Abstract
Cannabis use is increasing in the United States, as are its adverse effects. We investigated the genetics of an adverse consequence of cannabis use: cannabis-related aggression (CRA) using a genome-wide association study (GWAS) design. Our GWAS sample included 3,269 African-Americans (AAs) and 2,546 European Americans (EAs). An additional 89 AA subjects from the Grady Trauma Project (GTP) were also examined using a proxy-phenotype replication approach. We identified genome-wide significant risk loci contributing to CRA in AAs at the serotonin receptor 2B receptor gene (HTR2B), and the lead SNP, HTR2B*rs17440378, showed nominal association to aggression in the GTP cohort of cannabis-exposed subjects. A priori evidence linked HTR2B to impulsivity/aggression but not to cannabis response. Human functional data regarding the HTR2B variant further supported our finding. Treating an Htr2b−/− knockout mouse with THC resulted in increased aggressive behavior, whereas wild type mice following THC administration showed decreased aggression in the resident-intruder paradigm, demonstrating that HTR2B variation moderates the effects of cannabis on aggression. These concordant findings in mice and humans implicate HTR2B as a major locus associated with cannabis-induced aggression.
Introduction
Violent behavior is a major public health problem, resulting annually in approximately 1.43 million deaths worldwide1. Genetic factors explain 50-63% of the variance in aggressive behavior and several genetic variants that influence aggressive behavior have been identified2,3. Monoaminergic genes – identified via unbiased approaches or selected as biological candidates – have been extensively studied in relation to aggression-related traits3,4. An apparently unique mutation at the monoamine oxidase A locus (MAOA), which encodes an enzyme that catabolizes monoamines (including serotonin), was associated with aggressive behavior in a Dutch kindred5 after first being localized by genetic linkage analysis6. Caspi et al.7 subsequently reported that carriers of a different low-activity MAOA variant exhibited violent behavior only after exposure to moderate or severe levels of child abuse8. Sequencing of serotonin-system genes in a Finnish population of impulsive individuals revealed association between a stop codon in the serotonin 2B receptor gene (HTR2B Q20*) and risk of committing violent acts9. In a subsequent study, HTR2B Q20* carriers showed aggressive behavior, alcohol-related impulsivity, and emotional dysregulation10.
Aggressive behavior is influenced by a combination of genetic and environmental factors, including substance use. A high proportion of all crimes are committed under the influence of substances of abuse11, and thus aggression while intoxicated is an important subtype of all aggressive behavior. Cannabis, one of the most widely used drugs worldwide, has been linked to increased impulsivity12 and decreased behavioral inhibition13,14. The relationship between cannabis use and aggression has been established2,15-17; cannabis use is associated with a 7-fold risk of subsequent violent and aggressive behavior2. A recent study of 1,136 subjects from the McArthur Risk Assessment study also found that continuity of cannabis use is associated with increased risk of future violent behavior (OR=2.44)17.
We first conducted a genome-wide association study (GWAS) of physical aggression occurring under the influence of cannabis in African Americans (AA) and European Americans (EA) and a polygenic risk score (PRS) analysis to determine whether genetic risk factors for personality traits could predict cannabis-related aggression in humans. The GWAS implicated the serotonin 2B receptor gene (HTR2B), which was not previously considered a key cannabis target. We then evaluated the cannabis/Htr2b interaction in a mouse knockout model.
Methods
Subjects and Diagnostic Procedures
Discovery cohort
Our GWAS discovery sample included 2,185 AA and 1,362 EA subjects selected from the Yale-Penn sample, all of whom endorsed having used cannabis 10 or more times. The initial sample (Yale-Penn 1) consisted of small nuclear families and unrelated individuals recruited at five US clinical sites: Yale University School of Medicine (APT Foundation, New Haven, CT), the University of Connecticut Health Center (Farmington, CT), the University of Pennsylvania, Philadelphia, PA), the Medical University of South Carolina (Charleston, SC) and McLean Hospital (Belmont, MA). Subjects were recruited for studies of the genetics of drug (opioid or cocaine) or alcohol dependence18-21. All subjects provided written informed consent as approved by the institutional review boards at each site, and certificates of confidentiality were obtained from NIDA and NIAAA. Subjects were interviewed using the Semi-Structured Assessment for Drug Dependence and Alcoholism (SSADDA), which yield reliable DSM-IV and DSM-5 criteria and diagnoses. Cannabis-related aggression (CRA) was assessed with the question, “Did you ever get into physical fights while using marijuana?” Results from the Yale-Penn 1 sample were combined via meta-analysis with results from 1,084 AA and 1,184 EA cannabis-exposed subjects who were identically ascertained (Yale-Penn 2 sample). The study cohorts are described in Table 1.
Table 1.
Demographic characteristics of the study cohorts.
