Abstract
We report an anorexic adolescent girl with an intermittent Brugada syndrome. A 14-year-old anorexic girl with a body mass index (BMI) of 13.15 kg/m2 was admitted in the acute state of the disease with an ST elevation in V1 and V2, suggestive of Brugada syndrome. After 1 month of re-feeding, a control electrograph (ECG) was normal, but after an 8-month follow-up control with a nearly normal BMI, the ECG was again suggestive of Brugada syndrome. A genetic analysis of the gene SNC5A established a genetic change (p Leu 1582 pro), which provides the final explanation for the Brugada syndrome. Every rhythm problem in the acute state or during the re-feeding procedure deserves a strict follow-up to distinguish iatrogenic from heritable rhythm problems.
<Learning objective: (i) We report the first case of a patient with anorexia nervosa with an intermittent Brugada syndrome. (ii) Moderate hypothermia can decrease the depolarization of pacemaker cells and cause ST-segment changes. (iii) Every rhythm problem in the acute state or during the re-feeding procedure deserves a strict follow-up to distinguish iatrogenic from heritable rhythm problems. (iv) A genetic analysis can make the distinction and is necessary to give advice for the future lifestyle of the patient.>
Keywords: Anorexia nervosa, Brugada syndrome, Hypothermia, Weight loss, Iatrogenic
Introduction
The most common rhythm disorders in the anorexic population are extreme bradycardia <50 bpm with a prolonged QT interval (15–40%) and sudden death in up to 10%. Other electrograph (ECG) abnormalities seen in these patients are low voltage of P waves and QRS complexes, prolonged QTc, rightward QRS axis, non-specific ST-T changes, and the presence of U waves. Less frequent are ectopic atrial foci and second degree AV block Mobitz type I [1].
Minor T wave changes, such as T-wave inversion in V4 and T flattening, are also noticed.
ST depression is observed in patients with anorexia nervosa with extreme weight loss. The explanation of ST disturbances can be found in the early repolarization and acidosis due to hypothermia. Moderate hypothermia can decrease the depolarization of pacemaker cells and cause ST-segment changes.
Case report
A 14-year-old girl with anorexia nervosa was admitted to the department of eating disorders with a body mass index (BMI) of 13.15 kg/m2 (weight: 35.8 kg and height: 165 cm) and a weight loss of 26.94% in less than 6 months.
In the personal antecedents, she had no episodes of palpitations or suspicious syncopes. She once fainted following a tooth extraction. In childhood she had atopic eczema and there were no surgical procedures or hospitalizations.
The girl has an older brother and a sister age 22 and 20 years, respectively, and both had a syncope during a peripheral venous blood sample. No other suspicious episodes were noticed. Both parents are in good health. The father is an intense sportsman but never had any syncope. In the other family members we recorded neither had syncope nor sudden death.
During the physical examination, a cachectic girl (BMI: 13.15 kg/m2, core temperature: 34.9 °C) presented with the typical features of anorexia nervosa namely lanugo hairs, acrocyanosis, hypotension (87/46 mm Hg) and bradycardia 39 bpm. A syncope was not observed, neither chest pain and no medication were taken.
Routine blood chemistry (creatinine, blood urea nitrogen, potassium, sodium, bicarbonate, magnesium and calcium) and thyroid hormones were normal. There was a high ferritin level (258 ng/ml) (N: 5.0–122 ng/ml) and a slight elevation of the liver transaminases [aspartate aminotransferase (58 U/L) (N: 10–30 U/L), alanine aminotransferase (128 U/L) (N: 17–44 U/L)] and troponin I 0.0039 ng/ml (N: <0.0034 ng/ml).
Creatinine kinase and lactate dehydrogenase levels were normal. The levels of luteinizing hormone (LH), follicle-stimulating hormone (FSH), and insulin-growth factor-1 were low.
In the acute phase, an ECG together with a 2-D-echocardiography with Doppler was obtained.
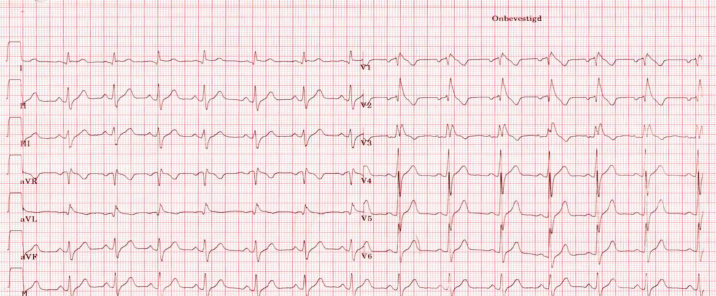
The ECG showed ST elevation in V1, V2 and less in V3 (suggestive of Brugada syndrome) (Fig. 1). The rhythm was 49 bpm, axis 11°, PR interval: 144 ms, QRS: 114 ms, QT: 422 ms, and QTc 384 ms. Echocardiography also showed a pericardial effusion of 0.9–1.1 cm (apex) and a decreased left ventricular mass/height2.7: 17.57 g/m2.7
Fig. 1.
