood throughout the circulatory system, dispersing oxygen, nutrients, and biochemical factors to both distal organs as well as itself.19 This contraction is contingent upon the sarcomere, a dynamic protein complex that serves as the basic mechanical unit of muscle. A sarcomere is composed of actin-based thin filaments and myosin-based thick filaments as illustrated in Fig. 9.1; these filaments slide past one another to create contraction. These structures are cross-linked together by Z-disks. Each of these sarcomeric components is critical in regulating how muscle performs mechanical work, maintains passive tension,20 undergoes biochemical signaling,21 and facilitates mechanotransduction.22 In vertebrates, the expression of these molecules during development is highly coordinated23 and mutations or alternative splicing of genes encoding these proteins are associated with a variety of congenital defects and cardiomyopathies24 that manifest in aberrant molecular, cellular, or organ-level structure.
4.1. Cardiac structure and mechanosignaling
Sarcomeric contraction and subsequent force production begins with an action potential. Depolarization of the cardiac membrane results in calcium influx from the extracellular space via L-type calcium channels located in structural membrane invaginations known as the transverse-tubules.25 Local increases in calcium concentration are detected by proximal ryanodine receptors in the sarcoplasmic reticulum (SR), causing the latter to release additional calcium ions into the cytosolic space. This event is known as a “calcium spark”26,27 and is part of a calcium-induced calcium-release mechanism central to cardiac excitation–contraction coupling. Though each spark is spatiotemporally constrained, it can result in a significant elevation of global cytoplasmic calcium concentration when summed (~>104 sparks). This elevation is transient, as sarco/endoplasmic reticulum ATP-ase pump and the sodium–calcium exchanger will transport calcium back into the SR and interstitium, respectively. This rise and fall of calcium is referred to as the calcium transient.
Myosin thick filaments bind to actin-based thin filaments transiently, and as they bind and unbind, they undergo a power stroke where the myosin head moves forward relative to the actin filament. Myosin’s cyclic binding is known as cross-bridge cycling, and this process creates the net contraction of the sarcomere. Binding can be blocked in the absence of calcium by the troponin–tropomyosin complex28 where troponin C can cover up myosin’s binding site on the thin filament. The probability of acto-myosin binding is therefore associated with increased intracellular calcium concentration.29 Changes in the calcium transient directly affect contraction and relaxation dynamics and short- and long-term power output of the heart, making it a potent regulator of transient and long-term cardiac mechanotransduction.30
In addition to perturbations in calcium handling, sarcomeric function is also modulated by changes in absolute gene expression,31 alternative splicing,24,32 impaired protein homeostasis,33,34 decreased protein quality,35 and altered ultrastructure of its subcomponents. Biochemical and mechanical cell stress can affect the quantity, quality, and integrity of the sarcomeres. In kind, molecules in the sarcomere are also capable of acting as stress sensors that are capable of nuclear signaling, allowing the myocyte to respond.36,37
The Z-disc was once thought to be a static architectural support for the myofilaments, responsible for anchoring and transversely cross-linking adjacent thin filaments. However in recent years, an intricate complex of force sensing and signaling molecules have been identified within Z-discs, and many of these proteins have critical roles in development and disease.30 To briefly outline the contents of the Z-disc, it is primarily composed of α-actinin, a spectrin-family protein required for actin-filament anchoring to the Z-disc.38,39 Nonsarcomeric clustering of α-actinin is a marker of sarcomere degeneration and is associated age-related dilated cardiomyopathy.40
In addition to α-actinin, Z-discs also contain a significant amount of titin, which helps maintain resting passive tension and longitudinal stiffness in the cell. Titin also acts as a molecular spring which spans the length of the Z-disk to the M-line.41–43 Embryonic titin is considerably shorter than adult titin, suggesting that transverse elasticity interplays with the changes in cardiac morphology and mechanics during development.44 On the other hand, maladaptive cross-linking and differential splicing of titin have been shown to modulate resting sarcomere length and perturb sarcomere force production.45 In addition to its mechanical properties, titin has several binding sites for ankyrin-repeat proteins (ARPs).46 ARP signaling proteins are capable of translocating to the nucleus, where they presumably alter the transcriptome of the cell.46 In addition to binding α-actinin at its N-terminus, titin forms a complex with muscle LIM protein (MLP), which has been hypothesized to act as a stress and/or length tensor.36,47 MLP-null myocytes experience decreased longitudinal passive stiffness and muted response to stretch in the form of BMP expression48,49 as well as decreased power output.50 MLP mutations are also associated with diastolic dysfunction followed by dilated cardiomyopathy, suggesting time-dependent remodeling.51 Other LIM-domain-containing proteins, including myopodin52 and zyxin,53 localize at the Z-disc and further suggest a critical sensory role for these types of proteins. During myoblast differentiation, myopodin is localized to the nucleus but translocates to the nascent Z-disk as sarcomerogenesis progresses.54 Acute, short-term heat shock of differentiated myotubes results in reverse translocation of myopodin from mature Z-discs to the nucleus, suggesting that myopodin plays a role in stress-response signaling.52,54 Nebulin, a hypothesized sarcomeric “ruler,”55–57 is associated with nebulin-related anchoring protein (N-RAP), another well-known LIM protein and member of the Z-disk-associated mechanosensitive network.58,59 Myomesin is another sarcomeric mechanosensor that is thought to bind titin near its C-terminal and act as a molecular spring.60,61 In addition to its contributions to mature sarcomere stability, it is associated with myosin during the earliest stages of sarcomerogenesis, suggesting that it is crucial for performing mechanical work given the few proteins present in these early contractile structures.62
4.2. Sarcomere mutations, microenvironmental changes, and their impact
Mutations in vital myofilament proteins are associated with congenital defects and adult-onset cardiomyopathies and can arise due to increased or decreased sensitivity to calcium.36,63–73 These changes reflect a potential role of the myofilaments in cardiogenesis and maintenance of structure. A missense mutation in α-MHC can result in maladaptive hypertrophy, myofibrillar disarray, and fibrosis due to impaired calcium homeostasis in the SR.74,75 This process can be ameliorated by inhibition of L-type calcium channel activity upstream76 and potentially other negative-inotropic agents, suggesting that the mutation results in a maladaptive gain of function. Certain mutations in cardiac troponins are associated with restrictive cardiomyopathy,68,69,77 a condition in which diastolic and systolic dimensions dramatically decline. Recent studies show that such mutations in critical regions can expose the myosin-binding site on thin filaments without the need for calcium influx, resulting in cross-bridge formation independent of calcium release, elevated diastolic tension, cortical stiffening, and impaired fractional shortening.68,71,78 A mutation in troponin C resulting in calcium-independent sarcomeric perturbation has been shown to be rescued by an engineered molecule.79
Sarcomeres are also known to respond to alterations in the mechanical forces being presented to the heart, i.e., “outside-in” mechanotransduction. For example, application of stretch on cardiomyocytes in vitro results in increased sarcomerogenesis and hypertrophic signaling.80–82 The addition of new sarcomeres appears to occur at the intercalated disc, suggesting synergy between the two compartments in this phenomena.83 Developing embryonic chick cardiomyocytes has increased sarcomerogenesis from when plated on time-dependent, stiffening hydrogels as compared to static elastic substrates.84,85 The rate of MHC and thin filament actin turnover is stretch sensitive.86,87 Thus, sarcomerogenesis and maintenance of sarcomeric protein quality may depend upon the ability of the sarcomeres to act as a stress/strain sensor; perturbations of this structure could impair the resultant signaling.
5. OTHER INTRACELLULAR MECHANOSENSITIVE STRUCTURES
In order for sarcomeric shortening to translate into cellular contraction, and for the contractile apparatus to sense changes in external loads, they require mechanical coupling to the membrane. This is achieved via the sarcolemma, which consists of highly ordered junctions and a cortical cyto-skeletal network. Cardiomyocytes are coupled longitudinally by an electromechanically active junctional complex known as the intercalated disc (ICD) and transversely at the Z-disc by special focal adhesion plaques known as costameres.12 Sensation and application of external loads by the sarcomeres are enabled by these transmembrane contacts and their associated cytoskeletal networks, making them the primary responders to forces leading to and from the contractile apparatus. Here, we will discuss these structures, their influence on downstream signaling, and its impact on contractile function.
