Abstract
The objectives of this work were to evaluate the effects of catechin on cytochrome P450 2E1 (CYP2El)-dependent oxidative stress. Microsomes co-expressing human CYP2E1 with NADPH cytochrome P450 reductase and cytochrome b5 were incubated with NADPH and DTPA at pH 7.0. Superoxide anion generation was specifically detected by spin-trapping with DEPMPO. Generation of the DEPMPO-OOH adduct was not observed in the absence of CYP2E1 and in the presence of superoxide dismutase (SOD) or catechin, while catalase was ineffective. Reactive oxygen species generation was detected with 1-hydroxy-3-carboxy-2,2,5,5- tetramethylpyrrolidine (CPH) by the EPR-detection of its oxidation product, 3-carboxy-proxyl radical (CP●). CP● generation was not observed in the absence of CYP2E1 and in the presence of SOD, while catalase was ineffective. In contrast, catechin increased CPH oxidation, an effect that was not observed in the absence of CYP2E1 or in the presence of SOD (but not catalase), and was not associated with an increase in oxygen consumption. Catechin also increased the non-specific oxidation of the probes CPH and hydroethidine by the superoxide anion-generating system xanthine plus xanthine oxidase. Catechin oxidized CPH in the presence of horseradish peroxidase plus hydrogen peroxide, a catechin radical-generating system. In conclusion, catechin exhibits both antioxidant (superoxide-scavenging) and pro-oxidant effects under CYP2E1- dependent oxidative stress.
Keywords: Antioxidant, catechin, CYP2E1, microsomes, oxidative stress, reactive oxygen species
Introduction
Reactive oxygen species (ROS) are a group of oxidant byproducts of the partial reduction or activation of molecular oxygen, which includes superoxide anion (O2●− ), hydrogen peroxide (H2O2), hydroxyl radical (●OH) and singlet oxygen (1O2). Pro-oxidant or antioxidant refers to any endobiotic or xenobiotic compound that induces an increase or a decrease, respectively, in the steady state levels of ROS in biological systems. An imbalance favoring prooxidants and/or disfavoring antioxidants, potentially leading to damage, is defined as oxidative stress (Sies, 1997).
Flavonoids are polyphenols derived from plants that have a basic structure of two aromatic rings (A and B) connected by a three-carbon chain forming an oxygenated heterocycle (C ring). Different substitutions on this basic skeleton are the basis for the classification of flavonoids into six groups (Verstraeten et al., 2015). (+) Catechin (catechin) belongs to the flavan-3-ol group, characterized by hydroxyl groups at positions C3, C5 and C7 of the A-C rings, and C3 and C4 of the B ring (Verstraeten et al., 2015). Catechin occurs naturally in green tea leaves, wine, certain fruits and seeds, and chocolate (Arts et al., 2000a; 2000b). In vitro, catechin at micromolar levels prevented cytotoxicity and oxidative stress caused by ethanol in SK-Hep-1 human hepatoma cells (Lee et al., 2012) and in HepG2 human hepatoma cells overexpressing cytochrome P450 2E1 (CYP2E1) (Lee et al., 2005), an effect that correlated with antioxidant protection to lipids but not to proteins (Oliva et al., 2011). Catechin prevented lipid peroxidation in iron-loaded rat hepatocytes (Morel et al., 1993), and in rat liver microsomes in the presence of NADPH (Yang et al., 2001) or acetaminophen and NADPH (Letelier at al., 2011). The antioxidant activity of catechin is ascribed to effects including direct ROS scavenging(Bematoniene and Kopustinskiene, 2018), metal chelation abilities (Mira et al., 2002), regeneration of α-tocopherol (Pedrielli and Skibsted, 2002) and upregulation of the nuclear factor erythroid 2-related factor 2 (Nrf2) antioxidant signaling pathway (Jing et al., 2018).
In contrast, catechin at micromolar levels was a pro-oxidant in rat hepatocytes supplemented with iron and linoleic acid (Sugihara et al., 2001), and induced cytotoxicity in rat hepatocytes exposed to hydrogen peroxide, an effect that correlated with the loss of endogenous antioxidants (reduced glutathione and ascorbic acid) and was blocked by a cytochrome P450 inhibitor (Moridani et al., 2001a). Catechin induced cytotoxicity in murine hepatoma MH-22 cells, an effect that correlated with its redox potential (Maroziene et al., 2012). The pro-oxidant activity of catechin is attributed to autoxidation or peroxidase-based oxidation and generation of ROS including phenoxyl radicals (Nakayama et al., 1995; Mochizuki et al., 2002), or quinone- dependent oxidation of low molecular weight antioxidants (Chan etal., 1999; Galati et al., 2002). The results described above show that catechin may exert antioxidant or pro-oxidant effects in different in vitro models of hepatic oxidative stress, depending on factors that are not well characterized. Because CYP2E1 activity is a major source of hepatic oxidative stress via the NADPH-dependent reduction of O2 to superoxide anion and hydrogen peroxide (Caro and Cederbaum, 2004), the objectives of this work were to evaluate the effects of (+) catechin (anti- and/or pro- oxidant) on CYP2E1- dependent oxidative stress in microsomal membranes. Understanding the effects of catechin on CYP2E1-dependent oxidative stress is important for the development of effective therapies based on flavonoids against pathophysiological conditions that are causally linked to CYP2E1-dependent oxidative stress, such as alcoholic liver disease, diabetes, and non-alcoholic steatohepatitis (Caro and Cederbaum, 2004).
Materials and Methods
1. Chemicals
1-hydroxy-3-carboxy- 2,2,5,5-tetramethylpyrrolidine (CPH), hydroethidine (HE) and NADPH were from Santa Cruz Biotechnology (Santa Cruz, CA). Insect cell microsomes (supersomes) coexpressing human CYP2E1 with NADPH cytochrome P450 reductase and cytochrome b5, control supersomes coexpressing human NADPH cytochrome P450 reductase and cytochrome b5 without CYP2E1, and control supersomes devoid of human CYP2E1, NADPH cytochrome P450 reductase and cytochrome b5 were from Coming (Coming, NY). According to the manufacturer, the human CYP2E1 supersomes contained 370 pmol cytochrome P450/mg protein, and they exhibited a p-nitrophenol hydroxylase activity of 11 pmol product/pmol P450/min. 2-hydroxyethidium (2-OH-E+) was synthesized according with Zielonka et al., (2008) with C18 cartridge purification. All other chemicals were from Sigma-Aldrich (St. Louis, MO).
2. Superoxide anion-generating systems
Two superoxide anion-generating systems were used:
a) xanthine + xanthine oxidase: The xanthine + xanthine oxidase reaction system consisted of air-saturated 100 mM potassium phosphate buffer pH 7.0, 5 mM DTP A, 200 μΜ xanthine, and 20 mU/mL xanthine oxidase in a final volume of 100 μL, in the absence or presence of various additions as described under Results.
b) CYP2E1 + NADPH: The microsomal reaction system consisted of air-saturated 100 mM potassium phosphate buffer pH 7.0, 5 mM DTP A, 1 mM NADPH and 1 mg CYP2E1-expressing microsomal protein/mL in a final volume of 100 μL, in the absence or presence of various additions as described under Results.
3. Rate of superoxide anion generation
The rate of superoxide anion generation was determined by: a) spin-trapping of superoxide with DEPMPO, orb) the superoxide-specific oxidation of HE to 2-OH-E+.
Spin-trapping with DEPMPO: The superoxide anion-generating systems were supplemented with 50 mM DEPMPO, and after incubation at room temperature for specific times as indicated under Results, the mixtures were analyzed by EPR at room temperature. A MiniScope MS 5000 EPR (Magnettech, Berlin, Germany) was used with the following parameters: microwave frequency 9.5 GHz, modulation frequency 50 kHz, modulation amplitude 0.02 mT, microwave power 10 mW, and sweep time 60 s. The amplitude of the low field component of the EPR signal was used for quantification.
Oxidation of hydroethidine: The superoxide anion-generating systems were supplemented with 10 μΜ HE, and after incubation at room temperature for 30 min, proteins were precipitated with methanol (2 volumes) and removed from the mixture by centrifugation at 10,000 g for 10 min (Rezende et al., 2017). The supernatant was filtered, and analyzed by ion-pair HPLC with electrochemical detection (Fraccarollo et al., 2011). Isocratic elution was performed at a flow rate of 0.5 mL/min using a Hypersil BDS C18 HPLC column (150×3mm, particle size 3 μm) from Thermo Scientific (Waltham, MA), and a mobile phase (octanesulfonic acid 100 μΜ, NaH2P04 50 mM, pH 2.7) containing 35% acetonitrile (v/v), for HE, 2-OH-E+, and ethidium (E+) separation. Electrochemical detection at 0.00V and +0.45V was performed using a Dionex ECD-3000RS electrochemical detector from Thermo Scientific (Waltham, MA).