A 12-lead electrocardiogram – ST elevation in V1, V2 and less in V3. Rhythm: 49 bpm, axis 11°, PR interval: 144 ms, QRS: 114 ms, QT: 422 ms, and QTc 384 ms. Type I electrocardiogram.
Magnetic resonance imaging of the heart confirmed a moderate pericardial effusion, end diastolic volume of 54.04 ml, end-systolic volume of 26.47 ml, stroke volume of 1654.69 ml, and an ejection fraction of 51% (Fig. 2).
Fig. 2.

Magnetic resonance imaging of the heart: moderate pericardial effusion.
Compatible with the bradyphrenic character of the patient, magnetic resonance imaging of the brain showed “cerebral atrophy” in the frontotemporal and cerebellar region (Fig. 3).
Fig. 3.

Magnetic resonance imaging of the brain: “cerebral atrophy” in the frontotemporal and cerebellar region.
ECG at rest of both parents was normal. After 1 month of slow re-feeding BMI: 13.7 kg/m2 (to prevent the re-feeding syndrome), there was a normalization of the ECG with no ST elevation in V1 and V2 (Fig. 4). But after 8 months of re-feeding (BMI: 18.5 kg/m2) (weight: 51 kg and height: 166 cm), a control ECG demonstrated Brugada syndrome again in V1 and V2 (Fig. 5).
Fig. 4.
After 1 month of re-feeding: normalization of the electrocardiogram.
Fig. 5.
After 8 months of re-feeding: body mass index: 18.5 kg/m2 again Brugada syndrome typical type I electrocardiogram (ECG). Three different 12-lead ECGs are shown.
The patient did not receive any treatment but education and prevention of arrhythmia via lifestyle awareness (avoidance of certain medications, fever treated with antipyretics) was discussed with the patient and her parents. A genetic analysis of the gene SNC5A established a genetic change (p Leu 1582 pro), which provides an explanation for the Brugada sequence. The mother had a normal genetic analysis and a normal cardiologic examination. The father refused any further cardiologic or genetic analysis.
Discussion
This is the first case of an anorexia patient with Brugada syndrome. In the early follow-up after 1 month of re-feeding, she had a normal ECG and we thought that extreme weight loss and hypothermia were the explanation for the ST-changes. In the literature, we found several cases of a Brugada-like electrocardiography pattern induced by severe hypothermia 2, 3. The reason is that severe hyponatremia may be expected to have the same effect by reducing inward sodium current [4]. There were no electrolyte disturbances in our patient.
Instead of hypothermia, the association with fever and Brugada syndrome is more known. Adler et al. [5] showed that Brugada ECGs are more (20 times more) prevalent during fever. Fever also has a comparable preferred gender policy for the Brugada syndrome ECG and those patients with Brugada syndrome who have a loss of function SCN5 A mutation may exhibit a fever-sensitive form 6, 7. The mechanism remains unclear: one explanation is that the mutation alters the temperature sensitivity of fast inactivation of the sodium channel [8], whereas other observations have shown that the temperature-dependent properties of the wild-type sodium channel itself might lead to the typical Brugada ECG during fever [9]. The same mutation was discovered in our patient and although fever was not registered, but instead hypothermia with a core-temperature of 34.9 °C was found in the subject, we could consider that the mutation is temperature-sensitive.
Because Brugada syndrome is more prevalent in men than in women with an estimated ratio of 8–10:1 [10], an additional exception is our patient's gender. The larger number of Brugada cases in men has been explained by the presence of significantly higher density of Ito currents in males according to the repolarization theory, although a second hypothesis suggests that testosterone may be responsible for gender differences [11]. An explanation in our patient could be acquired amenorrheic state in anorexics because of their extreme weight loss (low LH, FSH levels). Matsuo et al. [12] reported that in their study the Brugada cases had a significantly lower BMI and lower body weight. Estradiol and the female hormones reduce the expression of Kv 4-3 channels, components of the Ito channels [13]. In patients with low visceral fat, there are low levels of insulin, leptin [14], as we observe in anorexic patients, so these patients are more prone to have Brugada-like ECGs. In the later follow-up period, precisely after 8 months of re-feeding, with a nearly normal BMI of 18.5 kg/m2 we noticed again an ECG with a Brugada-like sequence. With this intermittent appearance of the Brugada syndrome, a genetic analysis was performed and provided the final diagnosis with a genetic change (p Leu 1582 pro) mutation in the SCN5A gene which encodes for the α-subunit of the cardiac sodium channel [15].
