Abstract
Sex steroid hormones are major regulators of uterine and placental growth and functions, as well as many other biological processes. To examine the mRNA expression of nuclear estrogen (ESR1 and 2) and progesterone (PGRAB and B) receptors in different compartments of the uterus and placenta, tissues were collected in experiment 1, on days 16, 20 and 28 after natural mating (NAT) and on day 10 after estrus (non-pregnant controls [NP]); and in experiment 2, on day 22 of NAT, and pregnancies established after transfer of embryos generated through mating of FSH-treated ewes (NAT-ET), in vitro fertilization (IVF), or in vitro activation (IVA; parthenotes). In experiment 1, ESR1 expression in endometrial stroma (ES), endometrial glands (EG) and myometrial blood vessels (MBV), ESR2 in endometrial blood vessels (EBV), PGRAB in ES, and PGRB in ES, EG and MBV was greater in pregnant than NP depending on day of pregnancy. Day of pregnancy affected expression of ESR1 in MBV, ESR2 in EBV and MBV, and PGRAB in ES. In experiment 2, ESR1, PGRAB and PGRB in EG, but not in other compartments, was greater in NAT-ET than NAT, and PGRB was greater in NAT-ET than the IVF. These data demonstrated that the ESR and PGR expression was different in pregnant vs. NP ewes in selected compartments, and was affected by pregnancy stage or embryo origin in selected utero-placental compartments. Thus, sex steroid hormone mRNA expression is differentially regulated in a spatio-temporal manner in uterus and placenta, and is affected by application of assisted reproductive technology in sheep.
Keywords: Sex steroid receptors mRNA, Uterine and placental compartments, Laser capture microdissection, Assisted reproductive technology, Sheep
1. Introduction
Several processes which are critical for pregnancy establishment take place during early pregnancy including implantation, placentation, and initiation of fetal and placental growth [1–3]. We have recently demonstrated that expression of steroid hormone receptors and factors involved in the regulation of growth and angiogenesis change dramatically during early pregnancy, and are affected by pregnancy stage and embryo origin [4–9].
Sex steroids are major regulators of uterine and placental growth and function in mammalian species [10–12]. Steroid hormones exert their direct effects through nuclear and membrane receptors [10–12]. Uterine and placental expression of estrogen and progesterone receptors (ER and PR, respectively) has been well described for numerous species [3,8,9,10–14]. Steroid receptors are expressed in several uterine and placental compartments, and their expression is regulated by estradiol-17β (E2), progesterone (P4) and other regulatory factors [11]. However, the level of mRNA expression, and regulation of steroid receptor protein expression in selected ovine uterine and placental compartments during early pregnancy have not been investigated using laser capture microdissection (LCM) followed by quantitative RT-PCR. Therefore, we have applied LCM to collect tissue compartments separately and perform gene expression analysis in ovine uterine and placental tissues, as it was performed before for other species [15–21].
We hypothesized that the mRNA expression of sex steroid receptors will: 1) differ in uterine vs. placental tissue compartments, and thus between pregnant vs. non-pregnant animals; 2) change during early pregnancy, and 3) be affected by application of assisted reproductive technology (ART). Therefore, the purpose of this study was to determine mRNA expression of ESR1, ESR2, PGRAB and PGRB in uterine and placental compartments collected separately using LCM, including luminal epithelium (LE), endometrial blood vessels (EBV), endometrial stroma (ES), endometrial glands (EG), myometrial blood vessels (MBV), myometrium (MYO), and/or fetal membranes (FM) from 1) non-pregnant ewes on day 10 of the estrous cycle, 2) naturally bred (NAT) ewes on days 16, 20 and 28 of pregnancy, 3) pregnant ewes on day 22 after natural breeding (NAT), or after transfer of embryos generated through natural breeding of FSH-treated ewes (NAT - ET), in vitro fertilization (IVF), or in vitro activation (IVA, parthenotes), and 4) to compare gene expression (i) in pregnant vs. non-pregnant, and (ii) in NAT vs. ART.
2. Material and methods
2.1. Animals and experimental design
The Institutional Animal Care and Use Committee at NDSU approved all animal procedures in this study. Experimental design for this study has been described in detail [4–8]. All crossbred Western Range (primarily Rambouillet, Targhee, and Columbia) ewes used in this study had similar genetic background, were 3–5 years old, and were in 2nd-4th parity. For all sheep, estrus was synchronized during the breeding season using a CIDR device (MWI, Boise, ID) implanted for 14 days [4,6].
In experiment 1, 24 h after CIDR removal, ewes were exposed to a fertile ram and allowed to breed. The day of breeding (day 0) was established by using a breeding harness. Immediately after euthanasia, gravid uteri were obtained on days 16, 20, and 28 (n = 4/day) after mating, and from non-pregnant (day 10 after estrus) control (n = 4) ewes. Uterine and placental cross-sections were dissected, fixed in formalin and embedded in paraffin [4,5]. Selection of specific days of early pregnancy for this study was based on previously obtained results demonstrating that on day 16, cell proliferation and enhancement in expression of several angiogenic and growth factors was initiated; on day 20, cell proliferation, vascular development and expression of several angiogenic and growth factors was at relatively high levels or increasing, and on day 28 majority of changes in tissues growth and expression of selected factors were being maintained [4,5].
