Abstract
Background:
Cardiovascular (CV) risk is high in children with chronic kidney disease (CKD), and further compounded in those who are overweight. Children with CKD have a unique body habitus not accurately assessed by body mass index (BMI). Waist-to-height ratio (WHr), a better predictor of CV risk in populations with short stature, has not been investigated in children with CKD.
Methods:
Analysis of 1723 visits of 593 participants enrolled in the Chronic Kidney Disease in Children (CKiD) study was conducted. CKiD participants had BMI and WHr measured and classified as: 1) lean (WHr≤0.49, BMI <85th percentile), 2) WHr-overweight (WHr>0.49, BMI<85th percentile), 3) BMI-overweight (WHr≤0.49, BMI ≥85th percentile), or 4) overweight by both BMI and WHr. Left ventricular mass index (LVMI), fasting lipids, FGF23, blood pressure, and glucose were measured as markers of CV risk. Linear mixed-effects regression was used to evaluate differences in CV markers between overweight and lean groups.
Results:
Participants were 12.2 years old, 60% male, 17% African-American. Approximately 15% were overweight by WHr but not by BMI. Overweight status by WHr-only or both WHr and BMI was associated with lower HDL and higher LVMI, triglycerides, and non-HDL cholesterol compared to lean. CV markers of participants overweight by BMI-only were similar to those of lean children.
Conclusions:
WHr-adiposity is associated with an adverse CV risk profile in children with CKD. A significant proportion of children with central adiposity are missed by BMI. WHr should be utilized as a screening tool for CV risk in this population.
Keywords: obesity, body mass index, left ventricular mass index, dyslipidemia, hypertension, fibroblast growth factor 23
Introduction
Chronic kidney disease (CKD) is a life-long chronic illness in which risks to cardiovascular (CV) health begin early in the course of the disease. Major CV events are rare in children, and early abnormalities are not easily detectable by conventional monitoring. By the time children with CKD reach young adulthood, their risk of CV mortality is 30 fold higher compared to their healthy peers [1], indicating the need for better predictors and monitoring.
Obesity and abdominal adiposity in particular, has been strongly associated with higher CV risk [2, 3]. It is known that obesity-related morbidity is more closely linked to central, or visceral, fat distribution rather than total body fat [3, 4]. Although body mass index (BMI) is the most common measure of anthropometrics used in clinical practice, it has inherent limitations that can lead to misdiagnosis of obesity. Specifically, the inability of BMI to differentiate between lean and fat mass can result in the misclassification of lean, muscular individuals as overweight while those with excess fat mass and low muscle mass can have a BMI in the healthy range [5, 6]. To this point, a recent study showed that 55% of children who were characterized as overweight by BMI did not have central obesity and these children had normal cardiometabolic risk profiles [7].
Evidence shows that waist-to-height ratio (WHr), a measure of abdominal obesity, may be a better predictor of CV risk than BMI in populations with shorter stature and in the general pediatric population [4, 7–11]. As in the general population, obesity is an increasingly common problem among children with CKD. A recent report from the CKiD study indicated that 32% of those with non-glomerular disease and 46% of those with glomerular disease were overweight or obese, and 12% had severe short stature. The combination of impaired growth with normal or above average weight contribute to high prevalence of overweight and obesity in this population [12]. BMI may not accurately reflect body composition in children with kidney disease, due to their short stature and altered fat deposition patterns [13, 14]. On the other hand, waist circumference is also likely to be inaccurate for detection of obesity in pediatric CKD patients, as comparison to age-specific percentiles are inappropriate in the setting of short stature and delayed maturation. WHr, a measure of abdominal obesity that takes height into account, may be better suited to the unique needs of children with CKD. The measure of obesity that best predicts CV risk children with CKD has not been established. This is the first study to investigate the association of WHr with markers of CV risk in children with CKD.
Materials and Methods
Study design and population
The detailed methods of the Chronic Kidney Disease in Children (CKiD) study, an ongoing prospective multi-center cohort study of North American children with CKD, have been previously described [15]. The CKiD study (NCT00327860) was approved by an external study monitoring board appointed by the National Institute of Diabetes and Digestive and Kidney Diseases and by the institutional review board of each participating center, including Children’s National Health System. Informed consent of participants was obtained by each center and the study was conducted in accordance with the Declaration of Helinski. The present study includes 593 CKiD participants who contributed 1723 visits between June 2008 and September 2015 while they were between 4 and 17 years of age with eGFR between 30–90 ml/min/1.73m2. CKiD participants with a diagnosis of autosomal recessive polycystic kidney disease (ARPKD) were excluded from the analysis due to the likelihood of increased abdominal girth related to enlarged kidneys.
