Summary
Inorganic polyphosphate (polyP) is a polymer of three to hundreds of phosphate units bound by high-energy phosphoanhydride bonds and present from bacteria to humans. Most polyP in trypanosomatids is concentrated in acidocalcisomes, acidic calcium stores that possess a number of pumps, exchangers, and channels, and are important for their survival. In this work, using polyP as bait we identified > 25 putative protein targets in cell lysates of both Trypanosoma cruzi and T. brucei. Gene ontology analysis of the binding partners found a significant over-representation of nucleolar and glycosomal proteins. Using the polyphosphate-binding domain (PPBD) of Escherichia coli exopolyphosphatase we localized long chain polyP to the nucleoli and glycosomes of trypanosomes. A competitive assay based on the pre-incubation of PPBD with exogenous polyP and subsequent immunofluorescence assay of procyclic forms of T. brucei showed polyP concentration-dependent and chain length-dependent decrease in the fluorescence signal. Subcellular fractionation experiments confirmed the presence of polyP in glycosomes of T. brucei procyclic forms (PCF). Targeting of yeast exopolyphosphatase to the glycosomes of PCF resulted in polyphosphate hydrolysis, alteration in their glycolytic flux and increase in their susceptibility to oxidative stress.
Keywords: polyphosphate, glycolysis, oxidative stress, glycosomes, nucleolus, trypanosoma
Abbreviated summary
In this work we report that polyphosphate, a polymer of up to hundreds of phosphates linked by high-energy bonds, binds to a number of trypanosomal proteins among them proteins localized to the glycosomes, and to the nucleoli. Polyphosphate was localized by a cytochemical technique to the glycosomes and nucleoli of trypanosomes. Targeting of an enzyme that cleaves polyphosphate to the glycosomes resulted in increased consumption of glucose, suggesting an inhibitory effect of polyphosphate on glycolysis.
Graphical Abstract
Introduction
African trypanosomiasis, caused by the Trypanosoma brucei group of parasites, and Chagas disease, caused by T. cruzi, are neglected tropical diseases that affect millions of people, causing thousands of deaths and affecting the ability of people to earn a living. Vaccines are not available and drug treatments have serious side effects or are not completely effective. The study of metabolic pathways in these parasites that may be essential for their survival could provide information on potential new targets that could be exploited for development of new therapeutic approaches.
Trypanosomatids are characterized by the compartmentation of the first six or seven enzymes of the glycolytic pathway in a peroxisome-like organelle, which for this reason was named the glycosome (Opperdoes & Borst, 1977), and by their high content of inorganic polyphosphate (polyP) that accumulates in acidocalcisomes, acidic calcium stores also rich in other organic and inorganic cations (Docampo & Huang, 2016). PolyP is a polymer of three to hundreds of high-energy phospho-anhydride-bonded orthophosphate units, and is universally conserved (Kornberg, 1995).
Although polyP accumulates in acidocalcisomes and acidocalcisome-like vacuoles (Docampo et al., 2005) of eukaryotes, it has also been found in most cellular compartments including mitochondria (Lynn & Brown, 1963), cytosol (Kulaev & Kulakovskaya, 2000), endoplasmic reticulum (Vorisek et al., 1982), nucleus (Griffin et al., 1965), nucleolus (Jimenez-Nunez et al., 2012), plasma membrane (Kumble & Kornberg, 1995) and lysosomes (Pisoni & Lindley, 1992). Rat liver nuclei and plasma membranes have several times the polyP concentration found in cytosol, mitochondria and microsomes (Kumble & Kornberg, 1995). Early work proposed that polyP is covalently bound to non-histone nuclear proteins (Offenbacher & Kline, 1984), a concept that was revived by the demonstration in yeast that nucleolar proteins Nsr1 and Top1 can be polyphosphorylated in lysine residues (Azevedo et al., 2015). Interestingly, recent work reported the polyphosphorylation of 15 target proteins in yeast, including a conserved network of proteins of nucleolar localization involved in ribosome biogenesis (Bentley-DeSousa et al., 2018). Nucleolar polyP was also found in myeloma cells and proposed to regulate RNA polymerase I activity (Jimenez-Nunez et al., 2012).
Several studies have shown that bacteria or unicellular eukaryotes lacking polyP are more sensitive to different stress conditions, including heat shock, osmotic stress, starvation, and reactive oxygen species (ROS), among others (Moreno & Docampo, 2013, Rao et al., 2009). The reason for this was never clearly understood until recent studies shed light on one possible mechanism behind this phenomenon (Kampinga, 2014). PolyP was initially shown to suppress glyceraldehyde 3-phosphate dehydrogenase thermal aggregation without noticeable loss in enzymatic activity (Semenyuk et al., 2013) and was recently identified in bacteria as a global, highly effective chaperone, that stabilizes proteins, prevents protein aggregation both in vitro and in vivo, and maintains proteins in a refolding-competent form (Gray et al., 2014). These results help to explain the long known but largely unexplained role of polyP in protecting bacteria against stress conditions, and suggest that polyP may have served as one of nature’s first chaperones (Gray et al., 2014). On the other hand, polyP was also shown to have a remarkable efficacy in accelerating amyloid fibril formation, serving as an effective nucleation source for different amyloid proteins, increasing fibril stability and reducing the formation of toxic oligomeric species (Cremers et al., 2016).
In this work we used biotinylated polyP to identify polyP-binding proteins in lysates from T. cruzi and T. brucei and were able to identify > 25 proteins in each parasite as putative polyP interaction partners. Among these proteins there was a significant enrichment in nucleolar and glycosomal proteins, which correlated with the cellular localization of polyP in these organelles. Targeting of Saccharomyces cerevisiae exopolyphosphatase to the glycosomes of T. brucei procyclic forms resulted in polyP hydrolysis, increase of their glycolytic rate and increased susceptibility to oxidative stress.
Results
Lysates of T. brucei procyclic forms and T. cruzi epimastigotes were incubated with biotinylated polyP after which the polyP-protein complexes were pulled down using magnetic streptavidin-coated beads, followed by washing and elution of bound proteins with high salt buffer. The proteins were identified by mass spectrometry. Two independent experiments for each parasite were done. We report the proteins that were identified in the two experiments from each parasite (Table 1 and 2), as well as all the proteins identified in at least one experiment (Tables S1 and S2). Proteins found in samples using biotinylated heparin (another anionic polymer) as control for non-specific binding were subtracted as described under Materials and Methods.
Table 1.
Polyphosphate-binding proteins identified in Trypanosoma brucei.
| Mascot scorea |
Protein name | Gene IDb | aac | IPd |
|---|---|---|---|---|
| Glycosomal enzymes | ||||
| 214/473 | glycosomal phosphoenolpyruvate carboxykinase | Tb427.02.4210 | 525 | 8.75 |
| 145/106 | hypoxanthine-guanine phosphoribosyl transferase, putative |
Tb427.10.1390 | 234 | 9.77 |
| 165/47 | ATP-dependent phosphofructokinase | Tb427.03.3270 | 487 | 9.77 |
| 69/100 | glycerol 3-phosphate dehydrogenase [NAD+], glycosomal |
Tb427.08.3530 | 354 | 8.75 |
| 78/78 | fructose-1,6-bisphosphatase | Tb427tmp.211.0540 | 350 | 9.46 |
| 75/75 | glyceraldehyde-3-phosphate dehydrogenase, glycosomal |
Tb427.06.4280 | 359 | 9.74 |
| 74* | pyruvate phosphate dikinase | Tb427tmp.02.4150 | 913 | 8.82 |
| 59* | glycerol kinase, glycosomal | Tb427tmp.211.3540 | 512 | 8.36 |
| Nuclear/nucleolar/ribosomal proteins | ||||
| 247/660 | NHP2/RS6-like protein | Tb427tmp.160.3670 | 126 | 7.37 |
| 56/453 | ALBA2 | Tb427tmp.02.2030 | 117 | 9.80 |
| 94/401 | 40S ribosomal protein S15, putative | Tb427.07.2370 | 172 | 10.56 |
| 22/338 | 60S ribosomal protein L10a, putative | Tb427tmp.01.1470 | 214 | 10.22 |
| 74/287 | 40S ribosomal protein S24E, putative | Tb427.10.7330 | 137 | 11.74 |
| 138/269 | ribosomal protein L36, putative | Tb427.10.1590 | 109 | 12.17 |
| 133/230 | 60S ribosomal protein L6, putative | Tb427.10.11390 | 192 | 11.05 |
| 51/217 | 40S ribosomal protein S10, putative | Tb427.10.5360 | 172 | 10.83 |
| 243/162 | 40S ribosomal protein S8, putative | Tb427.08.6160 | 220 | 11.66 |
| 187/80 | histone H2B, putative | Tb427.10.10460 | 112 | 12.29 |
| 58/69 | histone H3, putative | Tb427.01.2430 | 133 | 11.60 |
| 60/64 | ATP-dependent DEAD/H RNA helicase, putative |
Tb427.04.2630 | 843 | 10.02 |
| 49/18 | fibrillarin, putative | Tb427.10.14750 | 304 | 10.44 |
| Proteins of other or unknown locations | ||||
| 155/426 | high mobility group protein, putative | Tb427.03.3490 | 271 | 10.24 |
| 197/162 | cyclophilin, putative | Tb427.08.2000 | 301 | 10.32 |
| 44/118 | nascent polypeptide associated complex alpha subunit, putative |
Tb427tmp.01.1465 | 101 | 10.07 |
| 92/80 | kinetoplast DNA-associated protein, putative | Tb427.10.8950 | 126 | 11.46 |
| 88/88 | kinetoplast DNA-associated protein, putative | Tb427.10.8890 | 209 | 11.33 |
| 77/88 | acyl-CoA binding protein, putative | Tb427.04.2010 | 93 | 10.82 |
| 67/67 | kinetoplast-associated protein, putative | Tb427.08.7260 | 1028 | 10.33 |
| 62/62 | calmodulin | Tb427tmp.01.4621 | 149 | 3.86 |
| 42/50 | trichohyalin, putative | Tb427tmp.01.3320 | 658 | 10.55 |
| 69/222 | hypothetical protein, conservede | Tb427.03.1820 | 246 | 12.03 |
| 55/132 | hypothetical protein, conservedf | Tb427tmp.02.3560 | 206 | 12.39 |
| 53/64 | hypothetical protein, conservedg | Tb427tmp.03.0720 | 174 | 11.16 |
| 60/101 | hypothetical protein, conserved | Tb427tmp.01.2800 | 348 | 9.99 |
| 41/72 | hypothetical protein, conserved | Tb427tmp.160.1100 | 198 | 11.07 |
| 34/53 | hypothetical protein, conserved | Tb427tmp.211.4200 | 337 | 10.97 |
| 94/18 | hypothetical protein, conserved | Tb427.10.10030 | 100 | 5.04 |
Mascot scores obtained from search using T. brucei Lister 427 database on TriTrypDB (Aslett et al., 2010) in two independent experiments.
Gene identifier from TriTrypDB.
Protein length in amino acids.
Isoelectric point.
The protein is annotated as “mitochondrial RNA binding complex 1 subunit (Tb927.3.1820)” in the reference strain TREU927 database.
The protein is annotated as “surfeit locus protein 6 (Tb927.11.5810)” in the reference strain TREU927 database.
The protein is annotated as “Fcf2 pre-rRNA processing, putative (Tb927.11.420)” in the reference strain TREU927 database.
Glycosomal proteins detected in only one experiment.
Table 2.
Polyphosphate-binding proteins identified in Trypanosoma cruzi.
| Mascot scorea |
Protein name | Gene IDb | aac | IPd |
|---|---|---|---|---|
| Glycosomal enzymes | ||||
| 643/719 | fructose-1,6-bisphosphatase, putative | TcCLB.506649.70 | 344 | 8.01 |
| 108/592 | glycosomal malate dehydrogenase, putative | TcCLB.506503.69 | 323 | 8.88 |
| 148/479 | ATP-dependent 6-phosphofructokinase, glycosomal | TcCLB.508153.340 | 485 | 9.29 |
| 71/108 | glyceraldehyde-3-phosphate dehydrogenase, putative | TcCLB.506943.50 | 359 | 9.20 |
| 36* | fructose-bisphosphate aldolase, glycosomal, putative | TcCLB.510301.20 | 372 | 8.78 |
| Nuclear/nucleolar/ribosomal proteins | ||||
| 516/1179 | nucleolar protein 56, putative (fragment) | TcCLB.511573.58 | 387 | 8.44 |
| 527/380 | casein kinase II, putative | TcCLB.510761.60 | 345 | 8.53 |
| 30/553 | ribosomal protein L38, putative | TcCLB.503575.34 | 82 | 11.09 |
| 22/462 | 40S ribosomal protein S8, putative | TcCLB.511903.110 | 221 | 11.41 |
| 80/291 | snoRNP protein GAR1, putative | TcCLB.510687.120 | 239 | 11.92 |
| 85/164 | fibrillarin, putative | TcCLB.509715.40 | 316 | 10.37 |
| 78/92 | 60S ribosomal protein L22, putative | TcCLB.504147.120 | 130 | 10.79 |
| 56/95 | ribosomal protein L36, putative | TcCLB.509671.64 | 114 | 12.07 |
| 79/76 | ribosomal protein S20, putative | TcCLB.508823.120 | 117 | 10.52 |
| 73/41 | small nuclear ribonucleoprotein sm d3 | TcCLB.508257.150 | 115 | 10.72 |
| Proteins of other or unknown locations | ||||
| 7900/1379 | histidine ammonia-lyase, putative | TcCLB.506247.220 | 534 | 8.00 |
| 438/503 | retrotransposon hot spot (RHS) protein, putative | TcCLB.503483.9 | 916 | 8.40 |
| 288/180 | tripartite attachment complex protein 102 | TcCLB.509207.40 | 1136 | 5.36 |
| 182/53 | eukaryotic translation initiation factor 5, putative | TcCLB.504105.20 | 379 | 8.24 |
| 68/125 | serine carboxypeptidase S28, putative | TcCLB.506425.10 | 631 | 6.52 |
| 76/66 | ARP2/3 complex subunit, putative | TcCLB.508737.194 | 180 | 7.90 |
| 30/85 | actin-related protein 2/3 complex subunit 1, putative | TcCLB.504215.40 | 383 | 7.61 |
| 27/17 | ARP2/3 complex subunit, putative | TcCLB.506865.10 | 328 | 9.57 |
| 63/52 | Mu-adaptin 1, putative | TcCLB.510533.40 | 432 | 7.84 |
| 33/102 | hypothetical protein, conserved | TcCLB.506857.30 | 508 | 7.53 |
| 83/28 | hypothetical protein | TcCLB.511439.40 | 455 | 8.95 |
Mascot scores obtained from search using T. cruzi CL Brener Esmeraldo-like and Non-Esmeraldo-like databases on TriTrypDB (Aslett et al., 2010) in two independent experiments.
Gene identifier from TriTrypDB.
Protein length in amino acids.
Isoelectric point.
Glycosomal protein detected in only one experiment.
