Abstract
Objective:
To compare therapeutic response to behavioral therapy for insomnia (BT-I) among hypnotic-dependent insomnia (HDI) patients with and without Cluster C personality disorders.
Participants:
Twenty-three adults with HDI (17 females), aged between 33 and 68 (M = 53; SD = 9.9) were included in the study.
Methods:
Participants completed a personality disorder assessment (baseline), as well as sleep diaries, polysomnography (PSG), and an insomnia severity assessment (baseline, post-treatment, and one-year follow-up). Treatment consisted of eight weeks of individual BT-I and gradual hypnotic medication withdrawal. Multilevel mixed-effects linear regression models examined the interaction between study visit and Cluster C personality disorders status on treatment response to BT-I.
Results:
Obsessive-compulsive personality disorder (OCPD) was the most prevalent of the Cluster C personality disorders with 38% (n=8) of participants meeting criteria. There were no significant treatment differences by OCPD status across time as measured by sleep diaries and insomnia severity status. However, there were significant treatment differences by OCPD status by one-year follow-up on PSG outcomes indicating patients with OCPD status had shorter and more disrupted sleep than patients without OCPD status.
Conclusions:
Based on self-reported sleep measures, patients with insomnia and features of OCPD responded equivalently to BT-I at one-year follow-up compared to patients without features of OCPD. However, polysomnography outcomes indicated objective sleep deteriorated in these patients, which may suggest greater vulnerability to relapse.
Keywords: hypnotic-dependent insomnia, behavioral therapy, obsessive-compulsive personality disorder, polysomnography, self-report
Acute insomnia is common, with a 9.5% prevalence rate in the U.S. population and nearly a 37% annual incidence (Ellis, Perlis, Neale, Espie, & Bastien, 2012). About 21% of acute insomnia cases develop into a chronic problem (Ellis et al., 2012). Spielman’s 3P Model of Insomnia posits three factors are involved in the development of chronic insomnia: characteristics increasing vulnerability (predisposing), events triggering insomnia (precipitating), and behaviors or cognitions maintaining or exacerbating sleep difficulties (perpetuating) (Spielman, Caruso, & Glovinsky, 1987; Spielman & Glovinsky, 1991). Identifying predisposing factors may aid in the identification of those who are at particular risk for developing insomnia. Personality may be a factor contributing to the development of chronic insomnia by increasing vulnerability and reactiveness to stress-related sleep disruption (Harvey, Gehrman, & Espie, 2014; Yang, Hung, & Lee, 2014). However, few studies have focused on understanding the progression of insomnia and its responsiveness to treatment in relation to personality traits or disorders.
Cognitive-emotional and physiological hyperarousability have been shown to be associated with insomnia (Bonnet & Arand, 2010; Fernandez-Mendoza et al., 2010; Spiegelhalder & Riemann, 2013). Personality traits, namely higher neuroticism and perfectionism, and lower agreeableness and conscientiousness have also been associated with chronic insomnia, greater insomnia severity, and complaints of nonrestorative sleep (Emert, Tutek, & Lichstein, 2017; Harvey et al., 2014; Regen et al., 2014; Sassoon, de Zambotti, Colrain, & Baker, 2014; van de Laar, Verbeek, Pevernagie, Aldenkamp, & Overeem, 2010; Williams & Moroz, 2009). Higher levels of neuroticism may increase an individual’s vulnerability to stress-related sleep disruption and subsequently contribute to the development of insomnia (Harvey et al., 2014; van de Laar et al., 2010; Williams & Moroz, 2009). Individuals high in traits related to neuroticism take longer to fall asleep, have a smaller percentage of slow-wave sleep, more transitions to light sleep (N1), have poorer sleep quality, shorter sleep overall, and have lower rapid eye movement (REM) density compared to those low in these traits (Fuller, Waters, Binks, & Anderson, 1997; Harvey et al., 2014; Williams & Moroz, 2009).
Cluster C Personality disorders, and Obsessive-Compulsive Personality Disorder (OCPD) in particular, are also associated with neuroticism, anxiety disorders, and chronic insomnia. (American Psychiatric Association [APA], 2000; 2013; Mahendran, Subramaniam, & Chan, 2007; Ruiter, Lichstein, Nau, & Geyer, 2012; Samuels et al., 2000; Sassoon et al., 2014). OCPD is defined by a pervasive pattern of preoccupation with orderliness, perfectionism, and mental and interpersonal control. This control is often at the expense of flexibility, openness, and efficiency (APA, 2013). Specific OCPD features, as well as traits which are commonly associated with OCPD (e.g., neuroticism, anxiety, hyperarousal), are often prevalent in insomnia disorder and may even be predisposing factors in the development of sleep disturbances (Fernandez-Mendoza et al., 2010). Those with OCPD were found to be significantly more likely to have trouble falling asleep, more disturbed sleep, and restless sleep (Zhang & Lu, 2013).
Our previous work indicated that individuals with chronic insomnia and hypnotic dependence (HDI), and features of OCPD had poorer insomnia-related daytime functioning and greater fatigue severity compared to patients without features with OCPD (Ruiter et al., 2012). Therefore the experience or perception of insomnia on daytime sequelae for these individuals appeared to be more intense than for those without OCPD features. To date, no studies have investigated whether personality traits affect response to effective treatments for insomnia, specifically multicomponent behavioral therapies for insomnia (BT-I). Determining the extent that OCPD status contributes to the success of insomnia treatment was the successive step.
This study aimed to determine whether Cluster C personality disorders, specifically features of OCPD, were associated with treatment response to BT-I for individuals with HDI. Because of the link between OCPD and related exacerbating symptoms of insomnia (e.g., increased anxiety, rigidity, neuroticism), we hypothesized that patients with chronic insomnia and features of OCPD would have a poorer response to BT-I compared to patients without features of OCPD. Identifying whether Cluster C personality disorder features, as predisposing factors in the development of insomnia, affect treatment trajectories may help clinicians with case conceptualization and developing individualized treatment plans for these patients.