In experiment 2, 24 h after CIDR removal, NAT ewes were exposed to a fertile ram and allowed to breed, as in experiment 1. Embryo transfer procedures, IVF and IVF have been described in detail previously [6,7,8]. Briefly, for donor ewes from NAT-ET, IVF and IVA groups, after CIDR removal, estrus was checked using a vasectomized ram. Beginning on day 13 of the estrous cycle, donor ewes from the NAT-ET group were treated with FSH for 3 days, whereas donor ewes from IVF and IVA groups were treated with FSH for 2 days. On day 15 of the estrous cycle, donor ewes from the NAT-ET group were exposed to a fertile ram. For donor ewes from the IVF and IVA groups, ovaries were collected on day 15, cumulus oocyte complexes (COC) were isolated from follicles >3 mm, matured, and then fertilized or activated in vitro. For IVF and IVA procedures, up to 30 COC/0.5 ml were incubated overnight in maturation medium. After denuding the oocytes, half from each sheep was used for IVF and the other half for IVA. For IVF, oocytes were cultured in fertilization medium in the presence of capacitated frozen-thawed sperm (0.5 to 1 × 106 sperm/ml) for 24 h followed by incubation in culture medium until embryo transfer (ET). For IVA, oocytes were incubated for 5 min in TCM199 medium containing 2% FBS (Sigma) and ionomycin (2.5 μM; Sigma), followed by a 3-h incubation with 6-dimethylaminopurine (DMAP; 2 mM; Sigma). The IVA oocytes were then transferred to culture medium and incubated until ET. For the NAT-ET group, on day 5 after mating embryos were flushed, and then transferred to synchronized recipients (3 embryos/recipient). For the IVF and IVA groups, embryos were transferred on day 5 after fertilization or activation to the synchronized recipient ewes (3 embryos/recipient). On day 22 after mating, fertilization, or activation, utero-placental tissues were collected from the NAT, NAT-ET, IVF and IVA groups (n=4/group), as described for experiment 1.
2.2. Laser capture microdissection (LCM)
Tissues were cut (8 μm sections), mounted on slides (two tissue sections/slide; two slides/sheep; MembraneSlide 1.0 PEN NF, Zeiss, Germany), deparaffinized, rehydrated and immediately used for microdissection. LCM of selected compartments was performed on a Zeiss Palm Microbeam UV laser capture microscope, equipped with PALM@Robo Software version 4.3 SP2 (Zeiss Inc., Thornwood, NY, USA). Samples were captured with cut energy set depending on the compartment and tissue (50–80%). Excised areas were captured in adhesive cap tubes filled with Qiagen PKD buffer (Qiagen, Valencia, CA). Uterine and placental compartments were independently microdissected from the same tissue sections in order to avoid the risk of RNA cross-contamination [19]. For each sheep, tissue elements were collected from 4 tissue sections. For each compartment (e.g., LE, EBV, ES, EG, MBV, MYO and FM), ~40–600 elements/ewe were microdissected and kept frozen (−80 C) in PKD buffer until RNA extraction. Fetal membranes/placental cell types were not identified in this study due to methodological difficulties, such as a lack of specific markers for these cell types in sheep or absence of some cell types in individual tissue sections; thus we collected the entire FM present in utero-placental cross-section for mRNA extraction. Furthermore, FM tissues did not contained any embryonic tissues.
2.3. Quantitative real-time reverse transcriptase-polymerase chain reaction
Total RNA was isolated with Qiagen’s RNeasy FFPE kit with the following modifications. A 3 minute incubation at 55oC before the proteinase K digestion was added, as well as elimination of the on-column DNAse treatment (was performed as a part of the cDNA synthesis protocol, see below), and a 10 minute incubation was added after 15 μL of RNase-free water was applied to the column for RNA elution before the final centrifugation step. Qiagen’s Quantitect Reverse Transcription kit was used to make cDNA. As per the kit instructions, 12 μL of undiluted RNA was treated with DNAse I to remove contaminating genomic DNA. However two protocol modifications were used. Instead of using the RT primer mix provided in the kit, 7.5 μM random hexamers (Integrated DNA Technologies, Coralville, IA) were utilized, and a 30 minute incubation time was used for the reverse transcriptions step instead of 15 minutes. qPCR was performed using iTaq™ 2X Universal SYBR® Green Supermix (Bio-Rad Laboratories, Inc., Hercules, CA) and 2 μl of undiluted cDNA in a 12.5 μl total reaction volume. For ESR1, ESR2 and PGRAB, primer sequences have been reported [8]. PRB only primers were designed for Accession # XM_012169082 (Forward: GTCCGCTCCAGCCAAAT, Reverse: GGATAGCGGGAGGACCA). Amplification of 18s rRNA served as an internal control, and primers were published before [21]. Gene expression was determined on a 7500 Fast Real-Time PCR System (Applied Biosystems, Waltham, MA), and data were analyzed using the comparative 2−oΔCT method [22].