4. Rate of ROS generation
The rate of generation of ROS (such as superoxide anion, peroxynitrite, hydroxyl radical and phenoxyl radical) was determined by the ROS-dependent oxidation of 1-hydroxy-3-carboxy- 2,2,5,5-tetramethylpyrrolidine (CPH) to the stable 3-carboxy-proxyl radical (CP●), which can be detected by electron paramagnetic resonance (Kuzkaya et al., 2003; Kozlov et al., 2007; Dikalov et al., 2008; Straface et al., 2012). The superoxide anion-generating systems were supplemented with 1 mM CPH, and after incubation at room temperature for specific times as indicated under Results, the mixtures were analyzed by EPR at room temperature. A MiniScope MS 5000 EPR (Magnettech, Berlin, Germany) was used with the following parameters: microwave frequency 9.5 GHz, modulation frequency 50 kHz, modulation amplitude 0.02 mT, microwave power 10 mW, and sweep time 60 s. The amplitude of the low field component of the EPR signal was used for quantification.
5. Enzymatic activities
Xanthine oxidase activity was determined by measuring the production of uric acid from the xanthine + xanthine oxidase system, indicated by an increase in the absorbance at 290 nm, as described by Cos et al. (1998).
CYP2E1 activity was determined by measuring the production of p-nitrocatechol from p- nitrophenol in the CYP2E1 +NADPH system, as described by Caro and Cederbaum (2005).
6. O2 consumption.
O2 consumption in reaction mixtures described under Results were determined using a Clark-type O2 electrode (Hansatech Oxygraph, Hansatech Instruments, Norfolk, England).
7. Detection of phenoxyl radicals
The phenoxyl radicals generated by the oxidation of catechin with hydrogen peroxide in the presence of horseradish peroxidase (HRP) were detected by EPR at room temperature after stabilization with zinc as described by Yamasaki and Grace (1998). A MiniScope MS 5000 EPR (Magnettech, Berlin, Germany) was used with the following parameters: microwave frequency 9.5 GHz, modulation frequency 50 kHz, modulation amplitude 0.1 mT, microwave power 10 mW, and sweep time 60 s (Peri et al., 2005).
8. Simulation of EPR spectra
Computer simulations of experimental EPR spectra were done using EPR-WinSim, a public software tool from NIEHS (http:/www.epr.niehs.nih.gov/). For DEPMPO-OOH, the simulation corresponds to an exchange between two conformers I and II with the parameters (Bolojan et al., 2012): conformer I (50%), aN= 13.01G, aP= 49.46G, aHβ= 10.63G, aHγ= 0.87 (1H), aHγ= 0.34 (6H); conformer II (50%), aN= 13.07G, aP= 50.68G, aHβ= 11.15G, aHγ= 0.96 (1H), aHγ= 0.41 (6H). For CPH, the simulation used the following parameter: aN = 16.2 G (He et al., 2013).
9. Statistics
Data are expressed as mean ± standard error of the mean from three to five independent experiments run in duplicate. One-way analysis of variance with subsequent post hoc comparisons were performed using the SigmaStat 2.0 software. A p < 0.05 was considered as statistically significant.
Results
1. Effect of catechin on superoxide anion generated by CYP2E1 in the presence ofNADPH.
Superoxide anion generated by CYP2E1 in the presence of NADPH was quantified by EPR with the spin trap DEPMPO via reaction (1):
| (1) |
Supersomes expressing human CYP2E1 (with NADPH cytochrome P450 reductase and cytochrome b5), were incubated with NADPH and DEPMPO in a buffer at pH 7.0 with DTPA to inhibit iron-catalyzed redox reactions. The EPR spectrum obtained after 10 min of incubation (Fig. 1A a) was identical to the simulated spectrum of DEPMPO-OOH (Fig. 1A b), indicating an efficient generation of O2●− by CYP2E1 + NADPH. The relatively high stability of the DEPMPO-OOH adduct in the presence of rat liver microsomes (Beziere et al., 2014) probably contributed to the effective accumulation and detection of the adduct. No EPR signal was detectable under the same conditions as described above but in the presence of superoxide dismutase (SOD) (Fig. 1A c ), or in the absence ofNADPH (Fig. 1A d ), supersomes (Fig. 1A e ) or CYP2E1 (i.e. in the presence of supersomes expressing NADPH cytochrome P450 reductase and cytochrome b5 without CYP2E1 (Fig. 1A f ), or in the presence of supersomes devoid of NADPH cytochrome P450 reductase, cytochrome b5 and CYP2E1 (Fig. 1A g )). The EPR signal did not change significantly in the presence of catalase (Fig. 1A h ). A superoxide generating system composed of xanthine plus xanthine oxidase, in the presence of DEPMPO in a buffer at pH 7.0 with DTPA, produced the DEPMPO-OOH spectrum after 10 min of incubation (Fig 1B a ); this signal was blocked by SOD (Fig. 1B b). The generation of the DEPMPO-OOH adduct was linear with incubation time up to 10 minutes both in the presence of CYP2E1 + NADPH or xanthine + xanthine oxidase (data not shown).
Figure 1.
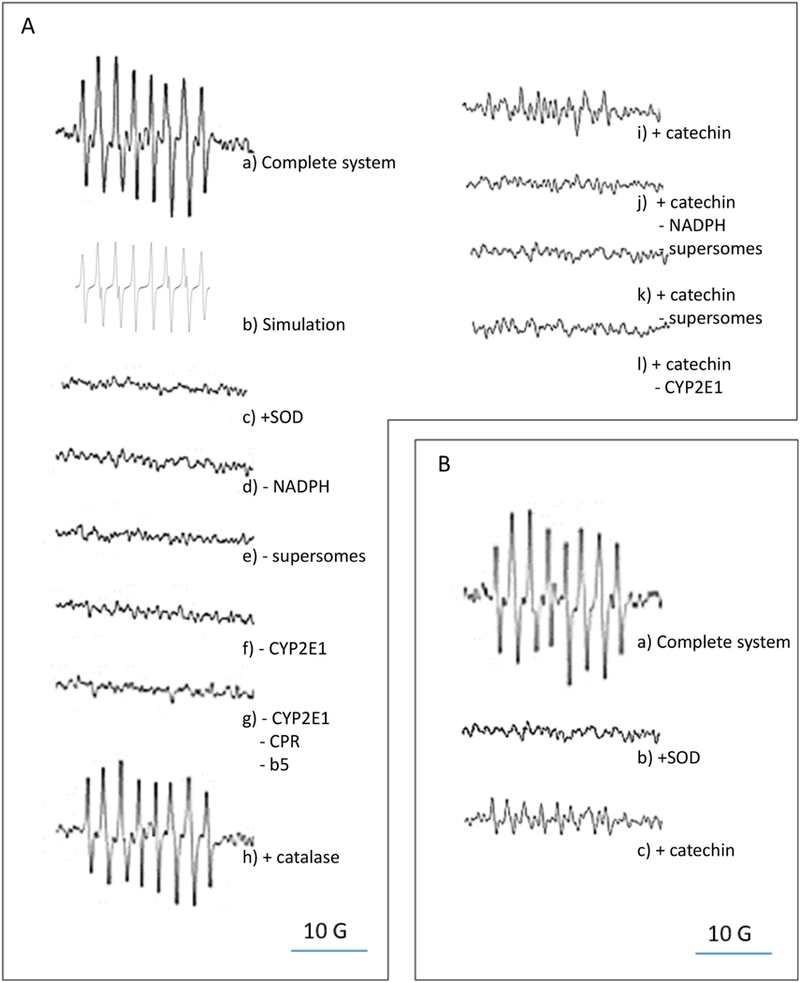
Effect of catechin on superoxide anion generation rate. A) EPR determination of superoxide anion generation by insect cell microsomes (supersomes) after 10 min of incubation. EPR spectra were recorded using the parameters specified under Materials and Methods, a) Complete system: the complete system consisted of air-saturated 100 mM potassium phosphate buffer pH 7.0, 5 mM DTPA, 1 mM NADPH, 50 mM DEPMPO, and 1 mg protein/mL of CYP2E1-expressing supersomes co-expressing NADPH cytochrome P450 reductase (CPR) and cytochrome b5 (b5); b) Simulation: a simulated DEPMPO-OOH spectrum was generated using the parameters described under Materials and Methods; c) + SOD: superoxide dismutase at 1000 U/mL was added to the complete system; d) -NADPH: NADPH was omitted from the complete system; e) - supersomes: supersomes were omitted from the complete system; f) - CYP2E1: supersomes expressing only CPR and b5 replaced CYP2E1-expressing supersomes in the complete system; g) -CYP2E1, -CPR, -b5: supersomes devoid of CYP2E1, CPR and b5 replaced CYP2E1-expressing supersomes in the complete system; h) + catalase: catalase at 1000 U/mLwas added to the complete system; i) + catechin: catechin at 0.1 mM was added to the complete system; j) + catechin, -NADPH, - supersomes: NADPH and supersomes were omitted from, and catechin at 0.1 mM was added to the complete system; k) + catechin, -supersomes: supersomes were omitted from, and catechin at 0.1 mM was added to the complete system; 1) + catechin, - CYP2E1: catechin was added to the complete system, and supersomes expressing NADPH cytochrome P450 reductase and cytochrome b5 replaced CYP2E1-expressing supersomes in the complete system. B) EPR determination of superoxide anion generation by xanthine and xanthine oxidase after 10 min of incubation, a) Complete system: the complete system 0 of air-saturated 100 mM potassium phosphate buffer pH 7.0, 5 mM DTP A, 50 mM DEPMPO, 200 μΜ xanthine, and 20 mU/mL xanthine oxidase; b) + SOD: superoxide dismutase at 1000 U/mL was added to the complete system; c) + catechin: catechin at 0.1 mM was added to the complete system.