Methods
Study Design and Sample Selection
Study data were acquired from a larger randomized clinical trial (2005–2010) that offered insomnia treatment and supervised hypnotic medication withdrawal to individuals with chronic HDI. Participants were local community members in Tuscaloosa and Birmingham, Alabama recruited through advertisements and clinical referrals. Key inclusion criteria included meeting quantitative sleep criteria, a complaint of difficulty initiating or maintaining sleep exceeding 30 minutes of sleep-onset latency (SOL) or wake after sleep onset (WASO) three or more times per week (Lichstein, Durrence, Taylor, Bush, & Riedel, 2003), meeting diagnostic criteria for an insomnia disorder (ICSD-II; AASM, 2005), and taking a prescription sleep medication three or more times per week for at least the previous six months. A full list of inclusion and exclusion criteria can be found in Table 1.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Ages: 21–69 years old | History of seizures |
| Met ICSD-II criteria for insomnia (AASM, 2005) | High levels of caffeine, nicotine, and/or alcohol use |
| Met quantitative sleep criteria for insomnia (Lichstein et al., 2003) | Use of other drugs with sleep-active properties |
| Report of mood, cognitive, or socio-occupational impairment | Apnea/hypopnea index (AHI) > 10/hour |
| Prescribed hypnotic use ≥ 4 nights/week for ≥ 6-months | Myoclonus arousals > 10/hour |
| Desire to quit hypnotic use with an inability to do so | Unstable medical/psychiatric status |
| Shiftwork |
Those interested in participation responded to community advertisements and clinical referrals and completed a 30-minute phone screening interview. Individuals who appeared to meet study criteria were mailed and asked to complete two-weeks of sleep diaries and daytime functioning questionnaires used to verify self-reported insomnia and hypnotic use. Once verified participants were scheduled for an in-person visit at one of two participating sleep centers, which included a diagnostic psychiatric and medical screening interview and a comprehensive sleep history. Potentially eligible participants were then scheduled for three nights of polysomnography (PSG) to rule out other sleep disorders, namely obstructive sleep apnea and periodic limb movement disorder. Urine screens were completed on each PSG night to document treatment effects on hypnotic use and rule out other drug contaminants. In combination with PSG, this provides an effective means of verifying participant report of diminished hypnotic use. The first PSG night served as an adaptation study. Bed and wake times were chosen by participants. Upon awakening each morning, participants completed sleep diaries for each night of the sleep study. In total, 2,129 community-based adults were screened for eligibility and of those 90 met criteria and were randomized to treatment.
The present analysis focused on participants randomized into the BT-I plus gradual hypnotic withdrawal treatment arm, comprised of eight weekly individual, 50-minute sessions. Participants whose hypnotic withdrawal exceeded 8 weeks, continued bi-weekly withdrawal monitoring. Therapy sessions were conducted by advanced clinical psychology graduate students. All participants commenced hypnotic withdrawal immediately upon completing baseline screening. Those assigned to the treatment group simultaneously began BT-I which combined training in relaxation, stimulus control, and sleep hygiene. The study was approved by The University of Alabama Institutional Review Board and all participants provided written consent.
Analytic Sample and Procedure
Study data were obtained from a diagnostic psychiatric interview at baseline, as well as an insomnia severity questionnaire, two weeks of sleep diaries, PSG sleep parameters, and hypnotic medication usage obtained at each time point. Of the 32 participants randomized to BT-I, only participants with complete diagnostic psychiatric interview data on personality disorders were included in our analysis (n = 23).
Measures
Personality Disorder Assessment.
Personality disorders were assessed using the Structured Clinical Interview for DSM-IV Personality Questionnaire (SCID-II-PQ), which is comprised of 119 yes/no questions used to screen for DSM-IV personality disorders. The SCID-II-PQ has shown good sensitivity, specificity, and internal consistency (Ball, Rounsaville, Tennen, &, Kranzler, 2001; Ekselius, Lindstrom, von Knorring, Bodlund, & Kullgren, 1994; Jacobsberg, Perry, & Frances, 1995; Nussbaum & Rogers, 1992). The SCID-II-PQ also has clinical cut-off scores for each personality disorder that demonstrate good agreement with diagnoses from the full SCID-II interview (Ekselius et al., 1994). Clinical cut-off scores for each of the Cluster C personality disorders were used as the primary predictor variables for this study.
Insomnia Severity Index.
Perceptions of insomnia severity were assessed with the Insomnia Severity Index (ISI; Morin, 1993) at baseline, post-treatment and one-year follow-up. The ISI is a seven-item self-report questionnaire which measures the severity of one’s insomnia problems over the previous two weeks. A total score is computed by summing all seven items, with higher scores indicating greater insomnia severity.
Sleep Diary.
Participants completed two-weeks’ of sleep diaries (given in Lichstein, Durrence, Riedel, Taylor, & Bush, 2004) at baseline, post-treatment, and one-year follow-up. Average total sleep time (TST), sleep efficiency (SE; ratio of TST to total time in bed multiplied by 100), SOL, WASO, and sleep quality (1 to 5 scale; 1 = poor, 5 = excellent) were calculated at each time point.
PSG.
Respironics’ computerized Alice 3 Infant and Adult and the Grass-Telefactor polysomnographic systems were used for overnight sleep recording (PSG). To score sleep stages and sleep-wake measures, electrode placements including two electroencephalography (EEG), two electro-oculography (EOG), and chin electromyography (EMG) were applied according to standard placements (Rechtschaffen & Kales, 1968). Supplementary channels included heart rate (EKG), oxygen saturation level, bilateral anterior tibialis EMG, thoracic strain gauge, and a nasal/oral thermistor. These supplementary channels were used to screen for other sleep disorders, particularly periodic limb movement disorder and sleep apnea.
Registered PSG technicians manually scored PSG records in 30-second epochs according to the American Academy of Sleep Medicine standard (Iber, Ancoli-Israel, Chesson, & Quan, 2007). Scored variables included absolute values for TST, SE, SOL, WASO, number of awakenings, and the percentage of sleep spent in each sleep stage (1, 2, 3, and REM). The mean of nights two and three for each sleep parameter were used for data analysis.