| Yale-Penn 1 |
Yale-Penn 2 |
GTP |
|||
|---|---|---|---|---|---|
| Characteristic | African American (n=2185) |
European American (n=1362) |
African American (n=1084) |
European American (n=1184) |
African American (n=89) |
| Age, mean (SD), y | 41 (8.4) | 36 (10.4) | 40 (10.6) | 37 (12.2) | 45 (10.7) |
| Female sex, No. (%) | 852 (39.0) | 501 (36.8) | 331 (30.5) | 374 (31.6) | 44 (49.4) |
| CRA, n (%) | 304 (13.9) | 147 (10.8) | 142 (13.1) | 99 (8.4) | N/A |
| CaD, n (%) | 895 (49.4) | 566 (55.2) | 503 (55.6) | 506 (61.3) | 76 (85.4)* |
| AD, n (%) | 1322 (71.2) | 865 (82.2) | 741 (80.4) | 787 (87.7) | 65 (73.0)* |
| CocD, n (%) | 1892 (87.6) | 1070 (82.1) | 675 (64.3) | 621 (61.1) | 42 (47.7)* |
| OD, n (%) | 637 (29.8) | 868 (65.9) | 295 (27.7) | 573 (55.2) | 5 (5.7)* |
| Age of onset cannabis use, mean (SD), y | 15 (4.6) | 14 (3.7) | 15 (5.6) | 14 (2.8) | N/A |
| More than 1 before 15, n (%) | 945 (43.3) | 720 (52.9) | 438 (40.4) | 527 (48.6) | N/A |
| Years exposed to cannabis, mean (SD), y | 21 (13.0) | 15 (11.7) | 19 (13.1) | 14 (12.8) | N/A |
| Frequency cannabis use (>100), n (%) | 1969 (90.1) | 1259 (57.6) | 970 (44.4) | 930 (42.6) | |
| Aggression (physical fights), n (%) | 1362 (62.6) | 791 (58.2) | 702 (64.8) | 611 (56.4) | N/A |
| Aggression (beat someone up), n (%) | |||||
| Never | N/A | N/A | N/A | N/A | 27 (30.3) |
| Once | N/A | N/A | N/A | N/A | 9 (10.1) |
| Several times | N/A | N/A | N/A | N/A | 35 (39.3) |
| Many times | N/A | N/A | N/A | N/A | 11 (12.4) |
| More than I can count | N/A | N/A | N/A | N/A | 7 (7.9) |
| ASPD, n (%) | 340 (15.9) | 232 (17.4) | 207 (19.6) | 213 (19.9) | N/A |
| CD, n (%) | 56 (2.6) | 49 (3.7) | 29 (2.7) | 33 (3.1) | N/A |
Abbreviations: SD, standard deviation; CRA, cannabis-related aggression; CaD, cannabis dependence; AD, alcohol dependence; CocD, cocaine dependence; OD, opioid dependence; ASPD, antisocial personality disorder; CD, conduct disorder; N/A, not applicable.
= Substance abuse and/or dependence.
Replication cohort: Grady Trauma Project (GTP)
The subjects for this study were part of a larger investigation of genetic and environmental factors that predict response to stressful life events in a predominantly African American, urban population of low socioeconomic status22. All subjects endorsed lifetime prevalence of cannabis use, assessed using the Structured Clinical Interview for DSM-IV (SCID)23. Aggressive behavior was assessed using the “Beat some up” item from the Aggressive Behavior Questionnaire (ABQ), a 48-item, self-report inventory of physical and verbal aggression, and criminal offenses including drug use and theft. This scale is based on the Conflict Tactics Scale, a commonly used measurement of relationship conflict behaviors24. The aggression measure was examined as a quantitative trait among these cannabis-exposed subjects. Additional details are provided in Table 1.
Genotyping and Quality Control
Discovery cohort
The Yale-Penn samples were genotyped using one of two genotyping arrays: 1) for the Yale-Penn 1 sample, the Illumina HumanOmni1-Quad v1.0 microarray was used (988,306 autosomal SNPs; Illumina, San Diego, CA, USA) at the Center for Inherited Research (CIDR) and the Yale Center for Genome Analysis (YCGA) and 2) for the Yale-Penn 2 sample, the Illumina Infinium Human Core Exome microarray (265,919 exome-focused SNPs and 243,345 tagging SNPs which allow genome-wide imputation) at our lab. All QC and subsequent analyses were performed separately within individuals genotyped on the two platforms. Genotypes were called using GenomeStudio software V2011.1 and genotyping module V1.8.4 (Illumina, San Diego, CA, USA). SNPs with significantly different allele frequencies (within population) across genotyping centers were set to missing prior to imputation. SNPs were filtered by minor allele frequency (MAF) < 0.01 and imputation score threshold of 0.8. After imputation, data cleaning, and QC, in AAs 15,101,332 SNPs and in EAs 8,365,931 SNPs were successfully meta-analyzed.
To verify and correct the misclassification of self-reported race, we compared the GWAS data from all subjects with the genotypes from the HapMap 3 reference CEU, YRI and CHB, and 1000 Genomes reference AFR, EUR, and ASN populations. Principle components (PC) analysis was conducted in the merged sample using Eigensoft25,26. The first 10 PCs were used to remove outliers and to distinguish AAs and EAs; these groups were subsequently analyzed separately. We then conducted PC analyses within the two remaining groups as previously described27, and the first three PCs were used in all subsequent analyses to correct for residual population stratification.
Grady Trauma Project (GTP) sample:
Quality control was performed using the Psychiatric Genomics Consortium PTSD Workgroup guidelines. Additional details are provided in Supplementary Notes.
Statistical Analysis Methods
Association tests were performed using logistic regression models embedded in generalized estimating equations (GEE) to correct for correlations among related individuals28. We modeled CRA as a binary variable, analyzed in a standard logistic regression and adjusted for age, sex, and three PCs of ancestry. Analyses were conducted separately within each population group, corrected for the genomic-inflation factor (λ) and combined by meta-analysis using the inverse variance method in the program METAL29. Conditional analysis was conducted to determine whether closely mapped genome-wide significant (GWS) SNPs represent independent signals. We conducted statistical testing as previously described, using a logistic regression model embedded in GEE with age, sex, three PCs, and the SNP of interest as covariates.
For the GTP cohort, we also conducted a logistic regression analysis to determine the association between rs17440378 and aggression-related trait in AA subjects with a lifetime prevalence of cannabis. The aggression-related trait used was “Beat someone up”, and modeled as an ordinal variable (Never=0, Once=1, Sometime=2, Many times=3, More than I can count=4) with age, sex, and first 3 PCs as covariates.