Brugada syndrome was named in 1992 after the Spanish/Belgian cardiologists Pedro Brugada and Josep Brugada. The syndrome is caused by ion channel abnormalities and is characterized by ST elevation and negative T waves in the right precordial leads and has a high incidence of sudden death 15, 16. This electrophysiological disorder is produced by the dysfunction of a cardiac ion channel involved in the generation of the action potential (AP) in a structurally normal heart. Mainly two hypotheses are proposed: the repolarization (channelopathy) hypothesis (1997) based on rebalancing of currents at the end of phase 1 of the AP which leads to accentuation of the AP notch in the epicardium of the right ventricle 17, 18; and the depolarization hypothesis based on the conduction delay in the right ventricle outflow tract (RVOT) 19, 20. Brugada syndrome is sensitive to body temperature and can lead to T wave alternans, ventricular tachycardia, and sudden death. Nishida et al. [21] proved in an in vivo canine model that there is a link between hypothermia and Brugada syndrome. They cooled the epicardium of the RVOT which resulted in a Brugada-like ECG pattern.
The explanation of ST disturbances can be found in the early repolarization and acidosis due to hypothermia. That is why in patients with already mild hypothermia, a sinus bradycardia or junctional bradycardia can be observed.
Moderate hypothermia can decrease the depolarization of pacemaker cells so that ST-segment changes can be seen. In severe hypothermia (body core temperature <32° C), atrial fibrillation can occur and when the body temperature further decreases ventricular fibrillation and sudden death can occur.
Conclusion
To our knowledge, this is the first case of an anorexic adolescent girl where we supposed that the ST elevation in the right precordial leads was the result of her extreme starvation and hypothermia, but her Brugada syndrome was shown to be inherited.
In Brugada syndrome, there is a high non-penetrance, so even if her father would have the mutation (he refused further genetic and cardiologic examination), this explained nothing about the damaging nature. Only Type 1 ECG pattern is diagnostic of Brugada syndrome [22].
Finally, we could say that every rhythm problem in the acute state in an anorexia patient deserves a good follow-up to distinguish iatrogenic from heritable rhythm problems.
Conflict of interest
Authors declare no conflict of interest.
Contributor Information
Martine K.F. Docx, Email: martine.docx@zna.be, docxmartine@skynet.be.
Bart Loeys, Email: bart.loeys@ua.ac.be.
Annik Simons, Email: annik.simons@zna.be.
Marc Gewillig, Email: marc.gewillig@uz.kuleuven.be.
Luc Mertens, Email: luc.mertens@sickkids.ca.
References
- 1.Bravender T., Kanter R., Zucker N. Anorexia nervosa and second-degree atrioventricular block (type I) Int J Eat Disord. 2006;39:612–615. doi: 10.1002/eat.20235. [DOI] [PubMed] [Google Scholar]
- 2.Fish J.M., Antzelevitch C. Link between hypothermia and brugada syndrome. J Cardiovasc Electrophysiol. 2004;15:942–944. doi: 10.1046/j.1540-8167.2004.04323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnemeier H., Mäuser W., Schunkert H. Images in cardiovascular medicine. Brugada-like ECG pattern in severe hypothermia. Circulation. 2008;118:977–978. doi: 10.1161/CIRCULATIONAHA.108.771329. [DOI] [PubMed] [Google Scholar]
- 4.Tamene A., Sattiraju S., Wang K., Benditt D.G. Brugada-like electrocardiography pattern induced by severe hyponatraemia. Europace. 2010;12:905–907. doi: 10.1093/europace/euq034. [DOI] [PubMed] [Google Scholar]
- 5.Adler A., Topaz G., Heller K., Zeltser D., Ohayon T., Rozovski U., Halkin A., Rosso R., Ben-Shachar S., Antzelevitch C., Viskin S. Fever-induced Brugada pattern: how common is it and what does it mean? Heart Rhythm. 2013;10:1375–1382. doi: 10.1016/j.hrthm.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postema P.G. Fever and the electrocardiogram: what about Brugada syndrome? Heart Rhythm. 2013;10:1383–1384. doi: 10.1016/j.hrthm.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 7.Chockalingam P., Rammeloo L.A., Postema P.G., Hruda J., Clur S.A., Wilde A.A. Fever-induced life-threatening arrhythmias in children harboring an SCN5A mutation. Pediatrics. 2011;127:e239–e244. doi: 10.1542/peds.2010-1688. [DOI] [PubMed] [Google Scholar]
