Abstract
Obesity is a worldwide public health epidemic that leads to increased morbidity, mortality, and cost burden to health care. Although bariatric surgery has been recognized as a standard invasive treatment for obesity, it is accompanied by relatively high morbidity and cost burden, as well as limited treatment outcome. Therefore, alternative treatments with lower morbidity and cost for surgery that target patients who are obese, but not morbidly obese, are needed. A minimally invasive trans-catheter procedure, named Bariatric Arterial Embolization or Bariatric Embolization (BAE), has been identified as a potential solution, based on its safety and preliminary efficacy profiles. The purpose of this review is to introduce up-to-date clinical data, and discuss future directions for BAE for the treatment of obesity.
Keywords: Obesity, bariatric, embolization, left gastric artery, weight loss
Introduction
Obesity, defined as having a Body Mass Index (BMI) ≥ 30 kg/m2, is one of the most prevalent public health issues of the 21st century [1]. It is now a major cause of morbidity and mortality worldwide, and has been recognized as a risk factor for several diseases, including type 2 diabetes, liver disease, heart disease, degenerative joint disease, obstructive sleep apnea, and even malignancy [2, 3]. In addition, mean BMI is increasing worldwide, bringing a heavy social and economic burden [4, 5]. Traditional therapies for obesity include lifestyle modifications (e.g., diet and exercise) and medical management [6–10].
Previous studies have demonstrated that lifestyle modifications can achieve an average weight loss of 5 to 10% in overweight (BMI between 25 and 29.9 kg/m2) and obese (BMI between 30 and 34.9 kg/m2) patients [6, 11]. Nevertheless Several studies have shown that patients may return to their original weight in as little as three years after initiating lifestyle changes [12, 13].
For severely obese patients or those with obesity-related comorbidity, more aggressive medical management such as bariatric surgery is often considered as a treatment option (Table 1) [14]. However, bariatric surgery is associated with a relatively high morbidity rate [15]. In addition, the cost of these surgical procedures is high, costing on average $38,000, and up to $64,000 in the U.S. and about $27,000 in Europe if complications occur [16–19].
Table 1.
Bariatric Surgery Options [54]
| Procedure | Advantages | Disadvantages | EWL |
|---|---|---|---|
| Adjustable gastric banding | Low complications Good weight loss | Reoperations Implanted device | About 19% |
| Sleeve gastrectomy | Continuous GI tract Good weight loss | Long staple line Nutritional deficit | 25–30% |
| Roux-en-Y gastric bypass | Better weight loss | Anastomoses Nutritional deficit | 33–36% |
| Biliopancreatic diversion duodenal switch | Best weight loss | Anastomoses Nutritional deficit | 34%+ |
EWL, estimated weight loss
Patients who fail to lose weight through lifestyle modifications, but are not candidates for bariatric surgery, have few other treatment options. There are currently six major U.S. Food and Drug Administration (FDA)-approved anti-obesity medications[20], most of which work through pathways in the central nervous system (CNS) that either reduce appetite, enhance satiety, or decrease the absorption of fat [20, 21]. However, pharmacotherapy can only achieve modest weight loss, with a range of 2.0 to 6.5 kg, according to previous data [22–24] (Table 2). Therefore, it is often regarded as an adjuvant therapy to lifestyle modifications [25]. Endoscopic bariatric therapies include intragastric balloons (space-occupying devices), endoscopic gastric plication, endoluminal duodenal-jejunal sleeve, and gastric pacer (Table 3) [26]. However, these endoscopic techniques have the potential to cause major complications [26–29].
Table 2.
Pharmacotherapy for Obesity [20]
| Medication | Mechanism | Side effects | Weight loss |
|---|---|---|---|
| Phentermine | Sympathomimetic amine | Increase in HR and/or BP, dizziness, dry mouth, constipation, insomnia, and irritability | 5.1% at 28 weeks |
| Orlistat | Pancreatic lipase inhibitor | Decreased absorption of fat-soluble vitamins, fecal urgency, fatty stool, fecal incontinence | Mean 5.0 kg vs. 3.8 kg with placebo |
| Phentermine/topiramate ER | Sympathomimetic, raises concentration of norepinephrine | Dizziness, insomnia, constipation, paresthesias | 9.3–10.5% vs. 1.8% loss of baseline weight in 2 years compared to placebo |
| Lorcaserin | Selective serotonin 2C receptor agonist | Headache, dizziness, fatigue, dry mouth, constipation | 5.0% vs. 1.5% total body weight loss in 1 year compared to placebo |
| Naltrexone/bupropion SR | Bupropion: dopamine/norepinephrine reuptake inhibitor Naltrexone: opiod receptor agonist | Nausea, constipation headache, and psychiatric and sleep disturbances | Up to 9.3% loss of initial body weight |
| Liraglutide | GLP-1 agonist | Nausea, hypoglycemia, and diarrhea, among others | 8.0% vs. 2.5% mean weight loss after 56 weeks compared to placebo |
HR, heart rate; BP, blood pressure; ER, extended release; SR, sustained release
Table 3.
