Abstract
We describe the case of a 61-year-old Japanese woman who developed acute heart failure 5 years after chemoradiotherapy for breast cancer. The patient received less than the cardiotoxic dose of docetaxel, epirubicin, cyclophosphamide, and fluorouracil and experienced no cardiovascular complications in the 5 years between the onset of chemoradiotherapy and the onset of acute heart failure. Cardiac catheterization was performed and elevation of end diastolic pressure of both ventricles was observed. Endomyocardial biopsy showed progressive replacement fibrosis in the subendocardium. Normal thickness of the right endocardium is <20 μm. Surprisingly, our patient had a fibrous subendocardium that was 100–200 μm thick. Ultrastructural abnormalities similar to those observed in anthracycline cardiotoxicity were evident on electron micrographs. This case report demonstrates the unique pathophysiology of heart failure in a patient who received less than the cardiotoxic dose of antineoplastic agents. Recent protocols have decreased the dosage of cardiotoxic agents; however, even these reduced doses might not be safe for all Japanese individuals and may cause subclinical cardiovascular damage and late-onset heart failure. Clinicians should monitor cancer survivors carefully, even if antineoplastic agents were administered under the cardiotoxic dose.
<Learning objective: Intensive chemotherapy is commonly used to treat cancer patients. Recent protocols have decreased the dose of cardiotoxic agents; however, even these reduced doses might not be safe and may cause subclinical cardiovascular damage and late-onset heart failure. Clinicians should monitor cancer survivors carefully, even if antineoplastic agents were administered under the toxic dose.>
Keywords: Breast cancer, Cardiotoxicity, Chemoradiotherapy, Subendocardial fibrosis
Introduction
In Japan, the incidence of breast cancer has increased in recent years, but the survival of patients with newly diagnosed breast cancer has improved [1]. With advancements in chemotherapy regimens, mortality rates are decreasing by 2.3% annually [2], and the 5-year survival rate for advanced breast cancer has increased to 30% [3]. In the past, cardiovascular complications were rare in breast cancer patients because the lifespan of a patient with advanced cancer was too short for the issue to manifest. However, more recent reports indicate that there are now a considerable number of cancer survivors who develop chemotherapy-related cardiac dysfunction and thus are under periodic observation by a cardiologist [4]. Here we describe a breast cancer patient who developed acute heart failure (HF) 5 years after chemoradiotherapy. We observed unique subendocardial fibrosis in the endomyocardial biopsy despite the fact that all antineoplastic agents had been delivered under the cardiotoxic dose.
Case report
A 61-year-old Japanese woman experienced shortness of breath in May 2012, 5 years after she had undergone a partial mastectomy and received subsequent chemoradiotherapy for breast cancer. Three weeks later, she developed dyspnea, general fatigue, and loss of appetite, and she was therefore admitted to our hospital with orthopnea. A chest radiograph revealed cardiomegaly, pulmonary congestion, and bilateral pleural effusion. At the time of hospital admission, the patient's weight had increased by 5 kg since her last regular medical check-up 4 weeks earlier. Physical examination showed moist rale in bilateral lower lung fields, and the patient was diagnosed with acute pulmonary edema.
The patient had been diagnosed with right breast cancer without metastasis in October 2006. The tumor was 5.3 cm in size and located in the lower medial region of the right chest (T3N1M0, stage IIIa). Initial chemotherapy with docetaxel (cumulative dose, 300 mg/m2) proved ineffective. After cardiovascular screening, she received epirubicin (cumulative dose, 240 mg/m2) in combination with cyclophosphamide and fluorouracil (cumulative dose, 2000 mg/m2 for each) from February to April 2007. She underwent a partial mastectomy in May 2007, followed by whole-breast irradiation of 60 Gy. The patient did not display any clinical symptoms of HF during chemoradiotherapy. Echocardiography showed normal left ventricular (LV) size and LV ejection fraction (LVEF; 5.2 cm and 70%, respectively). After these therapies, the concentration of the tumor markers CEA and CA15-3 decreased and then remained within their normal ranges.
On hospital admission in May 2012, an electrocardiogram showed poor R-wave progression and ST depression with inverted T in precordial leads (Fig. 1C). N-terminal pro-B-type natriuretic peptide was elevated, at 4684 pg/mL. A test for serum troponin T (Elecsys® troponin T STAT test; Roche, Basel, Switzerland) was negative, and creatine kinase level was normal. Echocardiography revealed a dilated and diffusely hypokinetic LV (LV size 6.3 cm and LVEF 34%).