We used publicly available bioinformatics tools to identify the functional effects of the GWS variants found in our CRA GWAS. Additional details are provided in Supplementary Notes.
Polygenic risk score analysis
To test for pleiotropy between the NEO personality factors and CRA, we conducted a PRS analysis using GWAS data from the five-factor model traits of neuroticism, extraversion, openness, agreeableness and conscientiousness, measured with the NEO Personality Inventory (NEO PI-R)30. The public summary data were downloaded from the website of the Genetics of Personality Consortium (GPC, http://www.tweelingenregister.org/GPC/). Additional details are provided in Supplementary Notes.
Htr2b knockout mice, THC administration and behavioral phenotyping
A total of 34 adult mice (20 wild type, 14 Htr2b−/− knockout) of 129SvPas background were used in the study; wild type (WT) mice were used as a control group. Saline or THC (10 mg/kg) was administered by intraperitoneal (i.p.) injection, 1 hour before test. Animals were randomly balanced in agreement to genotype and treatment during the experiment. The selected THC dose was based on a previous study showing that THC (10 mg/kg) decreases social interaction in mice31. Behavioral test, and animal care were conducted in accordance with the standard ethical guidelines (National Institutes of Health’s ‘Guide for the care and use of laboratory animals’, and European Directive 2010/63/UE). All of the experiments involving mice were approved by the local ethical committee (N°1170.02). Aggressive behavior was investigated using the resident-intruder test adapted from Koolhaas and colleagues32 and performed 1 hour after i.p. injection. Behavioral scoring was carried out by blinded investigators using the JWatcher™ software (University of California, LA, USA, and Macquarie University, Sidney, Australia). Additional details are provided in Supplementary Notes.
Behavioral data were analyzed using two-way ANOVA with genotype (knockout vs. WT) and treatment (saline vs. THC) as between-subjects factors. Tukey’s test was used for post hoc comparisons with significance set at p < 0.05. Statistical outliers, detected by Grubb’s test, were excluded from the final analysis. Analyses were carried-out using GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA).
Results
We studied two samples, Yale-Penn 1 and Yale-Penn 227. In Yale-Penn 1, the prevalence of cannabis-related aggression (CRA) was 13.9% in AAs and 10.8% in EAs, while in Yale-Penn 2 it was 13.1% in AAs and 8.4% in EAs (Table 1). GWAS results are summarized in Manhattan plots (Supplementary Figure 1), Table 2, and Supplementary Tables 1 and 2. Quantile-Quantile plots are shown in Supplementary Figures 2 and 3.
Table 2.
Results for HTR2B*rs17440378 from the analysis of cannabis-related aggression (CRA) and replication.
| Sample | n | n Cases | Direction | Effect | SE | MAF | MAF Controls |
MAF Cases |
P-value |
|---|---|---|---|---|---|---|---|---|---|
| Yale-Penn 1 | 2185 | 304 | + | 0.56 | 0.13 | 0.11 | 0.10 | 0.16 | 6.93E-06 |
| Yale-Penn 2 | 1084 | 142 | + | 0.59 | 0.17 | 0.12 | 0.11 | 0.16 | 8.36E-04 |
| Yale-Penn Meta | 3269 | 446 | + | 0.57 | 0.10 | 0.11 | 0.10 | 0.16 | 2.16E-08 |
| GTP | 89 | N/A | + | 0.48* | 0.27 | 0.13 | N/A | N/A | 4.00E-02** |
Abbreviations: SE, standard error; MAF, minor allele frequency; N/A, not applicable
This represents the odds ratio.
One-sided P-value
GWAS of cannabis-related aggression identified a GWS locus at HTR2B/PSMD1.
We identified one genome-wide significant (GWS) region on chromosome 2 in AAs (Regional Manhattan plot, Figure 1). The top SNPs included rs35750632 (β=0.54, P=1.79×10−8) in the proteasome 26S subunit, non-ATPase 1 gene (PSMD1) and rs17440378 (β=0.57, P=2.16×10−8) in HTR2B. The coding region of HTR2B is contained within that of PSMD1 (Figure 1); the lead variants are ~43 kb apart and in high LD (r2= 0.42, D’= 0.98). Conditional analysis confirmed that both SNPs reflect the same association signal. There was no GWS SNPs in EAs.
Figure 1. Association results for cannabis-related aggression (CRA) in African Americans.
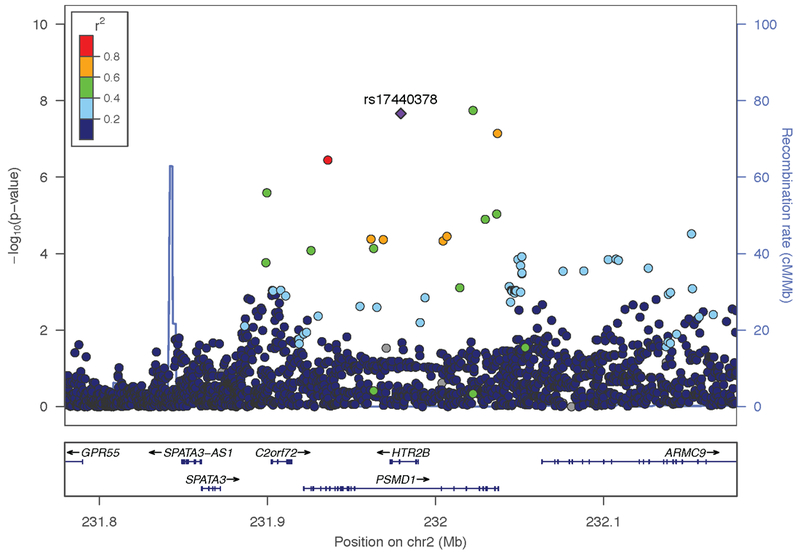
Regional association plot of 231.8- to 232.1-MB region on chromosome 2 encompassing HTR2B and PSMD1 in the Yale-Penn African-American participants after meta-analysis. This plot shows the genome-wide significant association between cannabis-related aggression and a single nucleotide polymorphism (SNP) rs17440378 (purple) at the HTR2B locus. Each circle represents a SNP, the left y-axis reflects the –log10(P value), and the light blue line and right axis the observed recombination rate. Color coding (dark blue, blue, green, yellow, and red) shows the degree of linkage disequilibrium (r2) between rs17440378 (purple, diamond shaped) and other SNPs in the region. Centimorgan (cM), megabase (Mb).