Endoscopic Bariatric Procedures [27]
| Procedure | Advantages | Disadvantages | EWL |
|---|---|---|---|
| Space-occupying devices | Easily placed endoscopically; restrict food consumption; well tolerated; effective; can be reversed/removed | Balloon may deflate over long term; can migrate, leading to perforation; FDA-required removal at 6 months with poor long-term weight loss | 39% at 1 year after removal |
| Restrictive procedures | Permanently reduces stomach capacity; effective; well tolerated | Not easily reversible; plication durability varies according to device | Up to 54% ± 40% at 12 months |
| Bypass liners | Pancreaticobiliary secretions can still travel along the sleeve, as opposed to surgical bypass | High risk of hepatic abscesses; not currently available in the U.S. because of complications | Up to 36% at 1 year |
| Gastric stimulation | Effective at treating moderate (class 1 and class 2) obesity | All use stimuli that are essentially not perceived by subjects, which may limit their long-term efficacy | Lose ≥15% of body weight at 1 year |
| Transpyloric shuttle | Does not take up considerable space, but results in delayed gastric emptying, and likely has additional mechanisms of action | High risk of gastric ulcer | 8.9% total body weight loss at 3 months, and 14.5% at 6 months |
EWL, excess weight loss; FDA, U.S. Food and Drug Administration
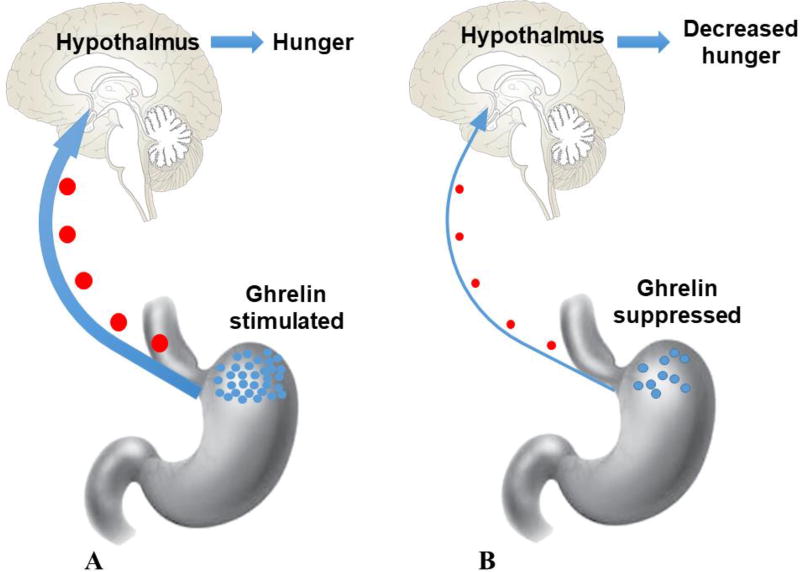
Bariatric Arterial Embolization (BAE) is a minimally invasive technique performed by interventional radiologists under imaging guidance. The procedure trans-arterially embolizes the gastric fundus through the left gastric artery (LGA) and, to a lesser extent, the gastroepiploic artery (GEA). The idea of using BAE to treat obesity originates from the fact that about 90% of ghrelin is produced by the fundus of the stomach [30]. The vascular supply to the gastric fundus is predominantly from the LGA (Fig. 1) [31]. Ghrelin is a 28-amino acid peptide that plays an important role in stimulating appetite, and promoting positive energy balance to gain weight [30, 32, 33]. Plasma ghrelin levels increase significantly before meals, and decrease after meals [33, 34]. Nevertheless, obese patients fail to suppress ghrelin levels after eating food, leading to overeating [35]. Another appetite regulating hormone, leptin, is produced by adipocytes to inhibit hunger signals. Plasma leptin levels decrease in response to reductions in body fat and are associated with the amount of peripheral fat stores [36]. Leptin is suppressed when fasting and is stimulated after food intake. BAE destroys ghrelin-producing cells by causing ischemia in the gastric fundus and decreasing ghrelin production, resulting in loss of body weight (Fig. 2). The safety and preliminary efficacy of BAE for obesity have been verified by several studies. Notably, some updated data from both animal and human studies have been recently reported. Here, our goal is to provide a comprehensive review of the newest clinical and pre-clinical data for BAE for the treatment of obesity.
Figure 1.
Angiography of the left gastric artery. Left gastric arterial branches cover a large part of the fundus of the stomach.
Figure 2.
