Abstract
Environmental and occupational exposures to cadmium increase the risk of various cancers, including lung cancer. The carcinogenic mechanism of cadmium, including its prevention remain to be investigated. Using fluorescence and electron spin resonance spin trapping, the present study shows that in immortalized lung cells (BEAS-2BR cells), exposure cadmium generated reactive oxygen species (ROS). Through ROS generation, cadmium increased the protein level of TNF-α, which activated NF-κB and its target protein COX-2, creating an inflammatory microenvironment. As measured by anchorage-independent colony formation assay, cadmium induced malignant cell transformation. Inhibition of ROS by antioxidants inhibited transformation, showing that ROS were important in the mechanism of this process. The inflammatory microenvironment created by cadmium may also contribute to the mechanism of the transformation. Using tandem fluorescence protein mCherry-GFP-LC3 construct, the present study shows that cadmium-transformed cells had a property of autophagy deficiency, resulting in accumulation of autophagosomes and increased p62. This protein upregulated Nrf2, which also upregulated p62 through positive feed-back mechanism. Constitutive Nrf2 activation increased its downstream anti-apoptotic proteins, Bcl-2 and Bcl-xl, resulting in apoptosis resistance. In untransformed BEAS-2BR cells, sulforaphane, a natural compound, increased autophagy, activated Nrf2, and decreased ROS. In cadmium-transformed BEAS-2BR cells, sulforaphane restored autophagy, decreased Nrf2, and decreased apoptosis resistance. In untransformed cells, this sulforaphane induced inducible Nrf2 to decrease ROS and possibly malignant cell transformation. In cadmium-transformed cells, it decreased constitutive Nrf2 and reduced apoptosis resistance. The dual roles of sulforaphane make this natural compound a valuable agent for prevention against cadmium-induced carcinogenesis.
Keywords: Cadmium, autophagy deficiency, sulforaphane, carcinogenesis
INTRODUCTION
Cadmium, a toxic heavy metal, is classified as a known human carcinogen (IARC, 1993). The major sources of cadmium exposures are food, cigarette smoking, and cadmium related industry, such as electroplating, pigment, and batteries (Rafati Rahimzadeh et al., 2017). Environmental and occupational exposures to cadmium cause inflammation and cancers of various organs, including cancer of the lung (Chen et al., 2015; Chen et al., 2016a; Chen et al., 2016b; Kim et al., 2017; Larsson et al., 2015). Although the mechanism of cadmium-induced carcinogenesis remains to be defined, ROS are considered the important mechanism in cadmium-induced carcinogenesis (Wang et al., 2016). ROS induce intracellular oxidative stress, which could damage macromolecules and eventually contribute to a variety of diseases including cancer (Wang et al., 2016). While carcinogenesis is a multiple step process, when discussing the known mechanisms of metal-induced carcinogenesis, we conceptually refer to two stages. In the first stage of cadmium-induced carcinogenesis (from normal cells to transformed cells), ROS play a major role in the malignant cells transformation of BEAS-2BR cells exposed to cadmium (Son et al., 2012; Xu et al., 2017). Inhibition of ROS using antioxidant [catalase (CAT) or superoxide dismutase (SOD)] is able to decrease cadmium-induced carcinogenesis (Son et al., 2012). Although the mechanism of the first stage of metal carcinogenesis is very extensively studied, the mechanism of the second stage of metal carcinogenesis (morphologically transformed cells progress into tumorigenesis) is not very well investigated. Our previous study (Son et al., 2014) showed that in cadmium-transformed cells, p62 and Nrf2 were constitutively activated and their downstream antioxidants and anti-apoptotic proteins were elevated. The final outcomes are a decrease in ROS, apoptosis resistance, and tumorigenesis (Son et al., 2014). A decrease of ROS generation in the second stage of metal-induced carcinogenesis is oncogenic, because it provides a favorable environment for the survival and tumorigenesis of transformed cells (Wang et al., 2016; Xu et al., 2017). Thus, a decrease of ROS generation in the first stage of cadmium carcinogenesis and upregulation of ROS generation in the second stage could be a strategy to inhibit cadmium induced carcinogenesis.
Persistent inflammation contributes to carcinogenesis and tumor progression by activating a series of inflammatory molecules and a creation of an inflammatory tumor microenvironment favorable for cancer growth (Sui et al., 2017). One of the pro-inflammatory cytokines, tumor necrosis factor alpha (TNF-α), activates cancer cell survival and proliferation pathway, triggers inflammatory cell infiltration of tumor, and promotes angiogenesis and tumor cell migration and invasion (Balkwill, 2010). TNF-α activates NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) pathway, which is important in carcinogenesis (Wu and Zhou, 2010). Activation of Cyclooxygenase-2 (COX-2) generates an inflammatory microenvironment, which is important for early-stage tumorigenesis (Echizen et al., 2018). Although, cadmium is able to induce inflammation, which is known to be involved in cancer initiation and progression (Kim et al., 2017; Olszowski et al., 2012; Phuagkhaopong et al., 2017). The role of inflammation in cadmium-induced carcinogenesis remains to be determined.