In addition, to the PSG parameters, all participants were asked to complete sleep diaries and the Spielberger State-Trait Anxiety Inventories, Form Y (STAI) for each PSG-recorded night. The STAI is a validated and reliable, 20-item measure of trait and state anxiety (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983). TST, SE, sleep quality ratings, and STAI scores were computed and averaged for nights two and three at each time point (i.e., baseline, post-treatment, follow-up).
Hypnotic Medication Usage.
Hypnotic medication usage, in terms of number of hypnotic medications, frequency consumed, and dosage converted into lowest recommended dose units was collected at each time point with the two-week sleep diaries as well as the diaries recorded for each PSG night. Mean dosage for each hypnotic medication consumed was computed separately for sleep diaries, and nights two and three of the PSG recordings. The mean dosages for all hypnotic medications consumed were then summed separately for sleep diary assessment and PSG assessment at each time point. This computation gives a dosage index of all hypnotic medications consumed for each participant across each time point.
Covariates.
Several variables with the potential to affect the outcome were considered as covariates, including: age, sex, education (highest level completed), number of reported nights per week insomnia was experienced (via sleep diaries), insomnia duration (self-reported), hypnotic medication duration in years (self-reported), and number of prescription medications taken at baseline (self-reported).
Statistical Analysis
Differences by Cluster C personality disorders status in baseline characteristics were analyzed with t tests for continuous variables and χ2 tests for categorical variables. Statistical significance was set at α = 0.05. Analyses were conducted using multilevel mixed-effects linear regression (intent-to-treat approach) examining the interaction between study visit (i.e., baseline, post-treatment, one-year follow-up) and Cluster C personality disorders clinical cut-off status on each sleep and medication dosage outcome. Multilevel models are robust to missing data allowing for inclusion of data from participants who did not complete the follow-up visit, thus reducing the probability of Type 1 errors. Data were adjusted for the aforementioned baseline covariates that differed by Cluster C personality disorder status by p < .1. Time was treated as a categorical variable to model its nonlinear effects and an unstructured covariance matrix was assumed. A random intercept and random slope for the sleep parameters were not included. Data was examined for potential outliers. If outliers were identified, sensitivity analyses were conducted to examine the change in effects when the outlier was removed. SPSS version 24 (IBM Corp.) was used for all analyses.
Results
Sample Characteristics
Twenty-three participants met criteria for this study and were included in the analysis. Mean age of the sample was 53.0 (SD = 9.9). About three quarters were women (n = 17). On average, participants had 16.0 (SD = 2.1) years of education completed. Of the three Cluster C personality disorders, 8 participants met or exceeded the clinical cut-off score for OCPD, only 1 participant met criteria for Avoidant Personality Disorder, and none met criteria for Dependent Personality Disorder. Thus, for the purposes of this study, only OCPD status was examined in subsequent analyses. The participant with features of Avoidant Personality Disorder did not meet criteria for OCPD. All analyses were examined with and without this participant. Baseline sleep and medication dosage characteristics of the sample can be found in Table 2. There were no significant differences (p > .1) between participants meeting or not meeting the clinical cut-off score for OCPD on the covariates examined except for education (t[20] = 1.93, p = .07) and total medications prescribed (t[21] = −2.11, p = .047). Patients with OCPD were less educated (M = 14.9, SD = 2.3 vs. M = 16.6, SD = 1.8) and reported taking more medications overall, hypnotic or otherwise (M = 5.8, SD = 2.6 vs. M = 3.8, SD = 1.9). Thus, only these covariates were adjusted for in subsequent analyses. By post-treatment, all 23 participants remained in the study though only 18 participants completed the PSG recordings of which 7 had features of OCPD (of original 8; 87.75% retention), whereas 11 participants had no OCPD (of original 15; 73.3% retention). By one-year follow-up the sample size was 20 participants though only 16 participants completed the PSG recordings of which 6 had features of OCPD (of original 8; 75% retention) whereas 10 participants had no OCPD (of original 15; 66.6% retention).
