Abstract
Objective:
The current study investigated the additive effect of oral lead (Pb) exposure and dietary iron (Fe) deficiency on intestinal lactobacilli, E. coli, and yeast in SD rats.
Methods:
Weanling rats were fed on control diet (CD) or iron deficient diet (ID) for 4 weeks, followed by oral Pb exposure for another 4 weeks. Lead exposure was withdrawn for 2 weeks, and then resumed after 2 weeks. Blood samples were collected to determine haemoglobin (Hb), serum iron, blood Pb and δ-Aminolevulenic acid dehydratase (ALAD) activity. Fecal samples were collected to enumerate the lactobacilli, E. coli and yeast population on selective agar media and determine Pb levels.
Results:
Hb and serum Fe levels decreased significantly in iron deficient rats. Pb exposed rats had a significant increase in blood Pb levels and decreased ALAD activity. The lactobacilli population was significantly decreased (p<0.05) in ID rats compared to the CD group. Further, a significant decrease in the lactobacilli population was observed in Pb exposed rats irrespective of the dietary regimen. Upon withdrawal of Pb exposure, lactobacilli increased significantly in both the CD+Pb and ID+Pb groups, whereas re-exposure to Pb decreased lactobacilli population. The E. coli and yeast populations were inconsistent among both the ID and Pb exposed rats compared to controls. Fecal Pb levels increased significantly in Pb exposed rats irrespective of diet.
Conclusion:
An additive effect of dietary Fe deficiency and oral Pb exposure resulted in greater reductions in the intestinal lactobacilli population compared to either treatment alone. In addition, transient withdrawal of Pb exposure led to improved lactobacilli population irrespective of Fe status.
Keywords: Dietary iron (Fe), E. coli, Lactobacilli, Oral lead (Pb) exposure, Yeast
Introduction
Iron (Fe) is one of the most important trace elements, and plays a major role in the metabolism of heme synthesis and regulation. Surveys conducted by the National Nutrition Monitoring Bureau (NNMB-2002) and National Family Health Survey (NFHS) -3 reported that the prevalence of anemia is 52 - 72 percent among Indian women and children1,2). This could be due to poor intake and absorption of iron from staple vegetarian diets consumed in India. It has been established that vegetarian diets contain phytates and polyphenols that inhibit Fe absorption due to their metal chelating ability3). In recent years, there has been a growing interest on prebiotics and probiotics. Coudray et al. have reported that prebiotics such as inulin increase the bioavailability of dietary calcium (Ca), magnesium (Mg), copper (Cu), zinc (Zn) and Fe4,5). Recent studies also suggest that these prebiotics have stimulatory effect on lactobacilli and bifidobacteria, which are the two important genera of intestinal flora6-8). Microorganisms require iron for their metabolic activities, which is derived from diet of their host. Studies on anemic (Hb ≤ 10.0 g%) young women (18 - 25 years) from southern India showed low levels of fecal lactobacilli as compared to normal (Hb ≥ 12.0 g%) women, although the intakes of energy, carbohydrates, fiber, Fe, and milk were similar in both groups9). Gastrointestinal resident microflora contains certain components that have ability to activate innate and adaptive immunity10,11). The presence of microflora and its colonization (e.g. by probiotics and prebiotics) offers a strategy to influence the development of mucosal and systemic immunity, which can play a central role in prevention and treatment of some diseases11,12).
On the other hand, lead (Pb) is ubiquitous environmental pollutant that enters into the body through the oral route (water, food and dust). It has been extensively shown that young children are more susceptible to Pb poisoning due to their hand-to-mouth activity, pica eating, and higher intestinal absorption (4-5 folds). Pb absorption in the gastrointestinal tract also depends on other factors such as the dietary intake of Ca, Fe, phosphorous, pectin etc.13,14). Unabsorbed Pb passes through the large intestine and is excreted.
The human gastrointestinal tract contains about 400 - 500 species of both aerobic and anaerobic microbes, such as Escherichia, Bacteroides, Bifidobacterium, and Lactobacillus genera15,16). Among normal intestinal flora, lactobacilli, E. coli, and Bacteroids play a major role in synthesizing the vitamins that are essential for human nutrition, and thus have a health promoting effect. These microorganisms prevent the colonization of pathogenic bacteria, by secreting bacteriocin compounds which eliminate pathogenic bacteria. Pb has been shown to have an inhibitory effect on normal intestinal microorganisms16). Iron deficiency may modulate Pb-mediated effects on intestinal microbes.
In light of the above and the higher prevalence of both subclinical Pb toxicity and anemia among Indians, we aimed to investigate the effect of oral Pb exposure on intestinal lactobacilli, E. coli, and yeast in iron deficiency, using the Sprague Dawley (SD) rat as experimental model.
Materials and Methods
Chemicals and reagents were obtained from Sigma Chemical Co. (Bangalore, India) unless otherwise specified. The chemicals for the AIN-93G diet (American Institute of Nutrition) were procured from Sd. Fine Chemicals, India. Cellulose, choline chloride, and vitamins (water and fat soluble) procured from Sigma-Aldrich (St. Louis, USA). Skimmed milk powder was purchased from Srikrishna Industries (Gujarat, India).
Preparation of the AIN-93G Diet
To induce iron deficiency in rats, the iron-deficient AIN-93G diet (American Institute of Nutrition) was prepared as described by Reeves et al.17). All of the components were weighed and mixed manually in plastic containers to avoid 'iron contamination'. The iron deficient diet was devoid of FeSO4, the source of iron, during the mineral mixture preparation, whereas 35 mg of FeSO4/kg of mineral mixture was used in the preparation of the control diet. The Fe content of control and iron deficient diets were determined as described below.
Induction of Iron Deficiency
A total of 48 weanling Sprague Dawley rats (24 males + 24 females) were obtained from the breeding facility of the National Centre for Laboratory Animal Sciences (NCLAS), ICMR - National Institute of Nutrition, Hyderabad, India. The animals were acclimatized for two days. Rats were randomized into two groups: the 'Control Diet' group (CD: 12 males + 12 females), which were fed on the AIN-93G diet supplemented by 35 mg of FeSO4/kg mineral mixture; and the 'Iron Deficient' Group (ID: 12 males + 12 females), which were fed on the AIN-93G diet lacking FeSO4 as source of iron for four weeks. The experimental protocol and procedures were approved by the 'Institutional Animal Ethics Committee' (IAEC) of ICMR-National Institute of Nutrition, Hyderabad, India.