For assay validation, two whole, non-dissected uterine or placental sections (n=4 ewes/day from experiment 1) were removed from the slides and transferred to a tube for RNA extraction serving as a “before dissection” control. Expression of ESR1, ESR2, PGRAB and PGRB gene (based on CT values) in these control non-dissected uterine or placental tissues was 3.5-, 3.2-, 15.8- and 5.1-fold greater (P<0.0001) than in any microdissected compartment, respectively, and was not affected by day of the estrous cycle or pregnancy, therefore data were combined for each gene. In addition, for non-pregnant controls and pregnant (day 20) sheep (n = 3/group), increasing numbers of EG (50, 100, 200 and 400) were collected using LCM followed by RNA extraction and qPCR. For all genes, in samples containing 50 EG, CT values were greatest (P<0.01–0.05), and gradually decreased to the lowest values in samples containing 400 EG (Table 1).
Table 1.
CT values for mRNA expression of ESR1, ESR2, PGRAB, PGRB, ACTB and 18S detected in 50, 100, 200 and 400 endometrial glands (EG)*.
| Gene | Number of EG | |||
|---|---|---|---|---|
| 50 | 100 | 200 | 400 | |
| ESR1 | 30.7±0.4a | 29.4±0.4ab | 29.0±0.5ab | 28.3±0.4b |
| ESR2 | 30.8±0.8a | 29.4±0.8 ab | 28.9±1.0ab | 28.5±0.8b |
| PRAB | 32.2±0.6a | 30.5±0.5 ab | 29.8±0.7bc | 29.4±0.6c |
| PRB | 34.4±1.0a | 32.5±1.0 ab | 32.0±1.1bc | 31.9±1.1c |
| ACTB | 32.8±0.6a | 31.8±0.5 ab | 31.4±0.62ab | 30.9±0.6b |
| 18S | 24.4±1.2a | 23.2±1.3 ab | 23.0±1.2ab | 22.4±1.2b |
Since level of expression of all genes was similar in non-pregnant and pregnant animals, data are combined.
P<0.01–0.05; values with different superscripts differ within a row.
P<0.01–0.05; values with different superscripts differ within a row.
P<0.01–0.05; values with different superscripts differ within a row.
2.4. Statistical Analysis
Data were analyzed using the general linear models procedure of SAS, with the main effect of uterine compartment, day of pregnancy (experiment 1) or pregnancy type (experiment 2), and their interactions. For validation experiment, the main effect included non-pregnant vs. pregnant stage, gene type and number of EG. When the F-test was significant (P<0.05), differences between specific means were evaluated by using the least significant difference test. Values tended to be statistically different are marked with P=0.05–0.1. Normality of data were assessed using residual diagnostics for PROC GLM (e.g., proc glm plots=diagnostics). Performed diagnoses indicated normality of errors, which aligns with ANOVA assumptions. The data are presented as means±SEM.
3. Results
In both experiments, the mRNA expression of four evaluated genes in uterine or placental compartments was affected by day of pregnancy or pregnancy type/embryo origin (Fig. 2–4). In experiment 1, mRNA expression of ESR1, ESR2, PGRAB and PGRB differed in pregnant compared to non-pregnant animals depending on the tissue compartment (Fig. 2 and 3), and in experiment 2, expression of ESR1, PGRAB and PGRB mRNA in EG was affected by ART application compared to NAT (Fig. 4).
Figure 2.
ESR1 (A), ESR2 (B), PGRAB (C) and PGRB (D) mRNA expression in different compartments including luminal epithelium (LE), endometrial blood vessels (EBV), endometrial stroma (ES), endometrial glands (EG), myometrial blood vessels (MBV) and myometrium (MYO) of ovine placenta on days 16, 20 and 28 of pregnancy (n=4 animals/d). Data are expressed as a fold change compared with non-pregnant controls (NP) for different compartments that were arbitrarily set as 1, and marked as a black horizontal line. *P < 0.020.0001, compared to NP. a,bP < 0.02–0.0001, mean ± SEM with different superscripts differ within a specific compartment.
Figure 4.
Expression of mRNA for ESR1 (A), ESR2 (B), PGRAB (C) and PGRB (D) in different compartments including luminal epithelium (LE), endometrial blood vessels (EBV), endometrial stroma (ES), endometrial glands (EG), myometrial blood vessels (MBV) and myometrium (MYO) of ovine placenta on day 22 of pregnancy established after 1) natural mating (NAT), or after transfer of embryos generated through 2) natural breeding of FSH-treated ewes (NAT - ET), 3) in vitro fertilization (IVF), or 4) in vitro activation (IVA; n=4 animals/group). Data are expressed as a fold change compared with NAT for different compartments that was arbitrarily set as 1, and marked as a black horizontal line. *P < 0.02–0.09, compared to NAT group. a,bP < 0.02–0.09, within specific compartment, mean ± SEM with different superscripts differ.