T. brucei polyP-binding protein identification
A Mascot search against T. brucei Lister 427 database led to the identification of 35 potential polyP-binding proteins identified in both experiments performed, from which 28 have been annotated as putative proteins with a predicted function, and 7 appear as hypothetical proteins (Table 1). The largest groups of identified proteins corresponded to nuclear/nucleolar/ribosomal proteins (13 proteins), and proteins of other or unknown locations (16 proteins), followed by glycosomal proteins (6 proteins). The protein with highest Mascot score in the second experiment was NHP2/RS6-like protein, followed by the glycosomal protein phosphoenolpyruvate carboxykinase, the cytosolic RNA-binding protein (TbAlba2) (Mani et al., 2011), a protein involved in signal transduction (high mobility group protein), and other ribosomal proteins (S15 and L10a), indicating a high relative abundance of these proteins in this proteome.
The list of potential T. brucei polyP-binding proteins found in this study is summarized in Table 1, where proteins were grouped by subcellular localization or functional relatedness. Table 1 also includes two additional glycosomal proteins identified in only one of the experiments (labeled with an asterisk). A complete list of T. brucei polyP-binding proteins identified in at least one experiment is in Table S1. The distribution according to gene ontology (GO) analysis of the proteins in Table 1 is shown in Fig. S1.
T. cruzi polyP-binding protein identification
To identify T. cruzi polyP-binding proteins a Mascot search was performed against T. cruzi CL Brener (Esmeraldo and non-Esmeraldo like) databases, as the genome sequence of T. cruzi Y strain is not yet available.
This search led to the identification of 25 potential polyP-binding proteins found in both experiments, from which 23 have been annotated as putative proteins with a predicted function, and 2 correspond to hypothetical proteins (Table 2).
It is important to mention that additional proteins (31 proteins) were found in T. cruzi pull downs but we are reporting here only the ones that were found in both T. cruzi experiments (25 proteins). A high number of proteins in T. cruzi polyP-binding proteome belong to the group of nuclear/nucleolar/ribosomal proteins (10 proteins), and proteins of other or unknown location (11 proteins) followed by glycosomal proteins (4 proteins). Mascot scores indicate that the most abundant proteins in this proteome are histidine ammonia lyase and nucleolar protein 56, followed by fructose-1,6-bisphosphatase. Two other proteins of the gluconeogenesis and glycolysis pathways exhibited a high score (> 300): malate dehydrogenase, and 6-phospho-1-fructokinase. Other high-score proteins found in this proteome were: ribosomal proteins L38 and S8, retrotransposon hot spot (RHS) protein and casein kinase II (Table 2). Some of these high-score proteins were also found in the T. brucei polyP-binding proteome, three of them being present in all four T. brucei and T. cruzi samples analyzed: phosphofructokinase, fructose-1,6-bisphosphatase, and ribosomal protein S8. The proteins glyceraldehyde-3-phosphate dehydrogenase, ribosomal protein L36, and fibrillarin were also found in T. brucei and T. cruzi samples. Table 2 also includes one additional glycosomal protein identified in only one of the experiments (labeled with an asterisk). Table S2 shows the complete list of T. cruzi polyP-binding proteins identified in at least one experiment. The distribution according to gene ontology (GO) analysis of the proteins in Table 2 is shown in Fig. S2.
GO analysis of the polyP-binding partners found a significant overrepresentation of nuclear/nucleolar/ribosomal and glycosomal proteins. We therefore investigated whether polyP was localized in these organelles using the polyphosphate-binding domain (PPBD) of E. coli exopolyphosphatase (PPX) (Saito et al., 2005). This technique has been used before to localize polyP in yeast (Saito et al., 2005), fungi (Saito et al., 2006), sea urchin eggs (Ramos et al., 2010), and mast cells (Moreno-Sanchez et al., 2012) vacuoles and myeloma cell nucleoli (Jimenez-Nunez et al., 2012). The original technique (Saito et al., 2005) used the recombinant PPBD of E. coli PPX containing an epitope tag at the N-terminal end (Xpress) that was detected with antibodies against the tag. Instead of using a tag and antibodies we labeled PPBD with Alexa Fluor 488 and directly detected the fluorescence signal of the polyP-bound PPBD.
Localization of polyP in T. brucei
We investigated the localization of polyP in procyclic (PCF) and bloodstream forms (BSF) of T. brucei grown in culture using super-resolution structured illumination microscopy. Figs. 1A and 1B show the staining with Alexa Fluor 488 PPBD of numerous intracellular vesicles randomly distributed in the cytosol of T. brucei PCF and staining of the nucleolus, the position of which was identified by the absence of DAPI staining (Landeira & Navarro, 2007). PolyP co-localizes in vesicles with antibodies against glycosomal phosphate pyruvate dikinase (PPDK) (Fig. 1A and Video S1) but not with antibodies against the acidocalcisome vacuolar proton pyrophosphatase (VP1) (Fig. 1B, and Video S2). There was also no co-localization with the mitochondrial marker MitoTracker, or the endoplasmic reticulum marker BiP as detected by immunofluorescence analysis (Fig. S3). The nucleolar localization was also confirmed by co-localization with an unknown nucleolar protein recognized by monoclonal antibody L1C6 (Devaux et al., 2007), which labels an area of the nucleolus distinct from those labeled by DAPI or PPBD (Fig. 1C).
Fig. 1.
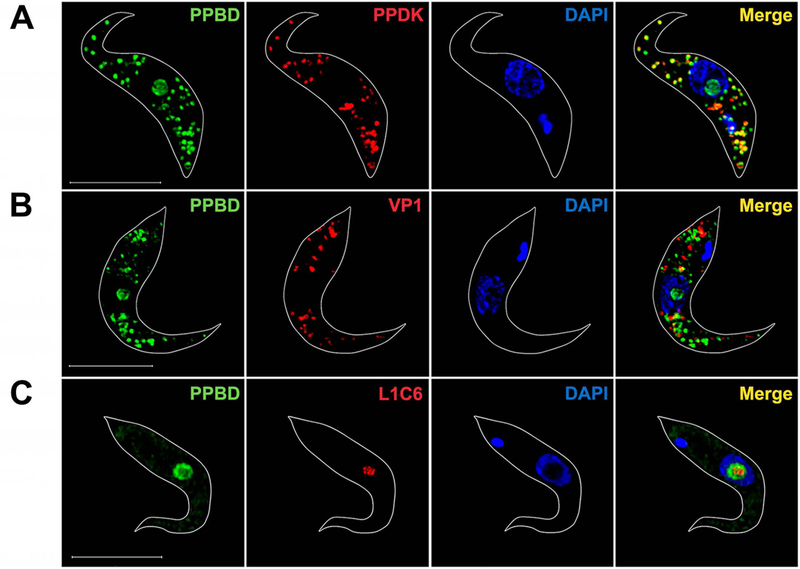
Super-resolution images of PPBD-labeled T. brucei PCF.
(A) PPBD (green) (8 µg/ml) localizes in the nucleolus, which is identified as the nuclear region not stained with DAPI (blue) and co-localizes (Merge, yellow) with antibodies against the pyruvate phosphate dikinase (PPDK, red) in the glycosomes.
(B) PPBD does not co-localize with antibodies against TbVP1 (red), the acidocalcisome marker.
(C) PPBD nucleolar localization coincides but does not superimpose to the labeling by nucleolar antibody L1C6 (red). The concentration of PPBD used in (C) was lower (2 µg/ml) to show only nucleolar labeling. Scale bars = 5 µm.
To further demonstrate that the PPBD is detecting longer chain polyP, we pre-incubated it with different concentrations of polyP100 and found a concentration-dependent decrease in nucleolar and glycosomal staining in T. brucei PCF (Fig. 2A,B). The nucleolus labeling was abolished when PPBD was pre-incubated with 0.1 and 1 mM (in phosphate units) of polyP100. Pre-incubation of PPBD with 1 mM of polyP100 reduced overall labeling by approximately 57% in relation to the control. Similarly, pre-incubation with fixed concentrations (1 mM) of longer chain polyP (polyP60, polyP100, and especially polyP700), but not polyP3, prevented labeling of PCF with PPBD (Figs. S4A,B). The labeling of the nucleolus disappeared after pre-incubations of PPBD with polyP60, polyP100 and polyP700. Most effectively, polyP700 decreased overall PPBD labeling by almost 78% compared to the control.
Fig. 2.
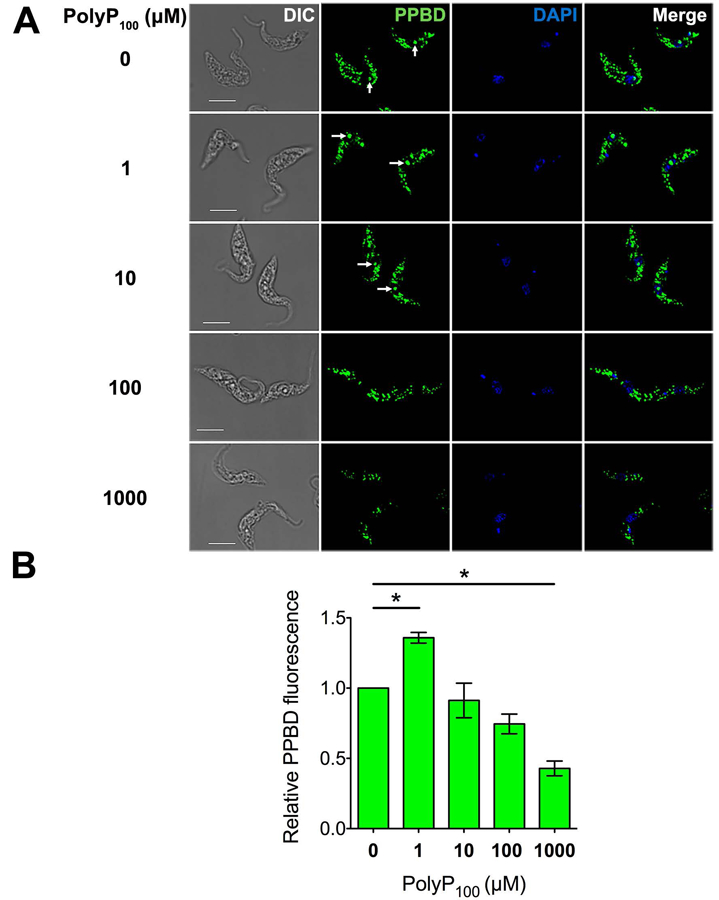
Fluorescence microscopy analysis of the effect of different concentrations of polyP100 on PPBD staining in T. brucei PCF.
(A) PPBD (green) was pre-incubated with 0 to 1.0 mM polyP100 (in phosphate units) for 1 h and then cell labeling was analyzed by fluorescence microscopy. Nucleolus labeling is indicated by white arrows. Differential interference contrast (DIC) images are shown on the left panel. DAPI staining is in blue. Scale bars = 5 µm.
(B) Quantification of the fluorescence of cells labeled with PPBD previously incubated with polyP100 as compared with control cells. A total of 471 cells were examined in three biological experiments. Values are means ± SEM (n = 3), * P < 0.05, One-Way ANOVA test with multiple comparisons.
In contrast with the results obtained with PCF, there was no labeling of the BSF nucleoli with PPBD and only partial co-localization with the glycosomal marker PPDK (Fig. 3A). However, as occurs with PCF, there was no co-localization of PPBD with VP1 in BSF (Fig. 3B).
Fig. 3.
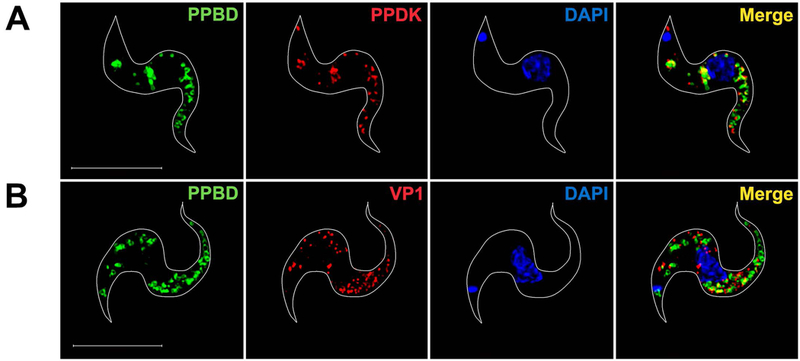
Super-resolution images of PPBD-labeled T. brucei BSF.
(A) PPBD (green) does not label the nucleolus and partially co-localizes (Merge, yellow) with antibodies against PPDK (red).
(B) PPBD does not co-localize with antibodies against TbVP1 (red). DAPI staining is in blue. Scale bars = 5 µm.
Localization of polyP in T. cruzi
We also investigated the localization of polyP in T. cruzi using PPBD. Figs. 4A,B show a strong nucleolar staining of epimastigotes with PPBD and a weak cytosolic staining that did not co-localize with antibodies against PPDK (Fig. 4A) or VP1 (Fig. 4B). Although cytosolic staining appears punctate this is because super-resolution microscopy eliminates the fluorescence coming from regions occupied by organelles, but this pattern is not apparent in regular fluorescent images (Fig. S5). In contrast, T. cruzi trypomastigotes (Figs. 4C,D) and amastigotes (Fig. 4E,F) showed strong nucleolar localization, partial co-localization with the glycosomal marker PPDK, and no co-localization with VP1.
Fig. 4.
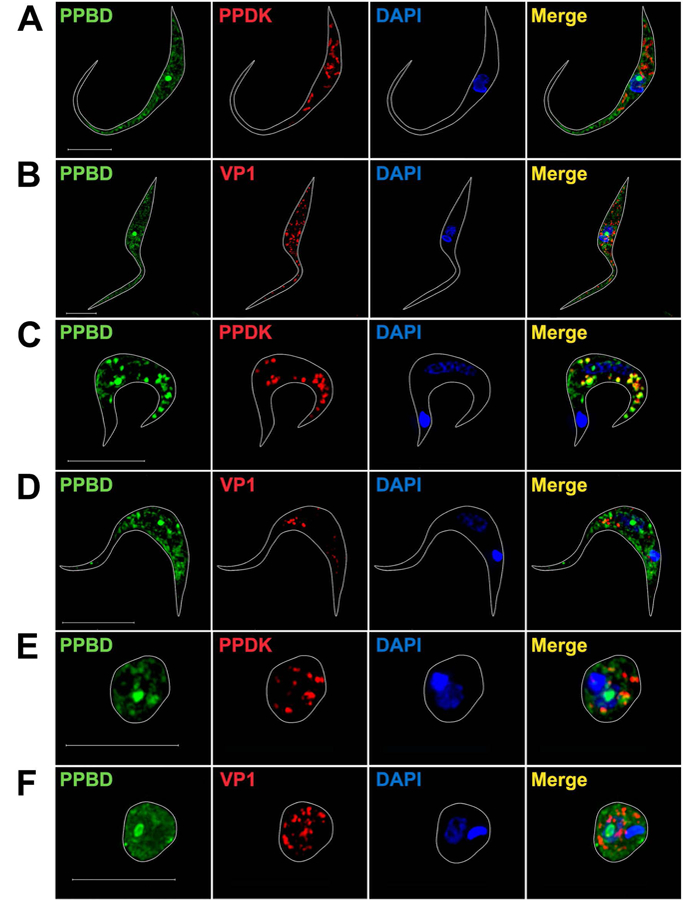
Super-resolution images of PPBD-labeled T. cruzi.