Evaluation and classification of overweight
Participants had weight, height, and waist circumference (WC) measured at annual study visits according to the CKiD study protocol, which have been previously described [15]. BMI was calculated as weight (kg) divided by height (m2) and was converted to age-and-sex specific percentiles and z-scores based on the 2000 Centers for Disease Control (CDC) growth charts, with BMI≥85th age-sex specific percentile defined as overweight [16]. WHr was calculated as waist circumference (cm) divided by height (cm). WHr-overweight status was defined by WHr > 0.49, based on a prior study of NHANES III data in children [4]. Participants were then classified into one of four groups: 1) non-overweight or lean (WHr≤0.49 and BMI <85th percentile), 2) overweight by WHr-only (WHr >0.49 and BMI <85th percentile), 3) overweight by BMI-only (WHr ≤0.49 and BMI ≥85th percentile), and those overweight by both methods (WHr>0.49 and BMI ≥85th percentile).
Evaluation of CV markers
Left ventricular mass index (LVMI), fasting lipid panel, c-terminal fibroblast growth factor 23 (FGF23), systolic and diastolic blood pressure, and fasting glucose were measured and included in the analyses as markers of CV risk. Left ventricular mass was measured every other year by echocardiography according to standard protocol and LVMI (g/m2.7) was calculated by indexing left ventricular mass to height2.7. Fasting triglycerides, HDL cholesterol, and non-HDL cholesterol (mg/dL) were measured every other year. C-terminal FGF23 (RU/mL) was measured every other year. Systolic blood and diastolic blood pressure (mm Hg) were measured annually and converted to z-scores [17]. Fasting glucose (mg/dL) was measured annually. The eGFR (mL/min/1.73m2) was determined annually based on the equation of Schwartz et al [18].
Statistical methods
The distribution of each marker of CV health was summarized using a box plot for each of four groups of participants: lean, overweight by WHr-only, overweight by BMI-only, and those overweight by both methods. Linear mixed-effects regression was used to evaluate whether there were any differences in markers of CV health between the three overweight groups compared to the lean group over time. Each linear mixed effects regression model was adjusted for the following covariates: age centered at 10 years, gender, race (African American vs. non-African American), concurrently measured eGFR centered at 50ml/min/1.73m2, CKD etiology (glomerular vs. non-glomerular), and pubertal status. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Study population
Demographic and baseline clinical characteristics of the study participants are presented in Table 1, overall and by overweight status. Median age of the 593 participants was 12.2 years, 60% were male, 17% African American, and 32% had glomerular disease. Median time of follow up was 3.2 years (IQR 2.0–5.2 years). The study cohort had a median eGFR of 60 ml/min/1.73m2 and median urine protein to creatinine ratio of 0.24, which did not differ significantly according to weight classification.