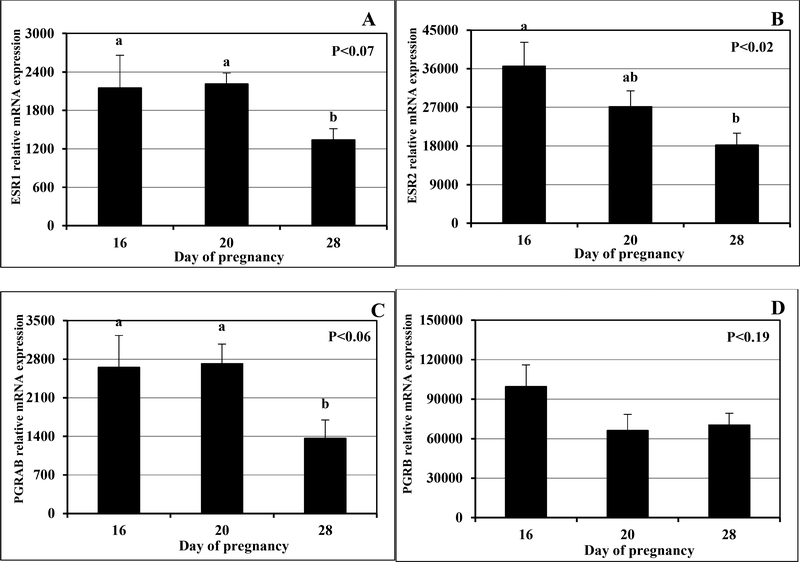
Figure 3.
Expression of mRNA for ESR1 (A), ESR2 (B), PGRAB (C) and PGRB (D) in fetal membranes (FM) on days 16, 20 and 28 of pregnancy (n=4 animals/d). a,bP < 0.02–0.07, mean ± SEM with different superscript differ.
In experiment 1, compared to NP controls, 1) mRNA expression of ESR1 was greater (P < 0.0001) in ES and EG on days 16 and 20, and MBV on day 20 (Fig. 2A); 2) expression of ESR2 was greater (P < 0.02) in EBV on day 16 (Fig. 2B); 3) expression of PGRAB was greater (P < 0.001) in ES on day 20 (Fig. 2C); and 4) expression of PGRB was greater (P < 0.001) in ES and EG on day 16 and MBV on day 20 (Fig. 2D). Furthermore, expression of 1) ESR1 mRNA in MBV was greater (P<0.0001) on day 16 than 28 of pregnancy (Fig 2A); 2) ESR2 mRNA in EBV was greater (P < 0.02) on day 16 than 28, and in MBV was greater on day 20 than 28 of pregnancy (Fig. 2B); and 3) PGRAB mRNA in ES was greater (P < 0.001) on day 20 than 28 of pregnancy (Fig 2C).
In experiment 1, in FM, ESR1 and PGRAB mRNA expression tended to be less (P < 0.07 and 0.06 respectively) on days 28 than on days 16 and 20 (Fig. 3A and C, respectively), ESR2 expression was greatest (P < 0.02) on day 16, less on day 20 and least on day 28 (Fig. 3B), and PGRB expression was similar in all days of pregnancy (Fig. 3D).
In experiment 2, compared to NAT, 1) mRNA expression of ESR1 was greater (P < 0.05) in EG in NAT-ET (Fig. 4A); 2) expression of ESR2 was similar in all groups (Fig. 4B); 3) expression of PGRAB in EG tended (P < 0.09) to be greater in the NAT-ET (Fig. 4C); and 4) expression of PGRB in EG was greater (P < 0.02) in NAT-ET (Fig. 3D). Furthermore, expression of PGRB mRNA was greater (P < 0.02) in EG in NAT-ET than IVF group (Fig. 4D). Expression of all steroid receptors in FM was similar in all pregnancy-type groups (data not shown).
4. Discussion
This study has demonstrated that the mRNA expression of steroid hormone receptors including ESR1, ESR2, PGRAB and PGRB was significantly different between non-pregnant and pregnant ewes, and was affected by stage of pregnancy and pregnancy type/embryo origin in selected placental compartments.
Differential expression of mRNA for selected genes in uterine or placental compartments has been demonstrated by others for sheep [13,23], humans [18,24], rhesus monkeys [15], and mouse [16,19,20,25]. In situ hybridization studies have shown that mRNA expression for ESR and PGR differed in LE, EG, ES and MYO depending on reproductive status (e.g., non-pregnant, pregnant or ovariectomized) in sheep [13,26]. For example, during early pregnancy, ESR and PGR mRNA expression was greater in EG, ES and MYO than LE [13]. Furthermore, microarray analysis of compartments collected using LCM has demonstrated that expression of genes was greater in LE than EG, and that LE and EG exhibit distinct genetic signatures in mouse uteri [20]. Similar to our results, level of ESR1 mRNA expression did not differ in human EG and ES dissected using LCM at proliferative phase of the estrous cycle [24]. Observed differences in the level of expression among uterine compartments are likely due to methods applied and species studied.
