Abstract
A 79-year-old male, with a history of percutaneous coronary intervention (PCI), was referred to our cardiovascular department for a detailed examination of blackout caused by sinus arrest only during meals. Ultrasound echocardiography showed normal cardiac contraction with no asynergy, irrespective of the remaining stenotic coronary lesion. An electrophysiological study revealed deteriorated atrioventricular nodal conduction at a Wenckebach point of 70 beats per minute. However, sinus node function was normal as demonstrated by a sinus node recovery time of 1369 ms. Coronary angiography showed triple-vessel disease including the remaining stenotic coronary lesion, and a PCI was performed on the right coronary artery. Nevertheless, sinus arrest during meals was unchanged. Swallow syncope was partially improved by dietary modification; however, pacemaker implantation (PMI) was performed eventually, and the patient became asymptomatic after PMI.
<Learning objective: Swallow syncope is a rare cause of syncope that belongs to the neurally mediated reflex syncopal syndromes, which can induce a variety of bradyarrhythmias: sinus bradycardia, sinus arrest, sinoatrial block, atrioventricular block, or atrial and ventricular asystole. In this case, we demonstrated that dietary modification or pacemaker implantation improved swallow syncope due to sinus arrest.>
Keywords: Swallow syncope, Sinus arrest, Pacemaker implantation
Introduction
Swallow syncope is a rare cause of syncope that belongs to the neurally mediated reflex syncopal syndromes. It occurs because of a vagal reflex during deglutition causing inhibition of the cardiac conduction system. Most cases with swallow syncope show an underlying abnormality of the esophagus or heart; however, some cases demonstrate no organic abnormality. Here, we report on a case of blackout caused by sinus arrest only during meals.
Case report
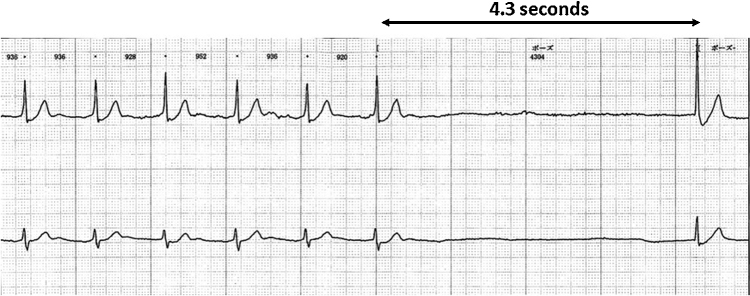
A 79-year-old male with diabetes mellitus and dyslipidemia had been suffering from blackout only during meals from the age of 75 years; however, no detailed examination had been performed. During these episodes, he usually stopped eating and the feeling of blackout spontaneously disappeared. He had a history of percutaneous coronary intervention (PCI), with the stent placed at the left anterior descending artery for unstable angina at the age of 72 years. PCI for the residual lesion at right coronary artery (RCA) and left circumflex artery was not performed because the patient refused to provide consent. The patient underwent laminectomy for cervical spondylosis at the orthopedic department in our hospital at the age of 79 years. Postoperative Holter electrophysiology (ECG) revealed sinus arrest with no ventricular escape beats for 4.3 s during hospital diet (Fig. 1). There were no associated symptoms of chest pain, dyspnea, diaphoresis, or palpitations. The patient had no history of medication for the suppression of heart rate (HR) such as beta-blockers, calcium blockers, and digitalis. A 12-lead ECG showed a first-degree atrioventricular (AV) block with a PQ interval of 240 ms, indicating aberrant AV conduction. Carotid sinus massage and Valsalva maneuvers did not reproduce symptoms or bradycardia. Neurological abnormality by spondylosis was observed; however, magnetic resonance imaging did not reveal any remarkable cause of blackout due to neurological abnormality. Upper gastrointestinal endoscopy showed no abnormal findings of the esophagus and stomach. Ultrasound echocardiography (UCG) revealed normal cardiac function (ejection fraction = 67%) with no asynergy irrespective of the history of ischemic heart disease. This patient had neck pain after the laminectomy for cervical spondylosis and the neck could not be suspended at an angle of 60°–80° to undergo head-up tilt test. Accordingly, we gave up performing head-up tilt test.