Each animal was placed in a separate polypropylene cage fitted with a stainless steel wire bottom and top, which held a stainless steel cup for the powdered diet. The diet and water (Milli Q) were provided ad libitum. The animals were maintained at 21±2°C, with 10-15 air changes per hour, and relative humidity was maintained between 50-55% with a 12-hour light/dark cycle. Food intake was monitored daily by weighing the remaining diet and waste, as well as the body weights of the animals which were recorded on alternating days. Malocclusion (overgrowth of incisors) among the rats was observed while recording the body weights. The incisors of affected rats were trimmed by a veterinarian once every 2 weeks, depending on the rate of growth. The Fe deficiency status was assessed by measuring the haemoglobin (Hb) and serum Fe levels of the rats at the end of the fourth week of feeding on the respective CD or ID diets.
Sub-clinical Pb Toxicity
An oral dose of 25 mg Lead Acetate / 4 ml Milli Q water containing 25 μl of acetic acid / l was administered per kg of rat body weight for induction of 'subclinical Pb toxicity'. Half of the rats from the CD group received lead acetate daily for a period of 4 weeks (CD + Pb); whereas the second subgroup received only Milli Q water with 25 μl of acetic acid / l as a control (CD). Similarly, the ID group was also divided into ID + Pb or ID only groups, which received either lead acetate or Milli Q water with acetic acid, respectively. Blood (1.0 ml) was collected from the retro-orbital plexus at the end of the 8th week to determine blood Pb levels, δ-aminolevulenic acid dehydratase activity (ALAD), Hb levels, and serum iron status as described below.
Sample collection and Laboratory Analysis
Fecal matter was collected aseptically by three fecal samples per rat at every time point into a 1.5 ml sterile vial and stored at −80°C until further analysis. The samples were collected from weanlings (n=20, 10 from each sex) during the acclimatization period, at the end of the 4th week (induction of Fe deficiency), the 8th week (subclinical Pb toxicity), the 10th week (Pb-withdrawal), and the 12th week (Pb re-exposure). At each time point two aliquots of fecal samples were collected. One aliquot was used for determination of intestinal lactobacilli, E. coli, and yeast populations; and the other was for estimation of Pb in feces. Similarly, about 1.0 - 1.5 ml of blood samples were collected at the end of induction of both 'Fe deficiency' and 'sub-clinical Pb toxicity' to determine the biomarkers of iron deficiency and Pb toxicity.
The concentration of Fe and Pb levels in biological samples, feces, and diet were determined by subjecting them to closed microwave acid digestion. Blood (0.5 ml), serum (0.3 ml), fecal matter (0.8 - 1.2 g) and AIN-93G diet (5.0 g in triplicate) samples were digested in Teflon tubes with 1.0 - 2.0 ml of Supra pure HNO3 (Merck) at a ramping temperature of 250°C / 15 min using a MARS Xpress microwave digester (CEM corporation, USA). Pb levels in the digested blood and fecal matter were quantified using Graphite Furnace Atomic Absorption Spectroscopy (GF-AAS), whereas serum and dietary Fe contents were determined using flame-AAS (Varian 220).
Blood Hb levels were estimated by the Cyanmethaemoglobin method. Briefly, 20 μl of blood was mixed with 5.0 ml of Drabkin's reagent (Potassium Ferric Cyanide Solution) and allowed to stand for 5 min to develop colour. The colour intensity was measured at 540 nm against known standard using spectrophotometer (Thermo UNICAM UV 300). ALAD activity was determined using the method described by Granick et al.18). Briefly, 50 μl of heparinized whole blood was added to Saponin buffer with and without Aminolevulenic acid as substrate, and incubated at 37°C for 1 hour, followed by termination of the reaction by the addition of Trichloroacetic-HgCl2 solution. An equal volume of modified Ehrlich reagent was added to the supernatant, and the absorbance was measured at 553 nm using a spectrophotometer (Thermo UNICAM UV 300).
Enumeration of Fecal Lactobacilli, E. coli, and Yeast
The lactobacilli, E. coli and yeast population in excreta were determined using the selective culture mediums lactobacilli MRS agar, Endo agar, and Sabouraud agar, respectively16). Known weights of wet fecal samples were suspended in sterile phosphate buffer saline (PBS: 10 mM, pH 7.2) and homogenized at 5000 rpm for 5 - 10 min at 4 - 10°C (Polytron Kinematica AG, # PT 1300D). The suspension was then serially diluted up to 106 times to obtain bacterial / yeast colonies in the countable range. A series of three consecutive serially diluted suspensions (100 μl) were transferred and spread uniformly on selective media in duplicate. Subsequently, the MRS agar plates were placed in 'anaerobic jar with gas pack' (Hi Media). The anaerobic jar containing MRS agar Petri dishes and Endo agar plates was incubated at 37°C for 24 - 48 h. Petri dishes containing Sabouraud agar were incubated at 25°C for 48 - 72 hours to develop yeast colonies. Bacterial and yeast colonies in a range of 10 - 200 colonies per plate were counted using the dark field colony counter (Quebeck, 3328, USA). The data was represented as the colony forming units (cfu) / g of wet weight fecal sample by applying the dilution factor. Lactobacilli, E. coli, and yeast were confirmed by the Gram Staining procedure.
Statistical Analysis
The mean and standard deviation (SD) / standard error of mean (SE) were calculated for all the variables. Between groups, statistical analysis was done by one-way Analysis of Variance (ANOVA), and pair wise significance was tested by the least significant difference (LSD) test. Wherever heterogeneity in the data of a particular group was observed, a non-parametric, Kruskal-Wallis and Mann-Whitney U test was performed to identify the difference at a 95% CI (p<0.05). The relationships were identified by means of Pearson's bivariate moment correlation coefficient. Results were considered significant at p<0.05. The data were analyzed using SPSS 15.0 Windows version software.