This and other studies have demonstrated differential protein expression of steroid receptors in uterine compartments depending on reproductive status in several species including sheep, cows and primates [9,13,15,27–29]. It seems that a pattern of expression of proteins and mRNA for steroid receptors differed in ovine uterine compartments in this and other experiments [9,13]. However, this is not surprising since complex and separate regulatory mechanisms control transcription and translation, and stability, half-lives, and the rates of mRNA and protein synthesis may be similar or differ substantially in mammalian cells [30].
In present study, expression of mRNA for ESR1 in ES, EG and MBV, ESR2 in EBV, PGRAB in ES and PGRB in ES, EG and MYO was greater in pregnant than non-pregnant ewes depending on the day of pregnancy. Comparably, Spencer and Bazer [13] have reported similar or greater ESR mRNA expression in ES and EG, and PGR in EG in pregnant vs. non-pregnant sheep. Differences in expression of steroid hormone receptors in selected compartments in pregnant vs. non-pregnant animals are likely associated with changes in circulating steroid concentration, local regulators such as immune, growth and angiogenic factors and the presence/absence of the conceptus [13,14]. Furthermore, we have detected expression of steroid receptors in EBV and MBV, and expression of mRNA for ESR1 in MBV, ESR2 in EBV and MBV differed among days of pregnancy. Similar to our study, estrogens and P4 steroid receptors have been detected in uterine and placental blood vessels of several species [9,28,31–35, 36]. Changes in ESR mRNA expression in blood vessels during early pregnancy are likely associated with the regulatory role estrogens play in vascular functions in the uterus and placenta such as regulation of blood flow, vascular tone, promotion of angiogenesis, blood vessel remodeling and others [32,37–40]. Overall, our results, which demonstrate differential mRNA expression and distribution of steroid receptors depending on uterine vs. placental compartments, support known phenomena that steroid hormones play different roles in the regulation of uterine and placental functions in pregnant vs. non-pregnant animals [10–12,41].
Sex steroid receptor mRNA was detected in FM in this study and expression of ESR and PGRAB mRNA was less on day 28 than 16 of pregnancy. Steroid hormone receptors have been also detected in embryos in other species including mice, cows, pigs, sheep, and primates [8,42–47]. Since the role of steroid receptors in regulation of fetal growth and development is unknown, future studies are warranted.
The current study has demonstrated that mRNA expression of ESR1, PGRAB and PGRB in EG, but not in other compartments, was enhanced in NAT-ET, but not in other ART groups when compared to NAT controls in experiment 2. These data support our previous observations that embryo origin may affect selected placental functions [6–8]. In our previous studies, ESR1 and PGR receptor mRNA in maternal ovine placenta were downregulated in ART groups, and ESR1 in FM was upregulated in IVF and IVA groups [8]. The differences in pattern of steroid receptors mRNA expression in these two studies are likely due to different methodologies used; in this experiment we extracted RNA from selected compartments of formalin fixed tissues, but in previous experiment, tissues were frozen immediately after collection, and then entire tissue was homogenized and used for RNA extraction. To clarify these discrepancies, in situ hybridization should be performed to localize mRNA expression in tissue compartments of intact placental slides. The effects of embryo origin on the maternal placenta including cell proliferation, vascularity, and expression of angiogenic factors and several other genes have been previously demonstrated in sheep [6,7,48]. This is not surprising since fetal membranes and maternal placenta are in constant interaction in order to maintain pregnancy [6,7,49,50].
In summary, our study has demonstrated that expression of sex steroid (E2 and P4) receptor mRNA: 1) differed in uterine (non-pregnant) vs. placental (pregnant) selected compartments; 2) was differentially regulated in selected placental compartments depending on a day of pregnancy; and 3) was affected by embryo origin in EG. This study is novel, since limited information was available concerning steroid receptor mRNA expression in uterine and placental compartments collected using LCM for any species. In addition, our results indicate that expression, and likely function, of steroid receptors changes depending on uterine or placental compartment, stage of pregnancy and embryo origin. However, it should be noted that all uterine/placental cell types/compartments interact with each other through complex mechanisms (e.g., juxtacrine, paracrine and endocrine) to maintain normal reproductive functions in order to provide optimal conditions for pregnancy establishment and maintenance.
Figure 1.
Schematic of cross section of utero-placental tissues indicating compartments collected using LCM that included fetal membranes (FM), luminal epithelium (LE), endometrial (Endo) glands (EG), endometrial stroma, endometrial blood vessels (BV), myometrium (Myo), and myometrial BV.
Highlights.