Table 1:
Demographic and Clinical Characteristics by BMI and WHr at Baseline Visit
| Weight Classification | Lean | WHr overweight only |
BMI overweight only |
Both WHr & BMI Overweight |
Overall |
|---|---|---|---|---|---|
| Number of children | 300 | 90 | 28 | 175 | 593 |
| Age, years | 12.3 [8.9, 15.5] | 9.7 [5.4, 15.5] | 13.0 [10.4, 15.6] | 12.9 [9.2, 16.0] | 12.2 [8.3, 15.7] |
| Male gender | 65% | 51% | 57% | 57% | 60% |
| African-American race | 16% | 12% | 36% | 19% | 17% |
| Glomerular diagnosis | 28% | 23% | 64% | 39% | 32% |
| Uncontrolled Blood Pressure | 20% | 27% | 14% | 27% | 23% |
| Peri/post pubertal status | 53% | 42% | 68% | 54% | 52% |
| Growth hormone usage | 6% | 10% | 0% | 6% | 6% |
| Short stature (height z score <−1.88) | 8% | 17% | 4% | 11% | 10% |
| eGFR, ml/min∣1.73m2 | 59.0 [47.1, 74.6] | 58.6 [44.1, 74.4] | 62.2 [48.9, 79.3] | 62.1 [47.3, 76.2] | 59.9 [46.8, 75.4] |
| Height z-score | −0.36 [−1.03, 0.30] | −1.00 [−1.63, −0.12] | 0.11 [−0.55, 1.06] | −0.23 [−0.86, 0.67] | −0.36 [−1.10, 0.36] |
| Weight z-score | −0.35 [−1.04, 0.22] | −0.25 [−0.77, 0.28] | 1.09 [0.83, 1.56] | 1.61 [1.04, 2.01] | 0.19 [−0.63, 1.09] |
| Body mass index (BMI) z-score | −0.19 [−0.74, 0.31] | 0.52 [0.10, 0.78] | 1.19 [1.16, 1.39] | 1.80 [1.43, 2.20] | 0.49 [−0.30, 1.36] |
| Waist-height ratio (WHr) | 0.44 [0.42, 0.47] | 0.51 [0.50, 0.53] | 0.47 [0.46, 0.48] | 0.57 [0.53, 0.62] | 0.48 [0.44, 0.54] |
| Proteinuria (urine protein to creatinine ratio) |
0.18 [0.09, 0.53] | 0.35 [0.13, 1.03] | 0.31 [0.10, 0.95] | 0.30 [0.11, 0.89] | 0.24 [0.10, 0.70] |
Continuous variables are reported as median [IQR]
Categorical variables are reported as the percent (%) of patients within each weight class column and % of the overall population at baseline.WHr.
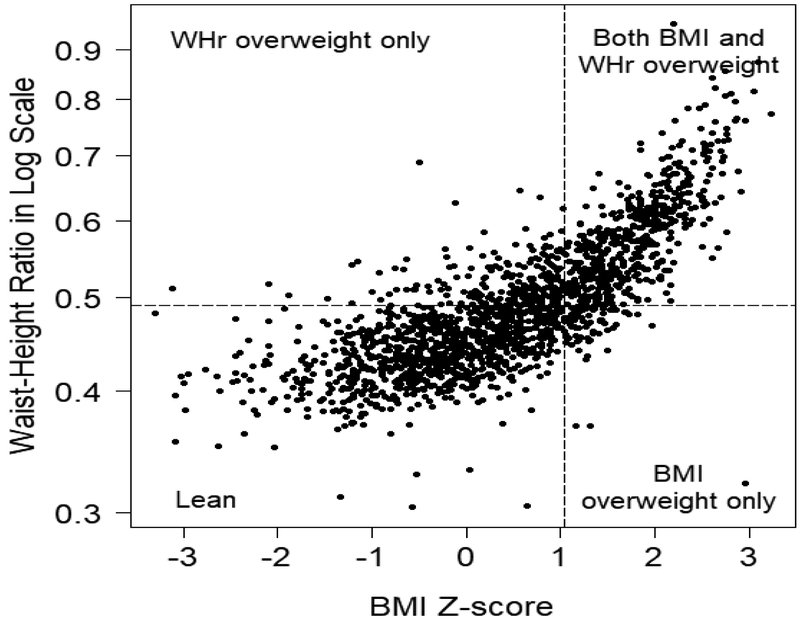
Of the 593 children, 50.6% (n=300) were lean, 15.2% (n=90) were overweight by WHr-only, 4.7% (n=28) were overweight by BMI-only, and 29.5% (n=175) were overweight by both BMI and WHr at baseline. A scatter plot depicting the distribution of BMI z-scores and WHr by overweight status over all visits is shown in Figure 1. Approximately 15% of the CKiD population was not identified as overweight by BMI, but were determined to be overweight by WHr. The subset of children identified as overweight by WHr-only were of shorter stature in comparison to all other categories (median height z-score of −1.00 for WHr-only overweight vs. −0.36, +0.11, and −0.23 for lean, BMI-only, and both BMI/WHr overweight groups, respectively), Kruskal-Wallis p<0.001. Short stature as defined by height z-score <−1.88 was significantly more prevalent in the WHr only group (21%), as compared to the lean (8%), BMI-only (3%) and combined BMI&WHr overweight (11%) groups (p=0.04 by Chi-square). The usage of growth hormone therapy among the study cohort was 6% and did not vary significantly by weight group (p=0.25 by Chi-square).