Table 2.
Analytic Sample Baseline Characteristics (n = 23) by OCPD status
| Overall Sample | OCPD (n=8) |
No OCPD (n=15) |
||
|---|---|---|---|---|
| Variable |
M (SD) or n (%) |
Range |
M (SD) or n (%) |
M (SD) or n (%) |
| Age | 53 (9.9) | 33 – 68 | 52.5 (11.5) | 53.3 (9.4) |
| Sex (female) | 17 (73.9) | -- | 5 (62.5) | 12 (80.0) |
| Education (years completed) | 16.0 (2.1) | 13 –20 | 14.9 (2.3) | 16.6 (1.8) |
| Total No. Medications (sleep + other) | 4.5 (2.3) | 1–11 | 5.8 (2.6) | 3.8 (1.9) |
| Sleep Medication duration (years) | 2.9 (2.4) | 0.5 – 10 | 3.8 (3.1) | 2.4 (2.0) |
| Insomnia Duration (years) | 10.2 | 0.7 – 52 | 10.5 (8.8) | 10.0 (13.6) |
| Insomnia Nights per week | 4.9 (1.5) | 2.5 – 7 | 4.4 (1.5) | 5.2 (1.5) |
| Insomnia Severity Index | 16.2 (4.6) | 7 – 25 | 15.8 (4.4) | 16.5 (4.9) |
| Sleep diary TST (min) | 363.4 (62.3) | 230.4 – 478.9 | 387.8 (74.8) | 350.5 (52.8) |
| Sleep diary SE | 74.3 (9.8) | 48.1 – 86.6 | 77.3 (9.5) | 72.7 (9.9) |
| Sleep diary SOL | 39.5 (21.7) | 15.0 – 107.1 | 33.1 (22.0) | 42.9 (21.5) |
| Sleep diary WASO | 49.2 (30.0) | 5.4 – 134.4 | 59.6 (38.0) | 43.6 (24.4) |
| Sleep diary quality rating | 2.6 (0.5) | 1.0 – 3.6 | 2.7 (0.5) | 2.6 (0.5) |
| PSG TST | 397.0 (46.2) | 299.5 – 501.8 | 407.7 (63.4) | 391.3 (35.3) |
| PSG SE | 85.4 (7.6) | 68.6 – 97.8 | 86.2 (10.5) | 85.0 (6.0) |
| PSG SOL | 16.3 (15.6) | 1.5 – 73.8 | 11.4 (8.2) | 19.0 (18.0) |
| PSG NWAK | 5.0 (3.2) | 1.0 – 15.5 | 4.3 (2.8) | 5.3 (3.5) |
| PSG WASO | 41.7 (35.9) | 8.8 – 101.8 | 37.6 (29.8) | 44.1 (21.4) |
| TST (sleep diary) during PSG nights | 371.2 (70.8) | 197.0–477.5 | 378.0 (74.7) | 367.3 (69.1) |
| SE (sleep diary) during PSG nights | 78.0 (12.7) | 41.1–93.8 | 77.2 (13.1) | 78.4 (12.5) |
| Sleep quality ratings (sleep diary) during PSG nights | 3.0 (0.9) | 1.0–5.0 | 2.9 (1.0) | 3.1 (0.9) |
| STAI scores during PSG nights | 30.8 (8.6) | 20–48.5 | 29.4 (8.3) | 31.5 (8.7) |
| N1% | 11.0 (7.9) | 2.5 – 36.1 | 11.2 (10.7) | 10.8 (6.3) |
| N2% | 67.5 (10.0) | 34.9 – 84.0 | 66.6 (13.7) | 67.8 (7.8) |
| N3% | 2.7 (5.3) | 0.0 – 23.2 | 1.7 (2.9) | 3.2 (6.2) |
| REM% | 19.0 (5.4) | 11.7 – 29.5 | 20.5 (5.7) | 18.6 (5.3) |
| Total dosage of all hypnotic medications in LRD units (diaries) | 2.3 (2.0) | 0.5 – 8.0 | 2.6 (2.7) | 2.2 (1.8) |
| Total dosage of all hypnotic medications in LRD units (PSG) | 2.6 (2.5) | 0.4 – 9.0 | 3.2 (3.1) | 2.2 (2.2) |
LRD = lowest recommended dose; NWAK = number of awakenings; REM = rapid eye movement; SE = sleep efficiency; SOL = sleep onset latency; STAI = State-Trait Anxiety Inventory; TST = total sleep time; WASO = wake after sleep onset
Self-Reported Sleep Variables
Results of the five sleep diary variables and ISI score are shown in Figure 1. The overall adjusted, OCPD status x time interactions were not significant for SOL (F2,19.81 = 2.72, p = 0.09), WASO (F2,18.94 = 0.50, p = 0.61), SE (F2,19.19 = 0.89, p = 0.43), TST (F2,18.0 = 1.23, p = 0.32), sleep quality (F2,18.45 = 0.21, p = 0.81), or ISI score (F2,16.29 = 0.72, p = 0.50). Polysomnography Sleep Continuity Variables
Figure 1. OCPD status by time for self-reported sleep onset latency, wake after sleep onset, total sleep time, sleep efficiency, Insomnia Severity Index score, sleep quality rating, and hypnotic medication dosage.
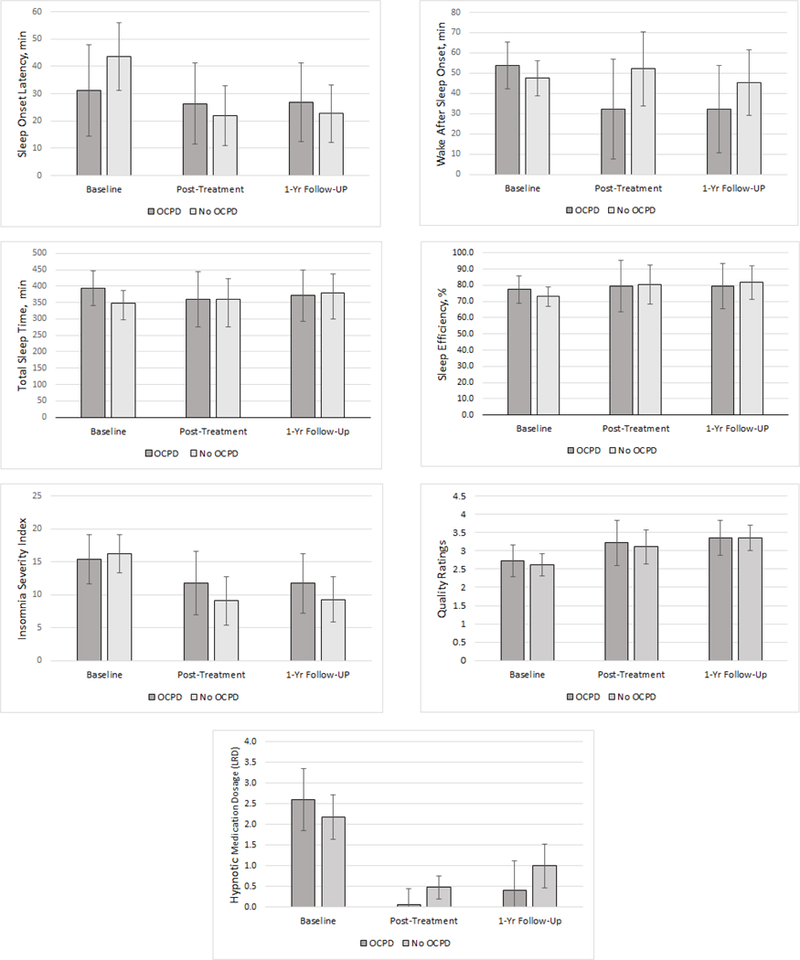
No significant OCPD status by time interactions were found. Note. Error bars show 95% CI with the exception of wake after sleep onset and hypnotic medication dosage, which show standard errors due to 95%CI that included zero. LRD = lowest recommended dosage units
Results of the PSG sleep continuity variables are shown in Figure 2. Significant, adjusted OCPD status x time interaction effects were found for TST (F2,14.74 = 6.27, p = 0.01), SE (F2,15.18 = 4.19, p = 0.04), and WASO (F2,15.19 = 6.78, p = 0.01), but not SOL (F2,16.55 = 2.76, p = 0.09) or number of awakenings (F2,16.64 = 1.07, p = 0.36). An analysis of simple effects found that participants with features of OCPD had significantly less TST (Mdiff = −127.9 minutes, SE = 39.6, p = .006), SE (Mdiff = −22.7%, SE = 8.4, p = .02) and more WASO (Mdiff = 53.1 minutes, SE = 16.0, p = .005) by one-year follow-up compared to participants without OCPD. Participants with features of OCPD also had significantly less TST at one-year follow-up compared to baseline (Mdiff = −159.2 minutes, SE = 29.3, p < .001), less SE at one-year follow-up compared to baseline (Mdiff = −22.9 minutes, SE = 6.7, p = .004) and post-treatment (Mdiff = −22.0 minutes, SE = 6.1, p = .003), and more WASO at one-year follow-up compared to baseline (Mdiff = 54.4 minutes, SE = 15.9, p = .003) and post-treatment (Mdiff = 50.0 minutes, SE = 13.2, p = .002).