The role of catechin as a superoxide anion scavenger is very well known, due to the relatively high rate constant (7.1 × 105 M−1s−1) for reaction (2) at pH 7 (where FlH2 represents reduced catechin and FLH● represents the one-electron oxidized catechin):
| 2 |
Considering that reaction (1) has a rate constant of 0.53 M−1s−1, we predicted that catechin at 0.1 mM would outcompete DEPMPO for CYP2E1-dependent O2●−, even if DEPMPO is used at a 500-fold excess (i.e. 50 mM) with respect to catechin. Catechin at 0.1 mM decreased the generation rate of DEPMPO-OOH in the presence of CYP2E1 + NADPH by 90% (Fig. 1A i), confirming our prediction. DEPMPO-OOH was non-detectable in the presence of DEPMPO and catechin alone (Fig. 1A j), or catechin + NADPH (Fig. 1A k), or catechin + NADPH + supersomes expressing NADPH cytochrome P450 reductase and cytochrome b5 without CYP2E1 (Fig. 1A 1). Catechin at 0.1 mM also blocked the generation of DEPMPO-OOH in the presence of xanthine + xanthine oxidase (Fig. 1B c). Catechin at 0.1 mM did not affect the enzymatic activities of xanthine oxidase or CYP2E1 (data not shown), confirming the weak inhibitory effect of non-gallated catechins on xanthine oxidase (Cos et al., 1998) and cytochrome P450 (Satoh et al., 2016).
2. Effect of catechin on ROS generated by CYP2E1 in the presence of NADPH.
To further explore the effect of catechin on CYP2E1-dependent ROS, CPH (a cyclic hydroxylamine spin probe) was used. Although CPH can be oxidized by O2●− to form the stable paramagnetic nitroxide CP● via reaction (3), CPH can also be oxidized to CP* by other one- electron oxidants including peroxynitrite, phenoxyl radicals and transition metals (Dikalov et al., 2008; Kuzkaya et al., 2003; Straface et al., 2012), supporting the use of CPH oxidation as a general indicator of ROS levels.
| 3 |
Supersomes expressing human CYP2E1 with NADPH cytochrome P450 reductase and cytochrome b5 were incubated with NADPH and the spin probe CPH, in a buffer with DTP A to inhibit iron-catalyzed redox reactions. The EPR spectrum obtained after 10 min of incubation (Fig. 2A a) was identical to the simulated spectrum of CP● (Fig. 2A b). The EPR signal decreased by more than 80% under the same conditions but in the presence of SOD (Fig. 2A c), or in the absence of NADPH (Fig. 2A d), supersomes (Fig. 2A e), or CYP2E1 (i.e. in the presence of supersomes expressing NADPH cytochrome P450 reductase and cytochrome b5 without CYP2E1 (Fig. 2A f), or in the presence of supersomes devoid of NADPH cytochrome P450 reductase, cytochrome b5 and CYP2E1 (Fig. 2A g)). The EPR signal did not change significantly in the presence of catalase (Fig. 2A h). The generation of CP● was linear with incubation time up to 10 minutes both in the presence of CYP2E1 + NADPH or xanthine + xanthine oxidase (data not shown).
Figure 2.
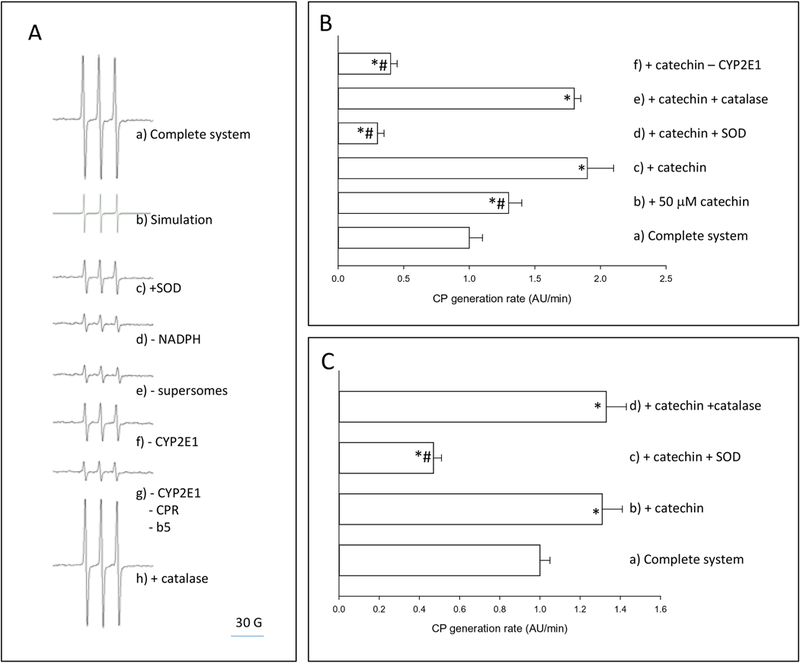
Effect of catechin on ROS generation rate. A) EPR determination of ROS generation by insect cell microsomes (supersomes) after 10 min of incubation. EPR spectra were recorded using the parameters specified under Materials and Methods, a) Complete system: the complete system consisted of air-saturated 100 mM potassium phosphate buffer pH 7.0, 5 mM DTP A, 1 mM NADPH, 1 mM CPH, and 1 mg protein/mL of CYP2E1-expressing supersomes coexpressing NADPH cytochrome P450 reductase (CPR) and cytochrome b5 (b5); b) Simulation: a simulated CPH spectrum was generated using the parameters described under Materials and Methods; c) + SOD: superoxide dismutase at 1000 U/mL was added to the complete system; d) - NADPH: NADPH was omitted from the complete system; e) - supersomes: supersomes were omitted from the complete system; f) - CYP2E1: supersomes expressing only CPR and b5 replaced CYP2E1-expressing supersomes in the complete system; g) -CYP2E1, -CPR, -b5: supersomes devoid of CYP2E1, CPR and b5 replaced CYP2E1-expressing supersomes in the complete system; h) + catalase: catalase at 1000 U/mL was added to the complete system. B) EPR determination of ROS generation rate by insect cell microsomes (supersomes). For quantification, the amplitude of the low field signal was expressed as arbitrary units (AU). a) Complete system: the complete system consisted of air-saturated 100 mM potassium phosphate buffer pH 7.0, 5 mM DTP A, 1 mM NADPH, 1 mM CPH, and 1 mg protein/mL of CYP2E1- expressing supersomes co-expressing NADPH cytochrome P450 reductase (CPR) and cytochrome b5 (b5); b) + 50 μΜ catechin: catechin at 50 μΜ was added to the complete system, c) + catechin: catechin at 100 μΜ was added to the complete system; d) + catechin, + SOD: catechin at 100 μΜ and SOD at 1000 U/ml was added to the complete system; e) + catechin, + catalase: catechin at 100 μΜ and catalase at 1000 U/mL was added to the complete system; f) + catechin, -CYP2E1: catechin at 100 μΜ was added to the complete system, and supersomes expressing NADPH cytochrome P450 reductase and cytochrome b5 replaced CYP2E1- expressing supersomes in the complete system. C) EPR determination of ROS generation rate by xanthine and xanthine oxidase. For quantification, the amplitude of the low field signal was expressed as arbitrary units (AU). a) Complete system: the complete system consisted of air- saturated 100 mM potassium phosphate buffer pH 7.0, 5 mM DTP A, 1 mM CPH, 200 μΜ xanthine, and 20 mU/mL xanthine oxidase; b) + catechin: catechin at 100 μΜ was added to the complete system; c) + catechin, + SOD: catechin at 100 μΜ and SOD at 1000 U/mL was added to the complete system; d) + catechin, + catalase: catechin at 100 μΜ and catalase at 1000 U/mL was added to the complete system. * p<0.05, ANOVA, with respect to the complete system; # p<0.05, ANOVA, with respect to + catechin.