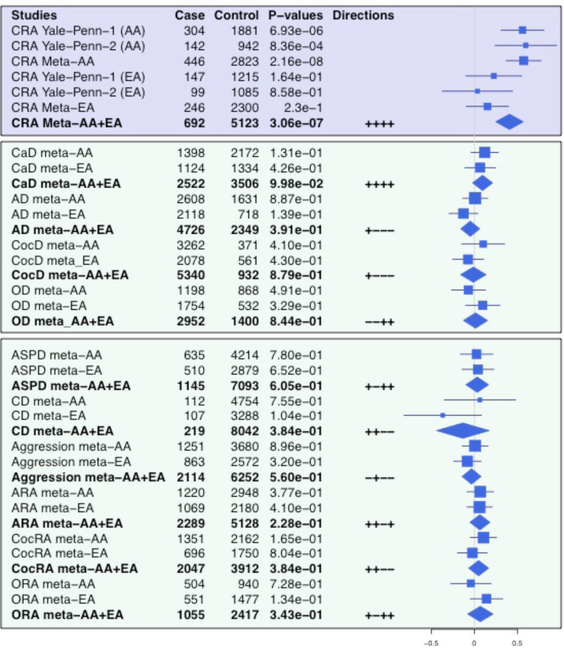
In AAs only, there was a dose-dependent relationship between CRA and HTR2B*rs17440378 genotype (Supplementary Figure 4), with TT genotype (MAF=0.1) associated with higher CRA: CC (n=2587), CRA=12.1%; CT (n=640), CRA=18.9%; TT (n=42), CRA=28.6%. Genome-wide meta-analysis of AA and EA samples showed no additional GWS SNPs. P-values of association, effect sizes, and effect directions of HTR2B*rs17440378 for CRA are shown in Figure 2 (upper panel).
Figure 2. Forest plot of rs17440378 (chr2:231979355), a genome-wide significant SNP for cannabis-related aggression (CRA).
The blue lines represent 95% confidence intervals of the effect size estimates. The blue rectangles are proportional to the square-root of the sample size. The blue diamonds represent the meta-analysis estimate of AAs and EAs. Abbreviations: Cannabis related aggression (CRA), cannabis dependence (CaD), alcohol dependence (AD), cocaine dependence (CocD), opioid dependence (OD), antisocial personality disorder (ASPD), conduct disorder (CD), alcohol-related aggression (ARA), cocaine-related aggression (CocRA), opioid-related aggression (ORA).
Annotation analysis was conducted using publicly available bioinformatics tools (Braineac, GTEx, and HaploReg v4.1) to identify the functional effects of the GWS variants found in the CRA GWAS. We found that HTR2B*rs17440378 is an eQTL for HTR2B and nearby genes (PSMD1, C2orf72, DIS3L2, SP140, B3GNT7) across several brain regions, (e.g., medulla, substantia nigra, and putamen) and peripheral tissue (Supplementary Tables 3 and 4; Supplementary Figures 5-7). Multiple epigenetic marks were also identified for HTR2B*rs17440378 (Supplementary Tables 5 and 6), suggesting a functional regulatory role for this GWS SNP.
Grady Trauma Project (GTP) sample:
A total of 89 AA subjects with data available for cannabis and aggression had previously been genotyped using the Illumina Omni-Quad 1M Array33; the HTR2B*rs17440378 variant was imputed (r2=0.99). HTR2B*rs17440378 show a significant association (p=0.04, one sided) with aggression in subjects with a lifetime prevalence of cannabis, with an effect size similar to that in the discovery sample (Table 2).
Polygenic risk score analysis for CRA and NEO factors.
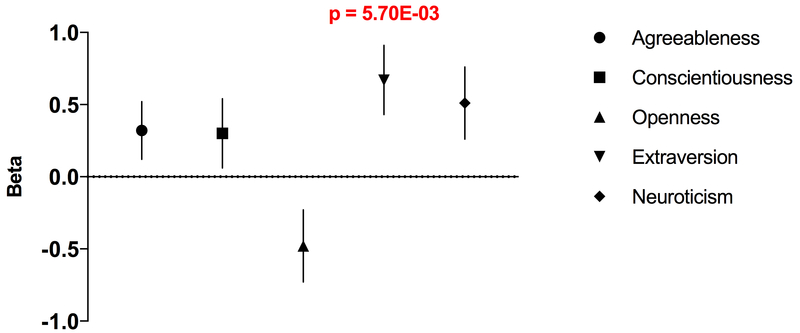
Using P-value thresholds of 0.001 and 0.005, polygenic risk scores (PRS) of extraversion were significantly associated with CRA, with the most significant result obtained for the SNP set (n=6,772) with a P-value threshold of 0.001 (Figure 3; Supplementary Table 7). Extraversion PRS predicted greater CRA risk (p=5.70×10−3, β=0.67, S.E.=0.24).
Figure 3. Results of polygenic risk score analyses predicting cannabis-related aggression (CRA) based on polygenic risk scores from NEO personality factors.