Results
Body Weights and Food Intake
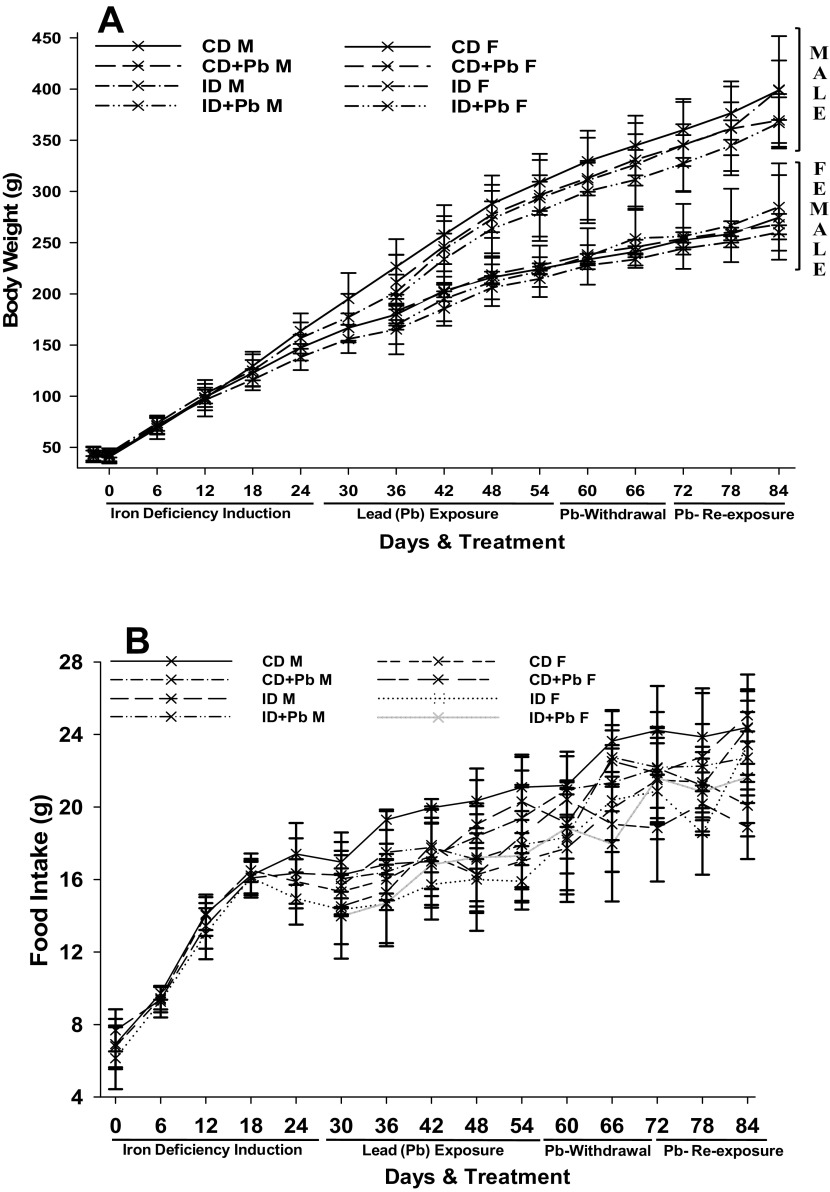
A proportionate increase in body weights of the male and female animals fed on ID and CD diets was observed. Male animals gained higher body weights than the females (Fig. 1A). By the end of the 4th week, male rats from the CD group gained significantly more body weight than male rats of ID group. Similarly, at all other time points male rats from the CD group had gained significantly higher body weight compared to male rats of ID+Pb group (Fig. 1A). However, no such consistent difference was observed among the female rats during the course of experiment (Fig. 1A).
Fig. 1.
Body weight of rats and food intake
Lines represent the body weight (A) and food intake (B) of rats during induction of Fe deficiency, Pb toxicity, Pb withdrawal and Pb re-exposure. CD = control diet; ID = iron deficient diet, M = male, F = female.
The mean iron levels of the iron deficient diet (ID) and control diets (CD) were 14.2 ± 0.812 μg/kg diet and 31.5 ± 3.64 μg/kg diet, respectively. A consistent increase of food intake by the animals fed on the ID and CD (both male and female) was observed (Fig. 1B). Food intake was comparable among the male and female rats between the groups at all time points except at the end of the 8th week (Fig. 1B). During the Pb exposure time points, a reduction in food intake was observed among ID+Pb and CD+Pb groups (Fig. 1A). During the course of 12 weeks of experimentation, 8.3% of rats (4 rats of the total 48) exhibited 'malocclusion', and were treated by trimming by a veterinary surgeon.
Induction of Iron Deficiency and Pb Toxicity
A significant (p<0.001) decrease in mean haemoglobin and serum Fe levels was observed among male and female rats of the ID group compared to the CD group at the end of 4th week (Table 1). The mean blood Pb levels increased significantly (p<0.01) in Pb exposed rats, irrespective of diet. The ALAD activity of male and female rats from the ID group was significantly (p<0.05) higher than their respective CD group (Table 1). Additionally, Pb exposed rats (CD+Pb and ID+Pb) had significantly (p<0.001) reduced ALAD activity compared to their respective controls (CD and ID). However, the extent of the decrease in ALAD activity in response to Pb exposure was higher in ID+Pb rats compared to the CD+Pb group (Table 1).
Table 1.
Serum Fe, Haemoglobin, blood Pb levels, and ALAD activity among iron deficient-Pb exposed rats.