5.1. Actin-associated intercalated disc and costameric proteins
Recent work has identified the intercalated disc and costameres, shown in Fig. 9.1 at the cell membrane facilitating cell–cell and cell–extracellular matrix (ECM) connections, as being remarkably mechanosensitive, i.e., capable of responding to and producing forces and enabling biochemical signaling through a variety of sensing molecules.88–93 Most of these studies have focused on in vitro systems89 or noncardiac cells,88,92 which have the same components but are organized differently. For example, Le Duc and coworkers applied cyclic stress to E-cadherin-coated magnetic beads bonded to endothelial cells and measured changes in bead deflection as the cell cytoskeleton responded.93 They found that cadherin-mediated binding is mechanosensitive and results in local stiffening, potentially driven by reinforcement actin of the cortical cytoskeleton. The adhesions eventually asymptote to a new stiffness, the rate and magnitude of which depends on the presence of the actin-binding, mechanosensitive molecule vinculin.90,93 In this way, the actin cytoskeleton downstream of cadherin–cadherin bonds reacts similarly to those proximal to integrin–ECM bonds.91,94 It is reasonable to assume that cadherin contacts perform similarly in cardiomyocytes as all the critical machinery exists and cell–cell contacts in cultured myocytes are known to remodel during cyclic and static stretch.95 However, the mechanosensing machinery is not always present or highly organized. In early development, cadherins are expressed ubiquitously throughout the membrane.12 It is only as sarcomerogenesis advances and cells elongate and hypertrophy that cadherins become polarized and constrained to the longitudinal ends. Once it is fully formed, the intercalated disc does not remain static; advancing age and dilation associates with convolution of the ICD ultrastructure and enrichment with mechanotransductive molecules.96,97 Remodeling of actin-binding mechanosensors is hypothesized to result in cortical or transverse stiffening during aging,38 though the ultimate impact of this event on cardiac function remains unclear.
Beyond normal changes with development and age, studies suggest that cortical remodeling from disease or mutation may play a functional role in modulating sarcomeric function. For example, Tangney and coworkers showed that vinculin-null neonatal mouse cardiomyocytes experience decreased cortical stiffness and increased interfilament spacing, the distance between thin and thick filaments in the myofilament lattice.98 Interfilament spacing impacts the calcium sensitivity and power output of the sarcomeres,99–101 perhaps due to a lack of cortical transverse compression upon the myofilaments. Cortical compression through osmotic loading in vitro has been shown to affect contractile function in rat myocardium.101,102
Lastly, it is important to consider mechanotransduction at focal adhesions, which play a leading role in contractility103,104 and mechanical induction of myogenic differentiation.88 In cardiomyocytes specifically, integrin expression and signaling, as well as focal adhesion and integrin-linked kinases, have been the focus of understanding age-related hypertrophic signaling.105–111 Integrin expression is regulated by both passive and active mechanical forces as well as the ligands and ligand density presented to the cell.112 Integrin clustering and isoform expression depend upon the ECM proteins and their arrangement.113–115 Another crucial component in lateral coupling to the membrane is the actin-binding dystrophin molecule, which is thought to stabilize the membrane and provide additional mechanical coupling to the cortical actin cytoskeleton.116–118 Dystrophin knockout or expression of a mutated isoform results in impaired sarcomeric force transmission to the membrane. Furthermore, without dystrophin as a structural support, conformational changes in stretch-activated channels are thought to occur, resulting in pathological intracellular leakage of calcium and eventual cell death.119–121
5.2. Intermediate filament and microtubule networks
While actin plays a dominant role in regulating cardiomyocyte development and function, both the intermediate filament (IF) and microtubule networks play important supporting roles. For example, IFs are extremely deformable, capable of being stretched to several times their slack length.122–124 One of these IFs, desmin, provides scaffolding around the Z-disk and links them laterally, binds to desmosomes at the intercalated disc, and bridges the nucleus to adjacent sarcomeres.125 Desmin is also believed to localize to costameres, perhaps conferring additional coupling between sarcomeres and the ECM.118 It is an early marker of cardiogenesis.126,127 Desminopathies result from mutations in the desmin gene and subsequent cytoplasmic aggregation of desmin and impaired myofibrillar assembly.128–130 Patients with arrhythmogenic right ventricular cardiomyopathy have been shown to have desmin mutations, suggesting that it may play a crucial role in EC coupling via longitudinal load bearing along the sarcomeres or by stabilizing gap junctions at the intercalated disc.131–133
Another IF protein prevalent in cardiomyocytes is lamin, which, as with desmin, is also extremely compliant. For example, during lineage specification of stem cells, lamin expression in stem cells scales with substrate stiffness134 and is a determinant of nuclear cytoskeleton deformability.135,136 Moreover, it may act as a universal transducer of mechanical signals into the nucleus to regulate gene expression.137 Thus, defects in lamin A/C can adversely affect signal transduction, making them less efficient. Defects in Lamin are associated with advanced aging,138 presumably due to genetic instability and accumulated DNA damage from external stressors,139 and dilated cardiomyopathy.15,140,141 The existence of mechanical couplings between the nucleus and the cytoskeletal network implies that some form of communication is required during development.142
Note that there exist structural overlaps in the actin and IF networks; ICD-localizing vinculin is also thought to stabilize gap junctions,134 and per turbations of actin-binding nesprins, found at the nucleus, are also known to mute biomechanical signaling and induce cardiovascular dysfunction.143,144 These findings suggest functional overlap in both cytoskeletal networks.
On the other hand, microtubules are crucial in guiding cytokinesis145 and directing vesicular transport. The microtubule network also serves several load bearing and signaling functions which impact cardiac function.146–148 Much focus has been placed on how signal transduction is altered following application of colchicine, a pharmacological agent that induces deconstruction of microtubule filaments, as well as how microtubule dysregulation occurs during cardiac hypertrophy.149–152 While it is unclear whether mechanical perturbation, impaired vesicular transport, or both are responsible for alterations in function, what is known is that micro-tubules contribute to passive stiffness20 and are remodeled during age38; both of these observations point to microtubules as being important regulators of cardiomyocyte function.
5.3. The cardiomyocyte membrane
In addition to intracellular and transmembrane mechanosensitive compartments, the cardiac myocyte membrane is also enriched with stretch-sensitive structures.153–157 Stretch-activated channels have been implicated in modulation of calcium handling and rhythmicity, although the strains required to observed stretch activation are often superphysiological.158 The membrane is partially buffered from stretch via caveolins, cytoskeletally regulated invaginations which can add additional material to the membrane through rapid disassembly under osmotic loads159 or stretch.160–163 Caveolin is known to alter its expression with age.164,165 Deletion of Cav3166,167 is associated with progressive dilated cardiomyopathy, while Cav1 appears to play a greater role in endothelial function.168,169
6. ECM AND MECHANOSENSING
The cardiac interstitium provides cardiomyocytes with avenues of mechanical and biochemical communication with their environment.22,115,170–176 In particular, the insoluble ECM is secreted by cardiac fibroblasts,170,171 which are the most abundant cells in the heart by number.177 This ECM plays a crucial role in providing architectural support for cardiomyocytes and allowing for efficient transmission of forces during contraction. ECM organization is known to direct integrin assembly and signaling and vice versa178 which can influence cell morphology and sarcomere alignment.1,179,180 This interstitium, like the cardiomyocytes, remodels during development12 and disease.170,171,181 Deposition of fibrillar ECM proteins, such as collagens, laminins, and fibronectin,182,183 is vital for the initiation of cardiogenesis and wound healing but is also associated with mal-adaptive hypertrophic growth. In addition to ECM deposition and paracrine signaling, cardiac fibroblasts are known to directly communicate with cardiomyocytes via connexins184,185 and N-cadherin,186,187 allowing for electromechanical coupling and modulation of action potential propagation.187 Cardiac fibroblasts also respond to external forces by altering their internal cytoskeletal expression,188 extracellular biochemical signaling,187,189–191 and increasing matrix production, which can lead to the induction of a smooth muscle phenotype.192 These “myofibroblasts” (Fig. 9.1, top) experience calcium-dependent contractility,193 elevated ECM deposition,171,194 and influence cardiac conduction.186,195 Increased substrate stiffness can also increase differentiation of fibroblasts into myofibroblasts,196–198 suggesting a positive feedback loop following ECM deposition.