Considering that reaction (3) has a rate constant of 3.2 × 103 M−1s−1, we predicted that if reaction (3) is the main pathway of CPH oxidation in a reaction system composed of CYP2E1-expressing microsomes together with NADPH and catechin, then catechin (at 0.1 mM) would outcompete CPH for CYP2E1-dependent O2●−, even if CPH is used at a 10-fold excess (i.e. 1 mM) with respect to catechin. Surprisingly, catechin at 0.1 mM increased the generation rate of CP● in the presence of CYP2E1 + NADPH by 61%; the increase in the generation rate of CP● was significant at concentrations of catechin higher than 50 μΜ (Fig. 2B). SOD significantly inhibited the CP● signal in the presence of CYP2E1 + NADPH + catechin, while catalase was ineffective (Fig. 2B). The CP● generation rate was significantly lower in a system composed of NADPH + catechin + supersomes expressing NADPH cytochrome P450 reductase and cytochrome b5 in the absence of CYP2E1 than in its presence (Fig. 2B). In addition, catechin at 0.1 mM increased the generation rate of CP● in the presence of xanthine + xanthine oxidase by 21% (Fig. 2C). SOD inhibited the generation of CP● in the xanthine + xanthine oxidase system in the presence of catechin by 68%, but catalase was ineffective (Fig. 2C).
To evaluate the possible contribution of autoxidation reactions to the increased oxidative stress by catechin in CYP2E1-expressing microsomes, the effect of catechin on oxygen consumption was determined. While CYP2E1-expressing microsomes in the presence of NADPH consume O2 during its catalytic cycle, autoxidation reactions mediated by catechin should produce an additional O2 consumption in this system, irrespective of the final product (superoxide or other ROS), as indicated by Murias et al., (2005). Supersomes expressing human CYP2E1 (with NADPH cytochrome P450 reductase and cytochrome b5) were incubated in an air-saturated buffer at pH 7.0 with DTP A. Under these conditions, basal O2 consumption was 2.2 μΜ/sec (Fig. 3 a). The subsequent addition of NADPH increased O2 consumption two-fold to 4.4 μΜ/sec (Fig. 3 a). No significant differences were observed if the buffer was supplemented with catechin up to 250 μΜ (Fig. 3 b). Increased O2 consumption in the presence of NADPH depended on CYP2E1 because NADPH did not increase O2 consumption in the presence of supersomes devoid of CYP2E1 (i.e. in the presence of supersomes expressing NADPH cytochrome P450 reductase and cytochrome b5 without CYP2E1 (Fig. 3 c), or in the presence of supersomes devoid of NADPH cytochrome P450 reductase, cytochrome b5 and CYP2E1 (Fig. 3 d)).
Figure 3.
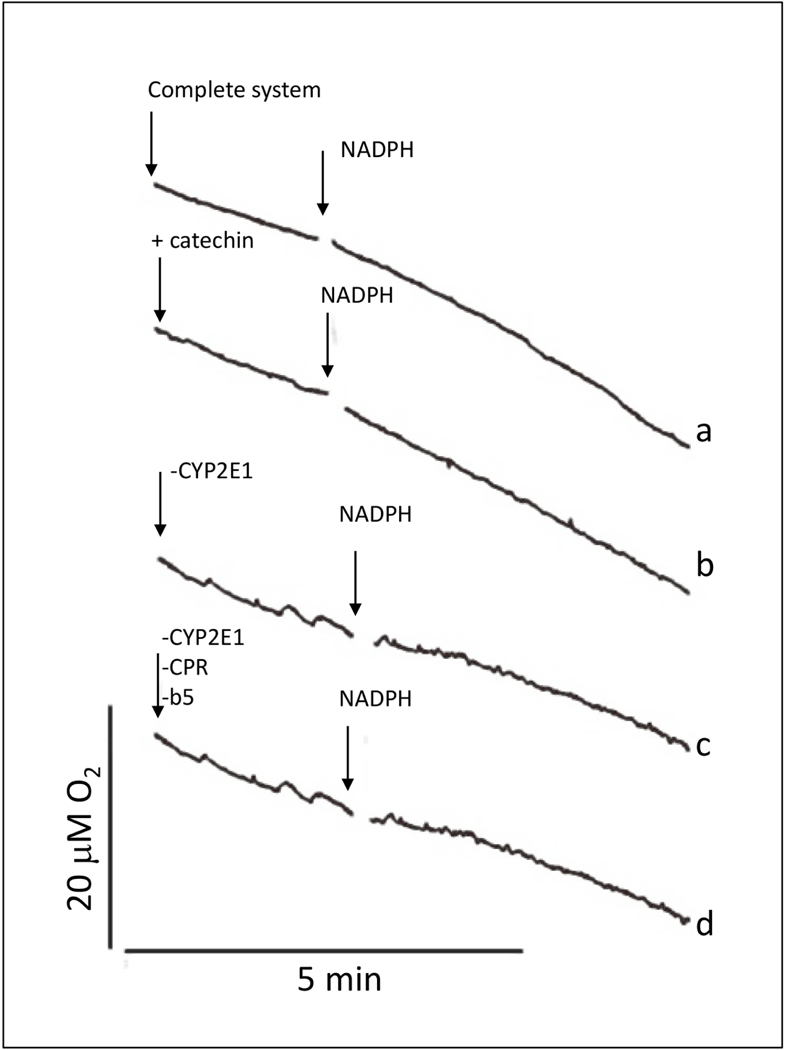
Effect of catechin on O2 consumption by the CYP2E1 + NADPH system, a) The complete system consisted of air-saturated 100 mM potassium phosphate buffer at pH 7.0 with 5 mM DTPA and 1 mg protein/mL of CYP2E1-expressing supersomes co-expressing NADPH cytochrome P450 reductase (CPR) and cytochrome b5 (b5); b) + catechin: catechin at 250 μΜ was added to the complete system; c) - CYP2E1: supersomes expressing only CPR and b5 replaced CYP2E1-expressing supersomes in the complete system; d) -CYP2E1, -CPR, -b5: supersomes devoid of CYP2E1, CPR and b5 replaced CYP2E1-expressing supersomes in the complete system. O2 concentration was recorded continuously using a Clark-type O2 electrode. In every experiment, NADPH at 2 mM was added at the time point indicated by the second arrow.
3. Oxidation of redox probes by catechin phenoxyl radical.
The increased CYP2E1-dependent oxidation of CPH in the presence of catechin might reflect the fast oxidation of catechin by CYP2E1-dependent superoxide, with the subsequent generation of catechin phenoxyl radical, which then oxidizes CPH. In order to test this possibility, catechin radical was generated with catechin, hydrogen peroxide and horseradish peroxidase and stabilized with Zn2+ (Yamasaki and Grace, 1998). In this system, a broad three- line EPR signal was observed (Fig. 4A a), which corresponds to the catechin radical spectrum as reported by Peri et al. (2005). The signal was not observed in the presence of catalase or in the absence of hydrogen peroxide, peroxidase, or Zn2+ (Fig. 4A b-e). Adding CPH to a system composed of catechin, hydrogen peroxide and horseradish peroxidase produced the oxidation product CP● (Fig. 4B a). Conditions that blocked the generation of catechin radical (i.e. presence of catalase, absence of hydrogen peroxide, or peroxidase) blocked the generation of CP● (Fig. 4B b-d), suggesting a reaction between CPH and catechin radical.
Figure 4.
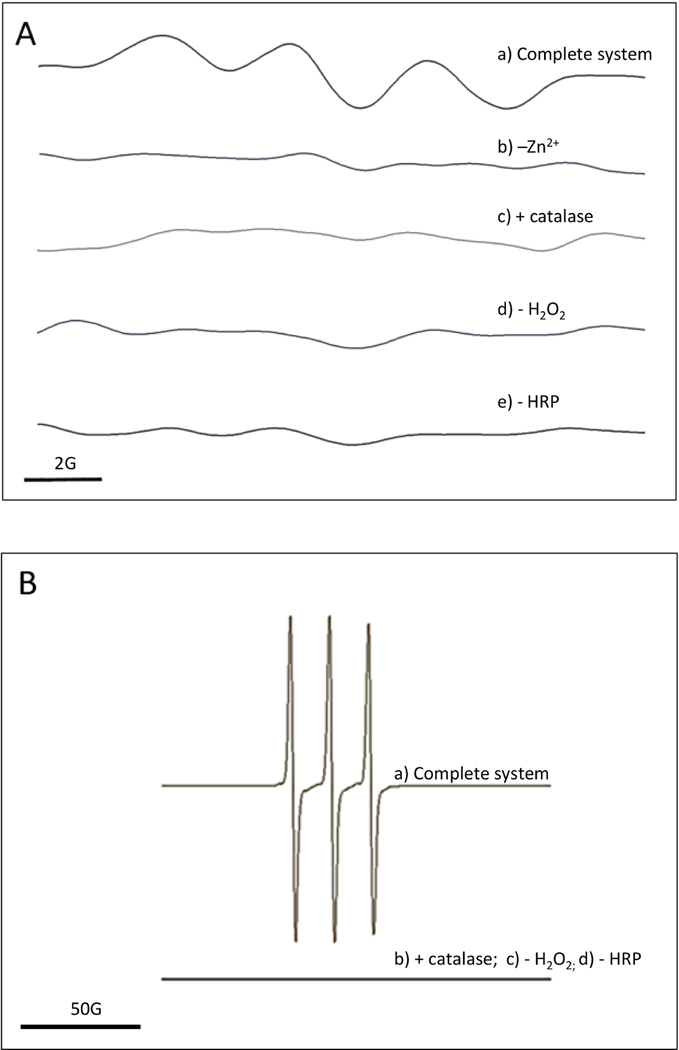
A) EPR detection of catechin phenoxyl radical. EPR spectra were recorded using the parameters specified under Materials and Methods, a) Complete system: the complete system consisted of 20 mM Mes buffer pH 5.5 with 200 mM ZnSCU, 1 mM H2O2, 0.5 U/mL horseradish peroxidase and 1 mM catechin; b) -Zn2+: ZnSCL was omitted from the complete system; c) + catalase: catalase at 1000 U/mL was added to the complete system; d) -H2O2: H2O2 was omitted from the complete system; e) -HRP: horseradish peroxidase was omitted from the complete system. B) EPR detection of CPH oxidation by catechin phenoxyl radical. EPR spectra were recorded using the parameters specified under Materials and Methods, a) Complete system: the complete system consisted of 20 mM Mes buffer pH 5.5, 1 mM H2O2, 0.5 U/mL horseradish peroxidase, 0.3 mM CPH and 1 mM catechin; b) + catalase: catalase at 1000 U/mL was added to the complete system; c) -H2O2: H2O2 was omitted from the complete system; d) - HRP: horseradish peroxidase was omitted from the complete system.