Figure 1. Distribution of weight status by study visit.
The distribution of WHr and BMI z-scores are plotted over all study visits (n=1723) and weight classified as lean (WHr≤0.49, BMI <85th percentile), WHr overweight only (WHr >0.49, BMI <85th percentile), BMI-overweight only (WHr ≤0.49, BMI ≥85th percentile), or overweight by both BMI and WHr (WHr >0.49, BMI ≥85th percentile).
Comparison of CV markers by weight classification
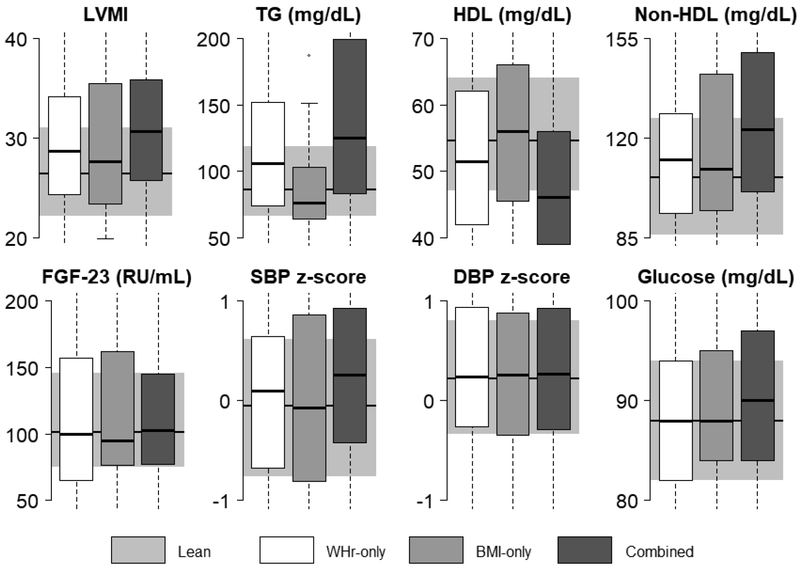
The distributions of each of the CV markers by the four exposure groups are illustrated in Figure 2. LVMI, triglycerides, HDL cholesterol, and non-HDL cholesterol median levels appear worse in the group overweight by WHr-only than by BMI-only, compared to the lean group. Those in the combined WHr and BMI-overweight group appear to have worse median LVMI, triglycerides, HDL cholesterol, and non-HDL cholesterol as well as higher glucose and SBP z-score compared to the lean group. Due to the longitudinal nature of the data, bivariate statistical tests of significance were not appropriate, and therefore Figure 2 represents a descriptive presentation of the data only. Associations of weight classification with CV markers were statistically confirmed by the linear mixed-effects regression analysis.
FIGURE 2. Distribution of CV markers by weight status.
CV markers (median and IQR) are illustrated by weight classification. Overweight by WHr-only, BMI-only, and combined (overweight by both WHr and BMI) groups are shown in the white, light gray, and dark gray bars, respectively, overlaying a shaded gray box representing the lean group. Due to the longitudinal nature of the data, bivariate statistical tests of significance were not appropriate; this figure represents a descriptive presentation of the data only.
Association of BMI and WHr with CV markers
Results of the multivariate analysis for associations of BMI and WHr with CV markers after adjusting for age, gender, race, eGFR, CKD etiology, and pubertal status are reported in Table 2. Overweight status by WHr-only or by both WHr and BMI was associated with higher LVMI, higher triglycerides, lower HDL cholesterol, and higher non-HDL cholesterol compared to lean. In addition, there was a stronger magnitude of association of adiposity defined by WHr alone with hypertriglyceridemia (+17.1% vs. +4%), low HDL-C (−3.71 vs. −0.42 mg/dL), and increased LVMI (+2.74 vs. +2.10 g/m2.7) compared with BMI alone, but this did not reach statistical significance. There were significantly stronger associations seen in those who were overweight by both WHr and BMI with hypertriglyceridemia (+43.6% vs. +4.0%, p<0.001) and low HDL cholesterol (−6.48 vs. −0.42, p=0.004), compared with those overweight by BMI alone. In those classified as being overweight by BMI-only, the LVMI, triglyceride, HDL cholesterol and non-HDL cholesterol levels were similar to those of the lean group. There were no significant interactions between BMI and WHr as related to CV markers. Participants classified as overweight by WHr-only or by BMI-only had similar FGF23, SBP z-scores, DBP z-scores, and glucose as lean participants. Being overweight by both WHr and BMI was associated with significantly higher SBP z-scores (0.34 higher; p<0.001) and higher glucose (2.9% higher; p=0.009).