| Activity | Male (n=24) | Female (n=24) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AIN-93G Diet | Control Diet (CD) (n=6) | Iron Deficient (ID) (n=6) | Control Diet (CD) (n=6) | Iron Deficient (ID) (n=6) | |||||
| Values represent the mean±SD. Different superscripts are significantly different between groups across the time point (p<0.05). For example, values marked by the superscript 'a' are statistically similar to each other, but significantly different from 'b', 'c' and 'd'. Hb = haemoglobin, ALAD = δ-Aminolevulenic acid dehydratase. | |||||||||
| End of 4th week | Hb (g%) | 13.6±1.00a | 8.1±1.41b | 13.4±0.93a | 8.7±1.03b | ||||
| Serum Fe (μg/dl) | 187.8±38.35a | 64.1±9.35b | 236.6±36.95c | 69.3±19.13b | |||||
| Pb Exposure | CD (n=6) | CD+Pb (n=6) | ID (n=6) | ID+Pb (n=6) | CD (n=6) | CD+Pb (n=6) | ID (n=6) | ID+Pb (n=6) | |
| End of 8th week | Hb (g%) | 14.2±2.01a | 12.6±2.02ac | 9.9±1.22b | 9.2±0.50b | 14.7±1.45a | 10.2±1.27b | 10.9±0.69bc | 9.7±0.90b |
| Serum Fe (μg/dl) | 201.8±55.30a | 191.3±49.38a | 64.1±19.35b | 83.6±18.12c | 214.6±23.72a | 207.5±13.92a | 81.1±21.02c | 101.5±19.61d | |
| Blood Pb (μg/dl) | 2.3±1.16a | 19.3±6.23b | 2.5±0.89a | 47.5±3.78c | 1.9±0.81a | 13.5±3.52b | 1.5±0.31a | 29.80±8.30d | |
| Blood ALAD Activity (nMole/h/ml) | 143.3±25.77a | 46.0±5.21b | 243.3±20.89c | 81.7±35.01b | 129.6±27.92a | 76.3±18.28b | 170.1±29.28c | 87.9±11.70b | |
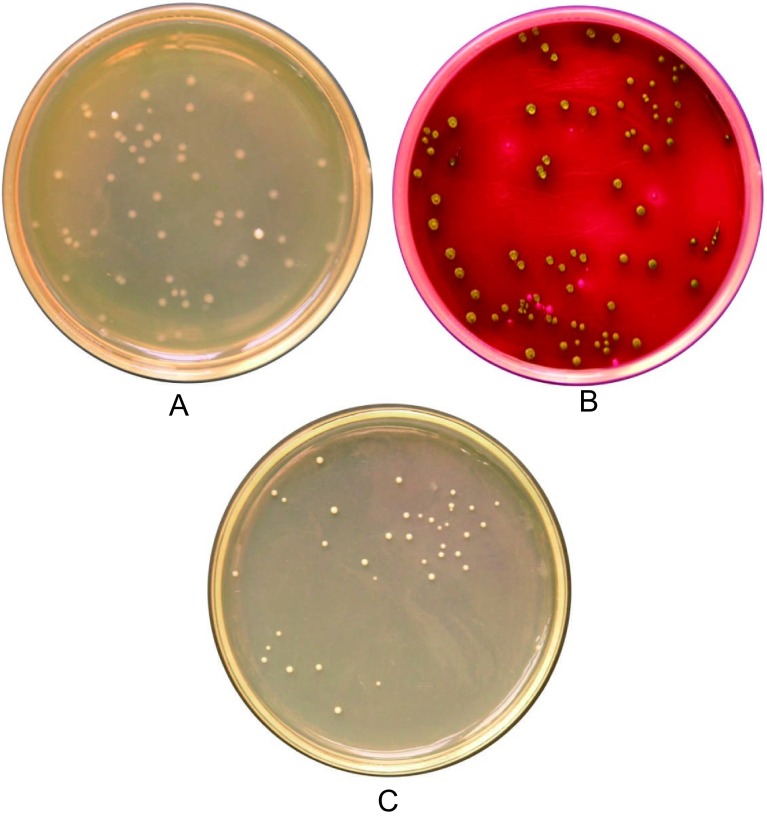
Colony Morphology and Enumeration of Fecal Lactobacilli, E. coli, and Yeast
Lactobacilli colonies were identified as creamy white and small colonies on lactobacilli MRS agar plates (Fig. 2A). In addition to colony morphology, lactobacilli were confirmed by microscopy, where they appear as Gram-positive bacilli found in pairs or chains. E. coli colonies exhibited a metallic sheen on Endo agar plates (Fig. 2B). Yeast colonies appeared as shiny white colonies on Sabouraud's agar medium (Fig. 2C). Gram staining was used to confirm E. coli, which appear as Gram-negative rods, and yeast, which appear as Gram-positive globules. Bacterial and yeast colonies were enumerated based on the typical colony morphological features on the selective media using a Quebeck colony counter.
Fig. 2.
Bacterial and Yeast Colonies on Selective Agar Culture Medium
Figure indicates the bacterial or yeast colonies on selective agar culture medium. (A) Small, white lactobacilli colonies on lactobacilli MRS agar medium, (B) E.coli colonies with a metallic sheen on an Endo agar plate, (C) small and shiny white Yeast colonies on a Sabouraud’s agar plate.
Intestinal Lactobacilli, E. coli, and Yeast Populations of Rats
At weanling age
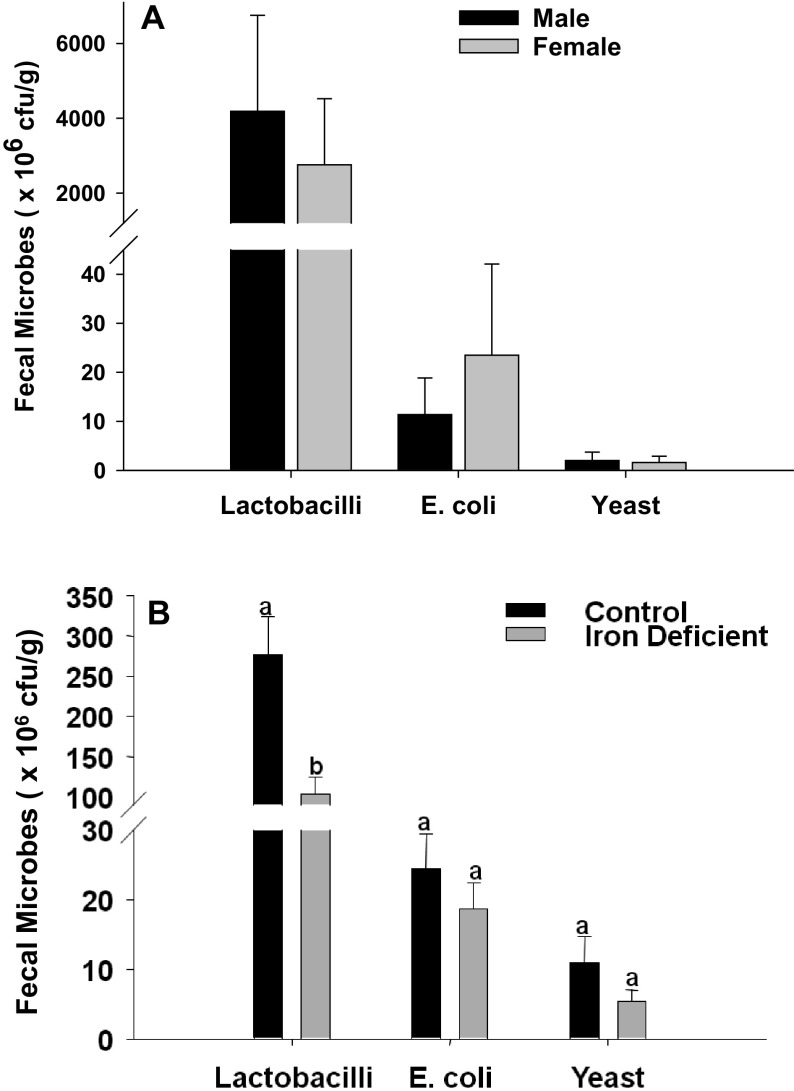
The weanling rat fecal lactobacilli, E. coli, and yeast populations are shown in Fig. 3A. The lactobacilli population varied widely in both male and female weanling rats. The mean population levels of lactobacilli, E. coli, and yeasts were comparable between male and female weanling rats at weanling age (Fig. 3A).