Further evidence of the generation of catechin phenoxyl radical by superoxide-generating systems was obtained using another redox probe, HE. HE is oxidized by superoxide anion specifically to 2-OH-E+; no other biologically relevant oxidant reacts with HE to form 2-OH-E+ (Zielonka et al., 2008). In contrast, HE is oxidized by other one-electron oxidants (such as heme proteins, peroxynitrite- or iron-derived oxidants) nonspecifically to E+ (Zielonka et al., 2009). In addition, the oxidation of HE to E+ by hydrogen atom transfer from semiquinone radicals derived from flavonoids such as catechin has been suggested to be a sensitive assay for the rapid quantification of the radical-forming activity of polyphenolic compounds (Quek and Huang, 2011).
A superoxide anion-generating system composed of xanthine plus xanthine oxidase was incubated with HE in a buffer at pH 7.0 with DTPA to inhibit iron-catalyzed redox reactions. After 30 min of incubation, the sample was processed for the HPLC determination of HE oxidation products. Identification of the peaks in the HPLC chromatograms was based on retention times and comparison with standards of HE (Fig. 5A a), 2-OH-E+ (Fig. 5 A b), and E+ (Fig. 5A c). Under these conditions, 2-OH-E+ was the only oxidation product detected (Fig. 5A d). In the presence of SOD, the generation of 2-OH-E+ was inhibited (Fig. 5A e), indicating a role for superoxide anion. Considering that the reaction of HE with O2●− has a rate constant of 6.2 × 103 M−1s−1 (Michalski et al., 2013), and that catechin was used at a 50-fold excess with respect to HE, it was expected that catechin would outcompete HE for xanthine + xanthine oxidase-dependent O2●−. In the presence of catechin, no 2-OH-E+ was detected, which confirmed our prediction (Fig. 5A f). However, in the presence of catechin, the product E+ was generated instead (Fig. 5A f), an event that was partially blocked in the presence of SOD (Fig. 5 A g). The generation of E+ in the xanthine + xanthine oxidase system supplemented with catechin depended on superoxide anion and xanthine oxidase activity, because the generation rate of E+ decreased by 64% in the presence of SOD and by 69% in the absence of xanthine oxidase (Fig. 5B). However, in the absence of xanthine oxidase, SOD did not decrease E+generation induced by catechin (Fig. 5B), suggesting that oxidation of HE under those conditions was superoxide-independent.
Figure 5.
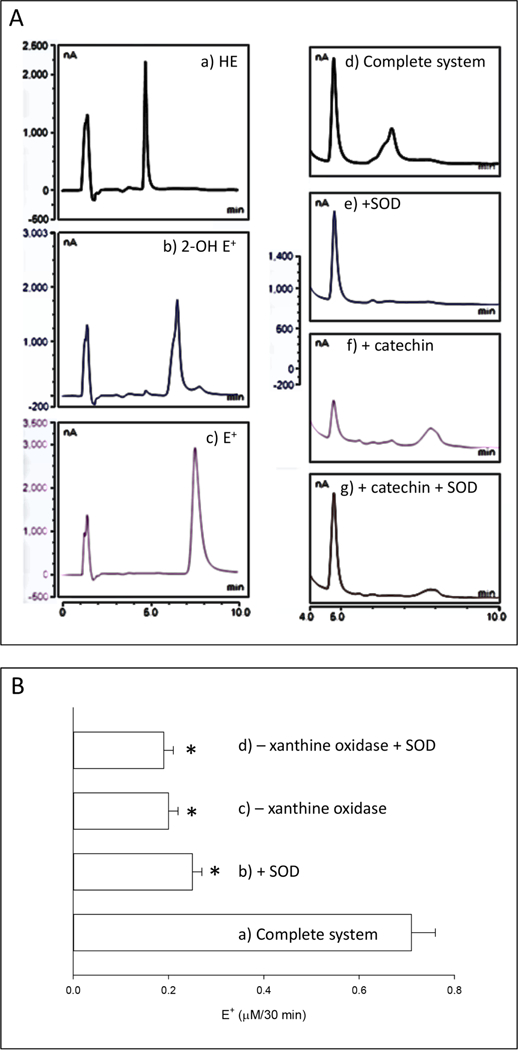
Effect of catechin on the oxidation of hydroethidine by superoxide anion. A) HPLC chromatograms of hydroethidine and oxidation products, a) HE: 1 μΜ hydroethidine standard; b) 2-OH E+: 1 μΜ 2-hydroxyethidium standard; c) E+: 1 μΜ ethidium standard; d) Complete system: the complete system consisted of air-saturated 100 mM potassium phosphate buffer pH 7.0, 5 mM DTP A, 10 μΜ hydroethidine, 200 μΜ xanthine, and 20 mU/mL xanthine oxidase; e) +SOD: SOD at 1000 U/mL was added to the complete system; f) + catechin: catechin at 0.25 mM was added to the complete system; g) + catechin, + SOD: catechin at 0.25 mM and SOD at 1000 U/mL was added to the complete system. In d-g, the reaction mixture was incubated for 30 min at room temperature and processed for HPLC analysis as indicated under Materials and Methods. B) Quantification of ethidium (E+) generation from the oxidation of hydroethidine (HE) by superoxide anion in the presence of catechin. a) Complete system: the complete system consisted of air-saturated 100 mM potassium phosphate buffer pH 7.0, 5 mM DTP A, 10 μΜ hydroethidine, 0.25 mM catechin, 200 μΜ xanthine, and 20 mU/mL xanthine oxidase; b) +SOD: SOD at 1000 U/mL was added to the complete system; c) - xanthine oxidase: xanthine oxidase was omitted from the complete system; d) - xanthine oxidase, + SOD: xanthine oxidase was omitted, and SOD at 1000 U/mL was added to the complete system. In a-d, the reaction mixture was incubated for 30 min at room temperature and processed for HPLC analysis as indicated under Materials and Methods. * p<0.05, ANOVA, with respect to the complete system.
Discussion
In conclusion, our data show that catechin at concentrations commonly used in in vitro models (50–250 μΜ) exhibits both anti- and pro-oxidant effects in microsomal membranes expressing active CYP2E1. Antioxidant effects are caused by the effective scavenging by catechin of CYP2E1-dependent superoxide anion, thereby inhibiting superoxide anion-dependent oxidative stress. Pro-oxidant effects are caused by the increased generation of catechin-derived oxidants after the initial scavenging of CYP2E1-dependent superoxide anion by catechin.
The identification of catechin as an antioxidant in microsomal systems expressing CYP2E1 is supported by the experimental observation that catechin prevented the generation of the superoxide-specific adduct of DEPMPO. Superoxide is much less reactive than other ROS, but some biological targets can react with it to propagate oxidative damage. For example, superoxide anion can oxidize iron-sulfur clusters in enzymes, and it can react with nitric oxide to form peroxynitrite (Benov, 2001); in addition, the protonated form of superoxide anion (HO2•) can initiate the peroxidation of polyunsaturated fatty acids (Halliwell et al., 1995). Scavenging of CYP2E1-dependent superoxide by catechin might therefore prevent these superoxide-dependent oxidative effects. Reports in the literature showing increased oxidation of redox probes in the presence of catechin (Kurisawa et al., 2003; Kim et al., 2004; Di Majo et al., 2014) probably reflect the unspecific oxidation of such probes not by superoxide anion but by other ROS generated by catechin. In our work, the determination of a superoxide-specific adduct (DEPMPO-OOH) precluded the unspecific detection of oxidants derived from catechin.