Table 2:
Mean differences in CV markers by BMI and WHr overweight status groups compared to the lean group by multivariate analysis
| CV Marker |
||||||||
|---|---|---|---|---|---|---|---|---|
| LVMI | Triglyceride (mg/dL)† |
HDL-C (mg/dL) |
NonHDL-C (mg/dL) |
FGF23 (RU/mL)† |
SBP z-score |
DBP z-score |
Glucose (mg/dL) |
|
|
Lean [REFERENCE] Mean(95%CI) |
27.4 (25.5-29.3) |
94.5 (85.5-104.6) |
57.9 (55.0-60.7) |
120.7 (113.8-127.7) |
128.8 (114.1-145.5) |
0.08 (−0.10-0.25) |
0.32 (0.17-0.47) |
89.1 (87.1- 91.0) |
|
WHr Overweight Mean difference vs. reference |
+2.74* p=0.004 |
+17.1%* p=0.001 |
−3.71* p=0.004 |
+6.04* p=0.043 |
−6.4% p=0.266 |
+0.08 p=0.301 |
−0.0003 p=0.996 |
+1.2% p=0.339 |
|
BMI Overweight Mean difference vs. reference |
+2.10 p=0.192 |
+4.0% p=0.620 |
−0.42 p=0.841 |
+6.33 p=0.192 |
−8.3% p=0.322 |
+0.01 p=0.961 |
−0.05 p=0.624 |
+1.3% p=0.526 |
|
Both WHr&BMI Mean difference vs. reference |
+4.51* p<0.001 |
+43.6%* p<0.001 |
−6.48* p<0.001 |
+15.40* p<0.001 |
−4.7% p=0.314 |
+0.34* p<0.001 |
+0.03 p=0.649 |
+3.0%* p=0.003 |
Difference in the CV parameter between the overweight group and the lean (reference) group is significant (p<0.05), adjusted for age centered at 10, eGFR centered at 50, glomerular diagnosis, black race, pubertal status and male gender
Differences in log transformed variables are presented as % change from reference group
Discussion
This is the first study to investigate the association of WHr with markers of CV risk in children with CKD. Sensitive methods for accurate detection of adiposity-associated CV risk are important in children with kidney disease due to their tendency to gain excess fat mass in combination with their high risk for CV morbidity and mortality. Our analysis demonstrated that WHr identifies overweight in a greater number of children, approximately 15% of whom are missed by BMI alone, and that WHr is strongly associated with certain markers of adverse CV health in the CKiD study population. The ability of WHr to detect adiposity-associated CV risk in our population was evidenced by significant associations of WHr with hypertriglyceridemia, low HDL cholesterol, increased LVMI, and increased non-HDL cholesterol compared with lean children. While the effects of WHr overweight alone were stronger than the effects of BMI overweight alone (+17.1% vs. +4.0%, p=0.179 for triglycerides and −3.71 vs. −0.42, p=0.150 for HDL cholesterol), the differences did not reach statistical significance because of the small percentage of overweight by BMI alone in our study population (5%). Furthermore, being overweight by both WHr and BMI was also strongly associated with adverse CV health and had stronger associations with hypertriglyceridemia and low HDL cholesterol compared with BMI alone. Thus, an important high-risk segment of the population is missed when BMI alone is used to assess obesity in pediatric CKD patients.