- 8.Dumaine R., Towbin J.A., Brugada P., Vatta M., Nesterenko D.V., Nesterenko V.V., Brugada J., Brugada R., Antzelevitch C. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ Res. 1999;85:803–809. doi: 10.1161/01.res.85.9.803. [DOI] [PubMed] [Google Scholar]
- 9.Keller D.I., Rougier J.S., Kucera J.P., Benammar N., Fressart V., Guicheney P., Madle A., Fromer M., Schläpfer J., Abriel H. Brugada syndrome and fever: genetic and molecular characterization of patients carrying SCN5A mutations. Cardiovasc Res. 2005;67:510–519. doi: 10.1016/j.cardiores.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 10.Jellins J., Milanovic M., Taitz D.J., Wan S.H., Yam P.W. Brugada syndrome. Hong Kong Med J. 2013;19:159–167. [PubMed] [Google Scholar]
- 11.Shimizu W., Matsuo K., Kobuko Y., Satomi K., Kurita T., Noda T., Nagaya N., Suyama K., Aihara N., Kamakura S., Inamoto N., Akahoshi M., Tomoike H. Sex hormone and gender difference – role of testosterone on male predominance in Brugada syndrome. J Cardiovasc Electrophysiol. 2007;18:415–421. doi: 10.1111/j.1540-8167.2006.00743.x. [DOI] [PubMed] [Google Scholar]
- 12.Matsuo K., Akahoshi M., Nakashina E., Seto S., Yano K. Clinical characteristics of subjects with the Brugada-type electrocardiogram: a case control study. J Cardiovasc Electrophysiol. 2004;15:653–657. doi: 10.1046/j.1540-8167.2004.03544.x. [DOI] [PubMed] [Google Scholar]
- 13.Song M., Helguera G., Eghbali M., Zhu N., Zarei M.M., Olcese R., Toro L., Stefani E. Remodeling of Kv 4.3 potassium channel gene expression under control of sex hormones. J Biol Chem. 2001;276:31883–31890. doi: 10.1074/jbc.M101058200. [DOI] [PubMed] [Google Scholar]
- 14.Collins S., Kuhn C.M., Petro A.E., Swick A.G., Chrunyk B.A., Surwit R.S. Role of leptin in fat regulation. Nature. 1996;380:677. doi: 10.1038/380677a0. [DOI] [PubMed] [Google Scholar]
- 15.Brugada P., Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome: a multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 16.Antzelevitch C., Brugada P., Brugada J., Brugada R., Towbin J.A., Nademanee K. Brugada syndrome: 1992–2002 a historical perspective. J Am Coll Cardiol. 2003;41:1665–1671. doi: 10.1016/s0735-1097(03)00310-3. [DOI] [PubMed] [Google Scholar]
- 17.Link M.S., Antzelevitch C., Waldo A.L., Grant A.O., DiMarco J.P., Josephson M.E., Marchlinski F.E., Garan H., Sager P.T., Reynolds D.W., Denes P., Scheinman M.M., Estes N.A., 3rd. Clinical cardiac electrophysiology fellowship teaching objectives for the new millennium. J Cardiovasc Electrophysiol. 2001;12:1433–1443. doi: 10.1046/j.1540-8167.2001.01433.x. [DOI] [PubMed] [Google Scholar]
- 18.Yan G.X., Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation. 1999;100:1660–1666. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 19.Meregalli P.G., Wilde A.A., Tan H.L. Pathophysiological mechanism of Brugada syndrome: depolarization disorder, repolarization disorder, or more? Cardiovasc Res. 2005;67:367–378. doi: 10.1016/j.cardiores.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Tukkie R., Sogaard P., Vleugels J., de Groot I.K., Wilde A.A., Tan H.L. Delay in right ventricular activation contributes to Brugada syndrome. Circulation. 2004;109:1272–1277. doi: 10.1161/01.CIR.0000118467.53182.D1. [DOI] [PubMed] [Google Scholar]
- 21.Nishida K., Fujiki A., Mizumaki K., Sakabe M., Sugao M., Tsuneda T., Inoue H. A canine model of Brugada syndrome using regional epicardial cooling of the right ventricular outflow tract. J Cardiovasc Electrophysiol. 2004;15:936–941. doi: 10.1046/j.1540-8167.2004.04041.x. [DOI] [PubMed] [Google Scholar]
- 22.Priori S.G., Wilde A.A., Horie M., Cho Y., Behr E.R., Berul C., Blom N., Brugada J., Chiang C.E., Huikuri H., Kannankeril P., Krahn A., Leenhardt A., Moss A., Schwartz P.J. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]