The identification of catechin as a pro-oxidant in microsomal systems expressing CYP2E1 is supported by the experimental observation that catechin increased the oxidation of the nonsuperoxide specific CPH probe. The mechanism by which catechin may increase probe oxidation in the presence of CYP2E1-expressing microsomes is proposed in Figure 6. Superoxide anion generated by the NADPH oxidase activity of CYP2E1 spontaneously dismutates (Fig. 6, reaction 1) or oxidizes catechin to its phenoxyl radical (Fig. 6, reaction 2). In the absence of any scavenger, the catechin phenoxyl radical dismutates (Fig. 6, reaction 3); but in the presence of CPH, the catechin phenoxyl radical readily oxidizes the CPH probe to its respective free radical, regenerating reduced catechin and closing the redox cycle (Fig. 6, reaction 4). In the presence of equimolar concentrations of CPH and catechin in a superoxide anion-generating system, the reaction of superoxide anion with catechin (Fig. 6, reaction 2) should prevail over the reaction of superoxide anion with CPH (Results, reaction 3), because the rate constant for the reaction of superoxide with catechin (k=7.1 × 105 M−1s−1) (Taubert et al., 2003) is 222 times higher than that with CPH (3.2 × 103 M−1s−1). This mechanism is supported by the following experimental observations: a) catechin radical, generated by hydrogen peroxide + HRP + catechin, oxidized CPH to its free radical; b) a superoxide scavenger (SOD) with a rate constant of 2.4 × 109 M−1s−1 blocked CPH oxidation in the presence of CYP2E1-expressing microsomes + NADPH + catechin, compatible with a much lower rate constant of superoxide scavenging by catechin (7.1χ 105 M−1s−1); c) microsomal systems devoid of CYP2E1 in the presence of NADPH generate significantly less superoxide and do not promote catechin-mediated oxidation of CPH; d) oxidation of the redox probe HE by a superoxide anion-generating system in the presence of catechin did not produce the superoxide-specific 2-OH-E+, but rather produced the phenoxyl radical-dependent E+, as shown in Fig. 6, reactions 5 and 6 (Quek and Huang, 2011). While O2 could react with CPH or catechin, these reactions were negligible because they are slow at pH 7 (Mochizuki et al., 2002; He et al., 2013). The oxidation of catechin radical with O2 should produce O2●− via reaction (4), where FL represents the two-electron oxidized catechin flavonoid:
| 4 |
Figure 6.
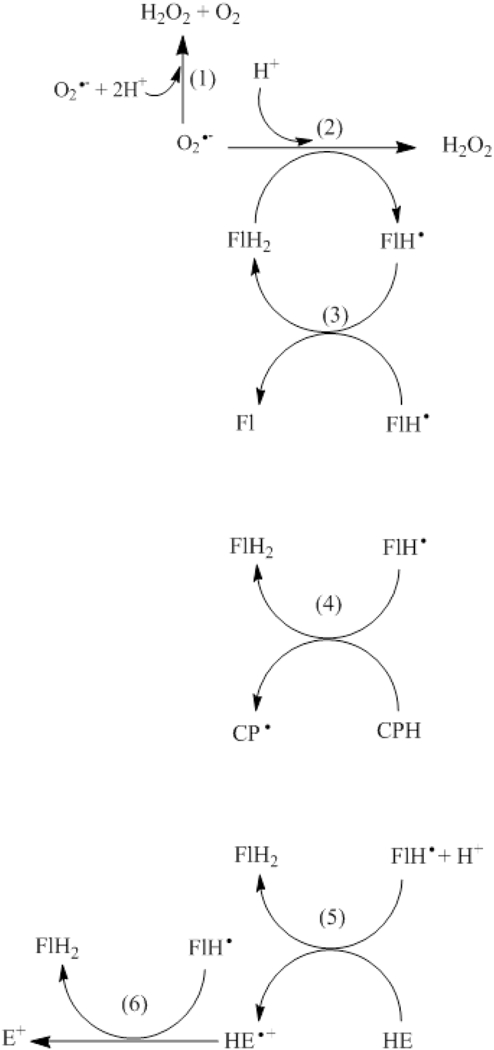
Proposed mechanism for increased redox probe oxidation in superoxide aniongenerating systems in the presence of catechin. Please see the Discussion section for details. FlH2, F1H● and FL represent the reduced, the one-electron oxidized and the two-electron oxidized catechin flavonoid, respectively.
However, this reaction was negligible because no significant increase in O2 consumption was observed in the CYP2E1 + NADPH system in the presence of catechin, and because the addition of catechin to superoxide- generating systems did not increase the generation of the superoxide adduct of DEPMPO. It should be noted that CYP2E1-related peroxidase or monooxygenase activities could potentially mediate catechin metabolism in the CYP2E1 + NADPH system, as suggested by Moridani et al., (2001a); however, the fact that SOD completely blocked the oxidation of CPH in the CYP2E1 + NADPH system incubated with catechin suggests that in our model catechin is metabolized directly by CYP2E1-dependent superoxide anion. While phenoxyl radicals derived from flavonoids could oxidize NADPH to , it has been suggested that the main pathway for NADPH oxidation in the presence of oxidized catechol ring-containing flavonoids such as catechin is its two-electron oxidation by the derived o-quinone: (Galati et al., 2002).
In vitro, catechin induced transition metal-dependent oxidation of DNA, lipids and proteins: catechin at 0.25 mM increased DNA breakage induced by (Fukuhara et al., 2002); catechin at 5–100 μΜ increased bovine serum albumin oxidation induced by hemoglobin + H2O2 (Lu et al., 2011); catechin at 0.5–1 mM increased the oxidation of linoleic acid initiated by Cu2+ (Fukumoto and Mazza, 2000); catechin at 10 μΜ increased the oxidation of DNA induced by Cu2+ (Oikawa et al., 2003). In our work, the use of millimolar levels of DTP A, a metal chelating agent elfective in blunting the metal’s redox activity in superoxide-generating systems (Buettner and Jurkiewicz, 1996), limited the contribution of contaminating transition metals to the pro-oxidant activity of catechin.
While the reports mentioned above recognize the pro-oxidant activity of catechin in other in vitro systems, several groups have manipulated the structure of catechin with the intention of decreasing its pro-oxidant activity: a) planar catechin, which was constrained by the formation of a bridge between the 3-OH group on ring C and C6’ on ring B, showed lower pro-oxidant activity in in vitro systems, probably due to the stabilization of free radicals derived from planar catechin (Fukuhara et al., 2002); b) poly(catechin), the product of enzyme-catalyzed oxidative coupling of monomeric catechin, showed lower pro-oxidant activity in in vitro systems (Kurisawa et al., 2003). The effect of these modified catechin molecules on CYP2E1-dependent oxidative stress is currently unknown.
Our work can provide a biochemical mechanism for in vivo effects of catechin in diseases associated with CYP2E1-dependent oxidative stress, such as alcoholic liver disease. For example, catechin administered orally at 50 mg/kg prevented alcoholic liver damage in rats via antioxidant effects (Bharrhan et al., 2011), an effect that might have been partially mediated by scavenging of CYP2E1-dependent superoxide anion. However, similar doses did not prevent alcohol liver damage in humans (Colman et al., 1980), and higher doses (300 mg/kg) increased liver fibrosis induced by ethanol and carbon tetrachloride in rats (Siegers et al., 1982), suggesting that other effects such as pro-oxidant effects might be more prevalent under these conditions.
The physiological relevance of the effects of catechin on microsomal CYP2E1-dependent oxidative stress depends on the actual in vivo concentration of catechin. Plasma concentrations of catechin after administration to experimental animals or humans are at low-micromolar levels (Donovan et al., 2006; Holt et al., 2002). However, several groups have manipulated the structure of catechin to ensure higher stability and bioavailability, such as conjugation with dextran (Vittorio et al., 2016) or encapsulation into micro- or nano-particles (Rodrigues et al., 2013). The effect of these modified catechin molecules on CYP2E1-dependent oxidative stress is currently unknown.
The pro-oxidant effect of catecholic flavonoids or non-flavonoids in microsomal systems expressing NADPH cytochrome P450 reductase and cytochrome P450 has been reported in the literature before: a) catechin (Moridani et al., 2001a) and the non-flavonoid catechols chlorogenic acid, caffeic acid, and dihydrocaffeic acid (Moridani et al., 2001b) induced the depletion of reduced glutathione in the presence of rat liver microsomes and NADPH, effects that were inhibited by a cytochrome P450 inhibitor; b) quercetin-o-quinone was univalently reduced to quercetin o-semiquinone by NADPH cytochrome P450 reductase (Metodiewa et al., 1999). Our work expands these observations by demonstrating that the specific CYP2E1- dependent metabolism of catechin increases the generation of oxidants capable of oxidizing the redox probe CPH. In addition, we provided experimental evidence for the role of CYP2E1- dependent superoxide anion in the increased generation of oxidants from catechin. Furthermore, evidence that these oxidants mainly represent catechin phenoxyl radicals included the oxidation of the redox probes CPH and HE with other enzymatic systems that generate catechin phenoxyl radical, such as catechin + HRP + H2O2, and catechin + xanthine + xanthine oxidase, respectively.