Figure 2. OCPD status by time for polysomnography-derived sleep onset latency, wake after sleep onset, number of awakenings, total sleep time, and sleep efficiency.
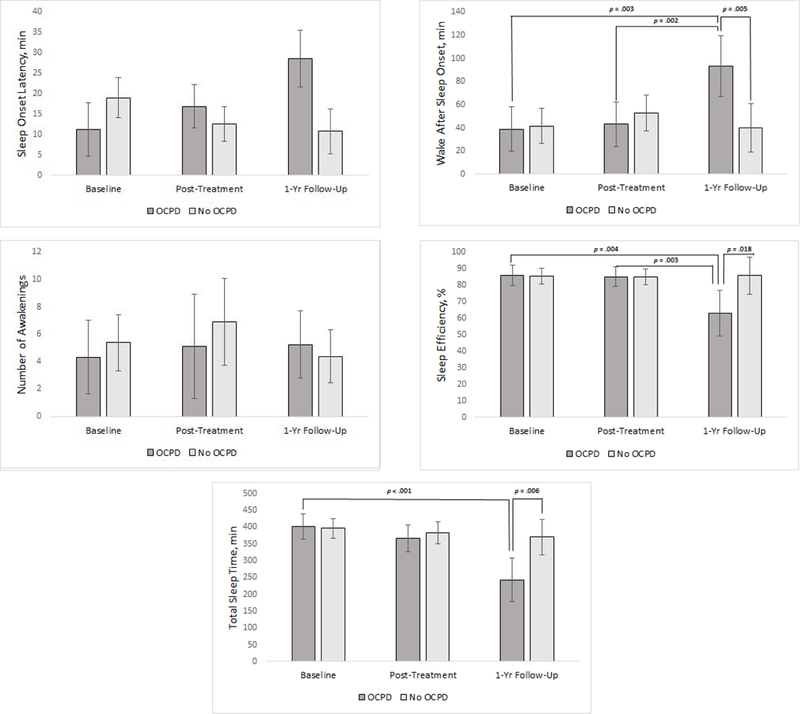
Significant OCPD status by time interaction effects were found for sleep onset latency, sleep efficiency, and total sleep time. Note. Error bars show 95% CI with the exception of sleep onset latency which shows standard error due to 95%CI that included zero.
Self-Reported Sleep During Polysomnography
Results of self-reported sleep (i.e., TST, SE%, sleep quality) and state anxiety (STAI scores) are shown in Figure 3. Significant, adjusted OCPD status x time interaction effects were found for TST (F2,15.14 = 9.01, p = 0.003), SE (F2,15.13 = 7.91, p = 0.004), sleep quality ratings (F2,15.09 = 29.63, p < .001), and STAI scores (F2,13.05 = 6.71, p = 0.01). Analyses of simple effects found that participants with features of OCPD reported significantly less TST (Mdiff = −165.2 minutes, SE = 65.3, p = .02) SE (Mdiff = −27.6%, SE = 12.9, p = .04), and lower sleep quality (Mdiff = 1.5, SE = 0.4, p = .002) by one-year follow-up compared to participants without OCPD. An analysis of simple effects found that participants with features of OCPD did not have significantly greater STAI scores than participants without OCPD at any time point. Yet participants with features of OCPD had greater STAI scores at one-year follow-up compared to their post-treatment assessment (Mdiff = 5.0, SE = 1.3, p = .003), and participants without OCPD has significantly less STAI scores at post-treatment (Mdiff = 5.8, SE = 2.1, p = .01), and one-year follow-up (Mdiff = 4.6, SE = 1.6, p = .01) compared to their baseline assessment
Figure 3. OCPD status by time for total sleep time, sleep efficiency, sleep quality ratings, and State Trait Anxiety Inventory scores during polysomnography recording nights.
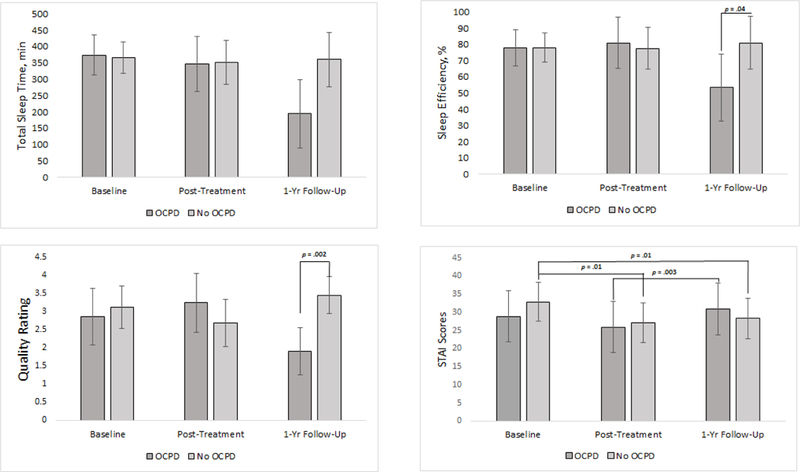
Significant OCPD status by time interaction effects were found for total sleep time, sleep efficiency, sleep quality ratings, and State Trait Anxiety Inventory scores. Note. Error bars show 95% CI.