Sex steroid receptors’ mRNA expression differed in uterine (non-pregnant) vs. placental (pregnant) compartments
mRNA expression of sex steroid receptors in selected placental compartments undergoes dynamic changes during early pregnancy
mRNA expression of selected steroid receptors in endometrial glands is affected by embryo origin
Acknowledgments
This project was partially supported by funds from grant #2007–01215 from the National Research Initiative of the U.S. Department of Agriculture, and fully supported by grant #R03HD076073 from the National Institutes of Health, as well as by hutch funds from the North Dakota Agricultural Experiment Station to Anna T Grazul-Bilska and Lawrence P Reynolds. The authors would like to thank Jim Kirsch, Terry Skunberg, the staff at NDSU Animal Nutrition and Physiology Center, and undergraduate students for assistance with animal care and handling.
This study was also partially supported by Thailand Research Fund (TRF) under Research and Researchers for Industries (RRI) and RIG 5980010, National Research Council of Thailand (NRCT), Increased Production Efficiency and Meat Quality of Native Beef and Buffalo Research Group, Khon Kaen University, and Department of Animal Science, Faculty of Agriculture, Khon Kaen University to Aree Kraisoon and Dr. Chainarong Navanukraw.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- [1].Reynolds LP, Borowicz PP, Caton JS, Vonnahme KA, Luther JS, Buchanan DS, et al. Utero-placental vascular development and placental function: An update. Intl J Dev Biol 2010;54:355–66. [DOI] [PubMed] [Google Scholar]
- [2].Reynolds LP, Borowicz PP, Palmieri C, Grazul-Bilska AT. Placental vascular defects in compromised pregnancies: effects of assisted reproductive technologies and other maternal stressors. Adv Exp Med Biol 2014;814:193–204. [DOI] [PubMed] [Google Scholar]
- [3].Bairagi S, Quinn KE, Crane AR, Ashley RL, Borowicz PP, Caton JS, et al. Maternal environment and placental vascularization in small ruminants. Theriogenology 2016;86:288–305. [DOI] [PubMed] [Google Scholar]
- [4].Grazul-Bilska AT, Borowicz PP, Johnson ML, Minten MA, Bilski JJ, Wroblewski R, et al. Placental development during early pregnancy in sheep: vascular growth and expression of angiogenic factors in maternal placenta. Reproduction 2010;140:165–74. [DOI] [PubMed] [Google Scholar]
- [5].Grazul-Bilska AT, Johnson ML, Borowicz PP, Minten M, Wroblewski R, Coupe LR, et al. Placental development during early pregnancy in sheep: Cell proliferation, global methylation and angiogenesis in fetal placenta. Reproduction 2011;141:529–40. [DOI] [PubMed] [Google Scholar]
- [6].Grazul-Bilska AT, Johnson ML, Borowicz PP, Baranko L, Redmer DA, Reynolds LP. Placental development during early pregnancy in sheep: Effects of embryo origin on fetal and placental growth, and global methylation. Theriogenology 2013;79:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Grazul-Bilska AT, Johnson ML, Borowicz PP, Bilski JJ, Cymbaluk T, Norberg S, et al. Placental development during early pregnancy in sheep: Effects of embryo origin on vascularization. Reproduction 2014;147:639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Reynolds LP, Haring JS, Johnson ML, Ashley RL, Redmer DA, Borowicz PP, GrazulBilska AT. Placental development during early pregnancy in sheep: estrogen and progesterone receptor messenger RNA expression in pregnancies derived from in vivo-produced and in vitro-produced embryos. Domest Anim Endocrinol 2015;53:60–9. [DOI] [PubMed] [Google Scholar]
- [9].Bairagi S, Grazul-Bilska AT, Borowicz PP, Reyaz A, Valkov V, Reynolds LP. Placental development during early pregnancy in sheep: Progesterone and estrogen receptor protein expression. Theriogenology 2018;114:273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Albrecht ED, Pepe GJ. Placental endocrine function and hormone action In Plant TM, Zeleznik AJ, eds. The Knobil and Neill’s Physiology of Reproduction, edn 4, New York, NY:Academic Press as an imprint of Elsevier; 2015:1783–834. [Google Scholar]
- [11].Binder AK, Winuthayanon W, Hewill SC. Steroid receptors in the uterus and ovary In Plant TM, Zeleznik AJ, eds. The Knobil and Neill’s Physiology of Reproduction, edn 4, New York, NY:Academic Press as an imprint of Elsevier; 2015:1099–193. [Google Scholar]
- [12].Mazur EC, Large ML, DeMayo FJ. Human oviduct and endometrium: Change over menstrual cycle In Plant TM, Zeleznik AJ, eds. The Knobil and Neill’s Physiology of Reproduction, edn 4, New York, NY:Academic Press as an imprint of Elsevier; 2015:1077–98. [Google Scholar]