Fig. 1.
Electrocardiograms before chemotherapy (October, 2006) (A), after chemotherapy (April, 2007) (B), at the time of hospital admission due to heart failure (May, 2012) (C), and 6 months later (November, 2012) (D) (10 mm/mV). Slight and non-specific ST segment depression was observed in (A) and (B), but there were no significant differences. Electrocardiogram at the time of heart failure admission showed diminished QRS amplitude, poor R waves in precordial leads, and ST segment depression associated with T-wave inversion in V5,6 (C). Six months later, the QRS amplitude almost normalized in precordial leads, but ST segment depression remained in II, III, aVF, V5,6 (D).
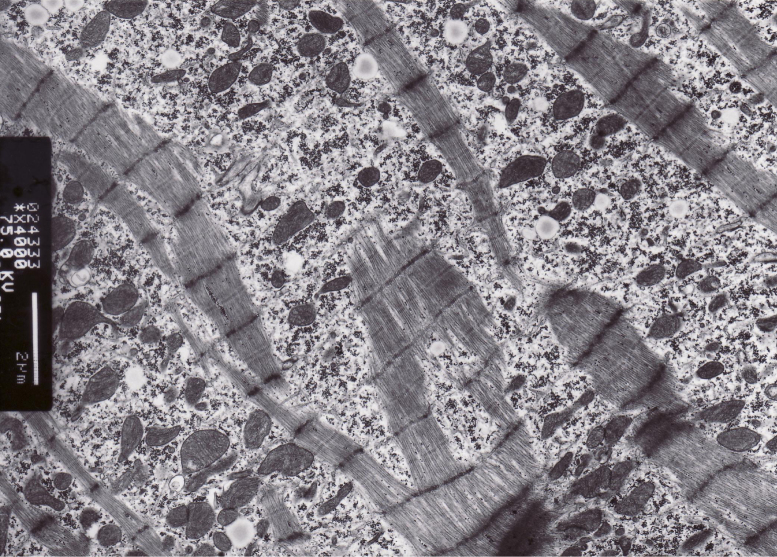
The patient was diagnosed with acute cardiogenic pulmonary edema and was administered oxygen, diuretics (40 mg/d of furosemide), and vasodilators (10 mg/d of nitroglycerin and 2.5 mg/d of enalapril). She recovered from dyspnea, and cardiac catheterization was performed on day 9 after hospital admission. Mean pulmonary artery wedge pressure, pulmonary artery pressure, and cardiac index were normalized at 13 mmHg, 20 mmHg, and 3.2 L/min/m2, respectively. However, end-diastolic pressure was elevated in the right and left ventricles (13 and 20 mmHg, respectively). Dip and plateau pattern of ventricular diastolic pressure was not observed. Left ventriculography indicated a dilated LV (131 mL/m2 of end diastolic volume index) with diffuse hypokinesis (LVEF 27%). Coronary arteries were normal. Endomyocardial biopsy from the right ventricle showed an extremely thickened subendocardium (100–200 μm thick) with replacement fibrosis, and some smooth muscle cells and degenerated myocytes were surrounded by collagen fibers (Fig. 2). There was a small amount of interstitial fibrosis and infiltration of mononuclear cells. Although myocytes appeared intact under a light microscope, injury to the ultrastructure of myocytes was evident in electron micrographs (Fig. 3).
Fig. 2.
Histopathological and immunohistochemical findings from the endomyocardial biopsy. (A) Subendocardial thickening was observed. Subendocardial thickness at the location indicated by the asterisk is 100 μm (hematoxylin–eosin staining, original magnification 200×). (B) Some degenerated myocytes were observed in the fibrous subendocardium (arrow). Smooth muscle cells were also observed in the subendocardium. Neither interstitial fibrosis nor edema was observed (Azan Mallory stain, original magnification 200×). (C) Subendocardial thickness reached 200 μm around some myocytes (arrows). The endothelium was observed as a red-violet-stained single layer (asterisk). The shape of myocytes appeared intact (Gitter stain, original magnification 200×). (D) Most myocytes were positive for anti-desmin stain (Dako), but myocytes in the subendocardial fibrotic tissue (arrows) were relatively less stained (original magnification 200×).
Fig. 3.