Fig. 3.
Lactobacilli, E. coli, and yeast populations among rats at weaning and after 4 weeks of feeding on an iron deficient diet
Bacterial and yeast levels in male and female rats at weaning (A) and after receiving an Fe-deficient AIN-93G diet for 4 weeks (B). No significant difference was noted among the bacterial and yeast population levels between male and females at weaning (A); hence at all other time points the gender-pooled data are presented (B). The bars represent the mean±SE of the bacterial or yeast population of 10 samples (measured in colony forming units [cfu]/g of wet weight of fecal matter). Different superscripts are significantly different (p<0.05); groups with the same superscripts are statistically similar.
At induction of iron deficiency
The population of lactobacilli was markedly decreased after 4 weeks of feeding on the AIN-93G diets (both CD and ID), compared to weanling rats (Fig. 3B). There was wide variation in the lactobacilli population among the rats in both the CD (125.2 to 635.8 × 106 cfu / g of wet weight) and ID groups (20.8 to 220.9 × 106 cfu / g of wet weight). The lactobacilli population was significantly decreased among ID rats compared to the CD group (Fig. 3B). Fecal E. coli and yeast population levels were also decreased in the ID group, although this difference was not significant.
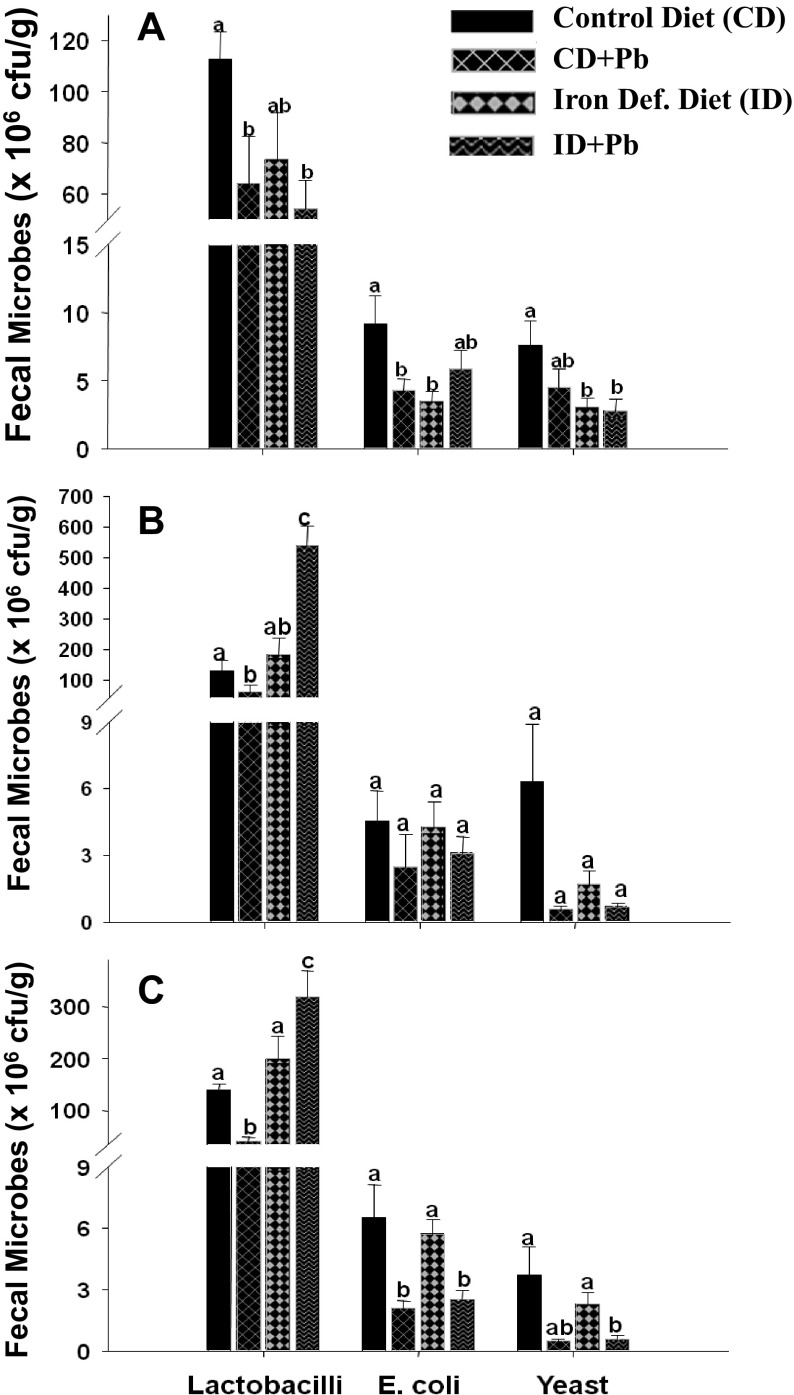
Oral Pb exposure
A significant (p<0.05) decrease in fecal lactobacilli and E. coli was observed in Pb exposed CD+Pb rats compared to the CD group (Fig. 4A). Similarly, lactobacilli levels were decreased in Pb exposed rats from the ID+Pb group compared to rats of ID group, although this decrease was not statistically significant. However, mean E. coli levels were higher among the ID+Pb group compared to the ID and CD+Pb groups, although this difference was not statistically significant (Fig. 4A). A significant decrease in yeast levels was noted among the ID+Pb and ID groups compared to the CD group; whereas no significant difference was observed between the CD and CD+Pb groups (Fig. 4A).