- [13].Spencer TE, Bazer FW. Temporal and spatial alterations in uterine estrogen receptor and progesterone receptor gene expression during the estrous cycle and early pregnancy in the ewe. Biol Reprod 1995;53:1527–43. [DOI] [PubMed] [Google Scholar]
- [14].Spencer TE, Bazer FW. Uterine and placental factors regulating conceptus growth in domestic animals. J Anim Sci 2004;82 E-Suppl:E4–13. [DOI] [PubMed] [Google Scholar]
- [15].Okulicz WC, Ace CI, Torres MS. Gene expression in the rhesus monkey endometrium: differential display and laser capture microdissection. Front Biosci 2003;8:d551–8. [DOI] [PubMed] [Google Scholar]
- [16].Hong SH, Nah HY, Lee JY, Gye MC, Kim CH, Kim MK. Analysis of estrogen-regulated genes in mouse uterus using cDNA microarray and laser capture microdissection. J Endocrinol 2004;181:157–67. [DOI] [PubMed] [Google Scholar]
- [17].Wiley AA, Kauffold J, Wähner M, Crean-Harris B, Miller DJ, Bagnell CA, Bartol FF. Laser microdissection of neonatal porcine endometrium for tissue-specific evaluation of relaxin receptor (RXFP1) expression in response to perinatal zearalenone exposure. Ann N Y Acad Sci 2009;1160:190–1. [DOI] [PubMed] [Google Scholar]
- [18].Evans GE, Martínez-Conejero JA, Phillipson GT, Sykes PH, Sin IL, Lam EY, et al. In the secretory endometria of women, luminal epithelia exhibit gene and protein expressions that differ from those of glandular epithelia. Fertil Steril 2014;102:307–317.e7. [DOI] [PubMed] [Google Scholar]
- [19].Field SL, Cummings M, Orsi NM. Epithelial and stromal-specific immune pathway activation in the murine endometrium post-coitum. Reproduction 2015;150:127–38. [DOI] [PubMed] [Google Scholar]
- [20].Filant J, Spencer TE. Cell-specific transcriptional profiling reveals candidate mechanisms regulating development and function of uterine epithelia in mice. Biol Reprod 2013;89:86,1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brooks K, Burns GW, Moraes JG, Spencer TE. Analysis of the uterine epithelial and conceptus transcriptome and luminal fluid proteome during the peri-implantation period of pregnancy in sheep. Biol Reprod 2016;95:88;1–9. [DOI] [PubMed] [Google Scholar]
- [22].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001;25: 402–408. [DOI] [PubMed] [Google Scholar]
- [23].Brooks K, Burns GW, Moraes JG, Spencer TE. Analysis of the uterine epithelial and conceptus transcriptome and luminal fluid proteome during the peri-implantation period of pregnancy in sheep. Biol Reprod 2016;95:88,1–17. [DOI] [PubMed] [Google Scholar]
- [24].Mohamed SA, Atta IS, Rowan BG, Desouki MM. ERα and ERK1/2 MAP kinase expression in microdissected stromal and epithelial endometrial cells. J Egypt Natl Canc Inst 2014;26:37–41. [DOI] [PubMed] [Google Scholar]
- [25].Walter LM, Rogers PA, Girling JE. Vascular endothelial growth factor-A isoform and (co)receptor expression are differentially regulated by 17beta-oestradiol in the ovariectomised mouse uterus. Reproduction 2010;140:331–41. [DOI] [PubMed] [Google Scholar]
- [26].Ing NH, Zhang Y. Cell-specific expression of estrogen-responsive genes in the uteri of cyclic, early pregnant and ovariectomized ewes. Theriogenology 2004;62:403–14. [DOI] [PubMed] [Google Scholar]
- [27].Okulicz WC1, Balsamo M. A double immunofluorescent method for simultaneous analysis of progesterone-dependent changes in proliferation and the oestrogen receptor in endometrium of rhesus monkeys. J Reprod Fertil 1993;99:545–9. [DOI] [PubMed] [Google Scholar]
- [28].Perrot-Applanat M, Deng M, Fernandez H, Lelaidier C, Meduri G, Bouchard P. Immunohistochemical localization of estradiol and progesterone receptors in human uterus throughout pregnancy: expression in endometrial blood vessels. J Clin Endocrinol Metab. 1994;78:216–24. [DOI] [PubMed] [Google Scholar]
- [29].Kimmins S, MacLaren LA. Oestrous cycle and pregnancy effects on the distribution of oestrogen and progesterone receptors in bovine endometrium. Placenta 2001;22:742–8. [DOI] [PubMed] [Google Scholar]
- [30].Schwanhäusser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, et al. Global quantification of mammalian gene expression control. Nature 2011;473:337–42. [DOI] [PubMed] [Google Scholar]
- [31].Bergqvist A, Bergqvist D, Fernö M. Estrogen and progesterone receptors in vessel walls. Biochemical and immunochemical assays. Acta Obstet Gynecol Scand 1993;72:10–6. [DOI] [PubMed] [Google Scholar]
- [32].Wu WX, Owiny J, Zhang Q, Ma XH, Nathanielsz PW. Regulation of the estrogen receptor and its messenger ribonucleic acid in the ovariectomized sheep myometrium and endometrium: the role of estradiol and progesterone. Biol Reprod 1996;55:762–8. [DOI] [PubMed] [Google Scholar]