Electron micrograph of the endomyocardial biopsy. Myofibrillar disruption and loss, different sized clusters of mitochondria, and distorted Z-bands are evident. Some of the mitochondria appear swollen with loss of compact cristae.
On day 12 after hospital admission, the patient was examined using myocardial single-photon emission computed tomography (SPECT) with 201Tl and 123I-β-methyl-iodophenyl-pentadecanoic acid (BMIPP) (Fig. 4A). Distribution of 201Tl and 123I-BMIPP was patchy and independent of coronary perfusion across the whole heart. Quantitative gated SPECT of the LV showed diffuse hypokinesis with LVEF of 30% (Fig. 4B).
Fig. 4.
Dual single-photon emission computed tomography (SPECT) with 201Tl and 123I-β-methyl-iodophenyl-pentadecanoic acid (123I-BMIPP) on day 12 after hospital admission. (A) Myocardial uptake of 201Tl and 123I-BMIPP was similar across the whole heart. Uptake was almost normal, but patches of slightly lower uptake were present, located independent of coronary perfusion. (B) A three-dimensional surface-rendered image of the left ventricle created by electrocardiogram-gated SPECT perfusion images. The green mesh represents the endocardium at end diastole, and the violet surface represents the endocardium at end systole. Left ventricular ejection fraction (LVEF) was quantified based on the volume change. ANT, anterior region; LAT, lateral region; SEPT, septal region.
The patient gradually recovered and was discharged on day 16 after hospital admission. Six months later, LV size had normalized, and LVEF had recovered to 47% in echocardiography, but ST segment depression remained in II, III, aVF, V5,6 in electrocardiogram (Fig. 1D). Although she needed diuretics and vasodilators for peripheral edema and pulmonary edema, she regained her social and familial activities.
Discussion
Despite receiving non-toxic doses of antineoplastic agents, our patient developed HF with progressive subendocardial fibrosis 5 years after the start of treatment for breast cancer. Normal thickness of the right endocardium is <20 μm [5], but our patient had a fibrous subendocardium that was 100–200 μm thick. Mortensen et al. [5] reported that 10 of the 11 patients with anthracycline-associated cardiotoxicity had subendocardial fibrosis, and the mean subendocardial thickness of the right ventricle in these 10 patients was 48 μm; hence, the subendocardial thickness in our patient surpassed that of the most severe case in the Mortensen et al. study, which was 60–100 μm in a 55-year-old woman with breast cancer who was treated with doxorubicin (575 mg/m2), cyclophosphamide, and irradiation, and died suddenly. Our case was treated with a modest amount of epirubicin, which was less toxic; therefore, the severity of subendocardial fibrosis in our patient was surprising.
In addition to subendocardial fibrosis, ultrastructural abnormalities similar to those of anthracycline cardiotoxicity were also observed in electron micrographs. However, the doses of chemotherapeutic agents that our patient had received were considerably lower than the cardiotoxic doses [6]. Docetaxel was stopped at 300 mg/m2 because of poor response. In a trial using 450 mg/m2 of docetaxel in combination with doxorubicin and cyclophosphamide, only 1.6% of the patients developed congestive HF in 5 years [7]. Our patient also received 240 mg/m2 of epirubicin in combination with 2000 mg/m2 cyclophosphamide and fluorouracil. Ryberg et al. [8] reported that the risk of HF was approximately 5% for a group of 60-year-old female breast cancer patients who had been treated with 700 mg/m2 epirubicin. The dose received by our patient was much lower than this dose. Notably, cyclophosphamide has acute cardiac toxicity at doses of 120–170 mg/kg [4], and our patient had received only one-third of the lower limit of this range.
The potential adverse effects of whole-breast irradiation should also be considered for our patient. She received 60 Gy of irradiation near the right heart 5 years prior to the onset of acute cardiogenic pulmonary edema. Radiation-induced damage to the heart and/or vasculature increases the subsequent rate of ischemic heart disease [9]. Recently, Darby et al. [10] reported that incidental exposure of the heart to radiotherapy for breast cancer increased the rate of major coronary events (myocardial infarction, coronary revascularization, or death from ischemic heart disease) by 7.4% per Gy, with no apparent threshold, and the increase started within 5 years of radiotherapy. For our patient, the combination of chemotherapy and radiotherapy was clearly effective for treating her cancer, but may have adversely affected her heart.