Fig. 4.
The effect of oral Pb exposure, withdrawal and re-exposure on the intestinal flora of rats
(A) Rats exposed to Pb through an oral route for 4 weeks after inducing Fe deficiency. (B) Pb exposure was subsequently withdrawn for 2 weeks. (C) Rats were then re-exposed to oral Pb for another 2 weeks. The bars represent the mean±SE of the bacterial or yeast population of 10 samples (measured in colony forming units [cfu]/g of wet weight of fecal matter). Different superscripts are significantly different (p<0.05); groups with the same superscripts are statistically similar.
Withdrawal of oral Pb exposure
The fecal lactobacilli, E. coli, and yeast levels at the end of the Pb withdrawal period are shown in Fig. 4B. The population of lactobacilli was increased in Pb withdrawn rats (end of 10th week) as compared to the end of the 8th week of Pb exposure. A significant (p<0.01) increase in lactobacilli levels among the ID+Pb rats was observed upon withdrawal of Pb exposure compared to the ID group (Fig. 4B). There was no significant difference among the E. coli and yeast levels in fecal samples collected from Pb withdrawn and unexposed pair fed groups.
Re-exposure to oral Pb
Upon re-exposure of oral Pb, the fecal lactobacilli levels decreased significantly (p<0.001) irrespective of diet (Fig. 4C). A decrease in the lactobacilli population was also noted within the ID+Pb group at the two different time points (i.e, Pb withdrawal and re-exposure, Fig. 4C and Fig. 4B). Upon Pb re-exposure, fecal E. coli and yeast levels were significantly decreased (p<0.007) in the CD+Pb and ID+Pb groups compared to their respective controls (Fig. 4C).
Fecal Pb Levels
Lead levels in fecal samples collected from Pb exposed rats were significantly (p<0.001) higher than the unexposed rats, irrespective of the dietary regimen (Table 2). The mean fecal Pb levels were comparable among ID+Pb and CD+Pb groups. A significant decrease in fecal Pb levels was noted after withdrawal of Pb exposure for 2 weeks, compared to fecal Pb levels at the end of 4 weeks of oral Pb exposure (Table 2).
Table 2.
Fecal Pb levels during Pb exposure, withdrawal and re-exposure
| Activity | Groups | ||||
|---|---|---|---|---|---|
| Pb exposure condition | Control Diet (CD) (n=10) | CD+Pb (n=10) | Iron Def. Diet (ID) (n=10) | ID+Pb (n=10) | |
| Fecal Pb levels were determined at the end of (1) induction of Pb toxicity, (2) Pb-withdrawal and (3) Pb re-exposure. The values are fecal Pb levels (μg/g of wet weight of feces) represented as the mean±SD. Different superscripts are significantly different between groups across the time point (p<0.05). For example, superscript 'a' means statistically similar to each other, but significantly different from 'b'. | |||||
| 1 | Pb-Exposure | 6.2±1.81a | 146.6±20.02b | 5.9±1.22a | 148.2±19.50b |
| 2 | Pb-Withdrawal | 4.8±1.03a | 16.3±4.29b | 3.2±2.15a | 12.9±3.21b |
| 3 | Pb-Re-exposure | 5.1±1.61a | 171.6±24.62b | 7.4±1.01a | 187.5±21.64b |
Upon re-exposure to oral Pb for two weeks, fecal Pb levels increased significantly in both CD and ID rats. There was no significant difference in the fecal Pb levels between ID and CD rats (Table 2). No correlations were observed between fecal Pb levels and the population of fecal lactobacilli, E. coli, or yeast at any of the time points.
Discussion
In a healthy human or animal, surface tissues (i.e., the skin and mucous membranes), are constantly in contact with environmental organisms, and are readily colonized by various microbial species, referred as normal flora or indigenous microbiota15). The normal flora of humans contains a few eukaryotic fungi and protists, but bacteria are the most numerous and obvious microbial contributors. They depend on their host for nutrients, a stable environment, protection, and transport; in turn, the host obtains certain nutritional and digestive benefits, stimulation of the immune system, and protection against colonization and infection by pathogenic microbes11,19). The bacterial flora of the gastrointestinal (GI) tract has been studied more extensively than that of any other site20,21). The composition differs both between species, and within individuals of the same species. In humans, differences in the composition of the flora are influenced by age, diet, cultural conditions, and the use of antimicrobial drugs.
Prebiotics and probiotics have positive effects against micronutrient deficiencies22). Studies in animals using fructo-oligosacherides (a combination of inulin, polidextrose, and arabig gum) as prebiotics have facilitated the absorption of several nutrients such as calcium, magnesium, iron, zinc and copper22,23). These prebiotics are known to stimulate the growth and metabolism of probiotic bacteria in the gut24). The metabolism of inulin leads to the generation of short chain free fatty acids. These are utilized as energy sources by the intestinal epithelium and other tissues, and are thought to play additional roles such as enhancing the solubilization and absorption of minerals25,26). In this study, there was no difference between the lactobacilli levels of male and female rats at weanling age, but a drastic decrease in the lactobacilli population was noted in both male and female rats after feeding the rats on an AIN-93G diet, whether ID or CD. Further, lactobacilli levels were significantly lower in the ID groups as compared to the CD groups. This observation suggests a role for Fe in maintaining intestinal lactobacilli levels, since the two diets were composed of same calories, fiber, carbohydrate, vitamins and mineral levels, except that the iron deficient diet is devoid of FeSO4, the primary source of iron. It is a well-established fact that microorganisms, animals, and humans require Fe for their metabolic activities27). During the process of evolution, every form of life has acquired a mechanism of iron acquisition or sequestration. We speculate that the decrease in the lactobacilli population may be an effect of iron deficiency, since at the weanling stage these rats had higher levels of lactobacilli. This finding is in agreement with a community study which found that anemic young women exhibit significantly lower fecal lactobacilli levels than normal young women9). Further, Morais et al. reported that a higher number of Escherichia coli R-6 was redistributed to the mesenteric lymph nodes in the anemic group as compared to control group28). In contrast, other studies have reported that ferric iron repletion or overload leads to decreased intestinal microflora29). The reason for decrease may be due to oxidation of largely anaerobic environments by ferric iron. Foster et al. reported that in the Caco-2 cell model, elevated enterocyte iron status increases susceptibility to infection and exacerbates the mucosal inflammatory response initiated by microbial invasion by increasing cytokine/chemokine expression30). Other intestinal microflora such as E. coli and yeast populations were not significantly influenced by the iron deficient diet.