Highlights.
CYP2E1-dependent superoxide anion was determined specifically by spin-trapping.
Catechin scavenged superoxide anion generated by CYP2E1.
Catechin increased the superoxide anion-dependent oxidation of the probes CPH and HE.
Catechin-derived phenoxyl radicals oxidized the probe CPH.
Catechin showed anti- and pro-oxidant traits under CYP2E1-dependent oxidative stress.
Acknowledgme nts
We thank Dr. Liz Gron and Dr. Thomas Goodwin for critical reading of the manuscript. This study was supported by Arkansas INBRE (National Institutes of Health, National Institute of General Medical Sciences Grant P20 GM103429), and the Byrd Chemistry Research Fund Award from Hendrix College.
Abbreviations
- b5
cytochrome b5
- CP●
3-carboxy-proxyl radical
- CPH
1-hydroxy-3-carboxy-2,2,5,5- tetramethylpyrrolidine
- CPR
NADPH cytochrome P450 reductase
- CYP2E1
cytochrome P450 2E1
- E+
ethidium;
- HE
hydroethidine
- 2-OH-E+
2-hydroxyethidine
- FlH2
reduced catechin
- FlH●
one-electron oxidized catechin
- Fl
two-electron oxidized catechin
- HRP
horseradish peroxidase
- ROS
reactive oxygen species
- SOD
superoxide dismutase
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arts IC, van de Putte B, Hollman PC. (2000a) Catechin contents of foods commonly consumed in The Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. Journal of Agricultural and Food Chemistry. 48:1746–1751. [DOI] [PubMed] [Google Scholar]
- Arts IC, van de Putte B, Hollman PC. (2000b) Catechin contents of foods commonly consumed in The Netherlands. 2. Tea, wine, fruit juices, and chocolate milk. Journal of Agricultural and Food Chemistry. 48:1752–1757. [DOI] [PubMed] [Google Scholar]
- Benov L (2001) How superoxide radical damages the cell. Protoplasma. 217:33–36. [DOI] [PubMed] [Google Scholar]
- Bernatoniene J, Kopustinskiene DM. (2018) The role of catechins in cellular responses to oxidative stress. Molecules. 23:965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beziere N, Hardy M, Poulhes F, Karoui H, Tordo P, Ouari O, Frapart YM, Rockenbauer A, Boucher JL, Mansuy D, Peyrot F. (2014) Metabolic stability of superoxide adducts derived from newly developed cyclic nitrone spin traps. Free Radical Biology and Medicine. 67:150–158. [DOI] [PubMed] [Google Scholar]
- Bharrhan S, Koul A, Chopra K, Rishi P. Catechin suppresses an array of signalling molecules and modulates alcohol-induced endotoxin mediated liver injury in a rat model. (2011) PLoS One. 6:e20635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolojan L, Takacs IM, Miclaus V, Damian G. (2012) An EPR study of superoxide radicals from potassium superoxide solutions. Applied Magnetic Resonance. 42:333–341. [Google Scholar]
- Buettner GR, Jurkiewicz BA. (1996) Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiation Research. 145:532–541. [PubMed] [Google Scholar]
- Caro AA, Cederbaum AI. (2004) Oxidative stress, toxicology, and pharmacology of CYP2E1. Annual Review of Pharmacology and Toxicology. 44:27–42. [DOI] [PubMed] [Google Scholar]
- Caro AA, Cederbaum AI. (2005) Inhibition of CYP2E1 catalytic activity in vitro by S-adenosyl- L-methionine. Biochemical Pharmacology. 69:1081–1093. [DOI] [PubMed] [Google Scholar]
- Chan T, Galati G, O’Brien PJ. (1999) Oxygen activation during peroxidase catalysed metabolism ofllavones or flavanones. Chemico-biological Interactions. 22:15–25. [DOI] [PubMed] [Google Scholar]
- Colman JC, Morgan MY, Scheuer PJ, Sherlock SH. (1980) Treatment of alcohol-related liver disease with (+)-cyanidanol-3: a randomised double-blind trial. Gut. 21:965–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cos P, Ying L, Calomme M, Hu JP, Cimanga K, Van Poel B, Pieters L, Vlietinck AJ, Berghe DV. (1998) Structure- activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. Journal of Natural Products. 61:71–76. [DOI] [PubMed] [Google Scholar]
- Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. (2008) Distinct roles of Noxl and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radical Biology and Medicine. 45:1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Majo D, La Guardia M, Leto G, Crescimanno M, Flandina C, Giammanco M. (2014) Flavonols and llavan-3-ols as modulators of xanthine oxidase and manganese superoxide dismutase activity. International Journal of Food Sciences and Nutrition. 65:886–892. [DOI] [PubMed] [Google Scholar]
- Donovan JL, Crespy V, Oliveira M, Cooper KA Gibson BB, Williamson G. (2006) (+)-Catechin is more bioavailable than (−)-catechin: relevance to the bioavailability of catechin from cocoa. Free Radical Research. 40:1029–1034. [DOI] [PubMed] [Google Scholar]
- Fraccarollo D, Berger S, Galuppo P, Kneitz S, Hein L, Schiitz G, Frantz S, Ertl G, Bauersachs J. (2011) Deletion of cardiomyocyte mineralocorticoid receptor ameliorates adverse remodeling after myocardial infarction. Circulation. 123:400–408. [DOI] [PubMed] [Google Scholar]
- Fukuhara K, Nakanishi I, Kansui H, Sugiyama E, Kimura M, Shimada T, Urano S, Yamaguchi K, Miyata N. (2002) Enhanced radical-scavenging activity of a planar catechin analogue. Journal of the American Chemical Society. 124:5952–5953. [DOI] [PubMed] [Google Scholar]
- Fukumoto LR, Mazza G. (2000) Assessing antioxidant and prooxidant activities of phenolic compounds. Journal of Agricultural and Food Chemistry. 48:3597–3604. [DOI] [PubMed] [Google Scholar]
- Galati G, Sabzevari O, Wilson JX, O’Brien PJ. (2002) Prooxidant activity and cellular effects of the phenoxyl radicals of dietary llavonoids and other polyphenolics. Toxicology. 177:91–104. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Murcia MA, Chirico S, Aruoma OI. (1995) Free radicals and antioxidants in food and in vivo: what they do and how they work. Critical Reviews in Food Science & Nutrition. 35:7–20. [DOI] [PubMed] [Google Scholar]
- He W, Kim HK, Warner WG, Melka D, Callahan JH, Yin JJ. (2013) Photogenerated charge carriers and reactive oxygen species in ZnO/Au hybrid nanostructures with enhanced photocatalytic and antibacterial activity. Journal of the American Chemical Society. 136:750–757. [DOI] [PubMed] [Google Scholar]
- Holt RR, Lazarus SA, Sullards MC, Zhu QY, Schramm DD, Hammerstone JF, Fraga CG, Schmitz HH, Keen CL. (2002) Procyanidin dimer B2 [epicatechin-(4P-8)-epicatechin] in human plasma after the consumption of a flavanol-rich cocoa. The American Journal of Clinical Nutrition. 76:798–804. [DOI] [PubMed] [Google Scholar]
- Jing X, Zhang J, Huang Z, Sheng Y, Ji L. (2018) The involvement of Nrt2 antioxidant signalling pathway in the protection of monocrotaline-induced hepatic sinusoidal obstruction syndrome in rats by (+)-catechin hydrate. Free Radical Research. 52:402–414. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Chung JE, Kurisawa M, Uyama H, Kobayashi S. (2004) Superoxide anion scavenging and xanthine oxidase inhibition of (+)-catechin-aldehyde polycondensates. Amplification of the antioxidant property of (+)-catechin by polycondensation with aldehydes. Biomacromolecules. 5:547–552. [DOI] [PubMed] [Google Scholar]
- Kozlov AV, Gille L, Miller I, Piskernik C, Haindl S, Staniek K, Nohl H, Bahrami S, Ohlinger W, Gemeiner M, Redl H. (2007) Opposite effects of endotoxin on mitochondrial and endoplasmic reticulum functions. Biochemical and Biophysical Research Communications. 352:91–96. [DOI] [PubMed] [Google Scholar]
- Kurisawa M, Chung JE, Kim YJ, Uyama H, Kobayashi S. (2003) Amplification of antioxidant activity and xanthine oxidase inhibition of catechin by enzymatic polymerization. Biomacromolecules. 4:469–471. [DOI] [PubMed] [Google Scholar]
- Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. (2003) Interactions of Peroxynitrite, Tetrahydrobiopterin, Ascorbic Acid, and Thiols. Implications for uncoupling endothelial nitric- oxide synthase. Journal of Biological Chemistry. 278:22546–22554. [DOI] [PubMed] [Google Scholar]