This finding is supported by a recent meta-analysis indicating that BMI has a low sensitivity to detect excess adiposity and fails to identify the presence of excess body fat in over 25 percent of children [6]. In the present analysis, CKiD participants who were classified as overweight by BMI-only had similar CV risk profiles to those of lean children. These findings are similar to those of Khoury et al. who reported that 55% of children who were characterized as overweight by BMI did not have central obesity and these children had essentially normal cardiometabolic risk profiles [7]. Our results are further supported by those of several large general pediatric population-based analysis comparing the ability of WHr and BMI to identify CV risk among children who participated in the NHANES study [4, 19]. An analysis by Kahn et al. demonstrated that WHr better identified youth with high heart rate, and elevated triglycerides, total cholesterol and LDL cholesterol, compared with BMI [4]. Another study showed that children and adolescents with central obesity measured by WHr had increased LVMI in comparison to those with no central obesity [20]. The possible reasons behind the stronger association of WHr with CV risk factors are multi-fold. Firstly, as WHr is a measure of abdominal adiposity, WHr-obesity signifies the accumulation of excess adipose tissue in the abdominal region. Even in the setting of a normal BMI, excess visceral adipose tissue can accumulate in the abdominal region when caloric intake exceeds the capacity of body utilization, resulting in an elevated WHr [21]. Continued excessive energy intake is then stored as triglyceride in the visceral adipocytes, eventually causing hypertrophy and hyperplasia, and finally leading to release of excess free fatty acids into circulation. The circulating free fatty acids then accumulate in the liver and heart, causing dysregulation of cholesterol metabolism and lipotoxic damage to cardiac muscle [22]. In addition, the epicardial fat surrounding cardiac muscle shuttles free fatty acids directly to the heart, resulting in cardiac fibrosis, myocardial hypertrophy, and increased left ventricular mass [23–25].
As noted above, in the CKiD study population, approximately 15% of children were overweight by WHr alone, despite having a normal BMI, and this subset of WHr-only overweight children had significantly shorter stature compared to the rest of the study cohort. Interestingly, WHr has been found to be more closely associated with cardiometabolic risk in Asian populations, which are characterized by shorter stature and greater tendency towards central obesity, in comparison to North American or European populations [26, 27]. Similarly, in the NHANES study, WHr preferentially identified obesity in children who were shorter, had increased central subcutaneous fat distribution characterized by higher subscapular/triceps skinfold ratio, and smaller mid-thigh circumference [4]. This body type preferentially identified by WHr in the general pediatric population closely mirrors the unique body habitus of children with CKD. The body composition of children with kidney disease has been characterized by central adiposity, short stature, reduced lean mass, high fat mass, and high trunk:leg fat mass ratio, as revealed by Dual X-ray Absorptiometry [13, 14]. Thus, WHr appears well-suited for the assessment of overweight in children with CKD.
Although waist circumference (WC) is another measure of central adiposity, WC-for-age percentiles likely underestimate abdominal obesity in children with CKD, due to the afore-mentioned issues with impaired growth, short stature, and delayed maturation, which do not allow for accurate comparisons to healthy age-matched peers. In fact, a recent study investigated associations of WC with CV parameters in the CKiD population, where WC z-scores and percentiles were determined based on age-matched reference data of healthy children [28]. The authors reported that central obesity as defined by WC provided no added benefit over BMI for identification of CV risk in this population. These findings are in contrast to the results of the present analyses which suggest that indexing WC to height adds predictive value in the identification of CV risk associated with central adiposity in children with CKD.
The major strength of this study was the use of CKiD study data, which was prospectively and longitudinally collected according to standardized protocols at 54 sites across North America. However, the analysis was limited by the inability to include indicators of subclinical CV dysfunction (carotid intima-media thickness and myocardial strain) due to a limited sample size for this particular data. Future studies investigating indicators of subclinical CV dysfunction with WHr will further elucidate the utility of this anthropometric measure. A novel aspect of this study was the exploration of FGF23 as a predictor of CV risk in overweight children with CKD. Emerging evidence suggests that FGF23 plays a role in the regulation of visceral adiposity [29], and is also a predictor of left ventricular hypertrophy (LVH) in children [30] and adults [31] with mild to moderate CKD. Whereas we did not identify FGF23 as a significant correlate of adiposity-associated CV risk in this CKiD study cohort, further investigation is warranted to explore the mechanisms that regulate the interplay between FGF23, adiposity, and CV risk in individuals with CKD.