Polygenic risk scores (PRS) were calculated using P-value thresholds of 0.00001 – 0.5. (A) We show the PRS of P-value threshold of 0.0001 for agreeableness, and 0.001 for conscientiousness, openness, extraversion, and neuroticism. Extraversion polygenic risk scores were significantly positively associated with CRA (p = 5.70 × 10−3, β= 0.67, S.E. = 0.24). Error bars represent mean ± standard error (S.E.).
THC increases aggressive behavior in Htr2b−/− mice.
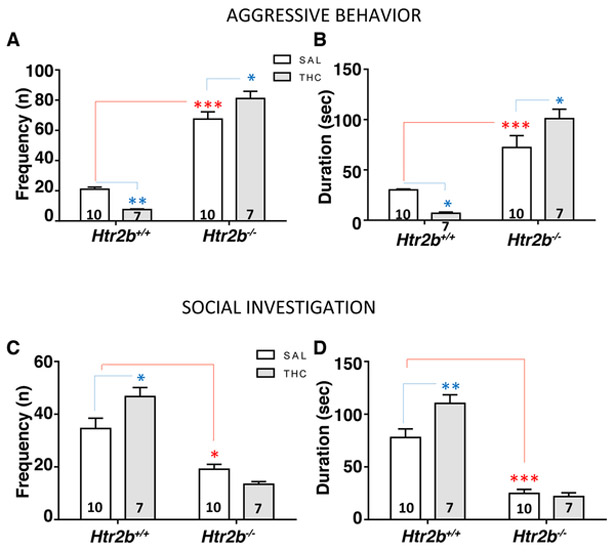
Htr2b−/− mice exhibited more impulsive behaviors and greater novelty-induced locomotion than wildtype (WT) mice9. In the present study, Htr2b−/− mice exhibited highly aggressive behavior (p<0.001 vs. WT), as shown by a significant effect of genotype on Aggressive Behavior (Fs(1,30) = 413.6 and 118.7, respectively for frequency and duration, P < 0.0001 for both) and had less social interaction (p<0.001 vs. WT) in the resident-intruder paradigm (Figure 4).
Figure 4. THC increases aggressive behavior in Htr2b−/− mice.
Effects of acute THC treatment (10 mg/kg, i.p.) on aggressive behavior and social interaction in Htr2b−/− and WT (Htr2b+/+) mice. (A-B) Htr2b−/− mice showed more aggressive behavior than WT. THC treatment significantly increased the aggressive response in Htr2b−/− mice; an opposite and significant effect was observed in WT mice. (C-D) THC treatment reduced social investigation in Htr2b−/− mice. Genotype effect: *** and * = P < 0.001 and 0.05, respectively. Treatment effect: ** and * = P < 0.01 and 0.05, respectively. Error bars represent mean ± standard error (S.E.).
A significant effect of the treatment × genotype interaction on both Aggressive Behavior (Fs(1,30) = 21.25 and 17.26, respectively for frequency and duration, P < 0.0001 and P =0.0002) and Social Investigation (Fs(1,30) = 7.553 and 6.017, respectively for frequency and duration, P < 0.0001 for both) showed a differential response to THC treatment for the two genotype groups. Indeed, acute THC treatment (10 mg/kg, i.p.) induced an opposite effect on aggressive response as a function of genotype: post-hoc comparisons showed increased aggression in Htr2b−/− mice (p<0.05 vs. Htr2b−/− saline control) and reduced aggression in WT mice (p<0.01 vs. WT saline control) (Figure 4A and B). Further, THC significantly increased the propensity to social investigation only in WT mice (p<0.05 vs. WT saline control; Figure 4C and D), but not in in Htr2b−/− mice.
Htr2b−/− mice were also hyperactive, as shown by the statistically significant interaction of treatment × genotype on crossing frequency (F(1,30) = 16.55, P = 0.0003; Supplementary Figure 8). Post-hoc analysis revealed that saline-treated Htr2b−/− mice crossed the cage surface more than saline-treated WT mice (p < 0.001). THC treatment decreased locomotion in both WT and Htr2b−/− mice (p <0.01 and p <0.001, respectively, vs. each respective saline control) (Supplementary Figure 8).
Discussion
In this first GWAS of CRA, we report GWS evidence linking HTR2B to cannabis response. We identified one GWS region mapped to HTR2B, which overlaps PSMD1. Functional analysis revealed genomic regulatory roles for HTR2B*rs17440378, mainly in brain tissue. HTR2B*rs17440378 also showed nominally significant association in the GTP sample with a related (but non-identical) phenotype. This specific locus is one of the best supported in previous studies of aggression phenotypes (that unlike the present study, used a candidate-locus approach)9,10 and the serotonin system is very well established as being related to traits involving violence and impulsivity. However, although serotonergic function is strongly implicated in cannabis response34, we are aware of no prior evidence implicating this particular receptor. PRS analysis showed that extraversion was significantly associated with CRA; higher extraversion predicted greater risk for CRA. Further, Htr2b−/− mice that received THC exhibited a greater aggressive response than WT mice, with the latter showing a reduced aggressive response, suggesting that Htr2b modulates aggression-related cannabis response.
Aggressive behavior is influenced by both genetic and environmental factors, including substance use. Cannabis use is associated with greater impulsive decision making12 and less behavioral inhibition14, critical contributors to risk-taking, substance use, and aggressive behavior. Cannabis use and cannabis withdrawal also contribute to aggression and related traits35; cannabis use is associated with increased subsequent violent behavior2,17. The identification of genetic factors contributing to the risk for aggressive behavior following cannabis use provides opportunities both for prevention and treatment.
