Abstract
The left internal mammary artery (LIMA) graft used in coronary artery bypass grafting (CABG) reliably revascularizes the left anterior descending artery (LAD) and has excellent long-term patency. Here, we report a rare case of ST-elevation myocardial infarction caused by acute occlusion of the LIMA in the late postoperative period. A 62-year-old man who underwent CABG for a myocardial infarction 15 years previously was transferred to our hospital for the recurrence of acute anterior myocardial infarction. Emergent cardiac catheterization revealed complete occlusion from the mid-portion of the LIMA to the LAD. We successfully treated this patient with primary stent implantation to the culprit LIMA lesion using intravascular ultrasound guidance.
<Learning Objective: The left internal mammary artery (LIMA) is commonly used as the conduit to bypass the left anterior descending artery (LAD) and has shown excellent long-term patency. However, we have experienced a rare case with acute myocardial infarction due to acute occlusion of LIMA in late postoperative period. We should carefully monitor, considering that the LIMA could possibly undergo arteriosclerotic changes.>
Keywords: Coronary artery bypass graft, Internal mammary artery, Acute myocardial infarction
Introduction
The left internal mammary artery (LIMA) is commonly used as the conduit to bypass the left anterior descending artery (LAD) and has shown excellent long-term patency 1, 2, 3. LIMA graft failure is caused by dissection, hematoma, spasm, or stenosis at the anastomosis, and mostly occurs in the early postoperative phase. Acute occlusion of the LIMA causing ST-elevation myocardial infarction (STEMI) in the late postoperative period is extremely rare, and its pathological mechanism is unclear.
Case report
A 62-year-old male patient had been admitted to another hospital 15 years previously for myocardial infarction. Coronary angiography showed total occlusion of the proximal LAD segment, for which emergent coronary artery bypass grafting (CABG) of the LIMA to the LAD was performed. After surgery, he was prescribed aspirin and statin. No adverse cardiac events occurred until he was transferred to our hospital because of acute chest oppression.
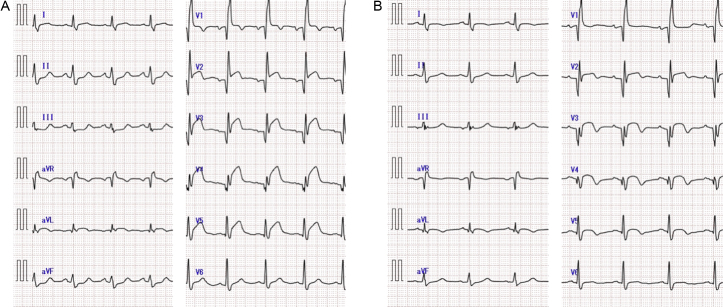
On admission to our hospital, the blood pressure was 110/75 mmHg, pulse rate was 98 beats/min, and the body temperature was 36.2 °C. The respiratory rate was 20 breaths/min and oxygen saturation was 96% in room air. Electrocardiography showed ST-elevation in the anterior leads and reciprocal changes in the inferior leads (Fig. 1A). The patient had no coronary risk factors except dyslipidemia, and the low-density lipoprotein level was 96 mg/dl after treatment with 10 mg of atorvastatin. Echocardiography showed impaired motion of the anterior wall of the left ventricle without mechanical complications.
Fig. 1.
(A) Electrocardiogram showing right bundle branch block with normal sinus rhythm and ST-elevation in the anterior leads and reciprocal changes in the inferior leads. (B) Electrocardiogram after stent implantation showing good resolution of ST segments.
