Abstract
TNF is a multifunctional cytokine that is critical to host defense against pathogens but can also drive the pathophysiology of inflammatory diseases. Inhibition of TNF occasionally causes exacerbation of some autoimmune diseases, suggesting a role for TNF in the regulation of immune homeostasis. Here, we demonstrate that human peripheral blood CD4+CD25+Foxp3+ regulatory T cells (Tregs) express membrane- bound TNF, a potent activator of the type 2 TNF receptor. While the type 1 TNF receptor can cause cell death and is expressed ubiquitously, the type 2 receptor promotes cell growth and its expression is limited mainly to immune and endothelial cells. When autocrine TNF is blocked in an in vitro culture without IL-2, activated Tregs stop proliferating. These data indicate a novel role for TNF as a Treg-derived autocrine growth factor.
Keywords: TNF, Treg, Foxp3, IL-2
Graphical abstract
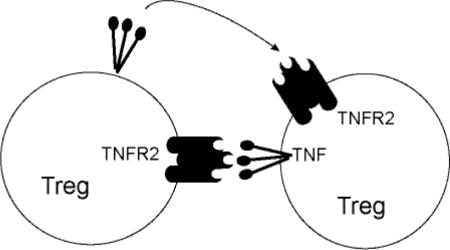
1. Introduction
Tumor Necrosis Factor (TNF, previously called TNF-α) is a multi-functional cytokine that plays a protective role in defense against microbes, but also exacerbates inflammation [1]. Clinical studies show that inhibition of TNF is a highly effective treatment for many inflammatory diseases [2]. In particular, neutralizing antibodies against TNF or TNF receptors greatly improve clinical outcomes in patients with rheumatoid arthritis and inflammatory bowel disease [3, 4].
While the anti-inflammatory effect of TNF inhibition is well established, TNF inhibition fails to ameliorate other autoimmune disorders such as multiple sclerosis and vitiligo [5, 6]. Moreover, inhibition of TNF causes adverse effects in some patients and even exacerbates autoimmune disorders [7]. Similarly, in TNF knockout mice, loss of TNF increases the extent of autoimmune disease rather than improving symptoms [8, 9]. A critical question is: how can decreased levels of TNF exacerbate, instead of ameliorate, inflammatory processes such as autoimmunity?
Contradictory data from different experimental and clinical settings suggest that TNF plays both immunosuppressive and immune-enhancing roles depending on the cell and receptor types stimulated by TNF. There are two types of receptors for TNF: TNFR1 and TNFR2. TNFR1 contains a death domain in the cytoplasmic region and can induce apoptosis when activated [1]. TNFR2 lacks the death domain and instead transduces signaling for cell proliferation. Previous reports suggest that TNFR2 plays protective roles in autoimmune disorders [10]. While many types of cells express TNFR1, expression of TNFR2 is limited mostly to endothelial and lymphoid cells [11]. Tregs express TNFR2 and require TNFR2 for functional stability [12]. Importantly, TNFR2 antagonists block proliferation of Tregs, but not effector T cells in vitro [13] and in vivo [14]. Moreover, TNF promotes proliferation of regulatory T cells [15] and aids in Treg differentiation by blocking differentiation of Th17 cells [16].
These data suggest that TNF supports the functions and/or homeostasis of Tregs via TNFR2. If so, then the manner in which Tregs receive TNFR2 signaling is an important component of immune regulation. TNF is initially expressed as a transmembrane protein and becomes soluble after proteolysis [17]. While soluble TNF (sTNF) activates both TNFR1 and TNFR2, membrane-bound TNF (mTNF) more potently activates TNFR2 [18]. Because of the significance of TNFR2 signaling in immune regulation and Treg proliferation, we investigated cell types that express mTNF in human PBMCs. A previous report demonstrated that mTNF on monocytes can activate Tregs through binding TNFR2 on Tregs [19]. In this study, we found that CD4+CD25+Foxp3+ regulatory T cells themselves express membrane-bound TNF. Additionally, our data show that TNF produced by Tregs plays a role in Treg proliferation when IL-2, a growth factor for Tregs, is limited.
2. Materials and Methods
2.1 Mononuclear Cell Isolation and Cell purification
Adult PBMCs were collected from healthy donors in heparin solution or sodium citrate, and mononuclear cells were isolated by density gradient centrifugation using Lymphocyte Separation Medium (Corning). Red blood cells were lysed with ACK lysis buffer (Gibco). Adult Tregs (CD4+CD127lowCD25+) and CD4+CD25− conventional T cells were enriched using the EasySep Human Regulatory T Cell Isolation Kit (Stem Cell Technologies).