In conclusion, WHr adiposity, either alone or in combination with increased BMI, is associated with a worse CV risk profile characterized by increased LVMI, higher triglycerides, lower HDL cholesterol, higher non-HDL cholesterol, higher systolic blood pressure and higher blood glucose in comparison to lean children with CKD. A significant proportion, approximately 15%, of at-risk children, are not recognized as overweight by BMI alone; in particular those of shorter stature are at risk of being missed. WHr captures these children and in combination with BMI is more strongly associated with increased triglycerides and low HDL cholesterol than BMI alone, whereas the CV risk profile of those overweight by BMI alone is similar to that of lean children. In addition, WHr does not require comparison to age and gender reference points, making it more convenient for clinical use than other methods. Therefore WHr, a measure of abdominal obesity that is well-suited to assess the unique body composition of pediatric patients with CKD, should be utilized as screening method to identify children at increased CV risk in this vulnerable population.
Acknowledgements:
Data in this manuscript were collected by the Chronic Kidney Disease in Children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri - Kansas City (Bradley Warady, MD) and Children’s Hospital of Philadelphia (Susan Furth, MD, PhD), Central Biochemistry Laboratory (George Schwartz, MD) at the University of Rochester Medical Center, and data coordinating center (Alvaro Muñoz, PhD) at the Johns Hopkins Bloomberg School of Public Health.
Funding: The CKiD Study is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U01DK-082194, U01-DK-66116). The CKiD website is located at http://www.statepi.jhsph.edu/ckid.
Footnotes
Conflict of Interest: none declared
References
- [1].Mitsnefes MM, Laskin BL, Dahhou M, Zhang X, Foster BJ, Mortality risk among children initially treated with dialysis for end-stage kidney disease, 1990–2010, JAMA 309(18) (2013) 1921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Elffers TW, de Mutsert R, Lamb HJ, de Roos A, Willems van Dijk K, Rosendaal FR, Jukema JW, Trompet S, Body fat distribution, in particular visceral fat, is associated with cardiometabolic risk factors in obese women, PloS one 12(9) (2017) e0185403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Caprio S, Hyman LD, McCarthy S, Lange R, Bronson M, Tamborlane WV, Fat distribution and cardiovascular risk factors in obese adolescent girls: importance of the intraabdominal fat depot, The American journal of clinical nutrition 64(1) (1996) 12–7. [DOI] [PubMed] [Google Scholar]
- [4].Kahn HS, Imperatore G, Cheng YJ, A population-based comparison of BMI percentiles and waist-to-height ratio for identifying cardiovascular risk in youth, The Journal of pediatrics 146(4) (2005) 482–8. [DOI] [PubMed] [Google Scholar]
- [5].Weber DR, Leonard MB, Zemel BS, Body composition analysis in the pediatric population, Pediatric endocrinology reviews : PER 10(1) (2012) 130–9. [PMC free article] [PubMed] [Google Scholar]
- [6].Javed A, Jumean M, Murad MH, Okorodudu D, Kumar S, Somers VK, Sochor O, Lopez-Jimenez F, Diagnostic performance of body mass index to identify obesity as defined by body adiposity in children and adolescents: a systematic review and meta-analysis, Pediatric obesity 10(3) (2015) 234–44. [DOI] [PubMed] [Google Scholar]
- [7].Khoury M, Manlhiot C, McCrindle BW, Role of the waist/height ratio in the cardiometabolic risk assessment of children classified by body mass index, Journal of the American College of Cardiology 62(8) (2013) 742–51. [DOI] [PubMed] [Google Scholar]
- [8].Maffeis C, Banzato C, Talamini G, Waist-to-height ratio, a useful index to identify high metabolic risk in overweight children, The Journal of pediatrics 152(2) (2008) 207–13. [DOI] [PubMed] [Google Scholar]
- [9].Mombelli G, Zanaboni AM, Gaito S, Sirtori CR, Waist-to-height ratio is a highly sensitive index for the metabolic syndrome in a Mediterranean population, Metabolic syndrome and related disorders 7(5) (2009) 477–84. [DOI] [PubMed] [Google Scholar]
- [10].Lo K, Wong M, Khalechelvam P, Tam W, Waist-to-height ratio, body mass index and waist circumference for screening paediatric cardio-metabolic risk factors: a meta-analysis, Obesity reviews : an official journal of the International Association for the Study of Obesity 17(12) (2016) 1258–1275. [DOI] [PubMed] [Google Scholar]