Ghrelin change pre- and post-Bariatric Arterial Embolization (BAE). A, Before BAE, ghrelin is secreted by X/A cells in the fundus of the stomach during the fasting state, initiating the hunger drive. B, After BAE, ghrelin-producing cells are destroyed by causing ischemia in the gastric fundus, decreasing ghrelin production and resulting in loss of body weight.
Pre-clinical studies
The first animal study of BAE was explored by Arepally et al. in 2007 [37]. The study showed ghrelin levels could be purposefully and significantly altered with chemical embolization of the gastric artery using morrhuate sodium. After this study, a series of early animal studies performed in growing swine and obese dogs showed a decrease in serum ghrelin levels, and either decreased weight gain or increased absolute weight loss when compared to the control group after embolization [38–45].
Clinical studies
Having been practiced for only a short period of time, available clinical data for BAE are limited. Excitingly, several clinical trials from different areas have been recently reported. The first clinical study focusing on this topic was performed by Gunn et al. in a retrospective fashion [46]. The patients included in this single-center study received LGA embolization, with the initial intention of stopping upper gastrointestinal bleeding (UGIB).The authors found that patients who underwent LGA embolization lost an average of 7.3% of their weight, which was significantly more than those who underwent embolization of other branches of the celiac axis (2%, p = 0.006). A similar retrospective study was performed by Anton et al. [47]. A group of 10 patients who underwent LGA embolization for UGIB unrelated to malignancy were compared to 22 patients who underwent embolizations of other mesenteric vessels for UGIB unrelated to malignancy. The BMI in the two groups was not significantly different (31 ± 6.8 kg/m2 for the LGA embolization group, and 28.4 ± 6.4 kg/m2 in the control group). The LGA embolization group had reduced their BMI by 9.8%, compared to the control group’s 4% loss (p = 0.042) at one month, and 11.7% compared to the control group’s gain of 0.1% (p = 0.033) at four months. The LGA embolization group continued to show greater weight loss up to one year, however not to a statistically significant extent. As with Gunn et al., the study was limited by its retrospective nature and small sample size.
The first prospective BAE study was carried out by Kipshidze et al. [48]. This single-arm study included five patients with a mean BMI of 42.2 kg/m2. BAE was performed using 300 to 500 µm microspheres (Biocompatibles UK Limited, Surrey, United Kingdom) mixed with contrast (1:1 ratio). In regards to safety, no periprocedural complications occurred, and a follow-up endoscopy found no significant alterations to the stomach mucosa. Three patients described mild transient epigastric discomfort after the procedure, though follow-up endoscopies found no gastric ulcers. Regarding efficacy, all five patients reported a reduction in their appetite. There was significant and continuous mean weight loss within the 24-month follow-up period, which decreased from a baseline of 128 kg to 106 kg (p < 0.05). There was a significant decrease in serum ghrelin levels at the one and three-month follow-up (by 29% and 36% from the baseline, respectively, p < 0.05). Although the serum ghrelin level increased at the six-month follow-up, as compared to the three-month follow-up, it remained 19% lower than at baseline (p < 0.05). This first prospective study for BAE showed a promising outcome. Some limitations for this study should be noted, such as the absence of detailed inclusion criteria, absence of details on the degree of embolization, and the early timing of endoscopy, which could exclude the later discovery of gastritis or ulcers.
Ongoing trials with published preliminary results
To date, there are three ongoing clinical trials focused on BAE, all of which have published their preliminary results [49–51]. Among them, the first reported study was the Gastric artery Embolization Trial for Lessening of Appetite Nonsurgically (GET LEAN) trial at Dayton Interventional Radiology and Ohio State University in the U.S., with FDA approval [49]. According to the study protocol of GET LEAN on clinicaltrials.gov (Identifier: NCT02248688), the purpose of this pilot study was to collect safety and efficacy data in patients undergoing left gastric artery embolization (LGAE) for morbid obesity in the U.S., with five patients included and undergoing a one-year follow up. The primary outcome measure was the safety assessment of BAE at one year post-procedure. The secondary outcome measures included changes in BMI, quality of life, appetite hormone levels, and change in overall weight of the subjects at one year post-procedure. The estimated primary completion date was October 2017.