7. THE INFLUENCE OF MECHANOTRANSDUCTION ON APPLICATIONS OF CARDIAC REGENERATION
In this chapter, we have broadly discussed how maintenance of cardiac structure and function is underlined by spatiotemporal patterning of biochemical and mechanical signaling. We have seen cases in which mutation, alternative splicing, maladaptive posttranslational modifications, and altered cytoskeletal and ECM assembly can dramatically alter morphology and function from the cell to the organ. One final consideration to note is how these changes, induced by development or disease, guide or can be used to guide regeneration.
The numerous heart shapes and sizes in the animal kingdom suggest that biology knows of many ways to build a heart. However, the sensitivity of the developing and aging human heart to genetic perturbations and external stresses further suggests that there is a narrow window to form a human tissue that is competently functional. Minor alterations in calcium handling or cytoskeletal ultrastructure and organization appear to have broad impacts on both basal state and response to mechanical and biochemical or pharmacological stress. For example, the use of time-dependent soluble cues appears to be sufficient for initial differentiation of cardiac lineage but insufficient for maturation.7,199,200 The latter can be assisted by the application of external loads which in turn promote intracellular remodeling.14,201–203 These mechanical cues may need to be dynamic; the limitations of static mechanical cues have become apparent in recent years. Attempts to revive cardiac function postinfarct via stem cell injection into the stiffened, infarcted niche, for example, result in differentiation into an osteogenic lineage instead of cardiac.204 Bulk hydrogels do little to provide mechanical support and may introduce arrhythmogenic defects by disrupting electrical coupling.205 Adult-like phenotype can be induced in developing cells through micro-patterning of ECM in 2D.206,207 In this way, a symmetry-breaking event can be used to guide integrin clustering and sarcomerogenesis downstream. However, this kind of ECM-mediated “boundary condition” guidance in cardiomyocytes is also dynamic in vivo, suggesting that the same will be required in 3D cultures of mature cardiac tissue, which cannot be created through current micropatterning technologies. All of these concerns are ameliorated if it is shown that, for a given concern, the response and function of engineered tissues are similar to those observed in vivo. Such criteria have held for the use of simpler animal models with otherwise limited homology to human structure and function.78,208–211
8. CONCLUSION
What is most evident from a review of current literature is that our understanding of precisely how the cardiomyocyte closes its mechanical feedback loop remains unknown; what are the basic signals that induce hypertrophy and self-assembly into a mature organ and what pathways signal the heart to cease or undergo aberrant growth? In the coming years, additional mechanosensitive molecules in cardiomyocytes will likely highlight cross talk between subcompartments, such as cell–cell and cell–matrix cooperation or myocyte–fibroblast communication. What is likely more important, however, is improving our understanding of the precise timing of mechanotransduction and its downstream pathways during the lifespan of the heart. Improved understanding of cardiac differentiation from stem cells and the mechanotransductive signaling that enables this may reveal indirect therapeutic targets and/or enable better direct engineering of cells and tissue for repair and regeneration.
REFERENCES
- 1.McCain ML, Parker KK. Mechanotransduction: the role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function. Pflugers Archiv. 2011; 462:89–104. [DOI] [PubMed] [Google Scholar]
- 2.Mammoto T, Mammoto A, Ingber DE. Mechanobiology and developmental control. Annu Rev Cell Dev Biol. 2013;29:27–61. [DOI] [PubMed] [Google Scholar]
- 3.Heisenberg CP, Bellaiche Y. Forces in tissue morphogenesis and patterning. Cell. 2013;153:948–962. [DOI] [PubMed] [Google Scholar]
- 4.Patwari P, Lee RT. Mechanical control of tissue morphogenesis. Circ Res. 2008; 103:234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez-Sanchez C, Garcia-Martinez V. Molecular determinants of cardiac specification. Cardiovasc Res. 2011;91:185–195. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Sanchez C, Garcia-Masa N, Ganan CM, Garcia-Martinez V. Movement and commitment of primitive streak precardiac cells during cardiogenesis. Int J Dev Biol. 2009;53:1445–1455. [DOI] [PubMed] [Google Scholar]
- 7.Yuasa S, Itabashi Y, Koshimizu U, et al. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotechnol. 2005;23:607–611. [DOI] [PubMed] [Google Scholar]
- 8.Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzahor E, Lassar AB. Wnt signals from the neural tube block ectopic cardiogenesis. Genes Dev. 2001;15:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dyer LA, Patterson C. A novel ex vivo culture method for the embryonic mouse heart. J Vis Exp. 2013;e50359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granados-Riveron JT, Brook JD. The impact of mechanical forces in heart morpho-genesis. Circ Cardiovasc Genet. 2012;5:132–142. [DOI] [PubMed] [Google Scholar]
- 12.Hirschy A, Schatzmann F, Ehler E, Perriard JC. Establishment of cardiac cytoarchitecture in the developing mouse heart. Dev Biol. 2006;289:430–441. [DOI] [PubMed] [Google Scholar]
- 13.Mollova M, Bersell K, Walsh S, et al. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci USA. 2013;110:1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCain ML, Sheehy SP, Grosberg A, Goss JA, Parker KK. Recapitulating maladaptive, multiscale remodeling of failing myocardium on a chip. Proc Natl Acad Sci USA. 2013;110:9770–9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaper J, Kostin S, Hein S, Elsasser A, Arnon E, Zimmermann R. Structural remodelling in heart failure. Exp Clin Cardiol. 2002;7:64–68. [PMC free article] [PubMed] [Google Scholar]
- 16.Torella D, Ellison GM, Nadal-Ginard B. Adult c-kit(pos) cardiac stem cells fulfill Koch’s postulates as causal agents for cardiac regeneration. Circ Res. 2014;114:e24–e26. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira-Martins J, Ogorek B, Cappetta D, et al. Cardiomyogenesis in the developing heart is regulated by c-kit-positive cardiac stem cells. Circ Res. 2012;110:701–715. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Hosoda T C-kit-positive cardiac stem cells and myocardial regeneration. Am J Cardiovasc Dis. 2012;2:58–67. [PMC free article] [PubMed] [Google Scholar]
- 19.Sonnenblick EH, Spotnitz HM, Spiro D. Role of the sarcomere in ventricular function and the mechanism of heart failure. Circ Res. 1964;15(suppl 2):70–81. [DOI] [PubMed] [Google Scholar]
- 20.Granzier HL, Irving TC. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J. 1995;68:1027–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicol RL, Frey N, Olson EN. From the sarcomere to the nucleus: role of genetics and signaling in structural heart disease. Annu Rev Genomics Hum Genet. 2000;1:179–223. [DOI] [PubMed] [Google Scholar]
- 22.Kresh JY, Chopra A. Intercellular and extracellular mechanotransduction in cardiac myocytes. Pflugers Archiv. 2011;462:75–87. [DOI] [PubMed] [Google Scholar]
- 23.Ehler E, Gautel M. The sarcomere and sarcomerogenesis. Adv Exp Med Biol. 2008; 642:1–14. [DOI] [PubMed] [Google Scholar]
- 24.Swank DM, Wells L, Kronert WA, Morrill GE, Bernstein SI. Determining structure/function relationships for sarcomeric myosin heavy chain by genetic and transgenic manipulation of Drosophila. Microsc Res Tech. 2000;50:430–442. [DOI] [PubMed] [Google Scholar]