Polysomnography Sleep Staging Variables
Results of sleep staging variables are shown in Figure 4. A significant, adjusted OCPD status x time interaction effect was found for N2 percentage (F2,12.66 = 5.33, p = 0.02), but not for N1 percentage (F2,14.5 = 1.46, p = 0.27), N3 percentage (F2,9.06 = 1.86, p = 0.21), or REM percentage (F2,14.07 = 2.05, p = 0.17). An analysis of simple effects found that participants with features of OCPD did not have a significantly greater percentage of N2 than participants without OCPD at any time point. Yet participants with features of OCPD had a greater percentage of N2 at one-year follow-up compared to their post-treatment assessment (Mdiff = 6.7%, SE = 2.5, p = .02), but not compared to their baseline assessment (Mdiff = 2.5%, SE =4.1, p = .55). This increase in N2 sleep percentage was not accompanied by significant decreases in the other sleep stages for participants with features of OCPD.
Figure 4. OCPD status by time for polysomnography-derived N1, N2, N3, and REM percentages.
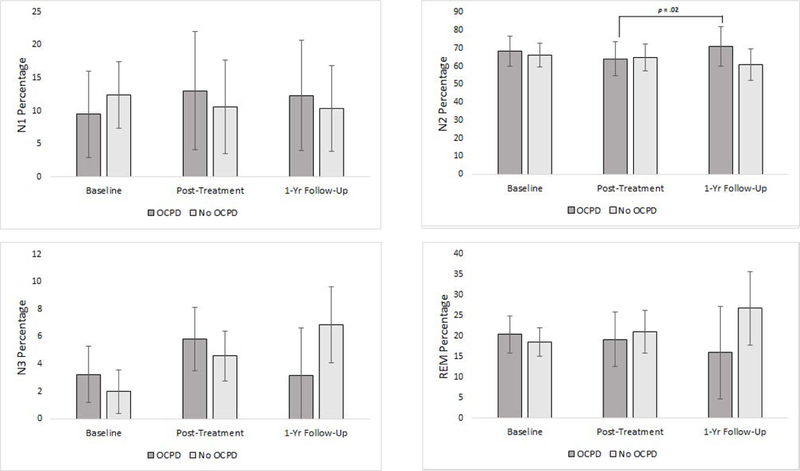
Significant OCPD status by time interaction effects were found for N2 percentage only. Note. Error bars show 95% CI with the exception of sleep onset latency which shows standard errors due to 95%CI that included zero.
Hypnotic Medication Dosage
Results of hypnotic medication dosage collected via two-week sleep diaries are shown in Figure 1. By post-treatment, only three participants continued to use hypnotic medications and by one-year follow-up, only four participants continued to use hypnotic medications but at lower doses than baseline (two were using anti-depressants, two were using benzodiazepines). The overall adjusted, OCPD status x time interaction was not significant for hypnotic medication usage as recorded on two-week sleep diaries (F2,18.80 = 0.48, p = 0.63). A multilevel mixed-effects linear regression model for hypnotic medication usage during the PSG recorded nights was not able to be computed due to a lack of hypnotic medication usage at post-treatment and one-year follow-up.
Sensitivity Analyses
Sensitivity analyses were conducted to discern any changes in effects when outliers in primary outcome measures were identified, and to ascertain whether the presence of a participant with features of Avoidant Personality Disorder in the group with no features of OCPD affected the results. Non-substantive changes in the findings occurred as a result of these analyses. The direction of simple effects remained constant in all models compared to the original models.
Discussion
In a BT-I trial among 23 patients with chronic HDI, data from subjective sleep diaries, questionnaire data, and reported hypnotic medication usage indicated that all patients, regardless of OCPD status, endorsed improvements in their overall sleep and reduced hypnotic mediation usage at post-treatment and reported maintaining these gains at one-year follow up. In conjunction with this, objective PSG outcomes indicated that participants with or without OCPD responded equivalently to BT-I at post-treatment. However, at one-year follow-up those with OCPD had less PSG-recorded TST, lower SE, and had greater WASO than their counterparts, and compared to their post-treatment levels, their percentage of N2 sleep significantly increased. These results are further reflected in their sleep diary responses during the PSG recordings. Participants with features of OCPD has significantly less TST, SE, and lower sleep quality ratings at one-year follow-up than participants without OCPD despite no differences in state anxiety experienced between the two groups during the PSG recordings. Overall, these results indicate that objective sleep of those with features of OCPD was shorter, more disrupted, and characterized by lighter sleep one year after BT-I, and this experience of poorer sleep was perceived accurately by these participants.
Research indicates that between 60–80% of subjects respond positively to cognitive-behavioral therapy for insomnia (CBT-I; i.e., BT-I plus a cognitive component) and have significantly better sleep outcomes (Morin & Wooten, 1996). Additionally, 50% of treated subjects exhibit clinically meaningful change and 30% of people administered CBT-I achieve sub-clinical to normal sleep parameters (Espie, Inglis, & Harvey, 2001). While OCPD patients did respond positively to BT-I via self-report, these individuals may have more difficulty in maintaining treatment gains over time as evidenced by a deteriorated, objective sleep profile at one-year follow-up compared to baseline assessment. This deterioration may indicate that patients with features of OCPD may be subject to particular predisposing factors, which may diminish treatment gains over time. For instance, those with OCPD tend to have higher rates of psychological disorders (e.g., depression, anxiety) and emotional problems (e.g., more negative affect, emotional dysregulation) which may make them more vulnerable to developing common, precipitating risk factors for insomnia (Smith, Shepard, Wiltgen, Rufino, & Fowler, 2017; Steenkamp, Suvak, Dickstein, Shea, & Litz, 2015). If so, then this patient population may be considered at-risk and greater clinical attention may be needed. What may increase vulnerability for relapse among insomnia patients with OCPD might be the hallmark characteristics of OCPD itself, namely perfectionism, rigidity, and preoccupation with details, all of which may increase the risk for perpetuating factors that exacerbate sleep difficulties such as maladaptive, ruminative cognitions and heightened physiological arousal at bedtime. Patients with OCPD may find difficulties with sleep particularly frustrating and demanding of anxious, cognitive, and behavioral fixation (Ruiter et al., 2012). These characteristics may compromise long-term adherence to treatment by increasing the risk of re-introducing perpetuating factors, or they may increase the risk of experiencing precipitating factors, both of which may increase vulnerability to relapse. Considering the ruminative nature of cognitions associated with the OCPD diagnosis, CBT-I rather than BT-I may be more appropriate for these patients, though further research is needed.