- Lee KM, Kang HS, Yun CH, Kwak HS. (2012) Potential in vitro protective effect of quercetin, catechin, caffeic acid and phytic acid against ethanol-induced oxidative stress in SK-Hep-1 cells. Biomolecules & Therapeutics. 20:492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Ho JN, Dong MS, Park CH, Kim HK, Hong B, Shin DH, Cho HY. (2005) Transfected HepG2 cells for evaluation of catechin effects on alcohol-induced CYP2E1 cytotoxicity. Journal of Microbiology and Biotechnology. 15:1310–1316. [Google Scholar]
- Letelier ME, Lopez-Valladares M, Peredo-Silva L, Rojas-Sepdlveda D, Aracena P. (2011) Microsomal oxidative damage promoted by acetaminophen metabolism. Toxicology in Vitro. 25:1310–1313. [DOI] [PubMed] [Google Scholar]
- Lu N, Chen P, Yang Q, Peng YY. (2011) Anti-and pro-oxidant effects of (+)-catechin on hemoglobin-induced protein oxidative damage. Toxicology in Vitro. 25:833–838. [DOI] [PubMed] [Google Scholar]
- Maroziene A, Nemeikaite-Ceniene A, Vidziunaite R, Cenas N. (2012) Correlation between mammalian cell cytotoxicity of flavonoids and the redox potential of phenoxyl radical/phenol couple. Acta Biochimica Polonica. 59:299–305. [PubMed] [Google Scholar]
- Metodiewa D, Jaiswal AK, Cenas N, Dickancaite E, Segura-Aguilar J. (1999) Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free Radical Biology and Medicine. 26:107–116. [DOI] [PubMed] [Google Scholar]
- Michalski R, Zielonka J, Hardy M, Joseph J, Kalyanaraman B. (2013) Hydropropidine: a novel, cell-impermeant fluorogenic probe for detecting extracellular superoxide. Free Radical Biology and Medicine. 54:135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira L, Tereza Fernandez M, Santos M, Rocha R, Helena Florencio M, Jennings KR. (2002) Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radical Research. 36:1199–1208. [DOI] [PubMed] [Google Scholar]
- Mochizuki M, Yamazaki SI, Kano K, Ikeda T. (2002) Kinetic analysis and mechanistic aspects of autoxidation of catechins. Biochimica et Biophysica Acta (BBA)-General Subjects. 1569:35–44. [DOI] [PubMed] [Google Scholar]
- Morel I, Lescoat G, Cogrel P, Sergent O, Pasdeloup N, Brissot P, Cillard P, Cillard J. (1993) Antioxidant and iron-chelating activities of the flavonoids catechin, quercetin and diosmetin on iron-loaded rat hepatocyte cultures. Biochemical Pharmacology. 45:13–19. [DOI] [PubMed] [Google Scholar]
- Moridani MY, Scobie H, Salehi P, O’Brien PJ. (2001a) Catechin metabolism: glutathione conjugate formation catalyzed by tyrosinase, peroxidase, and cytochrome p450. Chemical Research in Toxicology. 14:841–848. [DOI] [PubMed] [Google Scholar]
- Moridani MY, Scobie H, Jamshidzadeh A, Salehi P, O’Brien PJ. (2001b) Caffeic acid, chlorogenic acid, and dihydrocaffeic acid metabolisin: glutathione conjugate formation. Drug Metabolism and Disposition. 29:1432–1439. [PubMed] [Google Scholar]
- Murias M, Jager W, Handler N, Erker T, Horvath Z, Szekeres T, Nohl H, Gille L. (2005) Antioxidant, prooxidant and cytotoxic activity of hydroxylated resveratrol analogues: structure- activity relationship. Biochemical Pharmacology. 69:903–912. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Enoki Y, Hashimoto K. (1995) Hydrogen peroxide formation during catechin oxidation is inhibited by superoxide dismutase. Food Science and Technology International, Tokyo. 1:65–69. [Google Scholar]
- Oikawa S, Furukawa A, Asada H, Hirakawa K, Kawanishi S. (2003) Catechins induce oxidative damage to cellular and isolated DNA through the generation of reactive oxygen species. Free Radical Research. 37:881–890. [DOI] [PubMed] [Google Scholar]
- Oliva J, Bardag-Gorce F, Tillman B, French SW. (2011) Protective effect of quercetin, EGCG, catechin and betaine against oxidative stress induced by ethanol in vitro. Experimental and Molecular Pathology. 90:295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrielli P, Skibsted LH. (2002) Antioxidant synergy and regeneration effect of quercetin,(−)- epicatechin, and (+)-catechin on a-tocopherol in homogeneous solutions of peroxidating methyl linoleate. Journal of Agricultural and Food Chemistry. 50:7138–7144. [DOI] [PubMed] [Google Scholar]
- Peri L, Pietraforte D, Scorza G, Napolitano A, Fogliano V, Minetti M. (2005) Apples increase nitric oxide production by human saliva at the acidic pH of the stomach: a new biological function for polyphenols with a catechol group? Free Radical Biology and Medicine. 39:668–681 [DOI] [PubMed] [Google Scholar]
- Quek YL, Huang D. (2011) Hydroethidine as a probe for measuring superoxide formation rates during air oxidation of myricetin and quercetin. Tetrahedron Letters. 52:5384–5387. [Google Scholar]
- Rezende F, Prior KK, Lowe O, Wittig I, Strecker V, Moll F, Helfnger V, Schnutgen F, Kurrle N, Wempe F, Walter M. (2017) Cytochrome P450 enzymes but not NADPH oxidases are the source of the NADPH-dependent lucigenin chemiluminescence in membrane assays. Free Radical Biology and Medicine. 102:57–66. [DOI] [PubMed] [Google Scholar]
- F Rodrigues C, Ascencao K, AM Silva F, Sarmento B, BPP Oliveira M, C Andrade J. (2013) Drug-delivery systems of green tea catechins for improved stability and bioavailability. Current Medicinal Chemistry. 20:4744–4757. [DOI] [PubMed] [Google Scholar]
- Satoh T, Fujisawa H, Nakamura A, Takahashi N, Watanabe K. (2016) Inhibitory effects of eight green tea catechins on cytochrome P450 1A2, 2C9, 2D6, and 3A4 activities. Journal of Pharmacy & Pharmaceutical Sciences. 19:188–197. [DOI] [PubMed] [Google Scholar]
- Sies H (1997) Oxidative stress: oxidants and antioxidants. Experimental physiology. 82:291–295. [DOI] [PubMed] [Google Scholar]
- Straface E, Marchesi A, Gambardella L, Metere A, Tarissi de Jacobis I et al. (2012) Does oxidative stress play a critical role in cardiovascular complications of Kawasaki disease? Antioxidants & Redox Signaling. 17:1441–1446. [DOI] [PubMed] [Google Scholar]
- Siegers CP, Volpel M, Scheel G, Younes M. (1982) Effects of dithiocarb and (+)-catechin against carbon tetrachloride-alcohol-induced liver fibrosis. Agents and Actions. 12:743–748. [DOI] [PubMed] [Google Scholar]
- Sugihara N, Ohnishi M, Imamura M, Furuno K. (2001) Differences in antioxidative efficiency of catechins in various metal-induced lipid peroxidations in cultured hepatocytes. Journal of Health Science. 47:99–106. [Google Scholar]
- Taubert D, Breitenbach T, Lazar A, Censarek P, Harbinger S, Berkels R, Klaus W, Roesen R. (2003) Reaction rate constants of superoxide scavenging by plant antioxidants. Free Radical Biology and Medicine. 35:1599–1607. [DOI] [PubMed] [Google Scholar]
- Verstraeten SV, Fraga CG, Oteiza PF (2015) Interactions of flavan-3-ols and procyanidins with membranes: mechanisms and the physiological relevance. Food & Function. 6:32–40. [DOI] [PubMed] [Google Scholar]
- Vittorio O, Brandi M, Cirillo G, Kimpton K, Hinde E, Gaus K, Yee E, Kumar N, Duong H, Fleming C, Haber M. (2016) Dextran-Catechin: An anticancer chemically-modified natural compound targeting copper that attenuates neuroblastoma growth. Oncotarget. 7:47479–47493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, Grace SC. (1998) EPR detection of phytophenoxyl radicals stabilized by zinc ions: evidence for the redox coupling of plant phenolics with ascorbate in the H202-peroxidase system. FEBS Letters. 422:377–380. [DOI] [PubMed] [Google Scholar]
- Yang B, Kotani A, Arai K, Kusu F. (2001) Relationship of electrochemical oxidation of catechins on their antioxidant activity in microsomal lipid peroxidation. Chemical and Pharmaceutical Bulletin. 49:747–751. [DOI] [PubMed] [Google Scholar]
- Zielonka J, Vasquez-Vivar J, Kalyanaraman B. (2008) Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nature Protocols.3:8–21 [DOI] [PubMed] [Google Scholar]