- 25.Turczynska KM, Hellstrand P, Sward K, Albinsson S. Regulation of vascular smooth muscle mechanotransduction by microRNAs and L-type calcium channels. Commun Integr Biol. 2013;6:e22278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rovetti R, Cui X, Garfinkel A, Weiss JN, Qu Z. Spark-induced sparks as a mechanism of intracellular calcium alternans in cardiac myocytes. Circ Res. 2010;106: 1582–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stern MD, Rios E, Maltsev VA. Life and death of a cardiac calcium spark. J Gen Physiol. 2013;142:257–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chalovich JM, Chock PB, Eisenberg E. Mechanism of action of troponin·tropomyosin. Inhibition of actomyosin ATPase activity without inhibition of myosin binding to actin. J Biol Chem. 1981;256:575–578. [PMC free article] [PubMed] [Google Scholar]
- 29.Bogeholz N, Muszynski A, Pott C. The physiology of cardiac calcium handling. Wien Med Wochenschr. 2012;162:278–282. [DOI] [PubMed] [Google Scholar]
- 30.Judice CC, Marin TM, Franchini KG. Calcium and the mechanotransduction in cardiac myocytes. Front Biosci. 2009;1:189–199. [DOI] [PubMed] [Google Scholar]
- 31.O’Neill L, Holbrook NJ, Fargnoli J, Lakatta EG. Progressive changes from young adult age to senescence in mRNA for rat cardiac myosin heavy chain genes. Cardioscience. 1991;2:1–5. [PubMed] [Google Scholar]
- 32.Wiesner RJ, Ehmke H, Faulhaber J, Zak R, Ruegg JC. Dissociation of left ventricular hypertrophy, beta-myosin heavy chain gene expression, and myosin isoform switch in rats after ascending aortic stenosis. Circulation. 1997;95:1253–1259. [DOI] [PubMed] [Google Scholar]
- 33.Kiriazis H, Kranias EG. Genetically engineered models with alterations in cardiac membrane calcium-handling proteins. Annu Rev Physiol. 2000;62:321–351. [DOI] [PubMed] [Google Scholar]
- 34.Douglas PM, Dillin A. Protein homeostasis and aging in neurodegeneration. J Cell Biol. 2010;190:719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schips TG, Wietelmann A, Hohn K, et al. FoxO3 induces reversible cardiac atrophy and autophagy in a transgenic mouse model. Cardiovasc Res. 2011;91:587–597. [DOI] [PubMed] [Google Scholar]
- 36.Bos JM, Poley RN, Ny M, et al. Genotype-phenotype relationships involving hyper-trophic cardiomyopathy-associated mutations in titin, muscle LIM protein, and telethonin. Mol Genet Metab. 2006;88:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Guennec JY, Cazorla O, Lacampagne A, Vassort G. Is titin the length sensor in cardiac muscle? Physiological and physiopathological perspectives. Adv Exp Med Biol. 2000;481:337–348, discussion 348–351. [DOI] [PubMed] [Google Scholar]
- 38.Hein S, Kostin S, Heling A, Maeno Y, Schaper J. The role of the cytoskeleton in heart failure. Cardiovasc Res. 2000;45:273–278. [DOI] [PubMed] [Google Scholar]
- 39.Sorimachi H, Freiburg A, Kolmerer B, et al. Tissue-specific expression and alpha-actinin binding properties of the Z-disc titin: implications for the nature of vertebrate Z-discs. J Mol Biol. 1997;270:688–695. [DOI] [PubMed] [Google Scholar]
- 40.Mohapatra B, Jimenez S, Lin JH, et al. Mutations in the muscle LIM protein and alpha-actinin-2 genes in dilated cardiomyopathy and endocardial fibroelastosis. Mol Genet Metab. 2003;80:207–215. [DOI] [PubMed] [Google Scholar]
- 41.LeWinter MM, Wu Y, Labeit S, Granzier H. Cardiac titin: structure, functions and role in disease. Clin Chim Acta. 2007;375:1–9. [DOI] [PubMed] [Google Scholar]
- 42.Granzier H, Labeit S. Cardiac titin: an adjustable multi-functional spring. J Physiol. 2002;541:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Granzier H, Labeit D, Wu Y, Labeit S. Titin as a modular spring: emerging mechanisms for elasticity control by titin in cardiac physiology and pathophysiology. J Muscle Res Cell Motil. 2002;23:457–471. [DOI] [PubMed] [Google Scholar]
- 44.Kruger M, Babicz K, von Frieling-Salewsky M, Linke WA. Insulin signaling regulates cardiac titin properties in heart development and diabetic cardiomyopathy. J Mol Cell Cardiol. 2010;48:910–916. [DOI] [PubMed] [Google Scholar]
- 45.Lewinter MM, Granzier HL. Cardiac titin and heart disease. J Cardiovasc Pharmacol. 2014;63:207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller MK, Bang ML, Witt CC, et al. The muscle ankyrin repeat proteins: CARP, ankrd2/Arpp and DARP as a family of titin filament-based stress response molecules. J Mol Biol. 2003;333:951–964. [DOI] [PubMed] [Google Scholar]
- 47.Clark KA, Bland JM, Beckerle MC. The Drosophila muscle LIM protein, Mlp84B, cooperates with D-titin to maintain muscle structural integrity. J Cell Sci. 2007;120:2066–2077. [DOI] [PubMed] [Google Scholar]
- 48.Buyandelger B, Ng KE, Miocic S, et al. MLP (muscle LIM protein) as a stress sensor in the heart. Pflugers Archiv. 2011;462:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gunkel S, Heineke J, Hilfiker-Kleiner D, Knoll R. MLP: a stress sensor goes nuclear. J Mol Cell Cardiol. 2009;47:423–425. [DOI] [PubMed] [Google Scholar]
- 50.Clark KA, Lesage-Horton H, Zhao C, Beckerle MC, Swank DM. Deletion of Drosophila muscle LIM protein decreases flight muscle stiffness and power generation. Am J Physiol Cell Physiol. 2011;301:C373–C382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lorenzen-Schmidt I, Stuyvers BD, ter Keurs HE, et al. Young MLP deficient mice show diastolic dysfunction before the onset of dilated cardiomyopathy. J Mol Cell Cardiol. 2005;39:241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Faul C, Dhume A, Schecter AD, Mundel P. Protein kinase A, Ca2+/calmodulin-dependent kinase II, and calcineurin regulate the intracellular trafficking of myopodin between the Z-disc and the nucleus of cardiac myocytes. Mol Cell Biol. 2007;27:8215–8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Linnemann A, van der Ven PF, Vakeel P, et al. The sarcomeric Z-disc component myopodin is a multiadapter protein that interacts with filamin and alpha-actinin. Eur J Cell Biol. 2010;89:681–692. [DOI] [PubMed] [Google Scholar]
- 54.Weins A, Schwarz K, Faul C, Barisoni L, Linke WA, Mundel P. Differentiation- and stress-dependent nuclear cytoplasmic redistribution of myopodin, a novel actin-bundling protein. J Cell Biol. 2001;155:393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kontrogianni-Konstantopoulos A, Ackermann MA, Bowman AL, Yap SV, Bloch RJ. Muscle giants: molecular scaffolds in sarcomerogenesis. Physiol Rev. 2009;89: 1217–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Littlefield RS, Fowler VM. Thin filament length regulation in striated muscle sarcomeres: pointed-end dynamics go beyond a nebulin ruler. Semin Cell Dev Biol. 2008;19:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fowler VM, McKeown CR, Fischer RS. Nebulin: does it measure up as a ruler? Curr Biol. 2006;16:R18–R20. [DOI] [PubMed] [Google Scholar]
- 58.Ehler E, Horowits R, Zuppinger C, et al. Alterations at the intercalated disk associated with the absence of muscle LIM protein. J Cell Biol. 2001;153:763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Panaviene Z, Moncman CL. Linker region of nebulin family members plays an important role in targeting these molecules to cellular structures. Cell Tissue Res. 2007;327:353–369. [DOI] [PubMed] [Google Scholar]
- 60.Agarkova I, Perriard JC. The M-band: an elastic web that crosslinks thick filaments in the center of the sarcomere. Trends Cell Biol. 2005;15:477–485. [DOI] [PubMed] [Google Scholar]