Fig. 1.
Holter electrocardiogram showing a ventricular asystole of 4.3 s due to sinus arrest during a meal. The patient complained of lightheadedness during the event.
An electrophysiological study (EPS) demonstrated prolonged AH (157 ms) and normal HV intervals (50 ms) under HR of 60 beats per minute (bpm). Atrial burst pacing showed deteriorated AV nodal conduction by a Wenckebach block (WB) point at a pacing rate of 70 bpm. However, sinus node function was normal as demonstrated by a sinus node recovery time (SNRT) of 1369 ms. Ventricular burst pacing revealed a decremental ventriculoatrial (VA) conduction property and maximal VA one-to-one conduction was 100 bpm. Swallowing of a small amount of bread was performed; as a result, a shorter AH interval (137 ms), a prolonged WB point (80 bpm), an unchanged HV interval (50 ms), and SNRT (1362 ms) were observed. The amount of food swallowed during the hospital diet causing sinus arrest was larger than that during EPS; however, swallowing a larger amount of food in a supine position was not performed to avoid aspiration. Coronary angiography showed triple-vessel disease. We considered that a severe stenotic lesion of the RCA may have caused sinus arrest during the swallowing of food. Therefore, PCI at RCA was performed; however, sinus arrest after eating a normal amount of food (hospital diet) was unchanged. Sinus arrest was improved by modifying the diet to consume small amounts of food frequently, which was demonstrated by an unchanged SNRT during EPS. Nevertheless, pacemaker implantation (PMI) was performed with the patient's consent. The patient remained asymptomatic after PMI.
Discussion
Swallow syncope is a rare disorder, and its mechanism remains to be elucidated. The vasovagal reflex between the heart and esophagus plays an important role in the occurrence of syncope. The postulated mechanism involves aberrant conduction of the afferent and efferent impulses of the vagus nerve 1, 2. Swallow syncope is a dysautonomic syndrome associated with hypersensitive vagal activation induced by esophageal stimulation, producing the so-called upper gastrointestinal cardiac vagovagal reflexes, which by sympathetic inhibition can induce a variety of bradyarrhythmias: sinus bradycardia, sinus arrest, sinoatrial block, AV block, or atrial and ventricular asystole 3, 4, 5, 6, 7, 8.
Omi et al. [3] reviewed 63 reported cases of swallow syncope. Swallow syncope has been known to occur in patients with organic or functional disorders of the esophagus and heart, including esophageal spasms, stricture, achalasia, diverticula, cancer, and hiatal hernia. Swallow syncope has been observed in cardiac diseases, such as inferior or posterior myocardial infarction and rheumatic carditis, and in digoxin toxicity [3]. Some of the reported cases have been associated with ascending aortic aneurysm, thoracic surgery, advanced lung cancer, and transient hypoxia [3]. Therefore, it is important to evaluate the esophageal and cardiac structures of patients with swallow syncope. However, in a minority of patients, no underlying disease could be found 3, 9. There was no organic disease of the esophagus and heart in our case.
Bradycardia inducing blackout during meals was sinus arrest, not AV block although the EPS findings showed the deteriorated conduction system (WB point = 70 bpm) and normal sinus node function (SNRT = 1369 ms), which indicated that bradycardia occurred due to the vasovagal reflex induced by swallowing food, not the deteriorated AV conduction in this case. Previous studies reported that inferior or posterior myocardial infarction could be the cause of swallow syncope. UCG showed no asynergy irrespective of the history of PCI in this patient. PCI at RCA was performed considering that a stenotic coronary lesion may cause bradycardia during meals; however, the swallow syncope was unimproved, which may indicate that ischemic heart disease with no asynergy is not a cause of swallow syncope, and its treatment is not useful for the improvement of such symptoms.