2.2 Flow Cytometry
Antibodies used for flow cytometry experiments were anti-CD4-FITC or Pacific Blue (OKT4), CD25-APC/Fire 750 or Brilliant Violet 421 (M-A251), CD14 Pacific Blue (HCD14), CCR4-PE/cy7 (L291H4), CCR6-PerCP/cy5.5 (G034E3), Foxp3 AlexaFluor 647 (259D), TNF-α PE (Mab11), and IFN-γ AlexaFluor 488 (4S.B3) (Biolegend). Anti- CD127 conjugated to PE-cy5 (eBioRDR5) was purchased from eBioscience. Cells were blocked prior to surface staining with Human TruStain FcX blocking solution (Biolegend). After surface staining cells were fixed and permeabilized using the True Nuclear Transcription Factor Buffer set (Biolegend). Data were collected on FACS Canto II and LSR Fortessa (BD Biosciences) and analyzed by Flowjo v.10 software (Tree Star, Inc.).
2.3 Cell Stimulation
Cells were stimulated using PMA (50 ng/mL; Fisher Bioreagents) and ionomycin (1 μM; Sigma-Aldrich) in the presence of monensin (1 μM, Biolegend) for 4 hours at 37°C in 5% CO2. Cells were stimulated in RPMI 1640 (HyClone) supplemented with 10% fetal bovine serum (FBS). For surface TNF analysis, stimulation was performed without monensin. For antibody-based stimulation, Tregs and conventional T cells were stimulated for 16 hours by anti-CD3 (OKT3) and anti-CD28 (CD28.2) antibodies (Biolegend) coated on a 96 well non-tissue culture-treated plate, with monensin added for the final 4 hours.
2.4 Treg Proliferation Assay
Adult Tregs were isolated by magnetic sorting as previously described. Cells were labeled with CFSE according to manufacturer protocols (Biolegend) and 100,000 cells were added to each well of a 96-well non-tissue culture-treated plate coated with anti- CD3 (OKT3, Biolegend). Costimulation was provided by 5 μg/mL LEAF anti-CD28 (CD28.2, Biolegend). IL-2 (10 ng/mL; PeproTech) was provided to control wells only and all other conditions were treated with 2 μg/mL LEAF grade anti-IL-2 antibody (MQ1- 17H12, Biolegend). LEAF grade anti-TNF (MAb11) or isotype control LEAF mIgG1 (25 μg/mL) were added as indicated (Biolegend). After 5 days, live cells were counted in triplicate in 0.1% trypan blue solution in PBS. Average cell number was calculated from triplicate values and divided by cell number of untreated control to generate plotted data.
2.5 Statistical Analysis
Graphs were generated using GraphPad Prism software and analyzed by paired, two- tailed t-test unless otherwise specified. Figure 2A was analyzed by one-way ANOVA with Dunnett’s multiple comparisons test. The following designation was used: *p<0.05, **p<0.01, ***p<0.001. Error bars represent standard error of the mean unless otherwise specified.
Fig.2. Effect of TNF inhibition on Treg proliferation.
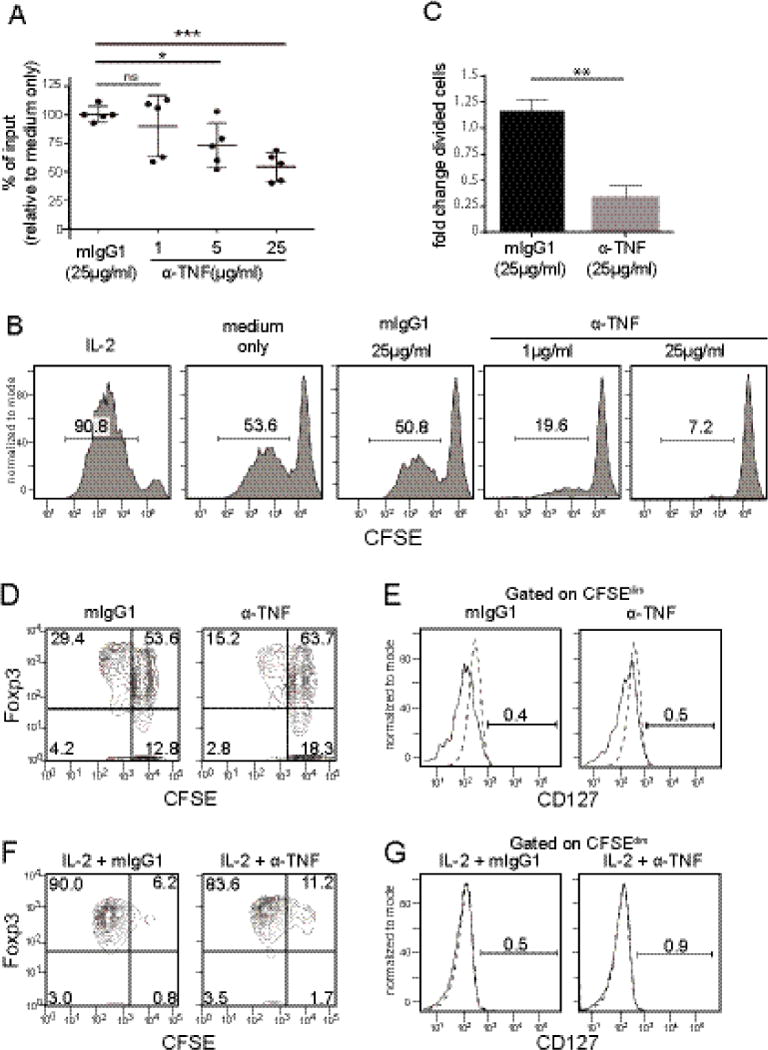