(A, B) In epimastigotes PPBD (green) labels the nucleolus and shows diffuse cytosolic labeling that does not co-localize (Merge, yellow) with antibodies against PPDK (red) (A) or with antibodies against TbVP1 (red) (B). Scale bars = 5 µm.
(C, D) In trypomastigotes PPBD labels the nucleolus and partially co-localizes (Merge, yellow) with antibodies against PPDK (red) (C) but not with antibodies against TbVP1 (red) (D). Scale bars = 5 µm.
(E, F) In amastigotes PPBD labels the nucleolus and partially co-localizes (Merge, yellow) with antibodies against PPDK (red) (E) but not with antibodies against TbVP1 (red) (F). DAPI staining is in blue. Scale bars = 5 µm.
Detection of long chain polyP in isolated glycosomes and acidocalcisomes
We isolated glycosomes and acidocalcisomes by a modification of an isolation procedure described previously (Huang et al., 2014). After grinding with silicon carbide to break the cells, the lysates were fractionated by differential centrifugation followed by density-gradient ultracentrifugation using high-density solutions of iodixanol (Fig. S6). The crude glycosomal fraction obtained from the first iodixanol gradient was applied to the 27% step of the second iodixanol gradient resulting in seven fractions. Fig. 5 shows protein abundance (Fig. 5A) as well as distribution of markers for glycosomes (hexokinase) (Fig. 5B) and acidocalcisomes (aminomethylenediphosphonate (AMDP)-sensitive vacuolar pyrophosphatase activity, TbVP1) (Fig. 5C). Glycosomes were enriched in fractions 1 and 2, while acidocalcisomes were enriched in fractions 3 and 5. We also evaluated our purification method by western blot analyses of the fractions using antibodies against a glycosomal marker (phosphate pyruvate dikinase, TbPPDK) and an acidocalcisomal marker (TbVP1). The crude glycosomal fraction (C) showed contamination with the acidocalcisome marker. However, fractions 1 and 2 of the second gradient were free of TbVP1 antibody reaction (Fig. 5D). Some pyrophosphatase activity was detected in fraction 2 (Fig. 5 C) but since no antibody reaction was detected (Fig. 5D) it can be attributed to the soluble inorganic pyrophosphatase described in trypanosomatids (Gomez-Garcia et al., 2004) or other non-specific pyrophosphatase activity. Fractions 1 and 2, or the acidocalcisome fractions obtained in the first iodixanol gradient (Fig. S6) were pooled and extracted for polyP analyses. Long chain polyP assayed by 30% polyacrylamide gel electrophoresis (PAGE), and staining with toluidine blue, showed the presence of long chain polyP in both glycosomal and acidocalcisomal fractions (Fig. 5E).
Fig. 5.
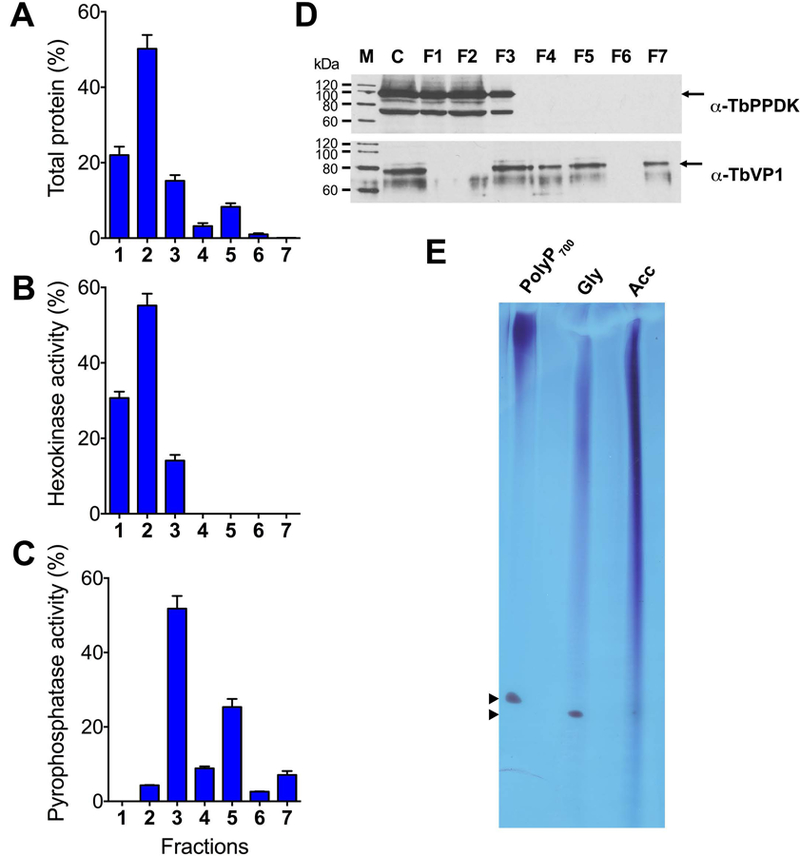
Distribution on iodixanol gradients of organellar markers from wild type PCF trypanosomes and detection of long chain polyP.
(A) Protein distribution.
(B) Glycosomal marker (hexokinase) distribution.
(C) Acidocalcisomal marker (pyrophosphatase) distribution.
In (A-C) the y-axis indicates relative distribution; the x-axis indicates fraction number from wild-type T. brucei PCF; bars show means ± SD from three independent experiments. In (B-C) the values are expressed as a percentage of the total recovered activity.
(D) Western blot analyses done with aliquots of fractions from wild-type T. brucei PCF, using antibodies against the glycosomal protein marker TbPPDK or the acidocalcisomal protein marker TbVP1, as described under Materials and Methods. C, crude glycosomes from the first iodixanol gradient. F1 to F7, fractions from the second iodixanol gradient, as indicated in Fig. S6. Molecular weight markers (M) and antibodies are shown at left and at right, respectively. Arrows show the bands corresponding to TbPPDK and TbVP1.
(E) Long chain polyP as detected by PAGE. Long chain polyP was extracted from glycosome (Gly) and acidocalcisome (Acc) subcellular fractions (Fig. S6). PolyP700 was used as a marker. Arrowheads indicate the orange G dye used in the sample buffer.
Expression of S. cerevisiae PPX in the glycosomes and E. coli PPX in the nuclei of T. brucei and phenotypic changes detected
To decrease the amount of polyP in PCF glycosomes, we expressed S. cerevisiae PPX1 (ScPPX1), which specifically hydrolyzes polyP to inorganic phosphate (Wurst & Kornberg, 1994), fused to a glycosomal targeting sequence (peroxisome-targeting sequence 2 [PTS2]) and enhanced yellow fluorescent protein (eYFP) (Bauer et al., 2013). The plasmid integrates into the tubulin locus and the procyclin repetitive acidic protein promoter (PARP) drives its constitutive expression (Bauer et al., 2013). Expression of PTS2-ScPPX1-eYFP in glycosomes was confirmed by fluorescence microscopy. Fig. 6A shows co-localization of the green fluorescent signal from the expressed protein with antibodies against PPDK, a glycosomal marker. Western blot analysis confirmed the expression of the protein (Fig. 6B, Fig. S7). We confirmed the activity of the expressed enzyme by measuring levels of Pi of cell lysates from cells transfected with the plasmid as compared to those of lysates from wild type cells. PolyP is very abundant in the cells and upon lysis it is released from different cellular compartments together with the glycosomally targeted ScPPX. When the lysates were incubated at 30oC there was an increase in the PPX activity of lysates from PTS2-ScPPX1-eYFP-expressing cells when compared to the endogenous PPX activity of lysates from WT cells (Fig. 6C) but this activity did not increase by adding exogenous polyP100 indicating that there was enough polyP in the medium to saturate the enzyme released from the glycosomes.
Fig. 6.
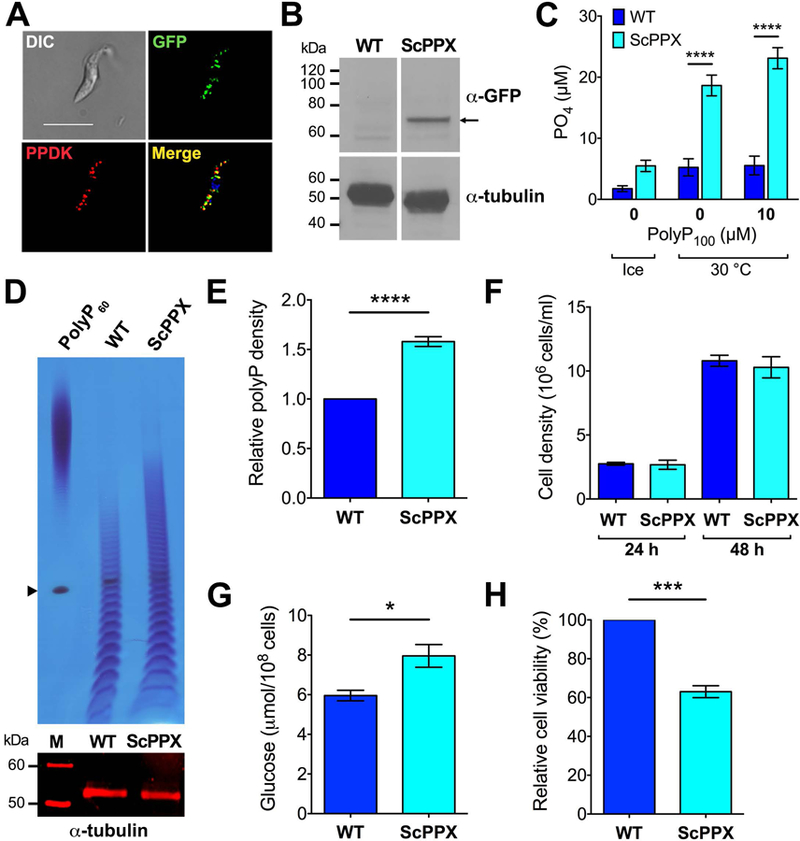
Glycosomal expression of ScPPX in T. brucei PCF and resulting phenotypic changes.
(A) PTS2-ScPPX1-eYFP (green) co-localizes (Merge, yellow) with antibodies against PPDK (red) (Pearson’s correlation coefficient = 0.5726). DIC, differential interference contrast. DAPI staining is in blue. Scale bar = 5 µm.
(B) Western blot analysis of PCF WT and PTS2-ScPPX1-eYFP-expressing cells using polyclonal antibody against GFP. Molecular weight markers are at left and arrow shows the band corresponding to PTS2-ScPPX1-eYFP (expected size: ~ 73 kDa). Tubulin was used as a loading control. Full gel in shown in Fig S7.
(C) Exopolyphosphatase assays in lysates. Lysates (4.5 × 107 cell equivalents) of WT or PTS2-ScPPX1-eYFP-expressing cells were incubated in ice or at 30ºC for 10 min in the absence or presence of 10 µM polyP100 (in phosphate units). ScPPX means PTS2-ScPPX1-eYFP-expressing cells. Values are means ± SEM (n = 3), ****P < 0.0001. Two-way ANOVA test with multiple comparisons.
(D) Short chain polyphosphate as detected by PAGE. Short chain polyP was extracted from WT and PTS2-ScPPX1-eYFP-expressing cells. PolyP60 was used as a marker. Arrowhead indicates orange G dye. Tubulin was used as a loading control.
(E) Densitometry of toluidine stained polyP from WT and PTS2-ScPPX1-eYFP-expressing cells. Values are means ± SEM (n = 4), ****P < 0.0001. Student’s t test.
(F) Growth of WT or PTS2-ScPPX1-eYFP-expressing cells at 24 and 48 h. No significant differences observed, n = 3.
(G) Glucose consumption in WT and PTS2-ScPPX1-eYFP-expressing cells after 6 h of incubation in culture medium at high cell density (108 cells/ml). Values are means ± SEM (n = 3), *P < 0.05. Student’s t test.
(H) Percentage of live cells following 24 h-incubation after treatment with 50 µM H2O2 for 1 h at 28ºC. Values are means ± SEM (n = 3), ***P < 0.001. Student’s t test.
To establish whether expression of ScPPX resulted in a decrease in polyP we measured total short and long chain polyP in wild type and PTS2-ScPPX1-eYFP-expressing cells using a biochemical method based on the Pi release by recombinant ScPPX, but we did not observe significant differences (Fig. S8). However, when we measured short chain polyP by 35.5% polyacrylamide gel electrophoresis (PAGE), toluidine blue staining revealed a higher accumulation of short chain polyP (lower than 60 Pi units) in PTS2-ScPPX1-eYFP-expressing cells as compared to wild type cells. These results are consistent with the hydrolysis of long chain polyP by glycosomally targeted ScPPX (Figs. 6D, E).
T. brucei PCF expressing glycosomal PPX grew at the same rate as control cells (Fig. 6F, Fig. S9). However, glucose consumption of PTS2-ScPPX1-eYFP-expressing cells after 6 h of incubation in culture medium was higher (Fig. 6G), and these cells were more sensitive to oxidative stress than control cells (Fig. 6H).
To decrease the levels of polyP in the nucleolus we tried to target E. coli PPX (EcPPX) to the nucleolus using a nucleolar localization signal (NoLS) reportedly used to target GFP to this organelle in T. brucei (Hoek et al., 2000). Expression of the protein NoLS-EcPPX-GFP was confirmed by western blot analysis (Fig. 7A) and it had nuclear but not nucleolar localization and failed to decrease PPBD staining of nucleolar polyP (Fig. 7B).
Fig. 7.
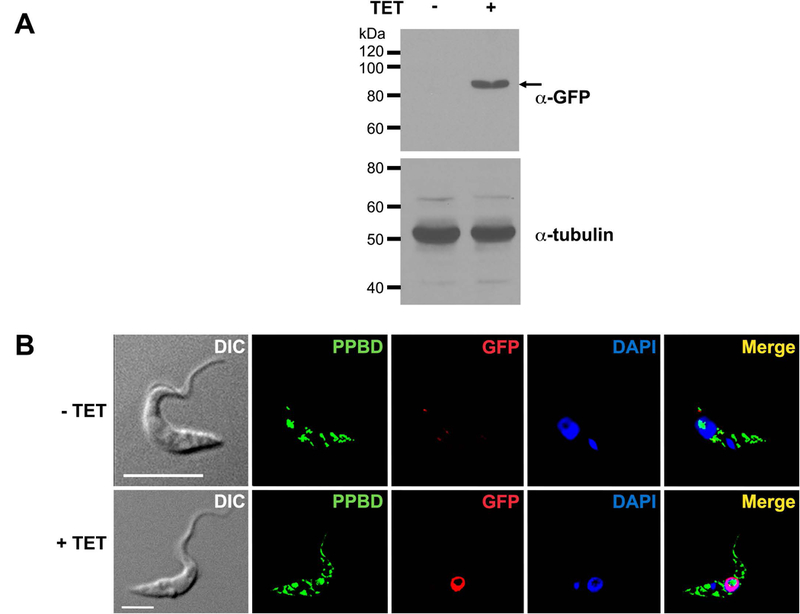
Western blot analysis and fluorescence microscopy of NoLS-EcPPX-GFP-expressing T. brucei PCF.