The treatment of swallow syncope has been reported to be atropine, oral anticholinergic medication, dietary modification, PMI, and so on [9]. Previous studies reported that atropine administration was effective for swallow syncope 4, 9. However, its efficacy is transient; therefore, the injection of atropine is for patients during hospitalization and not a definitive treatment for swallow syncope. Accordingly, the administration of atropine was not performed. Dietary modification, the frequent intake of small amounts of food in this patient, inhibited the appearance of bradycardia, which was demonstrated by shorter AH interval, prolonged WB point, and unchanged SNRT by EPS. The efficacy of oral anticholinergic medication, glycopyrrolate, was also reported previously [10]. Accordingly, the combination of glycopyrrolate and dietary modification was considered; however, deteriorated AV nodal conduction demonstrated by the WB point of 70 bpm and first-degree AV block was observed in this patient; hence, we estimated that PMI would be needed in the future. Furthermore, the patient desired to consume meals normally, which resulted in a PMI being performed. Kim et al. [11] reported that dietary modification involving the avoidance of cold beverages was useful for a 39-year-old male with swallow syncope. Therefore, dietary modification to avoid the specific food in the daily meals that cause swallow syncope may be useful to ameliorate the condition of the patient.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Levin B., Posner J.B. Swallow syncope. Report of a case and review of the literature. Neurology. 1972;22:1086–1093. doi: 10.1212/wnl.22.10.1086. [DOI] [PubMed] [Google Scholar]
- 2.Kalloo A.N., Lewis J.H., Maher K., Benjamin S.B. Swallowing. An unusual cause of syncope. Dig Dis Sci. 1989;34:1117–1120. doi: 10.1007/BF01536384. [DOI] [PubMed] [Google Scholar]
- 3.Omi W., Murata Y., Yaegashi T., Inomata J., Fujioka M., Muramoto S. Swallow syncope, a case report and review of the literature. Cardiology. 2006;105:75–79. doi: 10.1159/000089543. [DOI] [PubMed] [Google Scholar]
- 4.Kakuchi H., Sato N., Kawamura Y. Swallow syncope associated with complete atrioventricular block and vasovagal syncope. Heart. 2000;83:702–704. doi: 10.1136/heart.83.6.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang K.H., Cho W.H., Kim M.C., Chang H.J., Chung J.I., Won D.J. Cases of swallow syncope induced by the activation of mechanorecepters in the lower esophagus. Korean J Intern Med. 2005;20:68–71. doi: 10.3904/kjim.2005.20.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadish A.H., Wechsler L., Marchlinski F.E. Swallowing syncope: observations in the absence of conduction system or esophageal disease. Am J Med. 1986;81:1098–1100. doi: 10.1016/0002-9343(86)90418-3. [DOI] [PubMed] [Google Scholar]
- 7.Ausubel K., Gitler B. Swallow syncope in an otherwise healthy young man. Am Heart J. 1987;113:831–832. doi: 10.1016/0002-8703(87)90730-7. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa S., Hisanaga S., Kondoh H., Koiwaya Y., Tanaka K. A case of swallow syncope induced by vagotonic visceral reflex resulting in atrioventricular node suppression. J Electrocardiol. 1987;20:65–69. doi: 10.1016/0022-0736(87)90010-0. [DOI] [PubMed] [Google Scholar]
- 9.Mitra S., Ludka T., Rezkalla S.H., Sharma P.P., Luo J. Swallow syncope: a case report and review of the literature. Clin Med Res. 2011;9:125–129. doi: 10.3121/cmr.2010.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endean E.D., Cavatassi W., Hansler J., Sorial E. Deglutition syncope: a manifestation of vagal hyperactivity following carotid endarterectomy. J Vasc Surg. 2010;52:720–722. doi: 10.1016/j.jvs.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Kim C., Ko S.M., Kim N., Park S.M., Lee G.Y., Cho J.H., Kim J.S. A case of swallow syncope associated with cold beverage ingestion. Korean Circ J. 2012;42:212–215. doi: 10.4070/kcj.2012.42.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]