Tregs isolated from peripheral blood were labeled with CFSE and stimulated by plate- bound anti-CD3 and soluble anti-CD28 in the presence of anti-TNF or isotype control antibody (mIgG1) for 5 days. (A) Proliferation of Tregs in response to TNF inhibition. All conditions were cultured with anti-IL-2. Percent input was calculated by counting cell number in comparison to untreated control. Bars represent mean and standard deviation. Statistical analysis by one-way ANOVA with Dunnett’s multiple comparisons test (*p=0.011, ***p=0.0002). (B) Proliferation of Tregs measured by CFSE. Cells were treated with the addition of factors indicated above each panel. All conditions received anti-IL2 Ab except for the control with exogenous IL-2 added. Representative data from 4 donors. (C) Fold change of divided cells (CFSEdim) in response to TNF inhibition. Fold change calculated by percent divided cells in treated sample/untreated control (n=4) and analyzed by paired t-test; p=0.0066. (D) Proliferation of Tregs. After 5 days of treatment with isotype control (mIgG1) or anti-TNF (25 μg/mL), CFSE-labeled cells were stained for Foxp3 then analyzed by flow cytometry. Data are representative of two independent experiments (n=2). (E) Expression of CD127 among CFSEdim cells as gated in 2D. Dashed lines indicate isotype control (n=2) (F) Proliferation of Tregs with exogenous IL-2 and isotype control (mIgG1) or anti-TNF (25 μg/mL). After 5 days CFSE labeled cells were stained for Foxp3 and analyzed by flow cytometry. Data are representative of two independent experiments (n=2). (G) Expression of CD127 among CFSEdim cells as gated in 2F (n=2).
3. Results and Discussion
Activated monocytes/macrophages and T cells are considered to be the major producers of TNF [20]. TNF is expressed as a type II transmembrane protein and must be cleaved by proteolysis to produce the soluble form [21]. Both the membrane and soluble forms of TNF are active and can signal via TNF receptors, however, each form exhibits specific activity. Soluble TNF signals more readily via TNFR1, while mTNF has a greater affinity for TNFR2 [18]. TNFR1 contains an intracellular death domain and signals for cell cycle arrest and apoptosis, while TNFR2 lacks a death domain and promotes growth and proliferation. Human Foxp3+ Tregs express TNFR2 but not TNFR1 [12]. Due to the different effects mediated by the two different forms of TNF, we investigated cells in adult peripheral blood that express mTNF.
As previously reported, we detected mTNF staining on stimulated CD14+monocytes (sFig1A) [19]. In addition to monocytes, we found that a majority of activated, but not resting, peripheral blood CD4+CD25+CD127lowFoxp3+ Tregs express mTNF (Fig.1A, B). A previous study has shown mTNF expression on CD4+25+ T cells in patients with rheumatoid arthritis [22]. Our data show that healthy donor Tregs also express mTNF when activated. Only a small percentage of CD4+25−Foxp3− conventional T cells express mTNF at low levels compared to Tregs(Fig.1A, B). Intracellular TNF (total TNF, tTNF) in conventional T cells was present at comparable levels to activated Tregs, suggesting that conventional T cells release membrane TNF more efficiently than Tregs (Fig.1C, D). As a control, we examined the expression of another pro-inflammatory cytokine, IFN-γ, by Tregs. Unlike conventional Foxp3− T cells, Foxp3+ Tregs did not produce IFN-γ (Fig.1 C, D). Stimulation by anti-CD3 and anti- CD28 Abs also induces expression of TNF by Foxp3+ Tregs (sFig.1B). These data demonstrate that stimulated human Foxp3+CD4+ Tregs produce TNF.
Fig.1. Expression of TNF by human Foxp3+ Tregs.