- 61.Schoenauer R, Bertoncini P, Machaidze G, et al. Myomesin is a molecular spring with adaptable elasticity. J Mol Biol. 2005;349:367–379. [DOI] [PubMed] [Google Scholar]
- 62.Lange S, Agarkova I, Perriard JC, Ehler E. The sarcomeric M-band during development and in disease. J Muscle Res Cell Motil. 2005;26:375–379. [DOI] [PubMed] [Google Scholar]
- 63.Geske JB, Bos JM, Gersh BJ, Ommen SR, Eidem BW, Ackerman MJ. Deformation patterns in genotyped patients with hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2014;15:456–465. [DOI] [PubMed] [Google Scholar]
- 64.Witjas-Paalberends ER, Piroddi N, Stam K, et al. Mutations in MYH7 reduce the force generating capacity of sarcomeres in human familial hypertrophic cardiomyopathy. Cardiovasc Res. 2013;99:432–441. [DOI] [PubMed] [Google Scholar]
- 65.Cheng Y, Wan X, McElfresh TA, et al. Impaired contractile function due to decreased cardiac myosin binding protein C content in the sarcomere. Am J Physiol Heart and Circ Physiol. 2013;305:H52–H65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tian T, Liu Y, Zhou X, Song L. Progress in the molecular genetics of hypertrophic cardiomyopathy: a mini-review. Gerontology. 2013;59:199–205. [DOI] [PubMed] [Google Scholar]
- 67.Landstrom AP, Ackerman MJ. Beyond the cardiac myofilament: hypertrophic cardiomyopathy-associated mutations in genes that encode calcium-handling proteins. Curr Mol Med. 2012;12:507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davis J, Yasuda S, Palpant NJ, et al. Diastolic dysfunction and thin filament dys-regulation resulting from excitation-contraction uncoupling in a mouse model of restrictive cardiomyopathy. J Mol Cell Cardiol. 2012;53:446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parvatiyar MS, Pinto JR, Dweck D, Potter JD. Cardiac troponin mutations and restrictive cardiomyopathy. J Biomed Biotechnol. 2010;2010:350706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song W, Dyer E, Stuckey D, et al. Investigation of a transgenic mouse model of familial dilated cardiomyopathy. J Mol Cell Cardiol. 2010;49:380–389. [DOI] [PubMed] [Google Scholar]
- 71.Chang AN, Parvatiyar MS, Potter JD. Troponin and cardiomyopathy. Biochem Biophys Res Commun. 2008;369:74–81. [DOI] [PubMed] [Google Scholar]
- 72.Bos JM, Ommen SR, Ackerman MJ. Genetics of hypertrophic cardiomyopathy: one, two, or more diseases? Curr Opin Cardiol. 2007;22:193–199. [DOI] [PubMed] [Google Scholar]
- 73.Palmiter KA, Solaro RJ. Molecular mechanisms regulating the myofilament response to Ca2+: implications of mutations causal for familial hypertrophic cardiomyopathy. Basic Res Cardiol. 1997;92(Suppl 1):63–74. [DOI] [PubMed] [Google Scholar]
- 74.Kim SJ, Iizuka K, Kelly RA, et al. An alpha-cardiac myosin heavy chain gene mutation impairs contraction and relaxation function of cardiac myocytes. Am J Physiol. 1999;276:H1780–H1787. [DOI] [PubMed] [Google Scholar]
- 75.Blanchard E, Seidman C, Seidman JG, LeWinter M, Maughan D. Altered crossbridge kinetics in the alphaMHC403/+ mouse model of familial hypertrophic cardiomyopathy. Circ Res. 1999;84:475–483. [DOI] [PubMed] [Google Scholar]
- 76.Semsarian C, Ahmad I, Giewat M, et al. The L-type calcium channel inhibitor diltiazem prevents cardiomyopathy in a mouse model. J Clin Invest. 2002;109:1013–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Akhter S, Bueltmann K Jr, Huang X, Jin JP. Restrictive cardiomyopathy mutations demonstrate functions of the C-terminal end-segment of troponin I. Arch Biochem Biophys. 2013;552–553: 3–10. [DOI] [PubMed] [Google Scholar]
- 78.Viswanathan MC, Kaushik G, Engler AJ, Lehman W, Cammarato A. A Drosophila melanogaster model of diastolic dysfunction and cardiomyopathy based on impaired troponin-T function. Circ Res. 2014;114:e6–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu B, Lee RS, Biesiadecki BJ, Tikunova SB, Davis JP. Engineered troponin C constructs correct disease-related cardiac myofilament calcium sensitivity. J Biol Chem. 2012;287:20027–20036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frank D, Kuhn C, Brors B, et al. Gene expression pattern in biomechanically stretched cardiomyocytes: evidence for a stretch-specific gene program. Hypertension. 2008;51:309–318. [DOI] [PubMed] [Google Scholar]
- 81.De Deyne PG. Formation of sarcomeres in developing myotubes: role of mechanical stretch and contractile activation. Am J Physiol Cell Physiol. 2000;279:C1801–C1811. [DOI] [PubMed] [Google Scholar]
- 82.Dorn GW 2nd, Robbins J, Sugden PH. Phenotyping hypertrophy: eschew obfuscation. Circ Res. 2003;92:1171–1175. [DOI] [PubMed] [Google Scholar]
- 83.Wilson AJ, Schoenauer R, Ehler E, Agarkova I, Bennett PM. Cardiomyocyte growth and sarcomerogenesis at the intercalated disc. Cell Mol Life Sci. 2014;71:165–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Young JL, Tuler J, Braden R, et al. In vivo response to dynamic hyaluronic acid hydro-gels. Acta Biomater. 2013;9:7151–7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Young JL, Engler AJ. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials. 2011;32:1002–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simpson DG, Majeski M, Borg TK, Terracio L. Regulation of cardiac myocyte protein turnover and myofibrillar structure in vitro by specific directions of stretch. Circ Res. 1999;85:e59–e69. [DOI] [PubMed] [Google Scholar]
- 87.Simpson DG, Sharp WW, Borg TK, Price RL, Terracio L, Samarel AM. Mechanical regulation of cardiac myocyte protein turnover and myofibrillar structure. Am J Physiol. 1996;270:C1075–C1087. [DOI] [PubMed] [Google Scholar]
- 88.Holle AW, Tang X, Vijayraghavan D, et al. In situ mechanotransduction via vinculin regulates stem cell differentiation. Stem Cells. 2013;31:2467–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Spanjaard E, de Rooij J. Mechanotransduction: vinculin provides stability when tension rises. Curr Biol. 2013;23:R159–R161. [DOI] [PubMed] [Google Scholar]
- 90.Leerberg JM, Yap AS. Vinculin, cadherin mechanotransduction and homeostasis of cell-cell junctions. Protoplasma. 2013;250:817–829. [DOI] [PubMed] [Google Scholar]
- 91.Margadant F, Chew LL, Hu X, et al. Mechanotransduction in vivo by repeated talin stretch-relaxation events depends upon vinculin. PLoS Biol. 2011;9:e1001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Borghi N, Sorokina M, Shcherbakova OG, et al. E-cadherin is under constitutive actomyosin-generated tension that is increased at cell-cell contacts upon externally applied stretch. Proc Natl Acad Sci USA. 2012;109:12568–12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Le Duc Q, Shi Q, Blonk I, et al. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol. 2010;189:1107–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. [DOI] [PubMed] [Google Scholar]
- 95.Zhuang J, Yamada KA, Saffitz JE, Kleber AG. Pulsatile stretch remodels cell-to-cell communication in cultured myocytes. Circ Res. 2000;87:316–322. [DOI] [PubMed] [Google Scholar]
- 96.Perriard JC, Hirschy A, Ehler E. Dilated cardiomyopathy: a disease of the intercalated disc? Trends Cardiovasc Med. 2003;13:30–38. [DOI] [PubMed] [Google Scholar]
- 97.Schaper J, Froede R, Hein S, et al. Impairment of the myocardial ultrastructure and changes of the cytoskeleton in dilated cardiomyopathy. Circulation. 1991;83:504–514. [DOI] [PubMed] [Google Scholar]
- 98.Tangney JR, Chuang JS, Janssen MS, et al. Novel role for vinculin in ventricular myocyte mechanics and dysfunction. Biophys J. 2013;104:1623–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Konhilas JP, Irving TC, de Tombe PP. Myofilament calcium sensitivity in skinned rat cardiac trabeculae: role of interfilament spacing. Circ Res. 2002;90:59–65. [DOI] [PubMed] [Google Scholar]