Our CRA GWAS identified GWS loci at and near HTR2B. It is noteworthy that this GWAS, by nature hypothesis-free, landed on this gene, as it has previously been closely associated with impulsivity and aggressive behaviors in both humans and animal models. A stop codon variant of HTR2B (HTR2B Q20*) was associated to impulsivity in Finns9. A follow-up functional annotation analysis of rs17440378, the HTR2B intronic polymorphism, provided the best evidence of functionality based on its numerous regulatory effects in brain and peripheral tissues. HTR2B*rs17440378 is an eQTL for HTR2B and nearby genes. Multiple regulatory epigenetic marks were identified for rs17440378 in brain and peripheral tissue, including anterior caudate, involved in reward and cognitive function36, and cingulate gyrus, implicated in schizophrenia37 and threat processing in humans38. Based both on its demonstrated contribution to aggressive behavior and our functional annotation analysis, HTR2B appears to be the relevant gene rather than PSMD1.
The risk effect of HTR2B*rs17440378 appeared to be specific to individuals who are aggressive under the influence of cannabis, rather than being driven by drug dependence alone, aggression alone, or aggression referable to drugs other than cannabis (Figure 2). Decreased or null expression of HTR2B has been linked to impulsivity9,10, schizophrenia-like behaviors39, impaired social interaction39, and resistance to selective serotonin reuptake inhibitor antidepressants40. We extended these findings by showing that Htr2b−/− mice exhibit greater aggressive behavior and decreased social interaction than WT mice in the resident-intruder test. HTR2B variation may therefore interact with cannabis use to induce aggression by reducing brain monoaminergic tone. Interestingly, null expression or blockade of 5-HT2B receptors diminishes the reinforcing effects of psychoactive drugs by modulating serotonin41 and dopamine42 signaling. In this study, we found that THC induces opposite effects in aggressive behavior and social interaction by Htr2b status: THC increased aggression and produced no change in social investigation in Htr2b−/− mice, while it decreased aggression and increased social investigation in WT mice.
Because personality traits may contribute to the comorbidity of psychiatric disorders, we conducted a PRS analysis to estimate the genetic overlap of CRA with personality traits. PRS for extraversion was associated with CRA, such that higher extraversion showed a higher risk for cannabis-related aggression. Extraversion is positively associated with externalizing behavior 43. Further, extraversion has previously been linked to excitement- and attention-seeking behavior, as well as social and interpersonal dysfunction44.
Our human study is limited by the comparatively small sample size, especially among EAs. Significant results were observed in AAs only, an effect that may be population-specific. Although the association was not statistically significant in EAs, the effect direction was the same, such that a meta-analysis of both populations yielded a p-value of 3.06 × 10−7, i.e., it reduced significance. Thus, the effect may also be present in EAs, but a larger sample is needed to evaluate this possibility. Larger samples with additional phenotypic information would also be required to assess additional relevant correlated or confounding factors such as testosterone levels, psychosocial environment, and socioeconomic status, as well as impulsivity traits. In addition, human genomic and precision medicine research is limited by a lack of racial diversity, with 96% of GWAS participants reportedly being of European descent45. This limited our ability to identify an AA cohort of adequate size with the phenotypic assessment necessary to replicate our findings.
However, by using a proxy phenotype approach and a within-trait assessment, we found a significant association of HTR2B*rs17440378 and aggression in AA subjects with a history of cannabis use. This proxy approach supported out major GWAS finding, but is inherently limited (in this case, by both the phenotype definition and the sample size), and we emphasize the need for stricter replication. The lack of direct assessment of the effects of HTR2B*rs17440378 on protein function, including regulation, is a limitation of our study. Nonetheless, eQTL analysis enabled us to understand the functional effect of this risk variant on genomic regulation of HTR2B and nearby genes in peripheral and brain tissue. Further, there is clear biological relevance of the GWS locus, HTR2B, a gene previously shown to play an important role in aggression and impulsivity traits. The prior evidence for this gene and the findings from the animal model further support the role of the gene in CRA.
Our findings support a role of 5-HT2B receptors in the modulation of the aggression-related cannabis response, identify a specific mechanism that could result in violence in the context of cannabis use, and provide the first evidence that this is an important site for cannabis response. Medications acting at 5-HT2B receptors may be relevant in modifying such behaviors acutely. For example, an 5-HT2B receptor antagonist, such as the widely used antipsychotic aripiprazole, may, based on the evidence presented herein, exacerbate cannabis-related aggression, an interesting idea given its effects on the response to the acute administration of methamphetamine46 and in the treatment of depression47, schizophrenia48, and agitation in autism49.
Supplementary Material
Acknowledgments
We appreciate the work in recruitment and assessment provided at Yale University School of Medicine and the APT Foundation by James Poling, Ph.D.; at McLean Hospital by Roger Weiss, M.D., at the Medical University of South Carolina by Kathleen Brady, M.D., Ph.D., and Raymond Anton, M.D.; and at the University of Pennsylvania by David Oslin, M.D. We are grateful to Ann Marie Lacobelle and Christa Robinson for their excellent technical assistance, to the SSADDA interviewers who devoted substantial time and effort to phenotype the study sample, and to John Farrell and Alexan Mardigan for database management assistance. Assistance with data cleaning was provided by the National Center for Biotechnology Information. This study was supported by National Institutes of Health grants RC2 DA028909, R01 DA12690, R01 DA12849, R01 DA18432, R01 AA11330, R01 AA017535, and the VA Connecticut and Philadelphia VA MIRECCs; the Biological Sciences Training Program through Grant Number 5T32 MH14276 and the NARSAD Young Investigator Grant to JLMO. Funding support for genotyping, which was performed at the Johns Hopkins University Center for Inherited Disease Research, was provided by the NIH GEI (U01HG004438), the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and the NIH contract "High throughput genotyping for studying the genetic contributions to human disease" (HHSN268200782096C). LM and ID'A have been supported by funds from the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Université Pierre et Marie Curie, and by grants from the Fondation pour la Recherche Médicale "Equipe FRM DEQ2014039529", the French Ministry of Research (Agence Nationale pour la Recherche ANR-12-BSV1-0015 and ANR-17-CE16-0008 and the Investissements d'Avenir programme ANR-11-IDEX-0004-02). LM's team is part of the École des Neurosciences de Paris Ile-de-France network and of the Bio-Psy Labex and as such this work was supported by French state funds managed by the ANR within the Investissements d'Avenir programme under reference ANR-11-IDEX-0004-02.