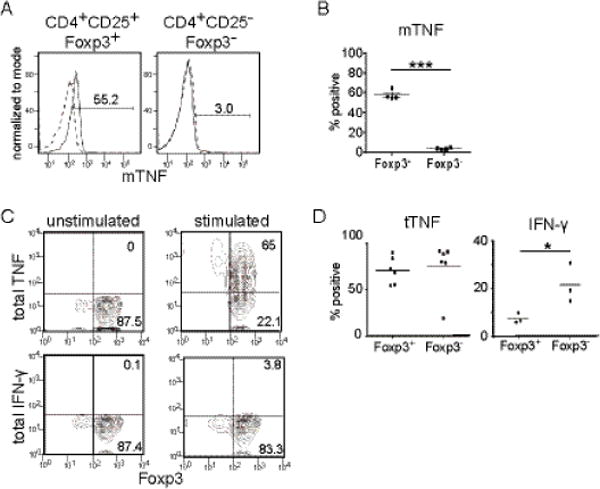
Adult peripheral blood Tregs and conventional T cells were stimulated by PMA and ionomycin for 4 hrs in the presence or absence of monensin (details described in materials and methods). The expression of cytokines was determined by intracellular staining or surface staining and analyzed by flow cytometry. (A) Expression of the membrane form of TNF (mTNF) by CD4+CD25+Foxp3+ (left) and CD4+CD25−Foxp3− T cells (right). Representative data from 4 donors. Dashed lines indicate unstimulated samples and solid lines indicate stimulated samples (B) Percentage of CD4+CD25+Foxp3+ (Foxp3+) and CD4+CD25−Foxp3− (Foxp3−) cells staining positive for mTNF. Paired t-test (n=4) p=0.0001. (C) Expression of total TNF (tTNF) (upper panels) and IFN-γ (lower panels) by unstimulated (left panels) or stimulated (right panels) Foxp3+ Tregs by intracellular staining. Representative data set from three samples. (D) Frequencies of tTNF (left) or IFN-γ (right) expressing cells among CD4+CD25+Foxp3+ (Foxp3+) cells and CD4+CD25−Foxp3− (Foxp3−) cells after stimulation. Student’s t-test; tTNF (n=6) p=0.758, IFN- γ (n=3) p=0.043.
We next addressed the function of TNF produced by Tregs. Since previous work by other groups indicates that TNF can play immunoregulatory roles, we hypothesized that TNF may be an autocrine growth factor for Tregs. It is well known that IL-2 plays a pivotal role in the maintenance of Foxp3+ Tregs. Mice that lack IL-2 or IL-2 receptors (α or β chain) show severe lymphoproliferative disorders and eventually develop lethal autoimmune diseases such as hemolytic anemia and IBDs [23, 24]. However, the symptoms of these mice are less severe compared to Foxp3 deficient mice (e.g. scurfy mice or Foxp3 KO mice). Indeed, there is a small fraction of Foxp3+ T cells present in the periphery of IL-2 or IL-2 receptor KO mice [25]. These data suggest that there are factors other than IL-2 that can support growth of Tregs. Based on these data, we hypothesized that TNF may provide a growth signal for Tregs in conditions where IL-2 availability is limited.
To test this hypothesis, we cultured purified Tregs without the addition of exogenous IL-2 (Fig.2). Treg purity after magnetic sorting was consistently >96% CD4+, and >90% of CD4+ cells were CD25+Foxp3+ (sFig2). An anti-IL-2 neutralizing antibody was also added to the purified Tregs to limit IL-2 signaling. In the absence of IL-2, Treg proliferation was limited and the cell number was maintained at the input level after 5 days of culture. When we added an anti-TNF neutralizing antibody, the cell number decreased in a dose-dependent manner (Fig.2A). The decrease in cell number is due to reduced proliferation of Foxp3+ Tregs as indicated by a reduction in CFSEdim cells in samples treated with anti-TNF Ab (Fig.2B). Tregs treated with anti-TNF antibody showed a significant decrease in cell division (Fig. 2C). The proliferating CFSEdim cells maintain the prototypic Treg phenotype of Foxp3+CD127low in the presence or absence of anti-TNF (Fig.2D-E). Proliferation of Tregs was minimally affected by anti-TNF when recombinant IL-2 was provided in the culture (Fig.2F), suggesting that TNF provides a growth signal for Tregs when IL-2 is limited. IL-2-treated Tregs proliferated and maintained high expression of Foxp3 and low expression of CD127 (Fig.2F-G).