- 100.Wang Y, Fuchs F. Interfilament spacing, Ca2+ sensitivity, and Ca2+ binding in skinned bovine cardiac muscle. J Muscle Res Cell Motil. 2001;22:251–257. [DOI] [PubMed] [Google Scholar]
- 101.McDonald KS, Moss RL. Osmotic compression of single cardiac myocytes eliminates the reduction in Ca2+ sensitivity of tension at short sarcomere length. Circ Res. 1995;77:199–205. [DOI] [PubMed] [Google Scholar]
- 102.Farman GP, Walker JS, de Tombe PP, Irving TC. Impact of osmotic compression on sarcomere structure and myofilament calcium sensitivity of isolated rat myocardium. Am J Physiol Heart Circ Physiol. 2006;291:H1847–H1855. [DOI] [PubMed] [Google Scholar]
- 103.Hannigan GE, Coles JG, Dedhar S. Integrin-linked kinase at the heart of cardiac contractility, repair, and disease. Circ Res. 2007;100:1408–1414. [DOI] [PubMed] [Google Scholar]
- 104.Bendig G, Grimmler M, Huttner IG, et al. Integrin-linked kinase, a novel component of the cardiac mechanical stretch sensor, controls contractility in the zebrafish heart. Genes Dev. 2006;20:2361–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Franchini KG. Focal adhesion kinase—the basis of local hypertrophic signaling domains. J Mol Cell Cardiol. 2012;52:485–492. [DOI] [PubMed] [Google Scholar]
- 106.Bettink SI, Werner C, Chen CH, et al. Integrin-linked kinase is a central mediator in angiotensin II type 1- and chemokine receptor CXCR4 signaling in myocardial hyper-trophy. Biochem Biophys Res Commun. 2010;397:208–213. [DOI] [PubMed] [Google Scholar]
- 107.DiMichele LA, Hakim ZS, Sayers RL, et al. Transient expression of FRNK reveals stage-specific requirement for focal adhesion kinase activity in cardiac growth. Circ Res. 2009;104:1201–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Umar S, van der Valk EJ, Schalij MJ, van der Wall EE, Atsma DE, van der Laarse A. Integrin stimulation-induced hypertrophy in neonatal rat cardiomyocytes is NO-dependent. Mol Cell Biochem. 2009;320:75–84. [DOI] [PubMed] [Google Scholar]
- 109.de Jonge HW, Dekkers DH, Houtsmuller AB, Sharma HS, Lamers JM. Differential signaling and hypertrophic responses in cyclically stretched vs endothelin-1 stimulated neonatal rat cardiomyocytes. Cell Biochem Biophys. 2007;47:21–32. [DOI] [PubMed] [Google Scholar]
- 110.Brancaccio M, Hirsch E, Notte A, Selvetella G, Lembo G, Tarone G. Integrin signalling: the tug-of-war in heart hypertrophy. Cardiovasc Res. 2006;70:422–433. [DOI] [PubMed] [Google Scholar]
- 111.Lu H, Fedak PW, Dai X, et al. Integrin-linked kinase expression is elevated in human cardiac hypertrophy and induces hypertrophy in transgenic mice. Circulation. 2006;114:2271–2279. [DOI] [PubMed] [Google Scholar]
- 112.McCain ML, Lee H, Aratyn-Schaus Y, Kleber AG, Parker KK. Cooperative coupling of cell-matrix and cell-cell adhesions in cardiac muscle. Proc Natl Acad Sci USA. 2012;109:9881–9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dabiri BE, Lee H, Parker KK. A potential role for integrin signaling in mechanoelectrical feedback. Prog Biophys Mol Biol. 2012;110:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chopra A, Lin V, McCollough A, et al. Reprogramming cardiomyocyte mechanosensing by crosstalk between integrins and hyaluronic acid receptors. J Biomech. 2012;45:824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bendall JK, Heymes C, Ratajczak P, Samuel JL. Extracellular matrix and cardiac remodelling. Arch Mal Coeur Vaiss. 2002;95:1226–1229. [PubMed] [Google Scholar]
- 116.Kabaeva Z, Meekhof KE, Michele DE. Sarcolemma instability during mechanical activity in Largemyd cardiac myocytes with loss of dystroglycan extracellular matrix receptor function. Hum Mol Genet. 2011;20:3346–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ervasti JM, Sonnemann KJ. Biology of the striated muscle dystrophin-glycoprotein complex. Int Rev Cytol. 2008;265:191–225. [DOI] [PubMed] [Google Scholar]
- 118.Kaprielian RR, Severs NJ. Dystrophin and the cardiomyocyte membrane cytoskeleton in the healthy and failing heart. Heart Fail Rev. 2000;5:221–238. [DOI] [PubMed] [Google Scholar]
- 119.Carlson CG. Spontaneous changes in acetylcholine receptor and calcium leakage activity in cell-attached patches from cultured dystrophic myotubes. Pflugers Archiv. 1999;437:371–380. [DOI] [PubMed] [Google Scholar]
- 120.Allen DG, Gervasio OL, Yeung EW, Whitehead NP. Calcium and the damage pathways in muscular dystrophy. Can J Physiol Pharmacol. 2010;88:83–91. [DOI] [PubMed] [Google Scholar]
- 121.Williams IA, Allen DG. Intracellular calcium handling in ventricular myocytes from mdx mice. Am J Physiol Heart Circ Physiol. 2007;292:H846–H855. [DOI] [PubMed] [Google Scholar]
- 122.Kreplak L, Herrmann H, Aebi U. Tensile properties of single desmin intermediate filaments. Biophys J. 2008;94:2790–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wagner OI, Rammensee S, Korde N, Wen Q, Leterrier JF, Janmey PA. Softness, strength and self-repair in intermediate filament networks. Exp Cell Res. 2007;313:2228–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Steinert PM, North AC, Parry DA. Structural features of keratin intermediate filaments. J Invest Dermatol. 1994;103:19S–24S. [DOI] [PubMed] [Google Scholar]
- 125.Pawlak A, Gil RJ, Walczak E, Seweryniak P. Desmin expression in human cardiomyocytes and selected clinical and echocardiographic parameters in patients with chronic heart failure. Kardiol Pol. 2009;67:955–961. [PubMed] [Google Scholar]
- 126.Auda-Boucher G, Bernard B, Fontaine-Perus J, Rouaud T, Mericksay M, Gardahaut MF. Staging of the commitment of murine cardiac cell progenitors. Dev Biol. 2000;225:214–225. [DOI] [PubMed] [Google Scholar]
- 127.van der Loop FT, Schaart G, Langmann H, Ramaekers FC, Viebahn C. Rearrangement of intercellular junctions and cytoskeletal proteins during rabbit myocardium development. Eur J Cell Biol. 1995;68:62–69. [PubMed] [Google Scholar]
- 128.Brodehl A, Dieding M, Klauke B, et al. The novel desmin mutant p.A120D impairs filament formation, prevents intercalated disk localization, and causes sudden cardiac death. Circ Cardiovasc Genet. 2013;6:615–623. [DOI] [PubMed] [Google Scholar]
- 129.Chang AN, Potter JD. Sarcomeric protein mutations in dilated cardiomyopathy. Heart Fail Rev. 2005;10:225–235. [DOI] [PubMed] [Google Scholar]
- 130.Paulin D, Huet A, Khanamyrian L, Xue Z. Desminopathies in muscle disease. J Pathol. 2004;204:418–427. [DOI] [PubMed] [Google Scholar]
- 131.Lorenzon A, Beffagna G, Bauce B, et al. Desmin mutations and arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol. 2013;111:400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Otten E, Asimaki A, Maass A, et al. Desmin mutations as a cause of right ventricular heart failure affect the intercalated disks. Heart Rhythm. 2010;7:1058–1064. [DOI] [PubMed] [Google Scholar]
- 133.Tsatsopoulou AA, Protonotarios NI, McKenna WJ. Arrhythmogenic right ventricular dysplasia, a cell adhesion cardiomyopathy: insights into disease pathogenesis from preliminary genotype–phenotype assessment. Heart. 2006;92:1720–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Swift J, Ivanovska IL, Buxboim A, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Capo-chichi CD, Cai KQ, Smedberg J, Ganjei-Azar P, Godwin AK, Xu XX. Loss of A-type lamin expression compromises nuclear envelope integrity in breast cancer. Chin J Cancer. 2011;30:415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.De Vos WH, Houben F, Hoebe RA, et al. Increased plasticity of the nuclear envelope and hypermobility of telomeres due to the loss of A-type lamins. Biochim Biophys Acta. 1800;2010:448–458. [DOI] [PubMed] [Google Scholar]