(A) Western blot analysis of uninduced (-TET) and induced (+TET) T. brucei PCF transfected with an expression vector containing EcPPX-GFP with a nucleolar localization signal (NoLS) using polyclonal antibody against GFP. Molecular weight markers are at left and arrow shows the band corresponding to NoLS-EcPPX-GFP (expected size: ~ 89 kDa). Tubulin was used as a loading control.
(B) Uninduced (-TET) and induced (+TET) T. brucei PCF transfected with NoLS-EcPPX-GFP plasmid were subjected to immunofluorescence analysis. Parasites were labeled with PPBD (green), antibodies anti-GFP (red), and DAPI (blue). Differential interference contrast (DIC) images are shown on the left panel. Merged images are shown on the right panel. In induced cells, EcPPX (red) exhibits nuclear localization and does not co-localize with nucleolar PPBD (green). Scale bars = 5 µm. TET: tetracycline.
Discussion
Inorganic polyP has multiple functions in both bacteria and eukaryotes. Some of these functions can be attributed to its chemical properties, such as its ability to store phosphate in an osmotically neutral form, to bind and store organic and inorganic cations, to store energy in the form of a few to hundreds of phosphoanhydride bonds, to combine with other polymers for the formation of channels and pumps, or to act as inorganic chaperone of proteins (Kornberg, 1995, Gray et al., 2014, Moreno & Docampo, 2013). Other more complex functions, however, such as its role in transcriptional control, and regulation of enzyme activity, motility, or resistance to stress response might depend on its interaction with proteins and cell signaling (Rao et al., 2009). However, besides its interaction with proteins of the blood coagulation cascade (Smith et al., 2006), or its ability to polyphosphorylate proteins (Azevedo et al., 2015, Bentley-DeSousa et al., 2018), little is known of other interactions with cellular proteins. Our proteomic studies are a first step in the identification of polyP-interacting proteins in eukaryotes.
The proteomic studies of polyP-binding proteins revealed an overrepresentation of nucleolar and glycosomal proteins in both T. cruzi and T. brucei and these results correlated with the localization of the polymer to the nucleolus and glycosomes. Interestingly, known polyP-associated proteins such as the components of the VTC complex involved in its synthesis (Lander et al., 2013, Ulrich et al., 2014, Fang et al., 2007a) and the exopolyphosphatase (PPX) (Fang et al., 2007b) and vacuolar soluble pyrophosphatase (VSP) (Lemercier et al., 2004, Yang et al., 2016), involved in its degradation, were not identified among the interacting partners. This could be explained because these enzymes are involved in short chain polyP synthesis and degradation while we used long chain polyP bound to biotin for the pull downs.
Besides several glycolytic enzymes (phosphofructokinase, fructose-bisphosphate aldolase, glyceraldehyde 3-phosphate dehydrogenase) other glycosomal enzymes (phosphoenolpyruvate carboxykinase, malate dehydrogenase, pyruvate phosphate dikinase, fructose 1,6-bisphosphatase, glycerol 3-phosphate dehydrogenase, glycerol kinase, hypoxanthine-guanine phosphoribosyl-transferase) were present in the pull downs. Multiple nuclear and nucleolar proteins (ribosomal subunits, snoRNP protein GAR1, small nucleolar ribonucleoprotein SmD3, fibrillarin, histones, high mobility group protein, casein kinase 2) were also detected. These results are in agreement with the localization of polyP in the nucleolus and glycosomes of the parasites. Interestingly, not all PPDK-stained glycosomes were detected by PPBD staining and many PPBD-positive particles were not recognized by anti-PPDK, suggesting the possibility of different populations of glycosomes.
The first report of the presence of PPDK in T. brucei (Bringaud et al., 1998) showed that the enzyme was not detectable in long slender BSF trypanosomes of Lister 427 or GUTat strain grown in rats and that short stumpy forms of GUTat strain grown in mice had low expression levels, as detected by western blot analyses. No PPDK was detected by immunofluorescence analysis of BSF trypanosomes. Our results suggest that either the BSF trypanosomes grown in culture that we used have stumpy-like characteristics or that the antibody we used was able to detect this enzyme using our standard IFA protocol.
It is interesting to note that many glycosomal proteins have the highest calculated positive charge within its family of homologous proteins (Wierenga et al., 1987). These observations led some authors to postulate the presence of “hot spots” of basic amino acids about 40 Å apart that could be important for the glycosomal import of these proteins (Wierenga et al., 1987). This idea was later discarded but the reason for the high positive charge of these proteins is still puzzling. It is tempting to speculate that polyP is the scaffold that maintains the tight packing of the glycosomal enzymes (Michels et al., 2000), and could be involved in the transfer of the negatively-charged glycolytic intermediates between the enzymes.
Another potential reason for the highest glycolytic rate of parasites targeted with ScPPX to the glycosomes is an inhibitory effect of polyP or a stimulatory effect of its hydrolytic products (Pi and PPi) on glycolytic activities. Glycosomes contain enzymes that can be inhibited by PPi such as the hexokinases of T. cruzi (Caceres et al., 2003) and L. mexicana (Pabon et al., 2007), one of the hexokinases of T. brucei (Chambers et al., 2008), and the phosphoenolpyruvate carboxykinase (PEPCK) of T. cruzi (Acosta et al., 2004), but their inhibition by polyP has not been tested. On the other hand binding of glycosomal enzymes to polyP could be explained by the presence of binding domains to polyP. Several glycosomal enzymes found in our proteome analyses, like hypoxanthine-guanine phosphoribosyl-transferase (HGPRT) (Shih et al., 1998) and pyruvate phosphate dikinase (PPDK) (Bringaud et al., 1998, Maldonado & Fairlamb, 2001, Shih et al., 1998) produce or utilize PPi. Phosphofructokinase (PFK) is an ATP-dependent enzyme but shows a high degree of sequence and structural similarity with PPi-dependent enzymes (Rodriguez E., 2009, Michels et al., 1997, McNae et al., 2009). Thus, interaction with potential PPi- or polyP-binding sites could explain their pull down by polyP. PolyP anions have been involved in stabilization of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Semenyuk et al., 2013), another glycolytic enzyme found in both polyP-binding proteomes. In that work the authors demonstrated that polyP suppresses thermal aggregation of the enzyme without affecting its activity. This could be a general mechanism for polyP-mediated regulation of glycolytic enzymes and it could explain the presence of polyP in T. brucei and T. cruzi glycosomes. Our results indicate that a detailed analysis of the effect of polyP on glycosomal enzymatic activities is warranted.
PolyP has been found in most subcellular compartments investigated so far but this is the first report of its presence in a peroxisome-related organelle. As glycosomes share not only similar biogenesis mechanisms, morphology, and some metabolic processes, but also have in common a dense matrix of proteins with pI values on average 1 to 2 pH unit higher than those in the cytosol (Michels & Opperdoes, 1991, Gabaldon et al., 2016), it is possible that polyP is present in peroxisomes of other organisms. In this regard, it has been indicated that peroxisomes seem to entirely lack proteinaceous chaperones and that it will be intriguing to test how the polyP pathway might play a role in the development of stress resistance in these organelles (Kampinga, 2014).
Only one polyP-synthesizing activity has been described in trypanosomes, which is catalyzed by the acidocalcisomal vacuolar transporter chaperone (VTC) complex (Lander et al., 2013). However, conditional knockout of VTC4, the catalytic subunit of the complex, in T. brucei did not affect the levels of long chain polyP suggesting the involvement of other enzymes in its synthesis (Ulrich et al., 2014). It is currently unknown whether polyP is synthesized within glycosomes or reach the organelles by piggy-backing on the glycosomal matrix enzymes that are synthesized in the cytosol and post-translationally imported via transient pores or by non-selective pores such as those that have been detected in T. brucei glycosomal (Gualdron-Lopez et al., 2012) or in different organisms peroxisomal (Antonenkov & Hiltunen, 2012) membranes. On the other hand, it has been reported that several NUDIX hydrolases in yeast and humans (Lonetti et al., 2011) possess polyP degrading activity and at least two NUDIX hydrolases (TbNH2 and TbNH3) have been detected in the glycosome proteome (Guther et al., 2014). Work is in progress to investigate their activity.
The interaction of polyP with ribosomal proteins has been previously reported in E. coli for two main processes: degradation of ribosomal protein through polyP-Lon protease complex (Kuroda et al., 2001) and as promoter of translation fidelity (McInerney et al., 2006). The ATP-dependent Lon protease forms a complex with polyP that degrades most of the ribosomal proteins, thereby supplying the amino acids required for response to starvation (Kuroda et al., 2001). On the other hand, polyP interacts with ribosomes to maintain optimal translation efficiency, as demonstrated in experiments measuring the in vivo translation rate in polyP kinase (ppk) mutants (McInerney et al., 2006). These studies provide an explanation for the abundance of ribosomal proteins observed in T. brucei and T. cruzi polyP-binding proteomes.
Transcription factors and RNA binding proteins represent another significant functional group in the T. brucei and T. cruzi polyP-binding proteomes. A previous work reported a role for polyP in transcription of myeloma plasma cells, where they accumulate higher levels of nucleolar polyP than normal plasma cells (Jimenez-Nunez et al., 2012). In that work they confirmed that nucleolar RNA polymerase I was modulated by polyP, opening up a broad spectrum of possibilities regarding the role of polyP as regulator of gene expression at the transcriptional level. In fact, another nucleolar enzyme that appeared with high score in T. cruzi, and also with a lower score in the T. brucei polyP-binding proteome, was the casein kinase II alpha subunit (CK2α). This enzyme has been characterized in T. brucei and it accumulates in the nucleolus, which is the site of ribosome biogenesis and where many of the CK2 substrates are present (Park et al., 2002). This isoform of the catalytic subunit prefers ATP over GTP as a substrate and modulators of its function are still unknown. It would be interesting to evaluate whether polyP regulates CK2 activity, as the anionic polymer and the enzyme localize to the nucleolus and are involved in transcription regulation. The report that many nucleolar proteins can be polyphosphorylated (Bentley-DeSousa et al., 2018) supports the presence of this polymer in the nucleoli.
Most polyP in trypanosomatids have been proposed to be concentrated in acidocalcisomes (Docampo, 2016). Early 31P-NMR studies of isolated acidocalcisomes from T. brucei PCF and T. cruzi epimastigotes found that the average chain lengths of polyP are 3.39 and 3.25 (Moreno et al., 2000), respectively, values that were in good agreement with the average phosphate chain lengths determined by X-ray microprobe analysis in T. cruzi (Scott et al., 1997, Moreno et al., 2000). This was considered consistent with the ability of the acidocalcisomal recombinant Vtc4 to synthesize short chain polyP in vitro (Lander et al., 2013). However, subcellular fractionation of T. cruzi epimastigotes detected both short and long chain polyP in acidocalcisome fractions (Ruiz et al., 2001). In this work we confirmed that acidocalcisomes, like glycosomes, possess considerable amounts of long chain polyP (Fig. 5E). This apparent discrepancy could be explained by the difficulty in detecting long chain polyP by 31P-NMR because of its broad signal, and the potential need of a membrane potential to efficiently synthesize and translocate long chain polyP in intact vacuoles (Hothorn M, 2009), in contrast to the in vitro synthesis by the recombinant enzyme. Acidocalcisomes, however, were not stained by PPBD. One potential explanation is that this highly charged polymer is known to bind Mg2+ and other cations in acidocalcisomes and form gels (Klompmaker et al., 2017) that could be inaccessible to PPBD. This might not occur in the glycosomes and nucleolus where polyP could be associated with proteins leaving free negatively charged residues that could more easily bind to PPBD. In vitro competitive binding assays previously showed that PPBD binds strongly to free long chain polyP and has been described as a reliable method for long chain polyP detection (Saito et al., 2005). Long chain polyP is very abundant in different trypanosomatids with reported values of 2.9, 0.8 and 0.13 mM in T. cruzi epimastigotes, trypomastigotes, and amastigotes, respectively (Ruiz et al., 2001); 57 mM in Leishmania major promastigotes (Rodrigues et al., 2002), and 6 mM in T. brucei procyclic forms (Lemercier et al., 2002).
We were not able to detect significant differences in short and long chain polyP content of total lysates from wild type and PTS2-ScPPX1-eYFP-expressing T. brucei procyclic forms. Therefore, we assayed polyP by PAGE in total cell extracts and we detected an increase in short chain polyP in PTS2-ScPPX1-eYFP-expressing cells. The results could be explained if only the glycosomal long chain polyP is hydrolyzed in PTS2-ScPPX1-eYFP-expressing cells, resulting in the increase in polyP of less than 60 Pi units, with no apparent decrease in short and long chain polyP levels in total cell extracts.
Our results using the PPBD of E. coli PPX suggest that the nucleolus and glycosomes possess long chain polyP. Targeting of yeast exopolyphosphatase to glycosomes of T. brucei resulted in decreased cellular polyP levels, alteration in their glycolytic flux, and increase in the susceptibility to oxidative stress. It is interesting to note that T. cruzi glycosomes only possess a glutathione peroxidase I, which decomposes hydroperoxides but not H2O2 (Wilkinson, 2002), and that polyP has been shown to protect cells from oxidative stress (Gray et al., 2014).
The nucleoli are membrane-less RNA/protein bodies within the nucleus where they function in ribosome subunit biogenesis. Despite the knowledge gained on their function there is still some lack of understanding of what holds the RNA and protein components together as a physical structure (Brangwynne, 2011). Recent evidence suggests that they have liquid-like properties, with an effective surface tension that minimizes surface area by viscous relaxation to a spherical shape and that may assemble by intracellular phase separation (Brangwynne, 2011). The nucleation properties of polyP (Cremers et al., 2016) could have a role in phase separation and RNA-protein interactions.
Targeting of E. coli exopolyphosphatase to the nucleolus did not reduce PPBD staining suggesting that nucleolar polyP could not be hydrolyzed because the enzyme could not localize to the nucleolus. The liquid droplet-like behavior of nucleoli could make them inaccessible to the enzymatic activity of EcPPX. In this regard, overexpression of EcPPX in yeast was also unable to reverse polyphosphorylation of target proteins (Bentley-DeSousa et al., 2018). Another possible explanation could be the reported masking of some polyP chains, which makes them resistant to the hydrolytic action of exopolyphosphatases (Rao et al., 2009). In this regard, derivatization of the terminal phosphates of polyP conferred resistance to exopolyphosphatase digestion (Choi et al., 2010). Furthermore, it has been suggested (Saiardi, 2012) that inositol hexakisphosphate (IP6) could be at the edge of polyP acting as a cap, like the one protecting mRNA for degradation, and protect polyP from the action of exopolyphosphatases.
In conclusion, our work reveals a novel localization of polyP to the glycosomes and nucleolus of trypanosomatids and that hydrolysis of glycosomal polyP results in an increased glycolytic rate and increased susceptibility to oxidative stress.