However, it is also plausible that after one year, participants with OCPD were more reactive to a sleep environment challenge (i.e., overnight sleep study) than those without OCPD. Vulnerability to stress-related sleep disruption may depend on personality and habitual coping style (Harvey et al., 2014). Personalities characterized by high levels of anxiety, such as OCPD, have generally been found to show greater disruption of sleep continuity and depth, especially in the first half of the sleep bout, a period of time characterized by deeper and more restorative sleep (Fuller et al., 1997; Smith, Shepard, Wiltgen, Rufino, & Fowler, 2017). After one-year, participants with OCPD may have an inherent increase in sleep reactivity to stress, a correlate of insomnia that identifies persons more susceptible to experiencing insomnia symptoms when stressful events occur (Drake, Friedman, Wright, & Roth, 2011; Drake, Pillai, & Roth, 2014; Jarrin, Chen, Ivers, & Morin, 2014). These stress related sleep disturbances may be further exacerbated by the unusual environment of a sleep study (Drake, Richardson, Roehrs, Scofield, & Roth, 2004). Increased sleep reactivity to a stressor may explain the response of the overnight PSG conducted in the lab at one-year follow-up for those with OCPD despite the lack of difference in state anxiety between groups. However, this result may not necessarily indicate that those with OCPD are vulnerable to relapse. Instead, it is possible that those with OCPD are more vulnerable to experiencing greater reactivity related to sleep environment challenges. It remains possible that the effects of BT-I are maintained outside of the lab, which is consistent with the results from the subjective sleep data. Information related to the effects of sleep challenges specific to those with OCPD is needed to understand and determine the responsible factors related to this difference.
This study had several strengths including it is one of few which have examined BT-I treatment response by a predisposing factor that is fairly prevalent in this population. Further, the study protocol included a validated clinical interview and robust sleep assessment including 3 nights of PSG to minimize the first night effect. Finally, participants were followed and retained for a long period after treatment ended. However, some limitations should be acknowledged. Primarily, the present study had a small sample size, which may have led to underestimation of OCPD effects due to low power. Further, the loss to follow-up for the PSG recordings needs to be considered in terms of generalizability. Results should be interpreted with caution. Additionally, OCPD status was determined using a screener rather than more rigorous diagnostic criteria.
Participants with features of OCPD responded equivalently on subjective measures across each time point, but objective differences seen at one-year follow up suggests greater vulnerability for relapse and/or sleep reactivity to stress. To substantiate this interpretation, further investigations should determine if increased sleep-reactivity to a stressful or environmental challenge is more prevalent in patients with OCPD and insomnia and if so, adapt BT-I or CBT-I accordingly. Another possibility is to gather objective sleep study data using ambulatory, at-home PSG. This may act to alleviate stress associated with environmental changes. Further, future studies should evaluate these questions in larger sample sizes and examine longer follow-up periods. Overall, features of OCPD as potential predisposing personality traits for insomnia do not appear to blunt treatment response to BT-I, though long-term maintenance of treatment gains may be vulnerable to deterioration.
Acknowledgments
We would like to thank Dr. Michael Todd for his support in affirming the soundness of the statistical models constructed.
Sources of Funding: NIH/NIDA R01 DA013574
References
- American Psychiatric Association; (2000). Diagnostic and Statistical Manual of Mental Disorders (DSM–V–TR) 4th ed Washington, DC. [Google Scholar]
- American Psychiatric Association; (2013). Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed; American Psychiatric Publishing: Washington, DC: Retrieved from http://psychiatryonline.org.libdata.lib.ua.edu/ [Google Scholar]
- American Academy of Sleep Medicine. (2005). International classification of sleep disorders: Diagnostic and coding manual, 2nd Ed Westchester: American Academy of Sleep Medicine. [Google Scholar]
- Bonnet MH, & Arand DL (2010). Hyperarousal and insomnia: State of the science. Sleep Medicine Reviews, 14, 9–15. [DOI] [PubMed] [Google Scholar]
- Drake CL, Friedman NP, Wright KP, & Roth T (2011). Sleep reactivity and insomnia: genetic and environmental influences. Sleep, 34, 1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CL, Richardson G, Roehrs T, Scofield H, & Roth T (2004). Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep, 27, 285–291. [DOI] [PubMed] [Google Scholar]
- Drake CL, Pillai V, & Roth T (2014). Stress and sleep reactivity: A prospective investigation of the Stress-Diathesis Model of insomnia. Sleep, 37, 1295–1304. doi: 10.5665/sleep.3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekselius L, Lindstrom E, von Knorring L, Bodlund O, Kullgren G (1994). SCID II interviews and the SCID Screen questionnaire as diagnostic tools for personality disorders in DSM-III-R. Acta Psychiatrica Scandinavica, 90,120–123. [DOI] [PubMed] [Google Scholar]
- Ellis JG, Perlis ML, Neale LF, Espie CA, & Bastein CH (2012). The natural history of insomnia: Focus on prevalence and incidence of acute insomnia. Journal of Psychiatric Research, 46(10), 1278–1285. doi: 10.1016/j.jpsychires.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Emert SE, Tutek J, & Lichstein KL (2017). Associations between sleep disturbances, personality, and trait emotional intelligence. Personality and Individual Differences, 107, 195–200. doi: 10.1016/j.paid.2016.11.050 [DOI] [Google Scholar]