Maintenance of Tregs is critical for homeostasis of the immune system. While activated T cells make IL-2, the availability of IL-2 in normal tissues is limited [26]. Our previous work using Cre knock-in of the IL-2 gene in mice also showed that, under healthy conditions, the cells that produce/produced IL-2 primarily exist in the gut mucosa-associated secondary lymphoid organs [27]. Therefore, tissue-resident Tregs may require factors other than IL-2 for growth, and Treg-derived autocrine TNF may be one of these growth factors.
If TNF is important for Tregs to grow in non-lymphoid tissues where IL-2 is limited, then we expected that these Tregs express chemokine receptors that promote their localization to non-lymphoid tissues. Recent reports suggest that human Tregs can be divided into subsets based on surface chemokine receptors [28]. Chemokine receptors can control localization of Tregs to suppress inflammation in a spatially controlled manner [29]. We found that TNF-producing Foxp3+ cells were predominantly CCR4+CCR6+ (Fig.3A, B, C), a phenotype previously considered to be Th17-like Tregs [30]. We also tested whether mTNF+Foxp3+ cells exhibited a similar phenotype. Significantly more mTNF+Foxp3+ Tregs co-express CCR4 and CCR6 compared to mTNF+Foxp3− conventional T cells(Fig.3D-E). We also observed a small population of mTNF+Foxp3− conventional T cells that were CCR4+CCR6+, suggesting that these mTNF+ conventional T cells may also impact Tregs in the same microenvironment. CCR4 ligands (CCL17, CCL22) are highly expressed in the thymus and lung while CCR6 ligands (CXCL9,10, and 11) are high in the liver and lung [31]. These results indicate that Foxp3+ TNF-producing cells have the potential to localize to tissues differently than Foxp3− conventional TNF-producing T cells. Additionally, these results suggest that TNF+ Tregs may have the potential to localize to non-lymphoid tissues.
Fig.3. Chemokine receptor expression by TNF producing Tregs.
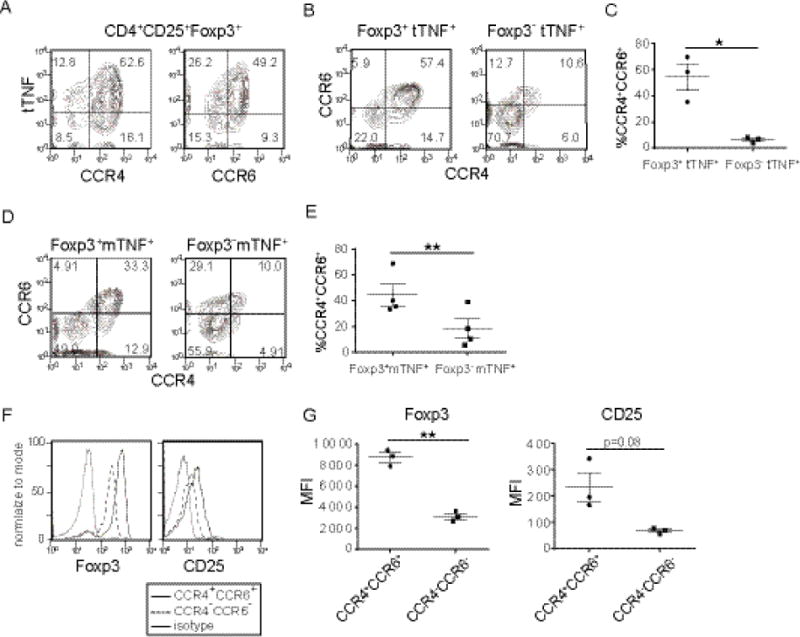
Tregs or conventional T cells from peripheral blood of healthy donors were stimulated and chemokine receptor expression was analyzed by flow cytometry. (A) Expression of chemokine receptors and tTNF by CD4+CD25+Foxp3+ cells. Tregs or conventional T cells were stimulated as in Fig.1 and analyzed for expression of total TNF, CCR4 and CCR6. A representative data set from three donors. (B) Expression of CCR4 and CCR6 by tTNF+CD4+CD25+Foxp3+ Tregs (left) or by tTNF+CD4+CD25−Foxp3− conventional T cells (right). (C) Percent of cells staining positive for CCR4 and CCR6 among CD4+CD25+Foxp3+tTNF+ (left) and CD4+CD25−Foxp3−tTNF+ (right) cells from three donors; paired t-test p=0.0339. (D) Expression of CCR4 and CCR6 by mTNF+CD4+CD25+Foxp3+ Tregs (left) or by mTNF+CD4+CD25−Foxp3− conventional T cells (right). Data are representative of three independent experiments (n=4). (E) Percent of cells staining positive for CCR4 and CCR6 among mTNF+CD4+CD25+Foxp3+ Tregs (left) or by mTNF+CD4+CD25−Foxp3− conventional T cells (right). Data from three independent experiments (n=4); paired t-test, p=0.0063(F) Expression of Foxp3 and CD25 by tTNF+CCR4+CCR6+ Tregs (solid lines) or by tTNF+CCR4−CCR6− Tregs (dashed lines). Dotted lines represent isotype control. Representative data from three donors. (G) Mean fluorescence intensity of Foxp3 (left) and CD25 (right) among tTNF+CD4+CCR4+CCR6+ and tTNF+CD4+CCR4−CCR6− Tregs from three donors. Paired t-test; Foxp3 p=0.0043; CD25 p=0.0807.