- 137.Talwar S, Jain N, Shivashankar GV. The regulation of gene expression during onset of differentiation by nuclear mechanical heterogeneity. Biomaterials. 2014;35:2411–2419. [DOI] [PubMed] [Google Scholar]
- 138.Smith ED, Kudlow BA, Frock RL, Kennedy BK. A-type nuclear lamins, progerias and other degenerative disorders. Mech Ageing Dev. 2005;126:447–460. [DOI] [PubMed] [Google Scholar]
- 139.Gonzalez-Suarez I, Gonzalo S. Nurturing the genome: A-type lamins preserve genomic stability. Nucleus. 2010;1:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Broers JL, Peeters EA, Kuijpers HJ, et al. Decreased mechanical stiffness in LMNA −/− cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Hum Mol Genet. 2004;13:2567–2580. [DOI] [PubMed] [Google Scholar]
- 141.Takeda N Cardiomyopathy: molecular and immunological aspects (review). Int J Mol Med. 2003;11:13–16. [PubMed] [Google Scholar]
- 142.Li F, Wang X, Gerdes AM. Formation of binucleated cardiac myocytes in rat heart: II. Cytoskeletal organisation. J Mol Cell Cardiol. 1997;29:1553–1565. [DOI] [PubMed] [Google Scholar]
- 143.Banerjee I, Zhang J, Moore-Morris T, et al. Targeted ablation of nesprin 1 and nesprin 2 from murine myocardium results in cardiomyopathy, altered nuclear morphology and inhibition of the biomechanical gene response. PLoS Genet. 2014;10:e1004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stroud MJ, Banerjee I, Veevers J, Chen J. Linker of nucleoskeleton and cytoskeleton complex proteins in cardiac structure, function, and disease. Circ Res. 2014;114:538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lee KY, Davies T, Mishima M. Cytokinesis microtubule organisers at a glance. J Cell Sci. 2012;125:3495–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Garcia L, Verdejo HE, Kuzmicic J, et al. Impaired cardiac autophagy in patients developing postoperative atrial fibrillation. J Thorac Cardiovasc Surg. 2012;143:451–459. [DOI] [PubMed] [Google Scholar]
- 147.Zhao Y, Xue T, Yang X, et al. Autophagy plays an important role in sunitinib-mediated cell death in H9c2 cardiac muscle cells. Toxicol Appl Pharmacol. 2010;248:20–27. [DOI] [PubMed] [Google Scholar]
- 148.Webster DR. Microtubules in cardiac toxicity and disease. Cardiovasc Toxicol. 2002;2:75–89. [DOI] [PubMed] [Google Scholar]
- 149.Gomez AM, Kerfant BG, Vassort G, Pappano AJ. Autonomic regulation of calcium and potassium channels is oppositely modulated by microtubules in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2004;286:H2065–H2071. [DOI] [PubMed] [Google Scholar]
- 150.Liu H, Ma A, Wang C, Liu Y, Tian H, Bai L. Variation and significance of microtubules in rat volume overload cardiac hypertrophy. Chin Med J. 2003;116:337–340. [PubMed] [Google Scholar]
- 151.Malan D, Gallo MP, Bedendi I, Biasin C, Levi RC, Alloatti G. Microtubules mobility affects the modulation of L-type I(Ca) by muscarinic and beta-adrenergic agonists in guinea-pig cardiac myocytes. J Mol Cell Cardiol. 2003;35:195–206. [DOI] [PubMed] [Google Scholar]
- 152.Palmer BM, Valent S, Holder EL, Weinberger HD, Bies RD. Microtubules modulate cardiomyocyte beta-adrenergic response in cardiac hypertrophy. Am J Physiol. 1998;275:H1707–H1716. [DOI] [PubMed] [Google Scholar]
- 153.Iribe G, Kohl P. Axial stretch enhances sarcoplasmic reticulum Ca2+ leak and cellular Ca2+ reuptake in guinea pig ventricular myocytes: experiments and models. Prog Biophys Mol Biol. 2008;97:298–311. [DOI] [PubMed] [Google Scholar]
- 154.Lammerding J, Kamm RD, Lee RT. Mechanotransduction in cardiac myocytes. Ann N Y Acad Sci. 2004;1015:53–70. [DOI] [PubMed] [Google Scholar]
- 155.Bett GC, Sachs F. Cardiac mechanosensitivity and stretch-activated ion channels. Trends Cardiovasc Med. 1997;7:4–8. [DOI] [PubMed] [Google Scholar]
- 156.Akay M, Craelius W. Mechanoelectrical feedback in cardiac myocytes from stretch-activated ion channels. IEEE Trans Biomed Eng. 1993;40:811–816. [DOI] [PubMed] [Google Scholar]
- 157.Bustamante JO, Ruknudin A, Sachs F. Stretch-activated channels in heart cells: relevance to cardiac hypertrophy. J Cardiovasc Pharmacol. 1991;17(Suppl 2):S110–S113. [DOI] [PubMed] [Google Scholar]
- 158.Senatore S, Ramieddy V, Semeriva M, Perrin L, Lalevee N. Response to mechanical stress is mediated by the TRPA channel painless in the Drosophila heart. PLoS Genet. 2010;6:e1001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sinha B, Koster D, Ruez R, et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell. 2011;144:402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Albinsson S, Nordstrom I, Sward K, Hellstrand P. Differential dependence of stretch and shear stress signaling on caveolin-1 in the vascular wall. Am J Physiol Cell Physiol. 2008;294:C271–C279. [DOI] [PubMed] [Google Scholar]
- 161.Zhang B, Peng F, Wu D, Ingram AJ, Gao B, Krepinsky JC. Caveolin-1 phosphorylation is required for stretch-induced EGFR and Akt activation in mesangial cells. Cell Signal. 2007;19:1690–1700. [DOI] [PubMed] [Google Scholar]
- 162.Bellott AC, Patel KC, Burkholder TJ. Reduction of caveolin-3 expression does not inhibit stretch-induced phosphorylation of ERK2 in skeletal muscle myotubes. J Appl Physiol. 2005;98:1554–1561. [DOI] [PubMed] [Google Scholar]
- 163.Kawabe J, Okumura S, Lee MC, Sadoshima J, Ishikawa Y. Translocation of caveolin regulates stretch-induced ERK activity in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2004;286:H1845–H1852. [DOI] [PubMed] [Google Scholar]
- 164.Fridolfsson HN, Patel HH. Caveolin and caveolae in age associated cardiovascular disease. J Geriatr Cardiol. 2013;10:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zou H, Stoppani E, Volonte D, Galbiati F. Caveolin-1, cellular senescence and age-related diseases. Mech Ageing Dev. 2011;132:533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hnasko R, Lisanti MP. The biology of caveolae: lessons from caveolin knockout mice and implications for human disease. Mol Interv. 2003;3:445–464. [DOI] [PubMed] [Google Scholar]
- 167.Woodman SE, Park DS, Cohen AW, et al. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J Biol Chem. 2002;277:38988–38997. [DOI] [PubMed] [Google Scholar]
- 168.Chow AK, Daniel EE, Schulz R. Cardiac function is not significantly diminished in hearts isolated from young caveolin-1 knockout mice. Am J Physiol Heart Circ Physiol. 2010;299:H1183–H1189. [DOI] [PubMed] [Google Scholar]
- 169.Murata T, Lin MI, Huang Y, et al. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med. 2007;204:2373–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ma Y, de Castro Bras LE, Toba H, et al. Myofibroblasts and the extracellular matrix network in post-myocardial infarction cardiac remodeling. Pflugers Arch. 2014;466:1113–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 2012;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jourdan-Lesaux C, Zhang J, Lindsey ML. Extracellular matrix roles during cardiac repair. Life Sci. 2010;87:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.McCurdy S, Baicu CF, Heymans S, Bradshaw AD. Cardiac extracellular matrix remodeling: fibrillar collagens and Secreted Protein Acidic and Rich in Cysteine (SPARC). J Mol Cell Cardiol. 2010;48:544–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zamilpa R, Lindsey ML. Extracellular matrix turnover and signaling during cardiac remodeling following MI: causes and consequences. J Mol Cell Cardiol. 2010;48:558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Goldsmith EC, Borg TK. The dynamic interaction of the extracellular matrix in cardiac remodeling. J Card Fail. 2002;8:S314–S318. [DOI] [PubMed] [Google Scholar]