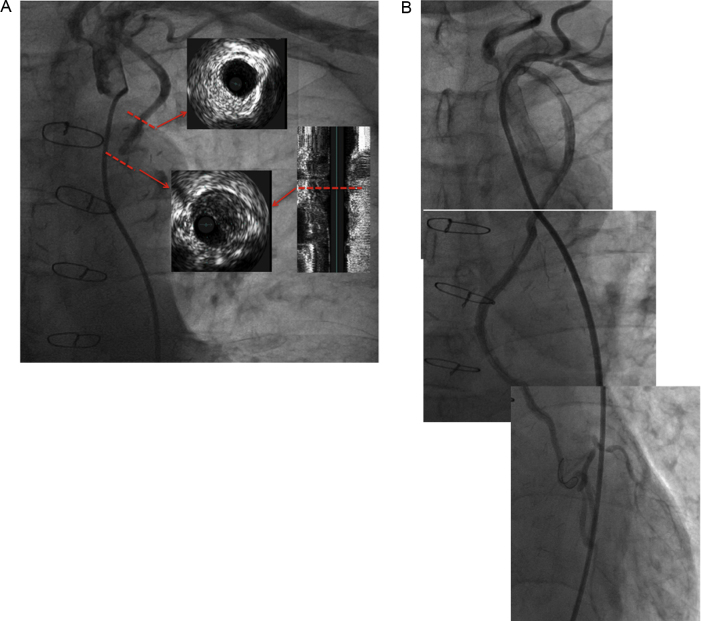
Emergent angiography showed total occlusion of the proximal LAD segment and total occlusion of the middle LIMA portion (Fig. 2A). We determined that the LIMA occlusion was causing anterior STEMI, and performed emergent intervention.
Fig. 2.
(A) Coronary angiogram of the left internal mammary artery–left anterior descending artery showing total occlusion of the middle portion. Intravascular ultrasonogram showing the culprit lesion containing significant plaque and demonstrating positive remodeling. (B) Final angiogram after stent implantation showing good dilatation and reperfusion.
Procedure
Using a 6 French JR 4 guiding catheter (Boston Scientific, Minneapolis, MN, USA) via a right femoral approach, a 0.014-in. floppy guide wire (Terumo, Tokyo, Japan) was advanced into the distal portion of the LIMA graft. Thrombolysis in myocardial infarction-3 flow was obtained by dilatation with a 2.0-mm balloon (Orbus Neich, Hong Kong, China). Intravascular ultrasonography (IVUS; Volcano Corporation, San Diego, CA, USA) revealed that the culprit lesion contained significant plaque and a mobile mass (Fig. 2A). We implanted a drug-eluting stent (Taxus Element® 2.5 mm × 32 mm; Boston Scientific). The final angiogram after stent implantation showed good dilatation of the lesion and good reperfusion (Fig. 2B). Electrocardiography after the procedure showed good resolution of ST segments (Fig. 1B). The patient's maximum creatine kinase level was 3520 IU/ml and his clinical course was good. He was discharged from the hospital after 10 days.
Discussion
We encountered a case of STEMI caused by acute LIMA occlusion 15 years after graft surgery. LIMA–LAD grafts have been associated with short- and long-term patency, and have improved survival rates compared with saphenous vein grafts 1, 2. The 10-year patency rate of LIMA grafts is approximately 90% if the graft is patent 1 week after the procedure [3]. Most LIMA occlusions occur in the early postoperative period and are associated with surgical complications such as dissection, hematoma, spasm, or stenosis at the anastomosis site. STEMI caused by occlusion of the LIMA in the late postoperative period is extremely rare; to our knowledge, only one case has been reported by Yong et al. [4]. They reported a STEMI case of LIMA occlusion at 7 years after CABG, and the patient died due to the recurrence of LIMA occlusion. The long-term patency of LIMA grafts has been reported to be related to the degree of competitive flow between the LAD and the LIMA [5]. However, the other reported case also presented with chronic total occlusion of LAD indicating that it may not be caused by competitive flow.
The endothelial layer of the LIMA generally has fewer fenestrations, lower intercellular junction permeability, greater amounts of anti-thrombotic molecules including heparin sulfate and tissue plasminogen activator, and higher endothelial nitric oxide production. The physiological properties of the LIMA include higher vasodilator sensitivity, lower vasoconstrictor sensitivity, rapid lipolysis, and less activation of lipid synthesis [6]. Arciniegas et al. [7] reported that only 2 of the 15 segments in the harvested LIMA were found to have atherosclerotic plaques. Since the above-mentioned features prevent arteriosclerotic changes in the LIMA, it is widely used as a graft and has excellent long-term patency. In our case, angiography revealed chronic proximal occlusion of the LAD and mid-portion occlusion of the LIMA. We determined that the LIMA occlusion was the culprit lesion and performed primary stent implantation using IVUS guidance. The IVUS image showed positive remodeling of the culprit lesion, significant soft plaque, and a mobile thrombus-like mass, which could indicate that plaque vulnerability at the lesion caused the acute LIMA occlusion. The patient did not receive lipid-lowering therapy even though the low-density lipoprotein cholesterol level was 146 mg/dl on admission. Although studies investigating lipid-lowering therapy and low-density lipoprotein cholesterol levels in CABG patients are limited, we think that lipid-lowering therapy with statin should also be recommended in CABG patients.