Some TNF-producing Foxp3+ cells were CCR4−CCR6−, and these cells differed in expression levels of Foxp3 and CD25 (Fig.3F-G). TNF+CCR4+CCR6+ Tregs express higher levels of Foxp3 and CD25 than TNF+CCR4−CCR6− Tregs. This CCR4+Foxp3hiCD25hi phenotype was also previously noted for TNFR2+ Tregs which display more potent suppressive activity compared to TNFR2− Tregs [32]. These data also agree with a previous report showing that exogenously added TNF increases the suppressive function of Foxp3+ Tregs [33].
Together these data demonstrate that activated CD4+Foxp3+ Tregs are a source of mTNF in human peripheral blood. As Tregs express TNFR2 but not TNFR1, mTNF expressed by Tregs may stimulate Tregs in an autocrine manner via TNFR2 and promote their growth and/or function. It should also be noted that mTNF can function as a receptor and transduce signals into mTNF-expressing cells and induce TGF-β expression [34]. An intriguing but untested possibility is that mTNF-expressing Tregs could communicate with TNFR1/TNFR2 expressing cells and receive yet undetermined signals to change their functions and/or growth.
Tregs may also impact other types of cells via TNF. Clinical studies show that patients with neurodegenerative disorders such as MS experienced exacerbation of symptoms following TNF inhibition [5, 7]. Some neuronal cells such as microglia and oligodendrocytes express TNFR2 [35]. TNFR2 expression by non-lymphoid cells was required for Treg functions in a mouse model of MS, experimental autoimmune encephalomyelitis (EAE) [36]. TNF promoted re-myelination by activating oligodendrocytes via TNFR2 [37] and a recent report showed that expression of TNFR2 by microglia but not by macrophages is required for effective neuronal protection in EAE [38]. An upcoming challenge will be to determine if TNF from Tregs functions in a paracrine manner during interactions with non-lymphoid cells to control local immune responses and tissue homeostasis.
Supplementary Material
Supplementary Fig.1 Expression of TNF by stimulated monocytes and anti- CD3/CD28 Ab stimulated Tregs
(A) Expression of mTNF by stimulated monocytes. Total adult PBMCs were stimulated with PMA and ionomycin for 4 hours and stained for mTNF. Representative histogram of mTNF expression gated on CD14+ cells. Unstimulated sample is shown with dashed line, and stimulated sample with the solid line (n=2). (B) CD4+CD127lowCD25+ Tregs and CD4+CD25− conventional T cells were enriched by magnetic bead sorting and stimulated by plate-bound anti-CD3/CD28 Abs for 16 hours, then treated with monensin for 4 hours. Cells were harvested, fixed, permeabilized and stained for Foxp3 and TNF. Representative data from three donors.
Supplemental Fig.2 Purity of Tregs enriched from peripheral blood mononuclear cells
Frequency of Tregs after enrichment from PBMCs. After enrichment by magnetic sorting (see methods) cells were stained for CD4, CD25, and Foxp3 and purity of Tregs assessed by flow cytometry. Gating on CD4+ cells (left) followed by expression of Foxp3 and CD25 by CD4+ cells (right) based on isotype controls. A representative sample from 5 donors.
Highlight.
Human Foxp3+ regulatory T cells produce membrane bound TNF.
Autocrine TNF supports growth of Tregs in the absence of IL-2.
TNF-producing Foxp3+ cells are predominantly CCR4+CCR6+.
Acknowledgments
The authors thank Drs. Katherine Knight and Caroline Le Poolefor critical reading of the manuscript. This work was supported by NIH RO1AI100129 and T32 AI007508.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests Disclosure: The authors declare no competing financial interests.