In the results of the GET LEAN study, four morbidly obese patients were included, one of which was diabetic. All of the patients underwent BAE through the right common femoral artery or left radial artery. The LGA and its branches were embolized using 300 to 500 µm Bead Block particles (BTG Interventional Medicine, West Conshohocken, PA) mixed with 5 mL of contrast medium. Complete cessation of flow (i.e., stasis) of the LGA and its branches was the endpoint of the procedure. Stasis was defined as visualization of contrast medium within the main LGA for at least five cardiac cycles. No major complications occurred. Three minor adverse events, including superficial gastric ulcerations, nausea, and vomiting were observed in three patients. These three patients required only nominal therapy without hospitalization, and all adverse events resolved within 30 days. Mean body weight loss among the four patients at six months post-procedure was 9.2 kg (p = 0.0775). Body weight loss as a percentage was 8.5%, and average excess body weight loss at six months was 17.2%. Among the four included patients, the first one had a weight loss of 17.2 kg representing 38.5% of excess body weight after one year, which seemed equivalent to the effect obtained with bariatric surgery. The diabetic patient showed a weight loss of 12.7 kg and 18.4% of excess body weight at six months post-procedure. The HBA1C level of this patient nearly normalized (7.4% pre-procedure to 6.3% at three months post-procedure), and remained at this level after six months. Serum ghrelin levels decreased in two patients and increased in another two patients after six months, with the mean increasing from 612 pg/mL at baseline to 645 pg/mL at six months. Leptin levels decreased overall, except in one patient who lost the least amount of weight.
The second reported study was the Bariatric Embolization of Arteries for the Treatment of Obesity (BEAT Obesity) trial carried out at the Johns Hopkins Hospital in the U.S. [50]. This study was a physician-sponsored Investigational Device Exemption (IDE) from the FDA, and Weiss et al. published the preliminary results in 2017. According to clinicallrials.gov (Identifier: NCT02165124), the BEAT Obesity trial aimed to observe weight loss at 12 months post-procedure as the primary outcome measure, and adverse event assessment as the secondary outcome measure within 30 days after BAE. The study aimed to include 20 patients at two centers, the Johns Hopkins Hospital in Baltimore, MD and the Mount Sinai Hospital in New York, NY.
The primary safety endpoint preliminarily reported was 30-day complications according to the American Society for Metabolic and Bariatric Surgery, and the secondary efficacy endpoint was weight loss at the three-month follow-up [52]. Five obese patients with a mean BMI of 43.8 ± 2.9 kg/m2 were included in the preliminary report. Notably, obese patients with diabetes were excluded from the study. Patients underwent BAE via a femoral artery approach. Embolization of one or more fundal arteries was performed with 300 to 500 µm Embosphere microspheres (Merit Medical, Dundalk, MD). The fundus was also embolized via the GEA, if needed. The results of this preliminary study showed that no major complications occurred during the follow-up. There were two minor adverse events. One patient had a transient, chemical pancreatitis that recovered and remained asymptomatic at the one-week follow-up visit. Another patient had a small asymptomatic superficial ulcer in the fundus/lesser curvature observed at the two-week follow-up endoscopy, which resolved by the time of the three-month follow-up endoscopy. In terms of efficacy, there was 5.9% excess weight loss at one month (n = 5) and 9.0% at three months (n = 4), respectively. Mean fasting serum ghrelin was relatively unchanged within the first two weeks post-procedure. It increased by 8.7% and decreased by 17.5% from baseline at one and three months, respectively. The preliminary results of the BEAT Obesity trial also demonstrated the safety and potential efficacy of BAE.
The latest published preliminary study of an ongoing trial was carried out by Bai et al. in China [51]. The protocol of this study on clinicaltrials.gov (Identifier: NCT02786108) showed that an estimated 50 patients would be included, with the weight loss at 12 months after BAE as the primary endpoint, and blood pressure, lipid profile, number of patients with adverse events, ghrelin levels, abdominal fat content, leptin levels, results of endoscopic examination, and quality of life as secondary endpoints. The trial included Chinese patients with BMI no less than 30 kg/m2. Patients underwent BAE via the superior-most branch of the LGA closest to the junction between the cardia and fundus, using 500 to 710 µm polyvinyl alcohol (PVA) particles (COOK Incorporated, Bloomington, IN). The PVA dosage used during embolization was based on real-time observation of the stasis of blood flow in the LGA. The follow-up period was nine months. The results showed no major complications occurred during the follow-up. A superficial linear ulceration below the cardia was observed in one patient at the three-day follow-up endoscopy, which resolved by the time of the 30-day follow-up endoscopy. The mean body weight showed significant and continuing loss during the follow-up (mean weight loss at three, six, and nine months was 8.28, 10.42, and 12.9 kg, respectively). Serum ghrelin levels decreased by 40.83%, 31.94%, and 24.82% at three, six, and nine months post-procedure, respectively. Magnetic resonance imaging showed that the subcutaneous adipose tissue decreased significantly during the follow-up period. Similar to previous studies, the preliminary results of this study verified the safety and potential efficacy of BAE.
Discussion
A systematic review published in 2016 gave the conclusion that data regarding the potential role of BAE in decreasing the ghrelin and potential weight loss is scarce [53]. As several pre- and early clinical studies of BAE have been carried out, more and more data support the safety and early effectiveness of this minimally invasive procedure (Table 4). Currently, there are future trials in the works that will explore BAE’s effects on different populations. A randomized control trial testing the efficacy of BAE has also recently begun in New Zealand, targeting morbidly obese patients not fit for bariatric surgery. The study will include 24 patients with BMI greater than 30 kg/m2. Primary outcome measures include weight loss at 60 months.