Percutaneous intervention for LIMA grafts has reported to be performed safely with higher success rates and lower in-hospital complication rates [8]. Reports have indicated that balloon angioplasty is associated with low restenosis rates for LIMA anastomosis sites but higher restenosis rates for LIMA body lesions 8, 9. Canham et al. [10] reported that the LIMA diameter is 1.9–2.6 mm with a wall thickness of 180–430 μm. Because of the small diameter of the LIMA and the high restenosis rate of balloon angioplasty, we chose a drug-eluting stent in this case. No adverse cardiac events have been reported 4 years after stent implantation.
In summary, acute occlusion of the LIMA in the late postoperative period is rare and its mechanism remains unknown; however, considering that the LIMA and saphenous vein graft could possibly undergo arteriosclerotic changes, the patient should be monitored by symptoms and exercise stress test, or computed tomography–angiography if needed.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Loop F.D., Lytle B.W., Cosgrove D.M., Stewart R.W., Goormastic M., Williams G.W., Golding L.A., Gill C.C., Taylor P.C., Sheldon W.C., Proudfit W.L. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314:1–6. doi: 10.1056/NEJM198601023140101. [DOI] [PubMed] [Google Scholar]
- 2.Cameron A., Davis K.B., Green G., Schaff H.V. Coronary bypass surgery with internal-thoracic-artery grafts—effects on survival over a 15-year period. N Engl J Med. 1996;334:216–219. doi: 10.1056/NEJM199601253340402. [DOI] [PubMed] [Google Scholar]
- 3.Goldman S., Zadina K., Moritz T., Ovitt T., Sethi G., Copeland J.G., Thottapurathu L., Krasnicka B., Ellis N., Anderson R.J., Henderson W., VA Cooperative Study Group Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol. 2004;44:2149–2156. doi: 10.1016/j.jacc.2004.08.064. [DOI] [PubMed] [Google Scholar]
- 4.Yong A., Groenestein P., Brieger D., Lowe H., Kritharides L. Late thrombotic occlusion of a left internal mammary artery graft causing ST-elevation myocardial infarction. Int J Cardiol. 2010;142:e42–e44. doi: 10.1016/j.ijcard.2008.11.184. [DOI] [PubMed] [Google Scholar]
- 5.Swillens A., De Witte M., Nordgaard H., Løvstakken L., Van Loo D., Trachet B., Vierendeels J., Segers P. Effect of the degree of LAD stenosis on “competitive flow” and flow field characteristics in LIMA-to-LAD bypass surgery. Med Biol Eng Comput. 2012;50:839–849. doi: 10.1007/s11517-012-0927-3. [DOI] [PubMed] [Google Scholar]
- 6.Motwani J.G., Topol E.J. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97:916–931. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 7.Arciniegas E., Fermin E., Tortoledo F., Vasquez J.R., Bello A. Characterization of the atherosclerotic plaque in the internal mammary artery. Cathet Cardiovasc Diagn. 1998;43:413–420. doi: 10.1002/(sici)1097-0304(199804)43:4<413::aid-ccd12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 8.Gruberg L., Dangas G., Mehran R., Hong M.K., Waksman R., Mintz G.S., Kent K.M., Pichard A.D., Satler L.F., Lansky A.J., Stone G.W., Leon M.B. Percutaneous revascularization of the internal mammary artery graft: short- and long-term outcomes. J Am Coll Cardiol. 2000;35:944–948. doi: 10.1016/s0735-1097(99)00652-x. [DOI] [PubMed] [Google Scholar]
- 9.Hearne S.E., Davidson C.J., Zidar J.P., Phillips H.R., Stack R.S., Sketch M.H., Jr. Internal mammary artery graft angioplasty: acute and long-term outcome. Cathet Cardiovasc Diagn. 1998;44:153–156. doi: 10.1002/(sici)1097-0304(199806)44:2<153::aid-ccd6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 10.Canham P.B., Finlay H.M., Boughner D.R. Contrasting structure of the saphenous vein and internal mammary artery used as coronary bypass vessels. Cardiovasc Res. 1997;34:557–567. doi: 10.1016/s0008-6363(97)00056-4. [DOI] [PubMed] [Google Scholar]