- [11].Savva SC, Tornaritis M, Savva ME, Kourides Y, Panagi A, Silikiotou N, Georgiou C, Kafatos A, Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index, International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity 24(11) (2000) 1453–8. [DOI] [PubMed] [Google Scholar]
- [12].Rodig NM, McDermott KC, Schneider MF, Hotchkiss HM, Yadin O, Seikaly MG, Furth SL, Warady BA, Growth in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children Study, Pediatr Nephrol 29(10) (2014) 1987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Johnson VL, Wang J, Kaskel FJ, Pierson RN, Changes in body composition of children with chronic renal failure on growth hormone, Pediatr Nephrol 14(7) (2000) 695–700. [DOI] [PubMed] [Google Scholar]
- [14].Rashid R, Neill E, Smith W, King D, Beattie TJ, Murphy A, Ramage IJ, Maxwell H, Ahmed SF, Body composition and nutritional intake in children with chronic kidney disease, Pediatr Nephrol 21(11) (2006) 1730–8. [DOI] [PubMed] [Google Scholar]
- [15].Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Munoz A, Warady BA , Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study, Clinical journal of the American Society of Nephrology : CJASN 1(5) (2006) 1006–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL, Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version, Pediatrics 109(1) (2002) 45–60. [DOI] [PubMed] [Google Scholar]
- [17].The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents, Pediatrics 114(2 Suppl 4th Report) (2004) 555–76. [PubMed] [Google Scholar]
- [18].Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, Furth SL, Munoz A, Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C, Kidney international 82(4) (2012) 445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Centers for Disease Control and Prevention. https://www.cdc.gov/growthcharts/index.htm.
- [20].Mehta SK, Waist circumference to height ratio and left ventricular mass in children and adolescents, Cardiology in the young 26(4) (2016) 658–62. [DOI] [PubMed] [Google Scholar]
- [21].Frayn KN, Adipose tissue as a buffer for daily lipid flux, Diabetologia 45(9) (2002) 1201–10. [DOI] [PubMed] [Google Scholar]
- [22].de Ferranti S, Mozaffarian D, The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences, Clinical chemistry 54(6) (2008) 945–55. [DOI] [PubMed] [Google Scholar]
- [23].Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mario U, Leonetti F, Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction, Obesity research 11(2) (2003) 304–10. [DOI] [PubMed] [Google Scholar]
- [24].Kankaanpaa M, Lehto HR, Parkka JP, Komu M, Viljanen A, Ferrannini E, Knuuti J, Nuutila P, Parkkola R, Iozzo P, Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels, The Journal of clinical endocrinology and metabolism 91(11) (2006) 4689–95. [DOI] [PubMed] [Google Scholar]
- [25].Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH, Lipotoxic heart disease in obese rats: implications for human obesity, Proceedings of the National Academy of Sciences of the United States of America 97(4) (2000) 1784–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ho SY, Lam TH, Janus ED, Waist to stature ratio is more strongly associated with cardiovascular risk factors than other simple anthropometric indices, Annals of epidemiology 13(10) (2003) 683–91. [DOI] [PubMed] [Google Scholar]
- [27].Diemer FS, Brewster LM, Haan YC, Oehlers GP, van Montfrans GA, Nahar-van Venrooij LMW, Body composition measures and cardiovascular risk in high-risk ethnic groups, Clin Nutr (2017). [DOI] [PubMed] [Google Scholar]
- [28].Patel HP, Saland JM, Ng DK, Jiang S, Warady BA, Furth SL, Flynn JT, Waist Circumference and Body Mass Index in Children with Chronic Kidney Disease and Metabolic, Cardiovascular, and Renal Outcomes, The Journal of pediatrics 191 (2017) 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hu X, Ma X, Luo Y, Xu Y, Xiong Q, Pan X, Xiao Y, Bao Y, Jia W, Associations of serum fibroblast growth factor 23 levels with obesity and visceral fat accumulation, Clin Nutr (2016). [DOI] [PubMed] [Google Scholar]
- [30].Mitsnefes MM, Betoko A, Schneider MF, Salusky IB, Wolf MS, Juppner H, Warady BA, Furth SL, Portale AA, FGF23 and Left Ventricular Hypertrophy in Children with CKD, Clin J Am Soc Nephrol 13(1) (2018) 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro OM, Kusek JW, Keane MG, Wolf M, FGF23 induces left ventricular hypertrophy, J Clin Invest 121(11) (2011) 4393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]