- 176.Borg TK, Rubin K, Carver W, Samarel A, Terracio L. The cell biology of the cardiac interstitium. Trends Cardiovasc Med. 1996;6:65–70. [DOI] [PubMed] [Google Scholar]
- 177.Nag AC. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios. 1980;28:41–61. [PubMed] [Google Scholar]
- 178.Coyer SR, Singh A, Dumbauld DW, et al. Nanopatterning reveals an ECM area threshold for focal adhesion assembly and force transmission that is regulated by integrin activation and cytoskeleton tension. J Cell Sci. 2012;125:5110–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Parker KK, Tan J, Chen CS, Tung L. Myofibrillar architecture in engineered cardiac myocytes. Circ Res. 2008;103:340–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Grosberg A, Alford PW, McCain ML, Parker KK. Ensembles of engineered cardiac tissues for physiological and pharmacological study: heart on a chip. Lab Chip. 2011;11:4165–4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Patterson NL, Iyer RP, de Castro Bras LE, et al. Using proteomics to uncover extracellular matrix interactions during cardiac remodeling. Proteomics Clin Appl. 2013;7:516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Konstandin MH, Volkers M, Collins B, et al. Fibronectin contributes to pathological cardiac hypertrophy but not physiological growth. Basic Res Cardiol. 2013;108:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Konstandin MH, Toko H, Gastelum GM, et al. Fibronectin is essential for reparative cardiac progenitor cell response after myocardial infarction. Circ Res. 2013; 113:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lu D, Soleymani S, Madakshire R, Insel PA. ATP released from cardiac fibroblasts via connexin hemichannels activates profibrotic P2Y2 receptors. FASEB J. 2012;26: 2580–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Doble BW, Kardami E. Basic fibroblast growth factor stimulates connexin-43 expression and intercellular communication of cardiac fibroblasts. Mol Cell Biochem. 1995;143:81–87. [DOI] [PubMed] [Google Scholar]
- 186.Thompson SA, Blazeski A, Copeland CR, et al. Acute slowing of cardiac conduction in response to myofibroblast coupling to cardiomyocytes through N-cadherin. J Mol Cell Cardiol. 2014;68:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Thompson SA, Copeland CR, Reich DH, Tung L. Mechanical coupling between myofibroblasts and cardiomyocytes slows electric conduction in fibrotic cell mono-layers. Circulation. 2011;123:2083–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Leslie KO, Taatjes DJ, Schwarz J, vonTurkovich M, Low RB. Cardiac myofibroblasts express alpha smooth muscle actin during right ventricular pressure overload in the rabbit. Am J Pathol. 1991;139:207–216. [PMC free article] [PubMed] [Google Scholar]
- 189.Dalla Costa AP, Clemente CF, Carvalho HF, Carvalheira JB, Nadruz W Jr, Franchini KG. FAK mediates the activation of cardiac fibroblasts induced by mechanical stress through regulation of the mTOR complex. Cardiovasc Res. 2010;86:421–431. [DOI] [PubMed] [Google Scholar]
- 190.Wang J, Chen H, Seth A, McCulloch CA. Mechanical force regulation of myofibroblast differentiation in cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2003;285:H1871–H1881. [DOI] [PubMed] [Google Scholar]
- 191.Herum KM, Lunde IG, Skrbic B, et al. Syndecan-4 signaling via NFAT regulates extra-cellular matrix production and cardiac myofibroblast differentiation in response to mechanical stress. J Mol Cell Cardiol. 2013;54:73–81. [DOI] [PubMed] [Google Scholar]
- 192.Lefebvre P, Nusgens BV, Lapiere CM. Cultured myofibroblasts display a specific phenotype that differentiates them from fibroblasts and smooth muscle cells. Dermatology. 1994;189(Suppl. 2):65–67. [DOI] [PubMed] [Google Scholar]
- 193.Pipelzadeh MH, Naylor IL. The in vitro enhancement of rat myofibroblast contractility by alterations to the pH of the physiological solution. Eur J Pharmacol. 1998;357:257–259. [DOI] [PubMed] [Google Scholar]
- 194.de Haas HJ, Arbustini E, Fuster V, Kramer CM, Narula J. Molecular imaging of the cardiac extracellular matrix. Circ Res. 2014;114:903–915. [DOI] [PubMed] [Google Scholar]
- 195.Kizana E, Chang CY, Cingolani E, et al. Gene transfer of connexin43 mutants attenuates coupling in cardiomyocytes: novel basis for modulation of cardiac conduction by gene therapy. Circ Res. 2007;100:1597–1604. [DOI] [PubMed] [Google Scholar]
- 196.Guvendiren M, Perepelyuk M, Wells RG, Burdick JA. Hydrogels with differential and patterned mechanics to study stiffness-mediated myofibroblastic differentiation of hepatic stellate cells. J Mech Behav Biomed Mater. 2013. http://dx.doi.org/10.1016/j.jmbbm.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J. 2007;93:4453–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Atance J, Yost MJ, Carver W. Influence of the extracellular matrix on the regulation of cardiac fibroblast behavior by mechanical stretch. J Cell Physiol. 2004;200:377–386. [DOI] [PubMed] [Google Scholar]
- 199.Ren Y, Lee MY, Schliffke S, et al. Small molecule Wnt inhibitors enhance the efficiency of BMP-4-directed cardiac differentiation of human pluripotent stem cells. J Mol Cell Cardiol. 2011;51:280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc Natl Acad Sci USA. 2007;104:18531–18536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Feinberg AW, Ripplinger CM, van der Meer P, et al. Functional differences in engineered myocardium from embryonic stem cell-derived versus neonatal cardiomyocytes. Stem Cell Rep. 2013;1:387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Feinberg AW, Alford PW, Jin H, et al. Controlling the contractile strength of engineered cardiac muscle by hierarchal tissue architecture. Biomaterials. 2012;33:5732–5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sheehy SP, Grosberg A, Parker KK. The contribution of cellular mechanotransduction to cardiomyocyte form and function. Biomech Model Mechanobiol. 2012;11:1227–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yoon YS, Park JS, Tkebuchava T, Luedeman C, Losordo DW. Unexpected severe calcification after transplantation of bone marrow cells in acute myocardial infarction. Circulation. 2004;109:3154–3157. [DOI] [PubMed] [Google Scholar]
- 205.Rane AA, Chuang JS, Shah A, et al. Increased infarct wall thickness by a bio-inert material is insufficient to prevent negative left ventricular remodeling after myocardial infarction. PLoS One. 2011;6:e21571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bray MA, Sheehy SP, Parker KK. Sarcomere alignment is regulated by myocyte shape. Cell Motil Cytoskeleton. 2008;65:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Grosberg A, Kuo PL, Guo CL, et al. Self-organization of muscle cell structure and function. PLoS Comput Biol. 2011;7:e1001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bier E, Bodmer R. Drosophila, an emerging model for cardiac disease. Gene. 2004;342:1–11. [DOI] [PubMed] [Google Scholar]
- 209.Hinits Y, Pan L, Walker C, Dowd J, Moens CB, Hughes SM. Zebrafish Mef2ca and Mef2cb are essential for both first and second heart field cardiomyocyte differentiation. Dev Biol. 2012;369:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fink M, Callol-Massot C, Chu A, et al. A new method for detection and quantification of heartbeat parameters in Drosophila, zebrafish, and embryonic mouse hearts. Biotechniques. 2009;46:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kaushik G, Zambon AC, Fuhrmann A, et al. Measuring passive myocardial stiffness in Drosophila melanogaster to investigate diastolic dysfunction. J Cell Mol Med. 2012;16:1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]