Footnotes
Conflict of interest statement
Although unrelated to the current study, HRK has been a consultant, advisory board member, or CME speaker for Indivior and Lundbeck. He is also a member of the American Society of Clinical Psychopharmacology's Alcohol Clinical Trials Initiative, which in the last three years was supported by Abbvie, Alkermes, Amygdala Neurosciences, Arbor, Ethypharm, Indivior, Lilly, Lundbeck, Otsuka, and Pfizer. No other authors declare possible conflicts.
References:
- 1.Siever LJ. Neurobiology of aggression and violence. Am J Psychiatry. 2008;165(4):429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoeler T, Theobald D, Pingault JB, Farrington DP, Jennings WG, Piquero AR, et al. Continuity of cannabis use and violent offending over the life course. Psychol Med 2016;46(8): 1663–1677. [DOI] [PubMed] [Google Scholar]
- 3.Veroude K, Zhang-James Y, Fernandez-Castillo N, Bakker MJ, Cormand B, Faraone SV. Genetics of aggressive behavior: An overview. American journal of medical genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2016;171B(1):3–43. [DOI] [PubMed] [Google Scholar]
- 4.Waltes R, Chiocchetti AG, Freitag CM. The neurobiological basis of human aggression: A review on genetic and epigenetic mechanisms. American journal of medical genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2016;171(5):650–675. [DOI] [PubMed] [Google Scholar]
- 5.Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262(5133):578–580. [DOI] [PubMed] [Google Scholar]
- 6.Brunner HG, Nelen MR, van Zandvoort P, Abeling NG, van Gennip AH, Wolters EC, et al. X-linked borderline mental retardation with prominent behavioral disturbance: phenotype, genetic localization, and evidence for disturbed monoamine metabolism. Am J Hum Genet 1993;52(6):1032–1039. [PMC free article] [PubMed] [Google Scholar]
- 7.Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–854. [DOI] [PubMed] [Google Scholar]
- 8.Weder N, Yang BZ, Douglas-Palumberi H, Massey J, Krystal JH, Gelernter J, et al. MAOA genotype, maltreatment, and aggressive behavior: the changing impact of genotype at varying levels of trauma. Biological psychiatry. 2009;65(5):417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bevilacqua L, Doly S, Kaprio J, Yuan Q, Tikkanen R, Paunio T, et al. A population-specific HTR2B stop codon predisposes to severe impulsivity. Nature. 2010;468(7327):1061–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tikkanen R, Tiihonen J, Rautiainen MR, Paunio T, Bevilacqua L, Panarsky R, et al. Impulsive alcohol-related risk-behavior and emotional dysregulation among individuals with a serotonin 2B receptor stop codon. Translational psychiatry. 2015;5:e681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karberg JCJ, D.J. Substance Dependence, Abuse, and Treatment of Jail Inmates, 2002. In: Statistics BoJ, ed2005. [Google Scholar]
- 12.Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, et al. Reflection impulsivity in adolescent cannabis users: a comparison with alcohol-using and non-substance-using adolescents. Psychopharmacology (Berl). 2012;219(2):575–586. [DOI] [PubMed] [Google Scholar]
- 13.Gruber SA, Dahlgren MK, Sagar KA, Gonenc A, Killgore WD. Age of onset of marijuana use impacts inhibitory processing. Neurosci Lett 2012;511(2):89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhattacharyya S, Atakan Z, Martin-Santos R, Crippa JA, Kambeitz J, Malhi S, et al. Impairment of inhibitory control processing related to acute psychotomimetic effects of cannabis. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2015;25(1):26–37. [DOI] [PubMed] [Google Scholar]
- 15.Renard J, Krebs MO, Le Pen G, Jay TM. Long-term consequences of adolescent cannabinoid exposure in adult psychopathology. Front Neurosci 2014;8:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barthelemy OJ, Richardson MA, Cabral HJ, Frank DA. Prenatal, perinatal, and adolescent exposure to marijuana: Relationships with aggressive behavior. Neurotoxicol Teratol 2016;58:60–77. [DOI] [PubMed] [Google Scholar]
- 17.Dugre JR, Dellazizzo L, Giguere CE, Potvin S, Dumais A. Persistency of Cannabis Use Predicts Violence following Acute Psychiatric Discharge. Frontiers in psychiatry. 2017;8:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelernter J, Kranzler HR, Sherva R, Koesterer R, Almasy L, Zhao H, et al. Genome-wide association study of opioid dependence: multiple associations mapped to calcium and potassium pathways. Biological psychiatry. 2014;76(1):66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gelernter J, Sherva R, Koesterer R, Almasy L, Zhao H, Kranzler HR, et al. Genome-wide association study of cocaine dependence and related traits: FAM53B identified as a risk gene. Molecular psychiatry. 2014;19(6):717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelernter J, Kranzler HR, Sherva R, Almasy L, Herman AI, Koesterer R, et al. Genome-wide association study of nicotine dependence in American populations: identification of novel risk loci in both African-Americans and European-Americans. Biological psychiatry. 2015;77(5):493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gelernter J, Kranzler HR, Sherva R, Almasy L, Koesterer R, Smith AH, et al. Genome-wide association study of alcohol dependence:significant findings in African- and European-Americans including novel risk loci. Molecular psychiatry. 2014;19(1):41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, et al. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry. 2009;31(6):505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.First MB, Spitzer RL, Gibbon M, JBW W. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I Research Version 2.0). New York: Biometrics Research; 1998. [Google Scholar]