There are several possible reasons for the severe injury to the subendocardium as presented by our patient. First, perfusion of chemotherapeutic agents into the subendocardium through the endocardium during chemotherapy might have negatively impacted adjacent myocytes. Second, compared with the epicardium, the subendocardium is more vulnerable to various stressors including ischemia, tachycardia, excessive preload, and cardiotoxicity. Although coronary arteriography of our patient appeared normal at the epicardium, the subendocardial microvasculature might have been injured by antineoplastic agents and radiation. We speculate that the combination of chemotherapy and radiotherapy may synergistically amplify their cardiotoxicities and inflict local damage on the most vulnerable region of heart, i.e. the subendocardium.
The incidence of breast cancer is increasing in Japan [1]. Chemotherapy is believed to be safe because improved protocols avoid high doses of cardiotoxic agents by combining them with antineoplastic agents. However, the cardiotoxic threshold doses of these agents in Japanese individuals remain uncertain because most clinical trials of breast cancer have been performed in the USA and have included few patients of Japanese ethnicity. Recent protocols might not be safe for ethnic Japanese individuals and may cause subclinical cardiovascular damage and late-onset HF. Therefore, clinicians should monitor cancer survivors carefully, even if all antineoplastic agents were administered under the toxic level. We recommend that all survivors have an annual check-up with electrocardiogram and chest radiograph for HF screening. Breast surgeons and cardiovascular specialists should work together for early detection of asymptomatic patients with LV dysfunction.
Financial support and disclosure
The authors report no financial or material support for this study and no affiliation or financial involvement with any organization or entity with a financial interest in the topic.
References
- 1.Cancer Statistics in Japan. Center for Cancer Control and Information Services; 2014. Estimated cancer incidence in Japan, 1975–2010, and 5-year relative survival rate by site in six cancer registries in Japan.http://ganjoho.jp/pro/statistics/en/table_download.html [Google Scholar]
- 2.Dawood S., Broglio K., Gonzalez-Angulo A.M., Buzdar A.U., Hortobagyi G.N., Giordano S.H. Trends in survival over the past two decades among white and black patients with newly diagnosed stage IV breast cancer. J Clin Oncol. 2008;26:4891–4898. doi: 10.1200/JCO.2007.14.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Cancer Society; Atlanta: 2012. Breast cancer facts and figures 2011–2012. [Google Scholar]
- 4.Floyd J.D., Nguyen D.T., Lobins R.L., Bashir Q., Doll D.C., Perry M.C. Cardiotoxicity of cancer therapy. J Clin Oncol. 2005;23:7685–7696. doi: 10.1200/JCO.2005.08.789. [DOI] [PubMed] [Google Scholar]
- 5.Mortensen S.A., Olsen H.S., Baandrup U. Chronic anthracycline cardiotoxicity: haemodynamic and histopathological manifestations suggesting a restrictive endomyocardial disease. Br Heart J. 1986;55:274–282. doi: 10.1136/hrt.55.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minami M., Matsumoto S., Horiuchi H. Cardiovascular side-effects of modern cancer therapy. Circ J. 2010;74:1779–1786. doi: 10.1253/circj.cj-10-0632. [DOI] [PubMed] [Google Scholar]
- 7.Martin M., Pienkowski T., Mackey J., Pawlicki M., Guastalla J.P., Weaver C., Tomiak E., Al-Tweigeri T., Chap L., Juhos E., Guevin R., Howell A., Fornander T., Hainsworth J., Coleman R. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 8.Ryberg M., Nielsen D., Cortese G., Nielsen G., Skovsgaard T., Andersen P.K. New insight into epirubicin cardiac toxicity: competing risks analysis of 1097 breast cancer patients. J Natl Cancer Inst. 2008;100:1058–1067. doi: 10.1093/jnci/djn206. [DOI] [PubMed] [Google Scholar]
- 9.Darby S.C., Cutter D.J., Boerma M., Constine L.S., Fajardo L.F., Kodama K., Mabuchi K., Marks L.B., Mettler F.A., Pierce L.J., Trott K.R., Yeh E.T., Shore R.E. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010;76:656–665. doi: 10.1016/j.ijrobp.2009.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darby S.C., Ewertz M., McGale P., Bennet A.M., Blom-Goldman U., Brønnum D., Correa C., Cutter D., Gagliardi G., Gigante B., Jensen M.B., Nisbet A., Peto R., Rahimi K., Taylor C. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]