- [33].Vermeirsch H, Simoens P, Lauwers H. Immunohistochemical detection of the estrogen receptor-alpha and progesterone receptor in the canine pregnant uterus and placental labyrinth. Anat Rec 2000;260:42–50. [DOI] [PubMed] [Google Scholar]
- [34].Corcoran JJ1, Nicholson C, Sweeney M, Charnock JC, Robson SC, Westwood M, Taggart MJ. Human uterine and placental arteries exhibit tissue-specific acute responses to 17βestradiol and estrogen-receptor-specific agonists. Mol Hum Reprod 2014;20:433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Brenner RM, Slayden OD. Steroid receptors in blood vessels of the rhesus macaque endometrium: a review. Arch Histol Cytol 2004;67:411–6. [DOI] [PubMed] [Google Scholar]
- [36].Grazul-Bilska AT, Thammasiri J, Kraisoon A, Reyaz A, Bass CS, Kaminski SL, Navanukraw C and Redmer DA. Expression of progesterone receptor in ovine uterus during the estrous cycle: Effects of nutrition, arginine and FSH-treatment. Theriogenology 2018;108:7–15. [DOI] [PubMed] [Google Scholar]
- [37].Boos A, Kohtes J, Janssen V, Mülling C, Stelljes A, Zerbe H, et al. Pregnancy effects on distribution of progesterone receptors, oestrogen receptor alpha, glucocorticoid receptors, Ki-67 antigen and apoptosis in the bovine interplacentomal uterine wall and foetal membranes. Anim Reprod Sci 2006;91:55–76. [DOI] [PubMed] [Google Scholar]
- [38].Boos A, Kohtes J, Stelljes A, Zerbe H, Thole HH. Immunohistochemical assessment of progesterone, oestrogen and glucocorticoid receptors in bovine placentomes during pregnancy, induced parturition, and after birth with or without retention of fetal membranes. J Reprod Fertil 2000;120:351–60. [PubMed] [Google Scholar]
- [39].Bracamonte MP, Miller VM. Vascular effects of estrogens: arterial protection versus venous thrombotic risk. Trends Endocrinol Metab 2001;12:204–9. [DOI] [PubMed] [Google Scholar]
- [40].Pastore MB, Jobe SO, Ramadoss J, Magness RR. Estrogen receptor-α and estrogen receptor-β in the uterine vascular endothelium during pregnancy: functional implications for regulating uterine blood flow. Semin Reprod Med 2012;30:46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Meikle A, Tasende C, Sosa C, Garófalo EG. The role of sex steroid receptors in sheep female reproductive physiology. Reprod Fertil Dev 2004;16:385–94. [DOI] [PubMed] [Google Scholar]
- [42].Gorski J, Hou Q. Embryonic estrogen receptors: do they have a physiological function? Environ Health Perspect 1995;103:Suppl 7, 69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hou Q, Gorski J. Estrogen receptor and progesterone receptor genes are expressed differentially in mouse embryos during preimplantation development. Proc Natl Acad Sci USA 1993:90:9460–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ying C, Yang YC, Hong WF, Cheng WT, Hsu WL. Progesterone receptor gene expression in preimplantation pig embryos. Eur J Endocrinol 2000;143:697–703. [DOI] [PubMed] [Google Scholar]
- [45].Haluska GJ, Wells TR, Hirst JJ, Brenner RM, Sadowsky DW, Novy MJ. Progesterone receptor localization and isoforms in myometrium, decidua, and fetal membranes from rhesus macaques: evidence for functional progesterone withdrawal at parturition. J Soc Gynecol Investig 2002;9:125–36. [PubMed] [Google Scholar]
- [46].Clemente M, de La Fuente J, Fair T, Al Naib A, Gutierrez-Adan A, Roche JF, et al. , Progesterone and conceptus elongation in cattle: a direct effect on the embryo or an indirect effect via the endometrium? Reproduction 2009;138:507–17. [DOI] [PubMed] [Google Scholar]
- [47].Goldman S, Weiss A, Almalah I, Shalev E. Progesterone receptor expression in human decidua and fetal membranes before and after contractions: possible mechanism for functional progesterone withdrawal. Mol Hum Reprod 2005;11:269–77. [DOI] [PubMed] [Google Scholar]
- [48].Johnson ML, Redmer DA, Reynolds LP, and Grazul-Bilska AT. Gap junctional connexin mRNA expression in the ovine uterus and placenta: Effects of estradiol-17β-treatment, stage of early pregnancy, and embryo origin. Domest Anim Endocr 2017;58:104–12. [DOI] [PubMed] [Google Scholar]
- [49].Sandra O, Mansouri-Attia N, Lea RG. Novel aspects of endometrial function: a biological sensor of embryo quality and driver of pregnancy success. Reprod Fertil Dev 2011;24:68–79. [DOI] [PubMed] [Google Scholar]
- [50].Sandra O, Constant F, Vitorino Carvalho A, Eozénou C, Valour D, Mauffré V, et al. Maternal organism and embryo biosensoring: insights from ruminants. J Reprod Immunol 2015;108:105–13. [DOI] [PubMed] [Google Scholar]