Table 4.
Treatment outcomes of early clinical studies
| Author /year |
AEs | Pre-procedure | Post-procedure | |
|---|---|---|---|---|
| Weight (kg) |
BMI (kg/m2) |
Weight (kg) | ||
| Gunn AJ/2014 [46] | Unknown | Unknown | 30.3 | Unknown |
| Anton K/2015 [47] | Unknown | 97.3 | 31 | Unknown |
| Kipshidze N/2015 [48] | Mild, transient epigastric discomfort | 128 | 42.2 | 106 |
| Syed MI/2016 [49] | Mild nausea, occasional vomiting, mild epigastric discomfort | 117.6 | 42.4 | 108.4 |
| Weiss CR/2017 [50] | Transient pancreatitis, asymptomatic superficial ulcer | 127.8 | 43.8 | 123.1 |
| Bai ZB/2017 [51] | Superficial linear ulceration, hematoma in puncture site | 102.02 | 38.1 | 89.12 |
AEs, adverse events; BMI, body mass index
Despite promising results and the addition of new studies, there are still several key questions that remain unanswered. First, the ideal candidate for BAE is unclear. The patients’ BMI in the GET LEAN and BEAT Obesity trials was no less than 40 kg/m2. However, patients with BMI no less than 30 kg/m2 were included in the latest trial in China [51]. Despite having different inclusion criteria for BMI, all three trials demonstrated positive preliminary results for weight loss. It is likely that BAE may be more effective in treating obese, but not severely or morbidly obese patients. In addition, the effect of BAE on diabetic or pre-diabetic patients is unknown. The GET LEAN and Chinese trials included diabetic, obese patients, while the BEAT Obesity trial excluded them. In the GET LEAN trial, the sole diabetic patient’s HbA1c dropped from 7.4 to 6.3 at three months, and remained at this level at six months. Due to the limited sample sizes of the trials, no statistically significant results were reported. Further long-term results with larger sample sizes are warranted to show the correlation between treatment efficacy and HbA1C.
Second, since prior animal studies have shown that weight and ghrelin levels trend toward baseline after BAE, it is important to identify the long-term treatment outcome of the procedure [37–40, 43]. However, the relative ages of the subjects have greatly differed in the pre-clinical and clinical trials. Most animals in the pre-clinical trials have been still-growing swine, possibly countering the effects of the procedure, while patients in the current clinical trials are generally past the period of maximum growth in humans.
Third, BAE may prove modestly effective on its own, but may have its efficacy enhanced if performed in combination with other therapies, lifestyle modifications, and/or pharmacotherapy. The rebound of weight and ghrelin levels may be due to re-vascularization of the gastric fundus [38, 43]. Another possibility may be a return of appetite not due solely to ghrelin, but recovery of the patient from ischemic injury. Therefore, technical points regarding BAE and studies including a diagnostic digital subtraction angiography (DSA) during follow-up, may need to be designed first to show whether a gastric fundus revascularization exists or not.
Fourth, the ideal embolic agents are uncertain. Several different kinds of embolic agents with various sizes have been used in both animal and clinical studies (Table 5). The GET LEAN and BEAT Obesity trials used 300 to 500 µm embolic agents, while the Chinese trial used 500 to 710 µm embolic agents. In addition, all three trials used different kinds of agents.
Table 5.
Characteristics of Early Clinical Studies
| Author /year |
Sample size |
Nature | Embolicagent | Embolic size (µm) |
Diabetes | Follow -up (months) |
Endoscopy | Primary endpoint |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Gunn AJ/2014 [46] | 19 | R | Coils, gelfoam, PVA | 300–500 | Unknown | 13.6 | Unknown | Weight loss |
| 500–710 | ||||||||
| 710–1000 | ||||||||
|
| ||||||||
| Anton K/2014 [47] | 10 | R | Coils, gelatin sponge pledget suspension | Unknown | Included | 12 | Unknown | Weight loss |
|
| ||||||||
| Kipshidze N/2015 [48] | 5 | P | Bead Block | 300–500 | Unknown | 24 | Day after procedure | Weight loss |
|
| ||||||||
| Syed MI/2016 [49] | 4 | P | Bead Block | 300–500 | Included | 6 | Baseline and 3 days post-procedure | Safety |
|
| ||||||||
| Weiss CR/2017 [50] | 5 | P | Embosphere | 300–500 | Excluded | 3 | Baseline, 2 weeks, and 3 months post-procedure | 30-day AEs |
|
| ||||||||
| Bai ZB/2017 [51] | 5 | P | PVA | 500–710 | Included | 9 | Baseline and 3 days post-procedure | Safety |
R, retrospective; P, prospective; PVA, polyvinyl alcohol; AEs, adverse events
Lastly, the ideal degree of embolization needs to be demonstrated. A previous animal study demonstrated that only animals in which all gastric arteries were embolized showed significant decreases in serum ghrelin levels. Additionally, a lower degree of embolization did not prevent gastric ulceration [42]. The three current trials have demonstrated different targets and endpoints for embolization. GET LEAN targeted all distal branches of the LGA, while BEAT Obesity targeted all arteries supplying the fundus, including the GEA if applicable, and the trial in China embolized only select branches of the LGA. What effect different degrees of embolization have, if any, has not been explored in clinical trials to date.