- 24.Straus MA, Douglas EM. A short form of the Revised Conflict Tactics Scales, and typologies for severity and mutuality. Violence Vict 2004;19(5):507–520. [DOI] [PubMed] [Google Scholar]
- 25.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nature genetics. 2006;38(8):904–909. [DOI] [PubMed] [Google Scholar]
- 26.Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS genetics. 2006;2(12):e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherva R, Wang Q, Kranzler H, Zhao H, Koesterer R, Herman A, et al. Genome-wide Association Study of Cannabis Dependence Severity, Novel Risk Variants, and Shared Genetic Risks. JAMA Psychiatry. 2016;73(5):472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1): 121–130. [PubMed] [Google Scholar]
- 29.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics (Oxford, England). 2010;26(17):2190–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costa PT Jr., McCrae RR. Stability and change in personality assessment: the revised NEO Personality Inventory in the year 2000. J Pers Assess 1997;68(1):86–94. [DOI] [PubMed] [Google Scholar]
- 31.Long LE, Chesworth R, Huang XF, McGregor IS, Arnold JC, Karl T. Transmembrane domain Nrg1 mutant mice show altered susceptibility to the neurobehavioural actions of repeated THC exposure in adolescence. The international journal of neuropsychopharmacology. 2013;16(1): 163–175. [DOI] [PubMed] [Google Scholar]
- 32.Koolhaas JM, Coppens CM, de Boer SF, Buwalda B, Meerlo P, Timmermans PJ. The resident-intruder paradigm: a standardized test for aggression, violence and social stress. Journal of visualized experiments : JoVE. 2013(77):e4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powers A, Almli L, Smith A, Lori A, Leveille J, Ressler KJ, et al. A genome-wide association study of emotion dysregulation: Evidence for interleukin 2 receptor alpha. Journal of psychiatric research. 2016;83:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller CP, Homberg JR. The role of serotonin in drug use and addiction. Behavioural brain research. 2015;277:146–192. [DOI] [PubMed] [Google Scholar]
- 35.Smith PH, Homish GG, Leonard KE, Collins RL. Marijuana withdrawal and aggression among a representative sample of U.S. marijuana users. Drug Alcohol Depend 2013;132(1–2):63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hikosaka O, Kim HF, Yasuda M, Yamamoto S. Basal ganglia circuits for reward value-guided behavior. Annu Rev Neurosci 2014;37:289–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bersani FS, Minichino A, Fojanesi M, Gallo M, Maglio G, Valeriani G, et al. Cingulate Cortex in Schizophrenia: its relation with negative symptoms and psychotic onset. A review study. Eur Rev Med Pharmacol Sci 2014;18(22):3354–3367. [PubMed] [Google Scholar]
- 38.Fiddick L. There is more than the amygdala: potential threat assessment in the cingulate cortex. Neurosci Biobehav Rev 2011;35(4): 1007–1018. [DOI] [PubMed] [Google Scholar]
- 39.Pitychoutis PM, Belmer A, Moutkine I, Adrien J, Maroteaux L. Mice Lacking the Serotonin Htr2B Receptor Gene Present an Antipsychotic-Sensitive Schizophrenic-Like Phenotype. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40(12):2764–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diaz SL, Narboux-Neme N, Boutourlinsky K, Doly S, Maroteaux L. Mice lacking the serotonin 5-HT2B receptor as an animal model of resistance to selective serotonin reuptake inhibitors antidepressants. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2016;26(2):265–279. [DOI] [PubMed] [Google Scholar]
- 41.Doly S, Bertran-Gonzalez J, Callebert J, Bruneau A, Banas SM, Belmer A, et al. Role of serotonin via 5-HT2B receptors in the reinforcing effects of MDMA in mice. PloS one. 2009;4(11):e7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doly S, Quentin E, Eddine R, Tolu S, Fernandez SP, Bertran-Gonzalez J, et al. Serotonin 2B receptors in mesoaccumbens dopamine pathway regulate cocaine responses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muris P, Meesters C, Blijlevens P. Self-reported reactive and regulative temperament in early adolescence: relations to internalizing and externalizing problem behavior and "Big Three" personality factors. J Adolesc 2007;30(6):1035–1049. [DOI] [PubMed] [Google Scholar]
- 44.Trull TJ, Widiger TA. Dimensional models of personality: the five-factor model and the DSM-5. Dialogues in clinical neuroscience. 2013;15(2): 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends Genet 2009;25(11):489–494. [DOI] [PubMed] [Google Scholar]
- 46.Stoops WW, Bennett JA, Lile JA, Sevak RJ, Rush CR. Influence of aripiprazole pretreatment on the reinforcing effects of methamphetamine in humans. Progress in neuro-psychopharmacology & biological psychiatry. 2013;47:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen J, Gao K, Kemp DE. Second-generation antipsychotics in major depressive disorder: update and clinical perspective. Curr Opin Psychiatry. 2011;24(1): 10–17. [DOI] [PubMed] [Google Scholar]
- 48.Fleischhacker WW, McQuade RD, Marcus RN, Archibald D, Swanink R, Carson WH. A doubleblind, randomized comparative study of aripiprazole and olanzapine in patients with schizophrenia. Biological psychiatry. 2009;65(6):510–517. [DOI] [PubMed] [Google Scholar]
- 49.Owen R, Sikich L, Marcus RN, Corey-Lisle P, Manos G, McQuade RD, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics. 2009;124(6):1533–1540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.