Experimental procedures
Chemicals and reagents
Monoclonal antibody L1C6 (Devaux et al., 2007) was provided by Dr. Keith Gull (Oxford University, UK), polyclonal antibody against TbBiP (Bangs et al., 1993) was provided by Dr. Jay Bangs (University at Buffalo, NY), polyclonal rabbit antibody against TbVP1 (Lemercier et al., 2002) was provided by Dr. Norbert Bakalara (University of Montpellier, France), monoclonal antibody against T. brucei pyruvate phosphate dikinase (TbPPDK) (Bringaud et al., 1998) was provided by Dr. Frédéric Bringaud (University of Bordeaux, France), the pXS2-AldoPTS2-eYFP vector (Bauer et al., 2013) was provided by Dr. Meredith T. Morris (Clemson University, NC), pTrc-PPBD plasmid (Saito et al., 2005) was provided by Dr. Katsuharu Saito (Shinshu University, Nagano-Ken, Japan), and pLEW100v5bld-BSD plasmid was provided by Dr. George Cross. PolyP60 was provided by Dr. Toshikazu Shiba (RegeneTiss Inc., Okaya, Japan). PolyP3 (Na5P3O10) was from Sigma-Aldrich (St. Louis, MO), and polyP100 and polyP700 were from Kerafast (Boston, MA). Aminomethylenediphosphonate (AMDP) was synthesized by Michael Martin (University of Illinois at Urbana-Champaign). Slide-A-Lyzer Dialysis Cassette, HisPur Ni-NTA Chromatography Cartridge and Slide-A-Lyzer Dialysis Cassette were from Thermo Scientific (Thermo Fisher Scientific, Walthman, MA). Alexa Fluor 488 Microscale Protein Labeling kit, MitoTracker Red CMXRos, and rabbit anti-GFP antibody were from Invitrogen Molecular Probes (Eugene, OR). Rabbit anti-HA was from Abcam (Cambridge, MA). Dynabeads MyOne Streptavidin T1 beads, blasticidin S HCL, and hygromycin B were from Invitrogen (Carlsbad, CA). Biotinylated heparin (Cat. No. 375054) was from Merck (Millipore Sigma, Burlington, MA). BCA Protein Assay kit and ECL Western Blotting Substrate were from Pierce Protein Biology (Thermo Fisher Scientific, Walthman, MA). Phusion High-Fidelity DNA polymerase and restriction enzymes were from New England Biolabs (Ipsich, MA). In-Fusion HD Cloning kit, and E. coli Stellar Competent Cells were from Clontech Laboratories (Mountain View, CA). Zymo 5α Mix & Go competent cells was from Zymo Research (Irvine, CA). LigaFast Rapid DNA Ligation System was from Promega (Madison, WI). G418 was from KSE Scientific (Durham, NC). Fluoromount-G mounting medium was from Southern Biotech (Homewood, AL). Benzonase was from Novagen (Merck Millipore, Burlington, MA). Mini-PROTEAN TGX Precast Protein Gel was from BioRad (Hercules, CA). MagicMark XP Western Protein Standard was from Life Technologies (Carlsbad, CA). D-Glucose Assay Kit (GOPOD Format) was from Megazyme Inc. (Chicago, IL). Protease inhibitor cocktail for use in purification of histidine-tagged proteins (Cat. No. P8849), protease inhibitor cocktail for use with mammalian cell and tissue extracts (Cat. No. P8340), polyclonal rabbit anti-tubulin and anti-IgG antibodies, and all other reagents of analytical grade were from Sigma-Aldrich (St. Louis, MO).
Cell cultures
For most studies T. brucei PCF and BSF (Lister 427) were grown as reported previously (Lander et al., 2013, Huang et al., 2014). Cell density was verified by counting parasites in a Neubauer chamber. PTS2-ScPPX1-eYFP-expressing PCF were grown in the presence of 10 µg/ml blasticidin. NoLS-EcPPX-GFP-expressing PCF were grown in the presence of 15 μg/ml G418, 50 μg/ml hygromycin, and 10 µg/ml blasticidin. For proteomic studies T. brucei PCF (29–13 strain) were grown at 28°C in SM medium (Cunningham & Honigberg, 1977) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 8 μg/ml hemin, 15 μg/ml G418 and 50 μg/ml hygromycin. Cultures were scale-up in glass sterile bottles under shaking until reaching a final volume of 3.0 L cell culture in exponential phase (1–1.5 × 107 cells/ml). T. cruzi epimastigotes (Y strain) were grown at 28°C in LIT medium (Bone & Steinert, 1956) supplemented with 10% heat-inactivated FBS. Cultures were scale-up in glass sterile bottles under shaking until reaching a final volume of approximately 1.0 L cell culture in exponential phase (~4 × 107 cells/ml). T. cruzi trypomastigotes and amastigotes (Y strain) were collected from the culture medium of infected Vero cells, using a modification of the method of Schmatz and Murray as described before (Moreno et al., 1994). Vero cells were grown in RPMI supplemented with 10% FBS and maintained at 37ºC with 5% CO2.
Cell lysis for proteomic studies
Approximately 4 × 1010 cells (T. brucei PCF and T. cruzi epimastigotes) were harvested separately by centrifugation at 1,000 × g for 15 min at room temperature (RT) and then washed twice with buffer A with glucose (BAG: 116 mM NaCl, 5.4 mM KCl, 0.8 mM MgSO4, 50 mM Hepes, pH 7.2, and 5.5 mM glucose). Cells were resuspended in 5 ml hypotonic lysis buffer plus protease inhibitors (50 mM Hepes, pH 7.0, protease inhibitor cocktail (P8340, 1:250 dilution), 1 mM PMSF, 2.5 mM TPCK and 100 μM E64) and then incubated for 1 h on ice. Three rounds of freeze-thaw were applied to the cells (5 min on dry ice/ethanol bath, 5 min at 37°C in water bath). Then cells were sonicated 3 times for 30 s at 40% amplitude, keeping them on ice for at least 1 min between pulses. Cell lysis was verified under light microscope and lysates were filtered through a 5 μm pore nitrocellulose membrane to remove cell ghosts. Protein concentration was determined by BCA Protein Assay kit. Cell lysates were stored at −80°C until processed for polyP-binding protein pull down within a week after lysis.
PolyP biotinylation
Biotinylated polyP was prepared as previously described (Choi et al., 2010). Briefly, medium size polyP (<1000 mers) was end-labeled by the covalent linkage of a primary biotinylated amine (amine-PEG2-biotin) to the terminal phosphates of polyP in the presence of 1-ethyl-3-[3-dimetyhlamino-propyl]carbodiimide (EDAC) (Choi et al., 2010).
PolyP-binding protein pull down
Twenty mg streptavidin-coated magnetic beads (Dynabeads MyOne Streptavidin T1) were washed three times with dynabeads wash buffer (1 M LiCl, 50 mM Tris-HCl, pH 7.4) in a 15 ml tube using a DynaMag™ magnet. Beads were incubated with 1 × 10−5 moles biotinylated polyP (resuspended in 5 ml dynabeads wash buffer) for 1 h, at RT under rotation. Then, beads were washed twice with 10 ml wash buffer and once with 10 ml 50 mM Tris-HCl, pH 7.4. Ten pull down rounds were performed as follows: 10 mg protein from T. cruzi (or T. brucei) total lysate were diluted in 10 ml wash buffer (50 mM Tris-HCl pH 7.4, 5 mM EDTA, 0.1% polyethylenglycol (PEG), 0.01% sodium azide) plus phosphatase inhibitors (2 mM imidazole, 1 mM sodium fluoride, 1.15 mM sodium molybdate, 1 mM sodium orthovanadate and 4 mM sodium tartrate) and filtered through a 0.22 μm pore nitrocellulose membrane. Diluted/filtered protein extracts were incubated with 10 mg polyP-coated beads on a rotator for 30 min at RT. Beads were washed 5 times with 10 ml wash buffer and polyP-binding proteins were eluted with 500 μl elution buffer (1 M NaCl, 50 mM Tris-HCl, pH 7.4). Eluates from 100 mg initial protein extract were combined and precipitated with trichloroacetic acid (TCA). Finally, polyP-binding proteins were resuspended in 500 μl 50 mM Tris-HCl pH 7.4 and quantified by BCA Protein Assay kit. As a control of binding specificity, biotinylated heparin was bound to streptavidin-coated magnetic beads following the same protocol, and then used for protein pull down from T. cruzi and T. brucei total protein extracts, as performed with polyP-coated beads.
Sample preparation
Samples containing T. brucei and T. cruzi polyP-binding proteins from two independent experiments were processed at the Protein Sciences Facility of University of Illinois (Urbana, IL) for liquid chromatography tandem mass spectrometry analysis (LC-MS/MS). Sample cleanup was performed using Perfect Focus according to manufacturer’s instructions. Protein samples were reduced in 10 mM DTT at 56°C for 30 min and alkylation was performed using 20 mM iodoacetamide for 30 min in the dark. Samples were digested with trypsin (G-Biosciences, St. Louis, MO) at a ratio of 1:10 – 1:50 using a CEM Discover Microwave Digestor (Mathews, SC) at 55˚C for 15 min. Digested peptides were extracted with 50% acetonitrile, 5% formic acid, dried under vacuum and resuspended in 5% acetonitrile, 0.1% formic acid for LC-MS/MS analysis.
Mass spectrometry
LC-MS/MS was performed using a Thermo Dionex Ultimate RSLC3000 operating in nano mode at 300 microliters/min with a gradient from 0.1% formic acid to 60% acetonitrile plus 0.1% formic acid in 120 min. The trap column used was a Thermo Acclaim PepMap 100 (100 µm x 2 cm) and the analytical column was a Thermo Acclaim PepMap RSLC (75 µm x 15 cm).
Data analysis
Xcalibur raw files were converted by Mascot Distiller interface (Matrix Science) into peak lists that were submitted to Mascot Server to search against specific protein databases: T. brucei Lister 427 and T. cruzi CL Brener (Esmeraldo and Non-Esmeraldo-like) on TriTrypDB (Aslett et al., 2010). Proteins found in control samples from T. cruzi and T. brucei pull downs using heparin-coated beads, with Mascot scores higher than 50, were considered unspecific binding proteins and subtracted from polyP-binding proteomes of T. cruzi and T. brucei, respectively.
PPBD conjugation to Alexa Fluor 488
E. coli TOP10 F’ harboring pTrc-PPBD plasmid (Saito et al., 2005) was grown in 550 ml of Luria-Bertani (LB) broth with 50 μg/ml ampicillin at 37ºC until reaching an OD600 of 0.6. The recombinant PPBD expression was induced with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) at 37ºC for 3 h. Cells were harvested by centrifugation at 10,000 x g for 10 min at 4ºC and resuspended in 30 ml of binding buffer (phosphate-buffered saline (PBS: 20 mM Na2HPO4, 300 mM NaCl), pH 7.4, plus 10 mM imidazole), containing protease inhibitor cocktail (P8849, 50 μl per g of pellet), 100 μg/ml lysozyme, and 75 U benzonase. The sample was incubated on ice for 30 min and then cells were lysed on ice for 2.5 min with 10 s-pulses (20 s between pulses) of a Branson Sonifier Cell Disruptor with Microtip. Cell debris were removed by centrifugation at 20,000 x g for 20 min at 4ºC. The supernatant was filtered through a 0.45-μm membrane filter and loaded. PPBD purification was performed at 4ºC using 1 ml HisPur Ni-NTA Chromatography Cartridge coupled to an ÄKTA Prime Plus chromatography system (GE Healthcare) selecting the standard program His Tag Purification His Trap. The column was washed with binding buffer and PPBD was eluted with elution buffer (PBS, pH 7.4, plus 300 mM imidazole). Both buffers were previously filtered through 0.22-μm membrane filter. The purity of PPBD in the FPLC fractions was determined by SDS-PAGE and the gel was stained with Coomassie Blue G-250. The purest PPBD fractions were combined and the buffer was exchanged to PBS, pH 7.4, using a 3–12 ml 10 kDa Slide-A-Lyzer Dialysis Cassette at 4ºC (a total of four buffer exchanges, including an overnight exchange). After dialysis, the sample was concentrated using an Amicon Ultra-15 10K device (Millipore) until reaching a volume of 1–1.5 ml. Protein concentration was quantified by using the BCA Protein Assay kit. Fluorescent labeling of PPBD (100 μg) was done using the Alexa Fluor 488 Microscale Protein Labeling kit as recommended by the manufacturer. Alexa Fluor 488-labeled PPBD was kept in 10% glycerol at −20ºC and protected from light for further experiments. Alexa Fluor 488-labeled PPBD concentration (in mg/ml) was determined by absorbance at 280 nm (A280) and at 494 nm (A494) according to the manufacturer’s manual.
Localization of polyP and co-localization with cellular markers in T. brucei and T. cruzi
Alexa Fluor 488-labeled PPBD was used for localization of polyP and co-localization with cellular markers in PCF and BSF of T. brucei, and in epimastigotes, trypomastigotes, and amastigotes of T. cruzi. Incubations with PPBD were always performed in tris-buffered saline (TBS: 100 mM Tris-HCl, 150 mM NaCl).
For co-localization of PPBD and mitochondria, PCF and BSF live cells were labeled for 30 min with 50 nM MitoTracker Red CMXRos in the corresponding culture medium before the immunofluorescence assays.
PCF (Lister 427), and Y strain epimastigotes and trypomastigotes/amastigotes were washed twice with BAG and then fixed with 4% paraformaldehyde in TBS, pH 7.4 (TBS1) at RT for 1 h. BSF (Lister 427) were washed with ice-cold TBS1 containing 1% glucose and then fixed with 1% paraformaldehyde in the same buffer at 4°C for 1 h. After fixation, all cells were washed twice with TBS1 and allowed to adhere to poly-L-lysine-coated coverslips for 30 min. The coverslips were washed three times with TBS1 and the fixed parasites were permeabilized with 0.3% Triton X-100 in TBS1 for 3 min for PCF, epimastigotes, and trypomastigotes/amastigotes or 0.1% Triton X-100 in TBS1 for 3 min for BSF. Coverslips were washed twice with TBS1 and blocked with TBS1 containing 5% goat serum, 50 mM NH4Cl, 3% BSA, 1% fish gelatin at 4ºC overnight.
After blocking, for co-localization with mitochondria, cells which were pre-incubated with MitoTracker were then incubated with Alexa Fluor 488-labeled PPBD (8 μg/ml) diluted in 1% BSA in TBS, pH 8.0 (TBS2), for 1 h, at RT in the dark.
For co-localization with other cellular compartments, after blocking cells were concomitantly incubated with Alexa Fluor 488-labeled PPBD (8 μg/ml) and one of the following primary antibodies diluted in 1% BSA in TBS2 for 1 h at RT in the dark: monoclonal mouse anti-TbPPDK antibody (glycosomal marker, 1:30 dilution), polyclonal rabbit anti-TbVP1 antibody (acidocalcisomal marker, 1:500 dilution for PCF, 1:1,000 dilution for BSF, epimastigotes, and trypomastigotes/amastigotes), or polyclonal rabbit anti-BiP antibody (endoplasmic reticulum marker, 1:500). All these coverslips were washed three times with 1% BSA in TBS2 and then incubated with Alexa Fluor 546-conjugated goat anti-rabbit or Alexa Fluor 546-conjugated goat anti-mouse secondary antibodies (1:1,000 dilution) diluted in 1% BSA in TBS2 for 1 h at RT in the dark.