- Fernandez-Mendoza J, Vela-Bueno A, Vgontzas AN, Ramos-Platon MJ, Olavarrieta-Bernardino S, Bixler EO, De la Cruz-Troca JJ (2010). Cognitive-emotional hyperarousal as a premorbid characteristic of individuals vulnerable to insomnia. Psychosomatic Medicine, 72, 397–403. doi: 10.1097/PSY.0b013e3181d75319 [DOI] [PubMed] [Google Scholar]
- Fuller KH, Waters WF, Binks PG, & Anderson T (1997). Generalized anxiety and sleep architecture: a polysomnographic investigation. Sleep, 20, 370–376. [DOI] [PubMed] [Google Scholar]
- Harvey CJ, Gehrman P, & Espie CA (2014). Who is predisposed to insomnia: A review of familial aggregation, stress-reactivity, personality and coping style. Sleep Medicine Reviews, 18(3), 237–247. doi: 10.1016/j.smrv.2013.11.004 [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson AL, & Quan SF (2007). The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. American Academy of Sleep Medicine: Westchester [Google Scholar]
- Jacobsberg L, Perry S, & Frances A (1995). Diagnostic agreement between the SCID-II screening questionnaire and the Personality Disorder Examination. Journal of Personality Assessment, 65, 428–433. doi: 10.1207/s15327752jpa6503_4 [DOI] [PubMed] [Google Scholar]
- Jarrin DC, Chen IY, Ivers H, & Morin CM (2004). The role of vulnerability in stress-related insomnia, social support and coping styles on incidence and persistence of insomnia. Journal of Sleep Research, 23, 681–688. doi: 10.1111/jsr.12172 [DOI] [PubMed] [Google Scholar]
- Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, & Riedel BW (2003). Quantitative criteria for insomnia. Behaviour Research and Therapy, 41, 427–445. [DOI] [PubMed] [Google Scholar]
- Lichstein KL, Durrence HH, Riedel BW, Taylor DJ, & Bush AJ (2004). Epidemiology of sleep: Age, gender, and ethnicity. Mahwah, NJ: Erlbaum [Google Scholar]
- Lichstein KL, Nau SD, Wilson NM, Aguillard RN, Lester KW, Bush AJ, & McCrae CS (2013). Psychological treatment of hypnotic-dependent insomnia in a primary older adult sample. Behaviour Research and Therapy, 51, 787–796. doi: 10.1016/j.brat.2013.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahendran R, Subramaniam M, & Chan YH (2007). Psychiatric morbidity in patients referred to an insomnia clinic. Singapore Medical Journal, 48(2), 163–165. [PubMed] [Google Scholar]
- Morin CM (1993). Insomnia: Psychological Assessment and Management. Guilford Press, New York. [Google Scholar]
- Nussbaum D, & Rogers R (1992). Screening psychiatric patients for Axis II disorders. The Canadian Journal of Psychiatry, 37, 658–660. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, & Kales A (Eds.). (1968). A manual of standardized terminology, techniques and scoring system of sleep stages in human subjects Los Angeles: Brain Information Service/Brain Research Institute, University of California. [Google Scholar]
- Regen W, Hertenstein E, Weil P, Kyle SD, Holz J, Baglioni C, … Spiegelhalder K(2014). Perfectionistic Tendencies in Insomnia Patients’ Behavior During Psychometric Testing. Behavioral Sleep Medicine, 0(0), 1–8. doi: 10.1080/15402002.2014.919918 [DOI] [PubMed] [Google Scholar]
- Roth T (2001). New developments for treating sleep disorders. Journal of Clinical Psychiatry, 62, 3–4. [PubMed] [Google Scholar]
- Ruiter ME, Lichstein KL, Nau SD, & Geyer JD (2012). Personality disorder features and insomnia status amongst hypnotic-dependent adults. Sleep Medicine, 13(9), 1122–1129. doi: 10.1016/j.sleep.2012.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels J, Nestadt G, Bienvenu OJ, Costa PT, Riddle MA, Liang K, Hoehn-Saric R, Grados MA, & Cullen BAM (2000). Personality disorders and normal personality dimensions in obsessive-compulsive disorder. British Journal of Pharmacology, 177, 457–462. doi: 10.1192/bjp.177.5.457 [DOI] [PubMed] [Google Scholar]
- Sassoon SA, de Zambotti M, Colrain IM, & Baker FC (2014). Association between personality traits and DSM-IV diagnosis of insomnia in peri- and postmenopausal women. Menopause, 21, 602–611. doi: 10.1097/GME.0000000000000192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelhalder K, & Riemann D (2013). Hyperarousal and insomnia. Sleep Medicine Clinics, 8, 299–307. doi: 10.1016/j.jsmc.2013.04.008 [DOI] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, & Jacobs GA (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Spielman AJ, Caruso LS, & Glovinsky PB (1987). A behavioral perspective on insomnia treatment. Psychiatric Clinics of North America, 10, 541–553. [PubMed] [Google Scholar]
- Spielman AJ, & Glovinsky PB (1991). The varied nature of insomnia In Hauri PJ (Ed.), Case Studies in Insomnia. (pp. 1–15). Springer Science & Business Media. [Google Scholar]
- Steenkamp MM, Suvak MK, Dickstein BD, Shea MT, & Litz BT (2015). Emotional functioning in obsessive-compulsive personality disorder: Comparison to borderline personality disorder and healthy controls. Journal of Personality Disorders, 29, 794–808. doi:http://dx.doi.org/101521pedi201428174 [DOI] [PubMed] [Google Scholar]
- Van de Laar M, Verbeek I, Pevernagie D, Aldenkamp A, & Overeem S (2010). The role of personality traits in insomnia. Sleep Medicine Reviews, 14(1), 61–68. doi: 10.1016/j.smrv.2009.07.007 [DOI] [PubMed] [Google Scholar]
- Williams PG, & Moroz TL (2009). Personality vulnerability to stress-related sleep disruption: Pathways to adverse mental and physical health outcomes. Personality and Individual Differences, 46(5–6), 598–603. doi: 10.1016/j.paid.2008.12.017 [DOI] [Google Scholar]
- Yang CM, Hung CY, Lee HC (2014). Stress-related sleep vulnerability and maladaptive sleep beliefs predict insomnia at long-term follow-up. Journal of Clinical Sleep Medicine, 10, 997–1001. doi: 10.5664/jcsm.4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, & Lu N (2013). The relationship between personality disorder and sleep quality in university students in China. Sleep Medicine, 14, e315. doi: 10.1016/j.sleep.2013.11.773 [DOI] [Google Scholar]