References
- 1.Brenner D, Blaser H, Mak TW. Regulation of tumour necrosis factor signalling: live or let die, Nature reviews. Immunology. 2015;15(6):362–74. doi: 10.1038/nri3834. [DOI] [PubMed] [Google Scholar]
- 2.Monaco C, Nanchahal J, Taylor P, Feldmann M. Anti-TNF therapy: past, present and future. International immunology. 2015;27(1):55–62. doi: 10.1093/intimm/dxu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elliott MJ, Maini RN, Feldmann M, Kalden JR, Antoni C, Smolen JS, Leeb B, Breedveld FC, Macfarlane JD, Bijl JA, Woody JN. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor α (cA2) versus placebo in rheumatoid arthritis. The Lancet. 1994;344(8930):1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- 4.Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362(15):1383–95. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 5.Toussirot E, Aubin F. Paradoxical reactions under TNF-alpha blocking agents and other biological agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD open. 2016;2(2):e000239. doi: 10.1136/rmdopen-2015-000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kollias G, Douni E, Kassiotis G, Kontoyiannis D. On the role of tumor necrosis factor and receptors in models of multiorgan failure, rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease. Immunol Rev. 1999;169:175–94. doi: 10.1111/j.1600-065x.1999.tb01315.x. [DOI] [PubMed] [Google Scholar]
- 7.Wendling D, Prati C. Paradoxical effects of anti-TNF-alpha agents in inflammatory diseases. Expert Rev Clin Immunol. 2014;10(1):159–69. doi: 10.1586/1744666X.2014.866038. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Oppenheim JJ. Contrasting effects of TNF and anti-TNF on the activation of effector T cells and regulatory T cells in autoimmunity. FEBS Lett. 2011;585(23):3611–8. doi: 10.1016/j.febslet.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Marino MW, Wong G, Grail D, Dunn A, Bettadapura J, Slavin AJ, Old L, Bernard CC. TNF is a potent anti-inflammatory cytokine in autoimmune-mediated demyelination. Nat Med. 1998;4(1):78–83. doi: 10.1038/nm0198-078. [DOI] [PubMed] [Google Scholar]
- 10.Faustman DL, Davis M. TNF Receptor 2 and Disease: Autoimmunity and Regenerative Medicine. Frontiers in immunology. 2013;4:478. doi: 10.3389/fimmu.2013.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walczak H. TNF and ubiquitin at the crossroads of gene activation, cell death, inflammation, and cancer. Immunological reviews. 2011;244(1):9–28. doi: 10.1111/j.1600-065X.2011.01066.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Wu X, Zhou Q, Howard OM, Netea MG, Oppenheim JJ. TNFR2 is critical for the stabilization of the CD4+Foxp3+ regulatory T. cell phenotype in the inflammatory environment. J Immunol. 2013;190(3):1076–84. doi: 10.4049/jimmunol.1202659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Baumel M, Mannel DN, Howard OM, Oppenheim JJ. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. Journal of immunology (Baltimore, Md.: 1950) 2007;179(1):154–61. doi: 10.4049/jimmunol.179.1.154. [DOI] [PubMed] [Google Scholar]
- 14.Torrey H, Butterworth J, Mera T, Okubo Y, Wang L, Baum D, Defusco A, Plager S, Warden S, Huang D, Vanamee E, Foster R, Faustman DL. Targeting TNFR2 with antagonistic antibodies inhibits proliferation of ovarian cancer cells and tumor-associated Tregs. Sci Signal. 2017;10(462) doi: 10.1126/scisignal.aaf8608. [DOI] [PubMed] [Google Scholar]
- 15.Myers L, Joedicke JJ, Carmody AB, Messer RJ, Kassiotis G, Dudley JP, Dittmer U, Hasenkrug KJ. IL-2-independent and TNF-alpha-dependent expansion of Vbeta5+ natural regulatory T cells during retrovirus infection. Journal of immunology. 2013;190(11):5485–95. doi: 10.4049/jimmunol.1202951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller PG, Bonn MB, McKarns SC. Transmembrane TNF-TNFR2 Impairs Th17 Differentiation by Promoting Il2 Expression. Journal of immunology. 2015;195(6):2633–47. doi: 10.4049/jimmunol.1500286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horiuchi T, Mitoma H, Harashima S, Tsukamoto H, Shimoda T. Rheumatology. 7. Vol. 49. Oxford; England: 2010. Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents; pp. 1215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G, Pfizenmaier K, Scheurich P. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83(5):793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen DX, Ehrenstein MR. Anti-TNF drives regulatory T cell expansion by paradoxically promoting membrane TNF-TNF-RII binding in rheumatoid arthritis. The Journal of experimental medicine. 2016;213(7):1241–53. doi: 10.1084/jem.20151255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beutler B, Cerami A. The biology of cachectin/TNF−a primary mediator of the host response. Annual review of immunology. 1989;7:625–55. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- 21.MacEwan DJ. TNF ligands and receptors−a matter of life and death. Br J Pharmacol. 