For the co-localization of PPBD and a nucleolus marker in PCF, following the blocking step, an immunofluorescence assay with three incubation steps was performed due to PPBD works well in TBS but not in PBS, and L1C6 antibody works well in PBS: (1) incubation with monoclonal mouse L1C6 antibody (1:200 dilution in PBS, pH 8.0, plus 1% BSA) followed by three washes with the same buffer; (2) incubation with the secondary antibody Alexa Fluor 546-conjugated goat anti-mouse (1:1,000 dilution in 1% BSA in PBS, pH 8.0) in the dark followed by three washes with TBS2 and (3) incubation with Alexa Fluor 488-labeled PPBD (2 μg/ml in TBS2 plus 1% BSA) in the dark; all incubations for 1 h at RT. In this case, PPBD was used at a lower concentration to minimize glycosomal labeling and show mainly the nucleolus labeling.
For all IFAs cited above, following the final incubation step with either PPBD or antibody, cells were washed three times with 1% BSA in TBS2, washed once with TBS2 and counterstained with DAPI (3 μg/ml) in Fluoromount-G mounting medium on the slides. Differential interference contrast and fluorescent optical images were taken with a 100X oil immersion objective (1.35 aperture) under nonsaturating conditions with a Photometrix CoolSnapHQ charge-coupled device camera driven by DeltaVision software (Applied Precision, Issaquah, WA) and deconvolved for 15 cycles using SoftWoRx deconvolution software. For super-resolution microscopy images were taken with a 100X oil immersion objective, a high-power solid-state 405 nm laser and EM-CCD camera (Andor iXon) under nonsaturating conditions in a Zeiss ELYRA S1 (SR-SIM) super-resolution microscope. Images were acquired and processed with ZEN 2011 software with SIM analysis module. Videos were done with Imaris Version 8.0 software using the Surface Reconstruction and Animation functions. For conventional fluorescent images of T. cruzi epimastigotes showing cytosolic and nucleolar PPBD labeling, images were acquired using a 100X oil immersion objective, with an Olympus BX60 fluorescence microscope coupled to Olympus DP70 digital camera, and processed with DP controller software.
Effect of polyP in PPBD labeling
T. brucei PCF Lister 427 parasites were washed twice with BAG and fixed with 4% paraformaldehyde in TBS1 for 1 h at RT. After fixation, cells were washed twice with the same buffer and allowed to adhere to poly-L-lysine-coated coverslips for 30 min. The coverslips were washed three times with TBS1 and incubated with 0.3% Triton X-100 in TBS1 for 3 min. Permeabilized cells were washed twice with TBS1 and blocked with the same buffer, containing 5% goat serum, 50 mM NH4Cl, 3% BSA, and 1% fish gelatin at 4ºC overnight. In separate tubes, Alexa Fluor 488-labeled PPBD (8 μg/ml) was pre-incubated in the absence or the presence of polyP diluted in TBS2 containing 1% BSA at RT for 1 h in the dark. Three independent experiments of two types of assays were done: (1) Varying the concentration of polyP100: 1, 10, 100, and 1000 μM (in phosphate units), and (2) Varying the polyP chain length: 1 mM polyP (in phosphate units) of polyP3, polyP60, polyP100, and polyP700. These samples were then incubated with the parasites-coated coverslips for 1 h at RT in the dark. In both cases, a control was done by incubation of the coverslips with Alexa Fluor 488-labeled PPBD that was not pre-incubated with polyP. The cells were washed three times with 1% BSA in TBS2, washed once with TBS2, and counterstained with DAPI (3 μg/ml) in Fluoromount-G mounting medium on the slides. Differential interference contrast and fluorescent optical images were taken as above. Cells were imaged and fluorescence intensity of each cell was quantified after subtracting background fluorescence, using an image processing software (FiJi, Image J, University of Wisconsin-Madison, WI). The averages of fluorescence intensities were calculated and the relative fluorescence (in comparison to the control, using PPBD that was not pre-incubated with polyP) was plotted using GraphPad Prism software. For the assay with variation of polyP100 concentration, we examined fluorescence intensity of at least 42 cells from at least 10 fields per replicate, totalizing 471 cells for three biological replicates. For the assay with different polyP chain lengths, at least 56 cells from at least 20 fields per replicate, totalizing 664 cells for three biological replicates.
Subcellular fractionation
Fractions enriched in glycosomes were isolated and purified using two iodixanol gradient centrifugations as described previously (Huang et al., 2014), with some modifications (Fig. S6). PCF trypanosomes (3–4 g wet weight) were washed twice with Buffer A with glucose (BAG), and once with cold isolation buffer (125 mM sucrose, 50 mM KCl, 4 mM MgCl2, 0.5 mM EDTA, 20 mM Hepes, 5 mM dithiothreitol (DTT)) supplied with Complete, EDTA-free, protease inhibitor cocktail (Roche) prior to lysis with silicon carbide in isolation buffer. Silicon carbide and cell debris were eliminated by a series of low speed centrifugations (100 x g for 5 min, 300 x g for 10 min, and 1,200 x g for 10 min). The supernatant was centrifuged at 17,000 x g for 10 min, and the pellet was resuspended in 2.2 ml isolation buffer and applied to the 20% step of a discontinuous gradient with 4 ml steps of 20, 24, 28, 34, 37 and 40% iodixanol (diluted in isolation buffer). The gradient was centrifuged at 50,000 g in a Beckman JS-24.38 rotor for 60 min at 4oC, and fractions were collected from the top. The fractions containing crude glycosomes or acidocalcisomes (Fig. S6) were combined and then washed twice with 25 ml isolation buffer by centrifugation at 17,000 g for 15 min at 4oC. The washed pellet containing acidocalcisomes was used for polyP extraction and analysis. The washed pellet containing crude glycosomes was resuspended in 700 µl isolation buffer and applied to the 27% step of another discontinuous gradient of iodixanol, with 1.4 ml of isolation buffer containing 10% w/v sucrose over-layered on the top and 1 ml steps of 27, 62 and 80% iodixanol, which were diluted from 90% w/v iodixanol with isolation buffer. To prepare 90% w/v iodixanol, 60% w/v iodixanol solution (Optiprep) was dried completely at 70oC and resuspended with isolation buffer. After the second gradient centrifugation at 50,000 g for 60 min at 4oC, fractions were collected from the top, washed twice with isolation buffer by centrifugation at 20,000 g for 15 min at 4oC, and analyzed by glycosome or acidocalcisome marker enzyme assays. The protein concentration was quantified by Bradford assay using a SpectraMax Microplate Reader. The fractions 1 and 2, containing approximately 70% (Fig. 5A) of total proteins with the highest hexokinase activity (Fig. 5B), were combined and used for polyP extraction and analyses. Hexokinase (glycosome marker) and pyrophosphatase (PPase) (acidocalcisome marker) activities and immuno-blots were assayed as described previously (Huang et al., 2014). Mouse antibodies against TbPPDK (1:200) or rabbit antibodies against TbVP1 (1:3,000) were used as indicated.
Molecular constructs and transfection
For the amplification of ScPPX1 (Saccharomyces cerevisiae exopolyphosphatase) gene (GeneBank ID L28711.1) by PCR, the following primers were designed: ScPPX1-F (5’-TACCCAACTCGCTAGCTCGCCTTTGAGAAAGACGGTTCCTG-3’), and ScPPX1-R (5’-CCTTGCTCACGCTAGCCTCTTCCAGGTTTGAGTACGCTTCC-3’), (NheI restriction sites are in bold), and pTrcHisB-ScPPX1 plasmid (Wurst et al., 1995) was used as DNA template. PCR analysis was done in a reaction volume of 100 μl using Phusion High-Fidelity DNA polymerase with 20 ng of DNA, as follows: initial denaturation for 30 s at 98ºC, followed by 30 cycles of 10 s at 98ºC, 30 s at 55ºC, 1 min at 72ºC and then a final extension for 10 min at 72ºC. The pXS2-AldoPTS2-eYFP vector (Bauer et al., 2013), which provides resistance to blasticidin, was digested with NheI-HF. The 1.2 Kb-insert ScPPX1 was then cloned into NheI-digested pXS2-AldoPTS2-eYFP vector using the In-Fusion HD Cloning kit, generating the pXS2-PTS2-ScPPX1-eYFP plasmid. The recombination product was transformed into E. coli Stellar Competent Cells following the manufacturer’s instructions. The recombinant construct was confirmed by digestion with restriction enzymes, and sequencing. For T. brucei transfection, pXS2-PTS2-ScPPX1-eYFP plasmid was linearized with MluI and purified with QIAGEN’s DNA purification kit.
To attempt to deliver EcPPX to the nucleolus of T. brucei, we designed the primers NoLS-EcPPX-F (5’-CCCAAGCTTAAACCACCATGCGTATCGGAGGGAGACGGAAGGCTAACCCTCACCTTTTGCGTGAGATAGCTGATGTGACGATGGAGTTGAAAAGATATAGGAAGGGTCGTAGTGGTCCAATACACGATAAATCCCCTCGTC-3’) and EcPPX-R (5’-GCTCTAGAAGCGGCGATTTCTGGTGTACTTTCTTC-3’); respective HindIII and XbaI restriction sites are in bold and a reported nucleolar localization signal (NoLS) sequence is underlined (Hoek et al., 2000). NLS-EcPPX-HA plasmid was used as DNA template. PCR analysis was done in a reaction volume of 100 μl using Phusion High-Fidelity DNA polymerase with 20 ng of DNA, as follows: initial denaturation for 30 s at 98ºC, followed by 30 cycles of 10 s at 98ºC, 30 s at 55ºC, 1 min at 72ºC and then a final extension for 10 min at 72ºC. The 1.6 Kb-insert NoLS-EcPPX and the vector pLEW100-MCS-GFP were digested with HindIII-HF and XbaI. The ligation of NoLS-EcPPX and pLEW100-MCS-GFP was done with LigaFast Rapid DNA Ligation System, originating NoLS-EcPPX-GFP plasmid. The recombination product was transformed into Zymo 5α Mix & Go competent cells according to manufacturer’s instructions. The recombinant constructs were confirmed by digestion with restriction enzymes, and sequencing. For T. brucei transfection, NoLS-EcPPX-GFP plasmid was linearized with NotI-HF and purified with QIAGEN’s DNA purification kit.
T. brucei PCF Lister 427 was used for transfection of PTS2-ScPPX1-eYFP plasmid. T. brucei PCF 29–13 was used for transfection of the plasmid NoLS-EcPPX-GFP. Cell transfections were done as reported previously (Huang et al., 2014). In brief, parasites in exponential phase were harvested by centrifugation at 1,000 x g for 7 min at RT, washed once with 10 ml of Cytomix buffer (2 mM EGTA, 5 mM MgCl2, 120 mM KCl, 0.15 mM CaCl2, 10 mM K2HPO4, 25 mM Hepes, 0.5 % glucose, 0.1 mg/ml bovine serum albumin, and 1 mM hypoxanthine, pH 7.6) and resuspended in 0.45 ml of the same buffer at a cell density of 1.5 × 108 cells/ml (7 × 107 cells per cuvette). The washed cells were mixed with 10 μg of plasmid in a 4 mm electroporation cuvette and subjected to two pulses from a Bio-Rad Gene Pulser Xcell electroporator set at 1,500 V, 25 μF, 200 Ω, with resting on ice for 1 min between pulses. Negative controls were flasks containing cells without plasmid DNA, submitted to the electroporation and flasks containing cells without plasmid DNA that were not submitted to electroporation. Transfectant PTS2-ScPPX1-eYFP were cultured in SDM-79 medium supplemented with 15% heat-inactivated FBS plus 10 μg/ml blasticidin until stable cell lines were obtained. The protein PTS2-ScPPX1-eYFP is constitutively expressed in these parasites. Transfectant NoLS-EcPPX-GFP-expressing cells were cultured in SDM-79 medium supplemented with 15% tetracycline-free and heat-inactivated FBS plus 50 μg/ml hygromycin, 15 μg/ml G418, and 10 μg/ml blasticidin until stable cell lines were obtained.
Western blot analyses
To confirm the expression of the protein PTS2-ScPPX1-eYFP, mid log phase parasites from PCF wild type and PTS2-ScPPX1-eYFP-expressing cells were harvested by centrifugation at 1,000 x g for 7 min and washed twice with BAG at RT. The pellet was resuspended in 200 μl of RIPA buffer (150 mM NaCl, 20 mM Tris-HCl, pH 7.5, 1 mM EDTA, 1% sodium dodecyl sulfate, and 0.1% Triton X-100) plus 2 mM phenylmethanesulfonyl fluoride (PMSF), and protease inhibitor cocktail (P8340, 1:250 dilution). The samples were incubated on ice for 1 h and then homogenized with a 1 mL-syringe. The lysates were kept at −80 ºC until further use. The protein concentration in the lysates was determined using the BCA Protein Assay Kit. Approximately 20 μg of each lysate were submitted to electrophoresis using 4–20% Mini-Protean TGX Precast Protein Gel. Magic Mark XP Western Protein Standard was applied on the gel. Electrophoresed proteins were transferred to nitrocellulose membranes using a Bio-Rad transblot apparatus for 1 hour at 100 V at 4ºC. Following transfer, the membrane blots were blocked with 5% non-fat dry milk in PBS containing 0.1% (v/v) Tween-20 (PBS-T) at 4ºC overnight. Blots were probed with polyclonal rabbit anti-GFP antibody (1:10,000 dilution in PBS-T) or polyclonal rabbit anti-tubulin antibody (1:20,000 dilution in PBS-T) for 1 h at RT. After washing three times with PBS-T, the blots were incubated with horseradish peroxidase conjugated anti-rabbit IgG antibody (1:20,000 dilution in PBS-T) for 1 h at RT. The membranes were washed three times with PBS-T, and western blot images were processed and analyzed using ECL Western Blotting Substrate according to the manufacturer’s instructions.
For expression of the protein NoLS-EcPPX-GFP, the culture was induced with 1 μg/ml tetracycline for 48 h. Lysates from non induced and induced cultures were analysed by western blot that were performed as above with the following modifications. Approximately 50 μg of each lysate were submitted to electrophoresis using 10% SDS-PAGE. Blots were probed with polyclonal rabbit anti-GFP antibody (1:10,000 dilution in PBS-T) or polyclonal rabbit anti-tubulin antibody (1:20,000 dilution in PBS-T) for 1 h at RT.
For loading control of Fig. 6D, the western blot was performed using rabbit anti-tubulin antibody (1:10,000 dilution) and IRDye 680RD goat anti-rabbit (1:20,000 dilution) and was analyzed with Li-Cor Odyssey CLx Imaging System.