In conclusion, BAE may be a promising method to treat obese patients via changes in hormonal balance. More and more pre-clinical and clinical studies have been carried out to explore the safety and preliminary efficacy of BAE to treat obesity. Nevertheless, no clinical trial with long-term follow-up and relatively large sample sizes has been reported. Some ongoing prospective trials may fill this gap in the near future. There are still many questions that remain to be answered. Once these key points are addressed, further randomized controlled trials should be performed to explore the efficacy of BAE compared to diet and exercise or sham embolization.
Acknowledgments
None
Funding
Dr. Weiss is funded through NIH/NIBIB R01EB017615, NIH/NIBIB T32EB006351, SIR Foundation (FSDG), Siemens Healthcare, BTG, Merit Medical, and Medtronic. Dr. Zhong receives funding through Fundamental Research Funds for the Central Universities, and the Scientific Research Innovation Program for College and University Graduates of Jiangsu Province (KYZZ16_0133). Funding sources have had no involvement in the financial support for the preparation of this article.
Footnotes
Conflict of interest
Dr. Weiss is a consultant for Medtronic and BTG.
References
- 1.Au N, Johnston DW. Too Much of a Good Thing? Exploring the Impact of Wealth on Weight. Health economics. 2015;24(11):1403–21. doi: 10.1002/hec.3094. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. Jama. 2003;289(1):76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 3.Bays HE, Toth PP, Kris-Etherton PM, Abate N, Aronne LJ, Brown WV, et al. Obesity, adiposity, and dyslipidemia: a consensus statement from the National Lipid Association. Journal of clinical lipidology. 2013;7(4):304–83. doi: 10.1016/j.jacl.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377(9765):557–67. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. Journal of health economics. 2012;31(1):219–30. doi: 10.1016/j.jhealeco.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Jones LR, Wilson CI, Wadden TA. Lifestyle modification in the treatment of obesity: an educational challenge and opportunity. Clinical pharmacology and therapeutics. 2007;81(5):776–9. doi: 10.1038/sj.clpt.6100155. [DOI] [PubMed] [Google Scholar]
- 7.Lowe MR. Self-regulation of energy intake in the prevention and treatment of obesity: is it feasible? Obesity research. 2003;11(Suppl):44S–59S. doi: 10.1038/oby.2003.223. [DOI] [PubMed] [Google Scholar]
- 8.DeMaria EJ. Bariatric surgery for morbid obesity. The New England journal of medicine. 2007;356(21):2176–83. doi: 10.1056/NEJMct067019. [DOI] [PubMed] [Google Scholar]
- 9.Jung Y. Role of Endoscopic Gastroplasty Techniques in the Management of Obesity. Clinical endoscopy. 2017;50(1):21–5. doi: 10.5946/ce.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vix M, Liu KH, Diana M, D'Urso A, Mutter D, Marescaux J. Impact of Roux-en-Y gastric bypass versus sleeve gastrectomy on vitamin D metabolism: short-term results from a prospective randomized clinical trial. Surgical endoscopy. 2014;28(3):821–6. doi: 10.1007/s00464-013-3276-x. [DOI] [PubMed] [Google Scholar]
- 11.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obesity research. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 12.Montesi L, El Ghoch M, Brodosi L, Calugi S, Marchesini G, Dalle Grave R. Long-term weight loss maintenance for obesity: a multidisciplinary approach. Diabetes, metabolic syndrome and obesity : targets and therapy. 2016;9:37–46. doi: 10.2147/DMSO.S89836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obesity research. 2004;12(Suppl):151S–62S. doi: 10.1038/oby.2004.282. [DOI] [PubMed] [Google Scholar]
- 14.NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Annals of internal medicine. 1991;115(12):956–61. [PubMed] [Google Scholar]
- 15.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, et al. Bariatric surgery versus intensive medical therapy for diabetes-3-year outcomes. The New England journal of medicine. 2014;370(21):2002–13. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dumon KR, Murayama KM. Bariatric surgery outcomes. The Surgical clinics of North America. 2011;91(6):1313–38. doi: 10.1016/j.suc.2011.08.014. x. [DOI] [PubMed] [Google Scholar]
- 17.Encinosa WE, Bernard DM, Chen CC, Steiner CA. Healthcare utilization and outcomes after bariatric surgery. Medical care. 2006;44(8):706–12. doi: 10.1097/01.mlr.0000220833.89050.ed. [DOI] [PubMed] [Google Scholar]