Pb is one of the ubiquitous environmental toxicants. In this study, when the rats were exposed to oral Pb, lactobacilli levels decreased considerably in both the CD and ID groups. It is well known that the gastrointestinal microflora is a complex ecological system, normally characterized by a flexible equilibrium. Bifidobacterium and lactobacilli are Gram-positive, lactic acid producing bacteria that constitute a major part of the intestinal microflora in humans and other mammals23,31). Similarly, Sadykov et al. have also reported that oral Pb exposure in rats resulted in a decrease of intestinal lactobacilli levels and a dramatic increase in the E. coli population16). In vitro studies mimicking the in vivo interactions between intestinal epithelial cells and lactobacilli and E. coli suggest that Pb inhibits the adhesion of lactobacilli to the epithelium, whereas the adhesion of E. coli cells was not inhibited but rather stimulated. In the current study, the associations between Pb and bacterial populations were not observed due to wide variation in lactobacilli and fecal Pb levels. No significant difference was observed in the E. coli or yeast levels among the Pb exposed rats fed either iron deficient or control diets.
Since workplace exposure is unavoidable, occupational management of the Pb acid battery industry utilizes a strategy of temporarily minimizing Pb exposure by shifting employees from an active exposure site to a work site with minimal or no exposure whenever their blood Pb levels rise above the permissible limit (>40 μg/dl)32). Therefore, in this study Pb exposure was temporarily halted in experimental animals (Pb - withdrawal), and then resumed. When Pb exposure was withdrawn, a significant increase in lactobacilli population was observed in ID rats which were previously exposed to Pb, suggesting the Pb has inhibitory effect on the lactobacilli population; whereas neither E. coli nor yeast populations were significantly altered when Pb was withdrawn. Fecal Pb levels in Pb-withdrawn rats also decreased significantly compared to the previous time point (at the end of the 4 weeks of Pb exposure). There was wide variation in the lactobacilli population and fecal Pb levels between individual rats in all of the experimental groups, which could explain the lack of statistical significance between fecal Pb levels and lactobacilli. It is possible that when Pb exposure is withdrawn, it may remove its inhibitory effect on the adhesion and multiplication of lactobacilli in intestines of these rats. Pb is known to inhibit such adhesion, thereby reducing the number of lactobacilli in the host intestine16). We speculate that when the Pb exposure was withdrawn, the native lactobacilli recovered their adhesive properties, improving their colonization abilities and ultimately increasing the population of lactobacilli.
Upon re-exposure to Pb, the lactobacilli population was decreased. Re-exposure of ID rats to Pb resulted in decreases in the lactobacilli population, although this effect was not significant. Since the ID rats had significantly higher levels of lactobacilli during the Pb withdrawal period, this was continued even in the Pb re-exposure phase, suggesting that oral Pb exposure leads to alterations in the gastrointestinal microbiota, particularly in beneficial lactobacilli. Similar observations have been reported following administration of oral antibiotics, which favors the growth of unwanted microorganisms16,33).
Conclusions
These results suggest that nutritional iron status influences intestinal lactobacilli levels. Concomitant oral Pb exposure results in further reduction of lactobacilli populations in iron deficient rats. Withdrawal of Pb exposure increases the lactobacilli population in iron deficient rats, suggesting that Pb plays a definite role in inhibiting the colonization of the intestinal epithelium by lactobacilli. Although not statistically significant, re-exposure to Pb decreased lactobacilli levels. However, no such effects were observed with respect to the E. coli and yeast populations.
Acknowledgments: We thank Dr. B. Sesikeran, the former Director, and Dr. Kalpagam Polasa, the former Director-In-Charge of the National Institute of Nutrition (NIN), Hyderabad for their interest in this study. YSR is supported by a Senior Research Fellow (SRF) from NIN- ICMR, Government of India.
Conflicts of interest: The authors declare that there are no conflicts of interest.
References
- 1). National Nutrition Monitoring Bureau (NNMB). . NNMB Micronutrient survey. Hyderabad: National Institute of Nutrition; 2002. [Google Scholar]
- 2).National Family Health Survey 3. (2007) India 2005-06; International Institute of Population Sciences, Mumbai, India and O RC Macro, Calverton, Maryland, USA. October 2007.