Immunofluorescence analyses
For immunofluorescence assays of PCF expressing PTS2-ScPPX1-eYFP, the cells were washed twice with BAG and fixed with 4% paraformaldehyde in PBS, pH 7.4, (PBS1) for 1 h at RT. After fixation, cells were washed twice with the same buffer and allowed to adhere to poly-L-lysine-coated coverslips for 30 min. The coverslips were washed three times with PBS1 and incubated with 0.3% Triton X-100 in PBS1 for 3 min. Permeabilized cells were washed twice with PBS1 and blocked with PBS1 containing 5% goat serum, 50 mM NH4Cl, 3% BSA, and 1% fish gelatin, at 4ºC overnight. After blocking, cells were incubated with the primary antibodies, polyclonal rabbit anti-GFP antibody (1:1,000 dilution), and monoclonal mouse anti-PPDK antibody (1:30 dilution) diluted in 1% BSA in PBS, pH 8.0 (PBS2) for 1 h at RT. The coverslips were washed three times with 1% BSA in PBS2 and then incubated with Alexa Fluor 488-conjugated goat anti-rabbit and Alexa Fluor 546-conjugated goat anti-mouse secondary antibodies (1:1,000 dilution) diluted in 1% BSA in PBS2 for 1 h at RT in the dark. The cells were washed three times with 1% BSA in PBS2, washed once with PBS2 and counterstained with DAPI (3 μg/ml) in Fluoromount-G mounting medium on the slides. Two negative controls were done, wild type PCF Lister 427, according to the protocol described above, and PTS2-ScPPX1-transfected PCF in the absence of primary antibodies. Differential interference contrast and fluorescent optical images were taken with DeltaVision system as described above. Pearson’s correlation coefficient was calculated using the SoftWoRx software by measuring the whole-cell images.
NoLS-EcPPX-GFP culture was induced with 1 μg/ml tetracycline for 24 h and IFA was performed as above with some modifications related to antibody incubations. A two-step IFA was chosen for NoLS-EcPPX-GFP-expressing cells due to PPBD works well in TBS but not in PBS, and rabbit anti-GFP antibody works well in PBS: until incubation with primary antibodies, PBS1 was used. Cells were then incubated with polyclonal rabbit anti-GFP antibody (1:2,000 dilution) diluted in 1% BSA in PBS2. Afterwards cells were washed three times with TBS2 and then concomitantly incubated with the secondary antibody Alexa Fluor 546-conjugated goat anti-rabbit, and PPBD (8 μg/ml), diluted in 1% BSA in TBS2. From this step all washes were done in TBS2. The negative control consisted in non-induced cells.
PPX activity determination
Parasites (4.5 × 107 cells) from wild type (Lister 427) and PTS2-ScPPX1-expressing PCF were harvested by centrifugation at 1,000 x g for 7 min at RT, washed twice with BAG at RT, and resuspended in 1 ml of resuspension buffer (150 mM NaCl, 20 mM Tris-HCl pH 7.5, 1 mM EDTA, 2 mM PMSF, and protease inhibitor cocktail (1:250 dilution, P8340)). Cells were lysed by 7 cycles of freezing and thawing (for freezing steps, the tubes were incubated in dry ice/ethanol bath, and for thawing steps, in water at RT) and then the lysates were homogenized using a 1 ml-syringe. In a 96-well plate, 20 μl of each lysate were incubated in the presence or the absence of 10 μM polyP100 (in phosphate units) in activity buffer (50 mM Tris-HCl pH 7.4, and 2.5 mM MgSO4) at 30ºC for 10 min, totalizing 100 μl of reaction volume. Negative controls were reaction buffers without lysates (blank) and lysates kept on ice (sample named “ice” on the graph). After the incubation time, the reactions were stopped by addition of 100 μl of freshly prepared malachite green solution (three parts of 0.045% malachite green and one part of 4.2% ammonium molybdate/4M HCl) (Lanzetta et al., 1979). Release of phosphate from polyP due to the PPX activity was measured in triplicate wells by reading the absorbance at 660 nm immediately on the plate reader Biotek Synergy H1. Three independent experiments were done. In all biological replicates, a standard curve of potassium phosphate was included in triplicate wells.
Short and long chain polyP extraction and analysis
Short chain polyP was extracted from wild type (Lister 427) and PTS2-ScPPX1-expressing PCF using a protocol reported previously (Ruiz et al., 2001). Long chain polyP was extracted from cells or from subcellular fractions according to a published protocol (Ault-Riche et al., 1998).
Quantification of short and long chain polyP (both extracted from 108 cells) was determined by measuring the amount of Pi released upon addition of recombinant ScPPX1 to the plate wells. For both short and long chain polyP quantification, 10 μl of polyP were incubated with 90 ng of recombinant ScPPX1 in activity buffer (50 mM Tris-HCl pH 7.4, and 2.5 mM MgSO4) at 30ºC for 1 h, in a final volume of 100 μl. A negative control was reaction buffer without enzyme. The rest of the protocol was followed as cited in the previous section. Three independent experiments were performed.
Short chain polyP was also resolved by PAGE. PCF (2 × 109 cells) were harvested by centrifugation at 1,000 x g for 7 min and washed twice with BAG. For loading controls, we collected from each sample 108 cells that were immediately lysed with RIPA buffer, the protein concentration measured using BCA Protein Assay kit (see methods for Western blot analysis) and used for western blot analysis with rabbit anti-tubulin antibodies. The other part of the cell cultures (1.9 × 109 cells) was centrifuged at 1,000 x g for 7 min and resuspended in 100 μl of ice-cold 1 M perchloric acid (HClO4) for polyP analysis. The samples were incubated on ice for 5 min and cell debris were removed by centrifugation at 18,000 x g for 5 min at 4ºC. The supernatants were transferred to new tubes and neutralized with 20 μl of neutralizing solution (0.6 M KHCO3, 0.72 M KOH). The pH was checked with pH strips and neutralized to 7.0–7.5, if necessary. The precipitated salt was removed by centrifugation at 18,000 x g for 5 min at 4ºC. An additional centrifugation step was carried out to eliminate remaining precipitates. The supernatants were immediately submitted to electrophoresis or kept at −80 ºC until further use. Short chain polyP was resolved by PAGE using 35.5% acrylamide/bis-acrylamide 19:1 gels in Tris/Borate/EDTA (TBE) buffer. Gels were stained with toluidine blue. PolyP density in each gel lane was quantified using FiJi software. Four independent experiments were done.
Analysis of long chain polyP in subcellular fractions from wild type PCF was done by PAGE using a 30% acrylamide/bis-acrylamide 19:1 gel stained with toluidine blue. Three independent biological experiments were done.
Effect of oxidative stress
Wild type (Lister 427) and PTS2-ScPPX1-expressing PCF (1.2 × 108 cells) were harvested by centrifugation at 1,000 x g for 7 min and resuspended in 4 ml of SDM-79 medium plus 10% heat-inactivated FBS (with addition of 10 μg/ml blasticidin to PTS2-ScPPX1-expressing PCF). Hydrogen peroxide (H2O2, 50 µM) was added and the cells were incubated at 28ºC for 1 h. Cultures were then diluted 10 times (with addition of blasticidin to the medium of PTS2-ScPPX1-expressing PCF) and incubated at 28ºC for 24 h. The cell density after 24 h was determined by counting parasites in a Neubauer chamber. Three independent experiments were done.
Glucose consumption
Wild type (Lister 427) and PTS2-ScPPX1-expressing PCF (108 cells/ml) were incubated in a modified SDM-79 medium (without phenol red and hemin plus 10% heat-inactivated FBS and addition of 10 μg/ml blasticidin to the PTS2-ScPPX1-expressing cells) for 6 h at 28 ºC. 100 μl of both cultures at time 0 and after 6 h of incubation were centrifuged at 14,000 x g for 1 min. Two μl of each supernatant were used to quantify glucose in triplicate plate wells, using the D-Glucose Assay Kit. After addition of 200 μl of glucose oxidase/peroxidase (GOPOD) reagent to each well, the plate was incubated at 48ºC for 20 min. The absorbance was read at 510 nm on the Biotek Synergy H1 plate reader. Three independent experiments were done. In all biological replicates, a standard curve of glucose was included in triplicate wells. The glucose consumption plotted in the graph was calculated by the difference in the amount of glucose in the supernatants at time 0 and after 6 h of incubation.
Statistical analyses
Values are expressed as means ± SEM, or means ± SD, as indicated. Significant differences between treatments were compared using unpaired Student’s t-test, and one-way and two-way ANOVA tests. Differences were considered statistically significant at P < 0.05, and n refers to the number of experiments performed. All statistical analyses were conducted using GraphPad Prism 6 (GraphPad Software, San Diego, CA).
Supplementary Material
Acknowledgements
We thank Norbert Bakalara, Jay Bangs, Frederic Bringaud, Keith Gull, Meredith Morris, Katsuharu Saito, George Cross, and Toshikazu Shiba for reagents, Stephen Vella for his help with the videos, and Muthugapatti Kandasamy and the Biomedical Microscopy Core of the University of Georgia for the use of microscopes. We also thank Peter Yau of the Roy J. Carver Biotechnology Center at the University of Illinois at Urbana-Champaign for performing the mass spectrometry. This work was funded by U.S. National Institutes of Health (grants AI0077538 and AI107633 to R.D., and R35 HL13582 to J.H.M.). R.S.N. was a postdoctoral fellow of the National Council for Scientific and Technological Development (CNPq, Brazil, 206380/2014–3). N.L. was a pre-doctoral fellow of the American Heart Association (ID Number: 12PRE12060508).
References
- Acosta H, Dubourdieu M, Quinones W, Caceres A, Bringaud F, and Concepcion JL (2004) Pyruvate phosphate dikinase and pyrophosphate metabolism in the glycosome of Trypanosoma cruzi epimastigotes. Comp Biochem Physiol B Biochem Mol Biol 138: 347–356. [DOI] [PubMed] [Google Scholar]
- Antonenkov VD, and Hiltunen JK (2012) Transfer of metabolites across the peroxisomal membrane. Biochim Biophys Acta 1822: 1374–1386. [DOI] [PubMed] [Google Scholar]
- Aslett M, Aurrecoechea C, Berriman M, Brestelli J, Brunk BP, Carrington M, Depledge DP, Fischer S, Gajria B, Gao X, Gardner MJ, Gingle A, Grant G, Harb OS, Heiges M, Hertz-Fowler C, Houston R, Innamorato F, Iodice J, Kissinger JC, Kraemer E, Li W, Logan FJ, Miller JA, Mitra S, Myler PJ, Nayak V, Pennington C, Phan I, Pinney DF, Ramasamy G, Rogers MB, Roos DS, Ross C, Sivam D, Smith DF, Srinivasamoorthy G, Stoeckert CJ Jr., Subramanian S, Thibodeau R, Tivey A, Treatman C, Velarde G, and Wang H (2010) TriTrypDB: a functional genomic resource for the Trypanosomatidae. Nucleic Acids Res 38: D457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault-Riche D, Fraley CD, Tzeng CM, and Kornberg A (1998) Novel assay reveals multiple pathways regulating stress-induced accumulations of inorganic polyphosphate in Escherichia coli. J Bacteriol 180: 1841–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo C, Livermore T, and Saiardi A (2015) Protein polyphosphorylation of lysine residues by inorganic polyphosphate. Mol Cell 58: 71–82. [DOI] [PubMed] [Google Scholar]
- Bangs JD, Uyetake L, Brickman MJ, Balber AE, and Boothroyd JC (1993) Molecular cloning and cellular localization of a BiP homologue in Trypanosoma brucei. Divergent ER retention signals in a lower eukaryote. J Cell Sci 105 ( Pt 4): 1101–1113. [DOI] [PubMed] [Google Scholar]
- Bauer S, Morris JC, and Morris MT (2013) Environmentally regulated glycosome protein composition in the African trypanosome. Eukaryot Cell 12: 1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley-DeSousa A, Holinier C, Moteshareie H, Tseng YC, Kajjo S, Nwosu C, Amodeo GF, Bondy-Chorney E, Sai Y, Rudner A, Golshani A, Davey NE, and Downey M (2018) A screen for candidate targets of lysine polyphosphorylation uncovers a conserved network implicated in ribosome biogenesis. Cell Rep 22: 3427–3439. [DOI] [PubMed] [Google Scholar]
- Bone GJ, and Steinert M (1956) Isotopes incorporated in the nucleic acids of Trypanosoma mega. Nature 178: 308–309. [DOI] [PubMed] [Google Scholar]
- Brangwynne CP, Mitchinson TJ, adn Hyman AA (2011) Active liquid-like behavior of nucleoli determines their size and shape in Xenopu laevis oocytes. Proc Natl Acad Sci U S A 108: 4334–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringaud F, Baltz D, and Baltz T (1998) Functional and molecular characterization of a glycosomal PPi-dependent enzyme in trypanosomatids: pyruvate, phosphate dikinase. Proc Natl Acad Sci U S A 95: 7963–7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres AJ, Portillo R, Acosta H, Rosales D, Quinones W, Avilan L, Salazar L, Dubourdieu M, Michels PA, and Concepcion JL (2003) Molecular and biochemical characterization of hexokinase from Trypanosoma cruzi. Mol Biochem Parasitol 126: 251–262. [DOI] [PubMed] [Google Scholar]
- Chambers JW, Kearns MT, Morris MT, and Morris JC (2008) Assembly of heterohexameric trypanosome hexokinases reveals that hexokinase 2 is a regulable enzyme. J Biol Chem 283: 14963–14970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Collins JN, Smith SA, Davis-Harrison RL, Rienstra CM, and Morrissey JH (2010) Phosphoramidate end labeling of inorganic polyphosphates: facile manipulation of polyphosphate for investigating and modulating its biological activities. Biochemistry 49: 9935–9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers CM, Knoefler D, Gates S, Martin N, Dahl JU, Lempart J, Xie L, Chapman MR, Galvan V, Southworth DR, and Jakob U (2016) Polyphosphate: a conserved modifier of amyloidogenic processes. Mol Cell 63: 768–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham I, and Honigberg BM (1977) Infectivity reacquisition by Trypanosoma brucei brucei cultivated with tsetse salivary glands. Science 197: 1279–1282. [DOI] [PubMed] [Google Scholar]
- Devaux S, Kelly S, Lecordier L, Wickstead B, Perez-Morga D, Pays E, Vanhamme L, and Gull K (2007) Diversification of function by different isoforms of conventionally shared RNA polymerase subunits. Mol Biol Cell 18: 1293–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo R (2016) The origin and evolution of the acidocalcisome and its interactions with other organelles. Mol Biochem Parasitol 209: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo R, de Souza W, Miranda K, Rohloff P, and Moreno SN (2005) Acidocalcisomes - conserved from bacteria to man. Nat Rev Microbiol 3: 251–261. [DOI] [PubMed] [Google Scholar]