- 18.Keating C, Neovius M, Sjoholm K, Peltonen M, Narbro K, Eriksson JK, et al. Health-care costs over 15 years after bariatric surgery for patients with different baseline glucose status: results from the Swedish Obese Subjects study. The lancet Diabetes & endocrinology. 2015;3(11):855–65. doi: 10.1016/S2213-8587(15)00290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiner JP, Goodwin SM, Chang HY, Bolen SD, Richards TM, Johns RA, et al. Impact of bariatric surgery on health care costs of obese persons: a 6-year follow-up of surgical and comparison cohorts using health plan data. JAMA surgery. 2013;148(6):555–62. doi: 10.1001/jamasurg.2013.1504. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava G, Apovian CM. Current pharmacotherapy for obesity. Nature reviews Endocrinology. 2017 doi: 10.1038/nrendo.2017.122. [DOI] [PubMed] [Google Scholar]
- 21.Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, et al. Pharmacological management of obesity: an endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2015;100(2):342–62. doi: 10.1210/jc.2014-3415. [DOI] [PubMed] [Google Scholar]
- 22.Maahs D, de Serna DG, Kolotkin RL, Ralston S, Sandate J, Qualls C, et al. Randomized, double-blind, placebo-controlled trial of orlistat for weight loss in adolescents. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2006;12(1):18–28. doi: 10.4158/EP.12.1.18. [DOI] [PubMed] [Google Scholar]
- 23.Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. The New England journal of medicine. 2010;363(3):245–56. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 24.Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341–52. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 25.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. The New England journal of medicine. 2002;346(21):1623–30. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 26.Verdam FJ, Schouten R, Greve JW, Koek GH, Bouvy ND. An update on less invasive and endoscopic techniques mimicking the effect of bariatric surgery. Journal of obesity. 2012;2012:597871. doi: 10.1155/2012/597871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abu Dayyeh BK, Edmundowicz S, Thompson CC. Clinical Practice Update: Expert Review on Endoscopic Bariatric Therapies. Gastroenterology. 2017;152(4):716–29. doi: 10.1053/j.gastro.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 28.Neylan CJ, Dempsey DT, Tewksbury CM, Williams NN, Dumon KR. Endoscopic treatments of obesity: a comprehensive review. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2016;12(5):1108–15. doi: 10.1016/j.soard.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Kumar N, Thompson CC. Endoscopic solutions for weight loss. Current opinion in gastroenterology. 2011;27(5):407–11. doi: 10.1097/MOG.0b013e328349e240. [DOI] [PubMed] [Google Scholar]
- 30.Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132(6):2116–30. doi: 10.1053/j.gastro.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 31.Venieratos D, Panagouli E, Lolis E, Tsaraklis A, Skandalakis P. A morphometric study of the celiac trunk and review of the literature. Clinical anatomy. 2013;26(6):741–50. doi: 10.1002/ca.22136. [DOI] [PubMed] [Google Scholar]
- 32.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 33.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. The Journal of clinical investigation. 2007;117(1):13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–9. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 35.English PJ, Ghatei MA, Malik IA, Bloom SR, Wilding JP. Food fails to suppress ghrelin levels in obese humans. The Journal of clinical endocrinology and metabolism. 2002;87(6):2984. doi: 10.1210/jcem.87.6.8738. [DOI] [PubMed] [Google Scholar]
- 36.Ravussin Y, Leibel RL, Ferrante AW., Jr A missing link in body weight homeostasis: the catabolic signal of the overfed state. Cell metabolism. 2014;20(4):565–72. doi: 10.1016/j.cmet.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arepally A, Barnett BP, Montgomery E, Patel TH. Catheter-directed gastric artery chemical embolization for modulation of systemic ghrelin levels in a porcine model: initial experience. Radiology. 2007;244(1):138–43. doi: 10.1148/radiol.2441060790. [DOI] [PubMed] [Google Scholar]
- 38.Arepally A, Barnett BP, Patel TH, Howland V, Boston RC, Kraitchman DL, et al. Catheter-directed gastric artery chemical embolization suppresses systemic ghrelin levels in porcine model. Radiology. 2008;249(1):127–33. doi: 10.1148/radiol.2491071232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bawudun D, Xing Y, Liu WY, Huang YJ, Ren WX, Ma M, et al. Ghrelin suppression and fat loss after left gastric artery embolization in canine model. Cardiovascular and interventional radiology. 2012;35(6):1460–6. doi: 10.1007/s00270-012-0362-8. [DOI] [PubMed] [Google Scholar]