- 3). Hu Y, Cheng Z, Heller LI, et al. Kaempferol in red and pinto bean seed (Phaseolus vulgaris L.) coats inhibits iron bioavailability using an in vitro digestion/human Caco-2 cell model. J Agric Food Chem 2006; 54 (24): 9254-9261. [DOI] [PubMed] [Google Scholar]
- 4). Coudray C, Demigné C, Rayssiguier Y. Effects of dietary fibers on magnesium absorption in animals and humans. J Nutr 2003; 133 (1): 1-4. [DOI] [PubMed] [Google Scholar]
- 5). Coudray C, Feillet-Coudray C, Tressol JC, et al. Stimulatory effect of inulin on intestinal absorption of calcium and magnesium in rats is modulated by dietary calcium intakes short- and longtermbalance studies. Eur J Nutr 2005; 44 (5): 293-302. [DOI] [PubMed] [Google Scholar]
- 6). Oozeer R, Leplingard A, Mater DD, et al. Survival of lactobacillus casei in the human digestive tract after consumption of fermented milk. Appl Environ Microbiol 2006; 72 (8): 5615-5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Tako E, Glahn RP, Welch RM, et al. Dietary inulin affects the expression of intestinal enterocyte iron transporters, receptors and storage protein and alters the microbiota in the pig intestine. Br J Nutr 2008; 99 (3): 472-480. [DOI] [PubMed] [Google Scholar]
- 8). Meyer D, Stasse-Wolthuis M. The bifidogenic effect of inulin and oligofructose and its consequences for gut health. Eur J Clin Nutr 2009; 63 (11): 1277-1289. [DOI] [PubMed] [Google Scholar]
- 9). Balamurugan R, Mary RR, Chittaranjan S, et al. Low levels of faecal lactobacilli in women with iron-deficiency anaemia in south India. Br J Nutr 2010; 104 (7): 931-934. [DOI] [PubMed] [Google Scholar]
- 10). Brisbin JT, Gong J, Sharif S. Interactions between commensal bacteria and the gut-associated immune system of the chicken. Anim Health Res Rev 2008; 9 (1): 101-110. [DOI] [PubMed] [Google Scholar]
- 11). Tlaskalová-Hogenová H, Stepánková R, Hudcovic T, et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett 2004; 93 (2-3): 97-108. [DOI] [PubMed] [Google Scholar]
- 12). Zhang W, Wen K, Azevedo MS, et al. Lactic acid bacterial colonization and human rotavirus infection influence distribution and frequencies of monocytes/macrophages and dendritic cells in neonatal gnotobiotic pigs. Vet Immunol Immunopathol 2008; 121 (3-4): 222-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Ballew C, Bowman B. Recommending calcium to reduce lead toxicity in children: a critical review. Nutr Rev 2001; 59 (3 Pt 1): 71-79. [DOI] [PubMed] [Google Scholar]
- 14). Ettinger AS, Hu H, Hernandez-Avila M. Dietary calcium supplementation to lower blood lead levels in pregnancy and lactation. J Nutr Biochem 2007; 18 (3): 172-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Baron MDS, Davis CP. Bacteriology. University of Texas Medical Branch at Galveston. pp. Chapter 6. Normal Flora. 1996. [PubMed] [Google Scholar]
- 16). Sadykov R, Digel I, Artmann AT, et al. Oral lead exposure induces dysbacteriosis in rats. J Occup Health 2009; 51 (1): 64-73. [DOI] [PubMed] [Google Scholar]
- 17). Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993; 123 (11): 1939-1951. [DOI] [PubMed] [Google Scholar]
- 18). Granick S, Sassa S, Granick JL, et al. Assays for Porphyrins, δ-Aminolevulinic-Acid Dehydratase, and Porphyrinogen Synthetase in Microliter Samples of Whole Blood: Applications to Metabolic Defects Involving the Heme Pathway. Proc Natl Acad Sci USA 1972; 69 (9): 2381-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Musa HH, Wu SL, Zhu CH, et al. The Potential Benefits of Probiotics in Animal Production and Health. J Anim Vet Adv 2009; 8 (2): 313-321. [Google Scholar]
- 20). Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science 2005; 308 (5728): 1635-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Savage DC. Microbial Ecology of the Gastrointestinal Tract. Annu Rev Microbiol 1977; 31 (1): 107-133. [DOI] [PubMed] [Google Scholar]
- 22). Tesan FC, Rubeglio E, Maciero E, et al. Experimental Studies in Rats on the Effects on Growth and Intestinal Microflora of a New Mix Combining Calcium, Iron, Zinc and a Prebiotic in a Milk-like Vehicle. Open Nutraceuticals J 2009; 2: 127-135. [Google Scholar]
- 23). Laparra JM, Glahn RP, Miller DD. Assessing Potential Effects of Inulin and Probiotic Bacteria on Fe Availability from Common Beans (Phaseolus vulgaris L.) to Caco-2 Cells. J Food Sci 2009; 74 (2): H40-H46. [DOI] [PubMed] [Google Scholar]
- 24). Roberfroid MB. Prebiotics and probiotics: are they functional foods? Am J Clin Nutr 2000; 71 (6 Suppl): discussion 1688S-1690S; 1682S-1687S. [DOI] [PubMed] [Google Scholar]
- 25). Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr 2002; 22: 283-307. [DOI] [PubMed] [Google Scholar]
- 26). Scholz-Ahrens KE, Schaafsma G, van den, Heuvel EG, Schrezenmeir J. Effects of prebiotics on mineral metabolism. Am J Clin Nutr 2001; 73 (2 Suppl): 459S-464S. [DOI] [PubMed] [Google Scholar]
- 27). Mascotti DP, Rup D, Thach RE. Regulation of iron metabolism: Translational effects medicated by iron, heme and cytokines. Annu Rev Nutr 1995; 15: 239-261. [DOI] [PubMed] [Google Scholar]
- 28). Morais MB, Menchaca-Diaz JL, Liberatore AMA, et al. Iron-deficiency anemia increases intestinal bacterial translocation in rats. Critical Care 2005; 9 (suppl): S24. [Google Scholar]
- 29). Tompkins GR, O'Dell NL, Bryson IT, et al. The Effects of Dietary Ferric Iron and Iron Deprivation on the Bacterial Composition of the Mouse Intestine. Curr Microbiol 2001; 43 (1): 38-42. [DOI] [PubMed] [Google Scholar]
- 30). Foster SL, Richardson SH, Failla ML. Elevated Iron Status Increases Bacterial Invasion and Survival and Alters Cytokine/Chemokine mRNA Expression in Caco-2 Human Intestinal Cells. J Nutr 2001; 131 (5): 1452-1458. [DOI] [PubMed] [Google Scholar]
- 31). Jonkers D, Stockbrügger R. Review article: Probiotics in gastrointestinal and liver diseases. Aliment Pharmacol Ther 2007; 26 Suppl 2: 133-148. [DOI] [PubMed] [Google Scholar]
- 32). Workplace Exposure Standards and Biological Exposure Indices. 4th Edition, PO Box 3705, Wellington 6011, New Zealand: Published by the Department of Labour; 2010. [Google Scholar]
- 33). Gus'kova T, Pushkina T, Radkevich T. Possibility of dysbacteirosis development upon using drugs belonging to different pharmacological groups. Pharm Chem J 2006; 7: 10-11. [Google Scholar]