- Docampo R, and Huang G (2016) Acidocalcisomes of eukaryotes. Curr Opin Cell Biol 41: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Rohloff P, Miranda K, and Docampo R (2007a) Ablation of a small transmembrane protein of Trypanosoma brucei (TbVTC1) involved in the synthesis of polyphosphate alters acidocalcisome biogenesis and function, and leads to a cytokinesis defect. Biochem J 407: 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Ruiz FA, Docampo M, Luo S, Rodrigues JC, Motta LS, Rohloff P, and Docampo R (2007b) Overexpression of a Zn2+-sensitive soluble exopolyphosphatase from Trypanosoma cruzi depletes polyphosphate and affects osmoregulation. J Biol Chem 282: 32501–32510. [DOI] [PubMed] [Google Scholar]
- Gabaldon T, Ginger ML, and Michels PA (2016) Peroxisomes in parasitic protists. Mol Biochem Parasitol 209: 35–45. [DOI] [PubMed] [Google Scholar]
- Gomez-Garcia MR, Ruiz-Perez LM, Gonzalez-Pacanowska D, and Serrano A (2004) A novel calcium-dependent soluble inorganic pyrophosphatase from the trypanosomatid Leishmania major. FEBS Lett 560: 158–166. [DOI] [PubMed] [Google Scholar]
- Gray MJ, Wholey WY, Wagner NO, Cremers CM, Mueller-Schickert A, Hock NT, Krieger AG, Smith EM, Bender RA, Bardwell JC, and Jakob U (2014) Polyphosphate is a primordial chaperone. Mol Cell 53: 689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JB, Davidian NM, and Penniall R (1965) Studies of phosphorus metabolism by isolated nuclei. VII. Identification of polyphosphate as a product. J Biol Chem 240: 4427–4434. [PubMed] [Google Scholar]
- Gualdron-Lopez M, Vapola MH, Miinalainen IJ, Hiltunen JK, Michels PA, and Antonenkov VD (2012) Channel-forming activities in the glycosomal fraction from the bloodstream form of Trypanosoma brucei. PLoS One 7: e34530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guther ML, Urbaniak MD, Tavendale A, Prescott A, and Ferguson MA (2014) High-confidence glycosome proteome for procyclic form Trypanosoma brucei by epitope-tag organelle enrichment and SILAC proteomics. J Proteome Res 13: 2796–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek M, Engstler M, and Cross GA (2000) Expression-site-associated gene 8 (ESAG8) of Trypanosoma brucei is apparently essential and accumulates in the nucleolus. J Cell Sci 113 ( Pt 22): 3959–3968. [DOI] [PubMed] [Google Scholar]
- Hothorn M,NH, Lenherr ED, Wehner M, Rybin V, Hassa PO, Uttenweiler A, Reinhardt M, Schmidt A, Seiler J, Ladurner AG, Herrmann C, Scheffzek K, and Mayer A. (2009) Catalytic core of a membrane-associated eukaryotic polyphosphate polymerase. Science 324: 513–516. [DOI] [PubMed] [Google Scholar]
- Huang G, Ulrich PN, Storey M, Johnson D, Tischer J, Tovar JA, Moreno SN, Orlando R, and Docampo R (2014) Proteomic analysis of the acidocalcisome, an organelle conserved from bacteria to human cells. PLoS Pathog 10: e1004555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Nunez MD, Moreno-Sanchez D, Hernandez-Ruiz L, Benitez-Rondan A, Ramos-Amaya A, Rodriguez-Bayona B, Medina F, Brieva JA, and Ruiz FA (2012) Myeloma cells contain high levels of inorganic polyphosphate which is associated with nucleolar transcription. Haematologica 97: 1264–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH (2014) Chaperoned by prebiotic inorganic polyphosphate molecules: an ancient transcription-independent mechanism to restore protein homeostasis. Mol Cell 53: 685–687. [DOI] [PubMed] [Google Scholar]
- Klompmaker SH, Kohl K, Fasel N, and Mayer A (2017) Magnesium uptake by connecting fluid-phase endocytosis to an intracellular inorganic cation filter. Nat Commun 8: 1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg A (1995) Inorganic polyphosphate: toward making a forgotten polymer unforgettable. J Bacteriol 177: 491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulaev I, and Kulakovskaya T (2000) Polyphosphate and phosphate pump. Annu Rev Microbiol 54: 709–734. [DOI] [PubMed] [Google Scholar]
- Kumble KD, and Kornberg A (1995) Inorganic polyphosphate in mammalian cells and tissues. J Biol Chem 270: 5818–5822. [DOI] [PubMed] [Google Scholar]
- Kuroda A, Nomura K, Ohtomo R, Kato J, Ikeda T, Takiguchi N, Ohtake H, and Kornberg A (2001) Role of inorganic polyphosphate in promoting ribosomal protein degradation by the Lon protease in E. coli. Science 293: 705–708. [DOI] [PubMed] [Google Scholar]
- Landeira D, and Navarro M (2007) Nuclear repositioning of the VSG promoter during developmental silencing in Trypanosoma brucei. J Cell Biol 176: 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander N, Ulrich PN, and Docampo R (2013) Trypanosoma brucei vacuolar transporter chaperone 4 (TbVtc4) is an acidocalcisome polyphosphate kinase required for in vivo infection. J Biol Chem 288: 34205–34216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzetta PA, Alvarez LJ, Reinach PS, and Candia OA (1979) An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem 100: 95–97. [DOI] [PubMed] [Google Scholar]
- Lemercier G, Dutoya S, Luo S, Ruiz FA, Rodrigues CO, Baltz T, Docampo R, and Bakalara N (2002) A vacuolar-type H+-pyrophosphatase governs maintenance of functional acidocalcisomes and growth of the insect and mammalian forms of Trypanosoma brucei. J Biol Chem 277: 37369–37376. [DOI] [PubMed] [Google Scholar]
- Lemercier G, Espiau B, Ruiz FA, Vieira M, Luo S, Baltz T, Docampo R, and Bakalara N (2004) A pyrophosphatase regulating polyphosphate metabolism in acidocalcisomes is essential for Trypanosoma brucei virulence in mice. J Biol Chem 279: 3420–3425. [DOI] [PubMed] [Google Scholar]
- Lonetti A, Szijgyarto Z, Bosch D, Loss O, Azevedo C, and Saiardi A (2011) Identification of an evolutionarily conserved family of inorganic polyphosphate endopolyphosphatases. J Biol Chem 286: 31966–31974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn WS, and Brown RH (1963) Synthesis of polyphosphate by rat liver mitochondria. Biochem Biophys Res Commun 11: 367–371. [DOI] [PubMed] [Google Scholar]
- Maldonado RA, and Fairlamb AH (2001) Cloning of a pyruvate phosphate dikinase from Trypanosoma cruzi. Mol Biochem Parasitol 112: 183–191. [DOI] [PubMed] [Google Scholar]
- Mani J, Guttinger A, Schimanski B, Heller M, Acosta-Serrano A, Pescher P, Spath G, and Roditi I (2011) Alba-domain proteins of Trypanosoma brucei are cytoplasmic RNA-binding proteins that interact with the translation machinery. PLoS One 6: e22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney P, Mizutani T, and Shiba T (2006) Inorganic polyphosphate interacts with ribosomes and promotes translation fidelity in vitro and in vivo. Mol Microbiol 60: 438–447. [DOI] [PubMed] [Google Scholar]
- McNae IW, Martinez-Oyanedel J, Keillor JW, Michels PA, Fothergill-Gilmore LA, and Walkinshaw MD (2009) The crystal structure of ATP-bound phosphofructokinase from Trypanosoma brucei reveals conformational transitions different from those of other phosphofructokinases. J Mol Biol 385: 1519–1533. [DOI] [PubMed] [Google Scholar]
- Michels PA, Chevalier N, Opperdoes FR, Rider MH, and Rigden DJ (1997) The glycosomal ATP-dependent phosphofructokinase of Trypanosoma brucei must have evolved from an ancestral pyrophosphate-dependent enzyme. Eur J Biochem 250: 698–704. [DOI] [PubMed] [Google Scholar]
- Michels PA, Hannaert V, and Bringaud F (2000) Metabolic aspects of glycosomes in trypanosomatidae - new data and views. Parasitol Today 16: 482–489. [DOI] [PubMed] [Google Scholar]
- Michels PA, and Opperdoes FR (1991) The evolutionary origin of glycosomes. Parasitol Today 7: 105–109. [DOI] [PubMed] [Google Scholar]
- Moreno B, Urbina JA, Oldfield E, Bailey BN, Rodrigues CO, and Docampo R (2000) 31P NMR spectroscopy of Trypanosoma brucei, Trypanosoma cruzi, and Leishmania major. Evidence for high levels of condensed inorganic phosphates. J Biol Chem 275: 28356–28362. [DOI] [PubMed] [Google Scholar]
- Moreno SN, and Docampo R (2013) Polyphosphate and its diverse functions in host cells and pathogens. PLoS Pathog 9: e1003230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno SN, Silva J, Vercesi AE, and Docampo R (1994) Cytosolic-free calcium elevation in Trypanosoma cruzi is required for cell invasion. J Exp Med 180: 1535–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Sanchez D, Hernandez-Ruiz L, Ruiz FA, and Docampo R (2012) Polyphosphate is a novel pro-inflammatory regulator of mast cells and is located in acidocalcisomes. J Biol Chem 287: 28435–28444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenbacher S, and Kline ES (1984) Evidence for polyphosphate in phosphorylated nonhistone nuclear proteins. Arch Biochem Biophys 231: 114–123. [DOI] [PubMed] [Google Scholar]
- Opperdoes FR, and Borst P (1977) Localization of nine glycolytic enzymes in a microbody-like organelle in Trypanosoma brucei: the glycosome. FEBS Lett 80: 360–364. [DOI] [PubMed] [Google Scholar]
- Pabon MA, Caceres AJ, Gualdron M, Quinones W, Avilan L, and Concepcion JL (2007) Purification and characterization of hexokinase from Leishmania mexicana. Parasitol Res 100: 803–810. [DOI] [PubMed] [Google Scholar]
- Park JH, Brekken DL, Randall AC, and Parsons M (2002) Molecular cloning of Trypanosoma brucei CK2 catalytic subunits: the alpha isoform is nucleolar and phosphorylates the nucleolar protein Nopp44/46. Mol Biochem Parasitol 119: 97–106. [DOI] [PubMed] [Google Scholar]
- Pisoni RL, and Lindley ER (1992) Incorporation of [32P]orthophosphate into long chains of inorganic polyphosphate within lysosomes of human fibroblasts. J Biol Chem 267: 3626–3631. [PubMed] [Google Scholar]
- Ramos IB, Miranda K, Pace DA, Verbist KC, Lin FY, Zhang Y, Oldfield E, Machado EA, De Souza W, and Docampo R (2010) Calcium- and polyphosphate-containing acidic granules of sea urchin eggs are similar to acidocalcisomes, but are not the targets for NAADP. Biochem J 429: 485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao NN, Gomez-Garcia MR, and Kornberg A (2009) Inorganic polyphosphate: essential for growth and survival. Annu Rev Biochem 78: 605–647. [DOI] [PubMed] [Google Scholar]
- Rodrigues CO, Ruiz FA, Vieira M, Hill JE, and Docampo R (2002) An acidocalcisomal exopolyphosphatase from Leishmania major with high affinity for short chain polyphosphate. J Biol Chem 277: 50899–50906. [DOI] [PubMed] [Google Scholar]
- Rodriguez E, Lander N Ramirez JL (2009) Molecular and biochemical characterisation of Trypanosoma cruzi phosphofructokinase. Memorias do Instituto Oswaldo Cruzi 104: 745–748. [DOI] [PubMed] [Google Scholar]
- Ruiz FA, Rodrigues CO, and Docampo R (2001) Rapid changes in polyphosphate content within acidocalcisomes in response to cell growth, differentiation, and environmental stress in Trypanosoma cruzi. J Biol Chem 276: 26114–26121. [DOI] [PubMed] [Google Scholar]
- Saiardi A, (2012) How inositol pyrophosphates control cellular phosphate homeostasis? Adv. Biol. Reg 52 351–359. [DOI] [PubMed] [Google Scholar]
- Saito K, Kuga-Uetake Y, Saito M, and Peterson RL (2006) Vacuolar localization of phosphorus in hyphae of Phialocephala fortinii, a dark septate fungal root endophyte. Can J Microbiol 52: 643–650. [DOI] [PubMed] [Google Scholar]
- Saito K, Ohtomo R, Kuga-Uetake Y, Aono T, and Saito M (2005) Direct labeling of polyphosphate at the ultrastructural level in Saccharomyces cerevisiae by using the affinity of the polyphosphate binding domain of Escherichia coli exopolyphosphatase. Appl Environ Microbiol 71: 5692–5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DA, Docampo R, Dvorak JA, Shi S, and Leapman RD (1997) In situ compositional analysis of acidocalcisomes in Trypanosoma cruzi. J Biol Chem 272: 28020–28029. [DOI] [PubMed] [Google Scholar]
- Semenyuk PI, Muronetz VI, Haertle T, and Izumrudov VA (2013) Effect of poly(phosphate) anions on glyceraldehyde-3-phosphate dehydrogenase structure and thermal aggregation: comparison with influence of poly(sulfoanions). Biochim Biophys Acta 1830: 4800–4805. [DOI] [PubMed] [Google Scholar]
- Shih S, Stenberg P, and Ullman B (1998) Immunolocalization of Trypanosoma brucei hypoxanthine-guanine phosphoribosyltransferase to the glycosome. Mol Biochem Parasitol 92: 367–371. [DOI] [PubMed] [Google Scholar]
- Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, and Morrissey JH (2006) Polyphosphate modulates blood coagulation and fibrinolysis. Proc Natl Acad Sci U S A 103: 903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich PN, Lander N, Kurup SP, Reiss L, Brewer J, Soares Medeiros LC, Miranda K, and Docampo R (2014) The acidocalcisome vacuolar transporter chaperone 4 catalyzes the synthesis of polyphosphate in insect-stages of Trypanosoma brucei and T. cruzi. J Eukaryot Microbiol 61: 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorisek J, Knotkova A, and Kotyk A (1982) Fine cytochemical localization of polyphosphates in the yeast Saccharomyces cerevisiae. Zentralbl Mikrobiol 137: 421–432. [PubMed] [Google Scholar]
- Wierenga RK, Swinkels B, Michels PA, Osinga K, Misset O, Van Beeumen J, Gibson WC, Postma JP, Borst P, Opperdoes FR, and et al. (1987) Common elements on the surface of glycolytic enzymes from Trypanosoma brucei may serve as topogenic signals for import into glycosomes. EMBO J 6: 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S, Meyer DJ, Taylor MC, Bromley EV, Miles MA, Kelly JM (2002) The Trypanosoma cruzi enzyme TcGPX1 is a glycosomal peroxidase and caan be linked to trypanothione reduction by glutathione or tryparedoxin,. J Biol Chem 277: 17062–17071. [DOI] [PubMed] [Google Scholar]
- Wurst H, and Kornberg A (1994) A soluble exopolyphosphatase of Saccharomyces cerevisiae. Purification and characterization. J Biol Chem 269: 10996–11001. [PubMed] [Google Scholar]
- Wurst H, Shiba T, and Kornberg A (1995) The gene for a major exopolyphosphatase of Saccharomyces cerevisiae. J Bacteriol 177: 898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ko TP, Chen CC, Huang G, Zheng Y, Liu W, Wang I, Ho MR, Hsu ST, O’Dowd B, Huff HC, Huang CH, Docampo R, Oldfield E, and Guo RT (2016) Structures of trypanosome vacuolar soluble pyrophosphatases: antiparasitic drug targets. ACS Chem Biol 11: 1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