- 40.Paxton BE, Kim CY, Alley CL, Crow JH, Balmadrid B, Keith CG, et al. Bariatric embolization for suppression of the hunger hormone ghrelin in a porcine model. Radiology. 2013;266(2):471–9. doi: 10.1148/radiol.12120242. [DOI] [PubMed] [Google Scholar]
- 41.Paxton BE, Alley CL, Crow JH, Burchette J, Weiss CR, Kraitchman DL, et al. Histopathologic and immunohistochemical sequelae of bariatric embolization in a porcine model. Journal of vascular and interventional radiology : JVIR. 2014;25(3):455–61. doi: 10.1016/j.jvir.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paxton BE, Arepally A, Alley CL, Kim CY. Bariatric Embolization: Pilot Study on the Impact of Gastroprotective Agents and Arterial Distribution on Ulceration Risk and Efficacy in a Porcine Model. Journal of vascular and interventional radiology : JVIR. 2016;27(12):1923–8. doi: 10.1016/j.jvir.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 43.Kim JM, Kim MD, Han K, Muqmiroh L, Kim SU, Kim GM, et al. Bariatric Arterial Embolization with Non-spherical Polyvinyl Alcohol Particles for Ghrelin Suppression in a Swine Model. Cardiovascular and interventional radiology. 2017;40(5):744–9. doi: 10.1007/s00270-017-1600-x. [DOI] [PubMed] [Google Scholar]
- 44.Pasciak AS, Bourgeois AC, Paxton BE, Nodit L, Coan PN, Kraitchman D, et al. Bariatric Radioembolization: A Pilot Study on Technical Feasibility and Safety in a Porcine Model. Journal of vascular and interventional radiology : JVIR. 2016;27(10):1509–17. doi: 10.1016/j.jvir.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 45.Diana M, Pop R, Beaujeux R, Dallemagne B, Halvax P, Schlagowski I, et al. Embolization of arterial gastric supply in obesity (EMBARGO): an endovascular approach in the management of morbid obesity. proof of the concept in the porcine model. Obesity surgery. 2015;25(3):550–8. doi: 10.1007/s11695-014-1535-0. [DOI] [PubMed] [Google Scholar]
- 46.Gunn AJ, Oklu R. A preliminary observation of weight loss following left gastric artery embolization in humans. Journal of obesity. 2014;2014:185349. doi: 10.1155/2014/185349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anton K, Rahman T, Bhanushali A, Nadal L, Pierce G, Patel A. Weight loss following left gastric artery embolization in a human population without malignancy: a retrospective review. Journal of Obesity & Weight Loss Therapy. 2015;5(6) [Google Scholar]
- 48.Kipshidze N, Archvadze A, Bertog S, Leon MB, Sievert H. Endovascular Bariatrics: First in Humans Study of Gastric Artery Embolization for Weight Loss. JACC Cardiovascular interventions. 2015;8(12):1641–4. doi: 10.1016/j.jcin.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 49.Syed MI, Morar K, Shaikh A, Craig P, Khan O, Patel S, et al. Gastric Artery Embolization Trial for the Lessening of Appetite Nonsurgically (GET LEAN): Six-Month Preliminary Data. Journal of vascular and interventional radiology : JVIR. 2016;27(10):1502–8. doi: 10.1016/j.jvir.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 50.Weiss CR, Akinwande O, Paudel K, Cheskin LJ, Holly B, Hong K, et al. Clinical Safety of Bariatric Arterial Embolization: Preliminary Results of the BEAT Obesity Trial. Radiology. 2017;283(2):598–608. doi: 10.1148/radiol.2016160914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bai ZB, Qin YL, Deng G, Zhao GF, Zhong BY, Teng GJ. Bariatric Embolization of the Left Gastric Arteries for the Treatment of Obesity: 9-Month Data in 5 Patients. Obesity surgery. 2018;28(4):907–15. doi: 10.1007/s11695-017-2979-9. [DOI] [PubMed] [Google Scholar]
- 52.Bayham BE, Bellanger DE, Hargroder AG, Johnson WD, Greenway FL. Racial differences in weight loss, payment method, and complications following Roux-en-Y gastric bypass and sleeve gastrectomy. Advances in therapy. 2012;29(11):970–8. doi: 10.1007/s12325-012-0062-4. [DOI] [PubMed] [Google Scholar]
- 53.Shoar S, Saber AA, Aladdin M, Bashah MM, AlKuwari MJ, Rizwan M, et al. Bariatric manipulation of gastric arteries: A systematic review on the potential concept for treatment of obesity. International journal of surgery. 2016;36(Pt A):177–82. doi: 10.1016/j.ijsu.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 54.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. Jama. 2004;292(14):1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]