2002;135(4):855–75. doi: 10.1038/sj.bjp.0704549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, van Dongen H, Scherer HU, Huizinga TW, Toes RE. Suppressor activity among CD4+, CD25++ T cells is discriminated by membrane-bound tumor necrosis factor alpha. Arthritis Rheum. 2008;58(6):1609–18. doi: 10.1002/art.23460. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJ, Ohashi PS, Griesser H, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science(New York, N.Y.) 1995;268(5216):1472–6. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 24.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3(4):521–30. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 25.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. The Journal of experimental medicine. 2002;196(6):851–7. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson J, Mochizuki D, Gillis S. T-cell growth factors: interleukin 2. Immunol Today. 1980;1(6):113–7. doi: 10.1016/0167-5699(80)90047-X. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto M, Seki Y, Iwai K, Ko I, Martin A, Tsuji N, Miyagawa S, Love RB, Iwashima M. Ontogeny and localization of the cells produce IL-2 in healthy animals. Cytokine. 2013;61(3):831–41. doi: 10.1016/j.cyto.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mailloux AW, Young MR. Regulatory T-cell trafficking: from thymic development to tumor-induced immune suppression. Crit Rev Immunol. 2010;30(5):435–47. doi: 10.1615/critrevimmunol.v30.i5.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells, Nature reviews. Immunology. 2011;11(2):119–30. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duhen T, Duhen R, Lanzavecchia A, Sallusto F, Campbell DJ. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood. 2012;119(19):4430–40. doi: 10.1182/blood-2011-11-392324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshie O, Imai T, Nomiyama H. Novel lymphocyte-specific CC chemokines and their receptors. Journal of leukocyte biology. 1997;62(5):634–44. doi: 10.1002/jlb.62.5.634. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Subleski JJ, Hamano R, Howard OM, Wiltrout RH, Oppenheim JJ. Co-expression of TNFR2 and CD25 identifies more of the functional CD4+FOXP3+ regulatory T cells in human peripheral blood. Eur J Immunol. 2010;40(4):1099–106. doi: 10.1002/eji.200940022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pierini A, Strober W, Moffett C, Baker J, Nishikii H, Alvarez M, Pan Y, Schneidawind D, Meyer E, Negrin RS. TNF-alpha priming enhances CD4+FoxP3+ regulatory T-cell suppressive function in murine GVHD prevention and treatment. Blood. 2016;128(6):866–71. doi: 10.1182/blood-2016-04-711275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szondy Z, Pallai A. Transmembrane TNF-alpha reverse signaling leading to TGF- beta production is selectively activated by TNF targeting molecules: Therapeutic implications. Pharmacol Res. 2017;115:124–132. doi: 10.1016/j.phrs.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 35.McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. Journal of neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsakiri N, Papadopoulos D, Denis MC, Mitsikostas DD, Kollias G. TNFR2 on non-haematopoietic cells is required for Foxp3+ Treg-cell function and disease suppression in EAE. Eur J Immunol. 2012;42(2):403–12. doi: 10.1002/eji.201141659. [DOI] [PubMed] [Google Scholar]
- 37.Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4(11):1116–22. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- 38.Gao H, Danzi MC, Choi CS, Taherian M, Dalby-Hansen C, Ellman DG, Madsen PM, Bixby JL, Lemmon VP, Lambertsen KL, Brambilla R. Opposing Functions of Microglial and Macrophagic TNFR2 in the Pathogenesis of Experimental Autoimmune Encephalomyelitis. Cell Rep. 2017;18(1):198–212. doi: 10.1016/j.celrep.2016.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig.1 Expression of TNF by stimulated monocytes and anti- CD3/CD28 Ab stimulated Tregs
(A) Expression of mTNF by stimulated monocytes. Total adult PBMCs were stimulated with PMA and ionomycin for 4 hours and stained for mTNF. Representative histogram of mTNF expression gated on CD14+ cells. Unstimulated sample is shown with dashed line, and stimulated sample with the solid line (n=2). (B) CD4+CD127lowCD25+ Tregs and CD4+CD25− conventional T cells were enriched by magnetic bead sorting and stimulated by plate-bound anti-CD3/CD28 Abs for 16 hours, then treated with monensin for 4 hours. Cells were harvested, fixed, permeabilized and stained for Foxp3 and TNF. Representative data from three donors.
Supplemental Fig.2 Purity of Tregs enriched from peripheral blood mononuclear cells
Frequency of Tregs after enrichment from PBMCs. After enrichment by magnetic sorting (see methods) cells were stained for CD4, CD25, and Foxp3 and purity of Tregs assessed by flow cytometry. Gating on CD4+ cells (left) followed by expression of Foxp3 and CD25 by CD4+ cells (right) based on isotype controls. A representative sample from 5 donors.