The role of autophagy in the mechanism of metal carcinogenesis is increasingly recognized. Autophagy is a self-degradative process and plays a housekeeping role in removing proteins, clearing damaged organelles, and eliminating intracellular pathogens (Glick et al., 2010). Owning to a key role in the preservation of intracellular homeostasis, autophagy constitutes a barrier against various degenerative processes that may affect healthy cells, including carcinogenesis (Chen and Karantza, 2011; Levine and Klionsky, 2004). Our previous studies showed that autophagy inhibited arsenic-induced ROS generation by facilitating mitochondrial turnover in untransformed BEAS-2BR cells (Zhang et al., 2012). Thus, autophagy can exhibit anti-oncogenic effects. During the autophagy process, cytosolic substrates are assimilated in double-membrane vesicles or autophagosome, and subsequently transferred to lysosome to be degraded and recycled in autolysosome (the fusion product of autophagosome and lysosome) (Nakahira et al., 2014). Cadmium treatment increased autophagy in untransformed BEAS-2BR cells, while cadmium-transformed cells are autophagy-deficient, which is a blockage of the fusion between autophagosomes and lysosomes to prevent autophagy from completion (Son et al., 2014). In autophagy competent cells, p62 binds directly to LC3 to facilitate degradation of p62 and ubiquitinated protein aggregates by autolysosomes (Pankiv et al., 2007). In autophagy deficiency cells, the inability of the cells to remove p62 causes accumulation of p62 (Son et al., 2014), which is oncogenic. Thus, the investigation of autophagy deficiency in cadmium-transformed cells will provide important information for understanding the mechanism of second stage of cadmium carcinogenesis.
In cadmium-transformed cells, high expression of p62 due to autophagy deficiency causes p62 to bind to Keap 1 (kelch-like ECH-associated protein 1), the inhibitor of Nrf2 (nuclear factor erythroid 2-related factor), leading to constitutive activation of Nrf2. Constitutively activated Nrf2 is translocated from cytosol to nucleus, where it binds to antioxidant response elements (ARE) in the promoter regions of Nrf2 downstream proteins, such as superoxide dismutase, catalase and anti-apoptotic proteins such as Bcl2 and Bcl-xl. The final results are the decrease in ROS, development of apoptosis resistance, and creation of a favorable environment for tumorigenesis of cadmium-transformed cells (Son et al., 2014). In addition, Nrf2 also upregulates p62 and has a positive feedback by binding directly to the ARE site of p62 (Jain et al., 2010). Nrf2 is likely to act as a double-edged sword in metal induced-carcinogenesis. In the first stage of metal carcinogenesis, the antioxidant property of Nrf2 decreases ROS to protect cells from malignant transformation. In the second stage of metal carcinogenesis, constitutive activation of Nrf2 leads to decrease in ROS generation and acquired apoptosis resistance, providing a favorable environment for the survival and tumorigenesis of transformed cells (Xu et al., 2017).
Sulforaphane (SFN), a natural dietary isothiocyanate produced by cruciferous vegetable such as broccoli, displays a very strong anti-cancer, anti-oxidant, and anti-inflammatory activities (Brandenburg et al., 2010; Sestili and Fimognari, 2015). Due to safety, efficiency, and practicability, foods containing bioactive phytochemicals are gaining significant attention as elements of chemoprevention strategies against cancer (Yang et al., 2016a). Previous studies indicate that sulforaphane reduced cadmium-induced toxic effects in different cells (Alkharashi et al., 2017; Alkharashi et al., 2018; Yang et al., 2016b). As potent naturally occurring inducers of Nrf2 signaling, sulforaphane exhibits its cytoprotective property through Nrf2 pathway (Yang et al., 2016a). Since in the first stage of cadmium carcinogenesis Nrf2 is anti-oncogenic, we hypothesized that induction of Nrf2 by sulforaphane decreased cadmium-generated ROS, leading to inhibition of cadmium-induced malignant transformation of BEAS-2BR cells. In the second stage of cadmium carcinogenesis, Nrf2 is constitutively activated through accumulation of p62 and lost its inducibility due to autophagy deficiency, leading to apoptosis resistance and tumorigenesis of cadmium-transformed cells. Previous studies also reported that sulforaphane increased autophagy flux in certain cells (Herman-Antosiewicz et al., 2006; Lee et al., 2014). Thus, it is very likely that sulforaphane may activate inducible Nrf2 in normal cells to reduce ROS and to decrease malignant cell transformation and that in cadmium-transformed cells sulforaphane may restore autophagy competence, leading to decrease of p62/Nrf2, apoptosis resistance and tumorigenesis of cadmium-transformed cells.
MATERIALS AND METHODS
Chemicals and cultureware
Unless specified otherwise all chemicals and culturewares were purchased from Sigma Chemical Co. (St Louis, Mo) and Falcon Labware (Bectone-Dickinson, Franklin Lakes, NJ), respectively. Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum (FBS), and antibiotic-antimycotic were purchased from Gibco Company (Gibco BRL, NY).
Cell lines and cell culture
The human bronchial epithelial cell line (BEAS-2BR cells) were obtained from the American Type Culture Collection and cadmium-transformed cells were generated as described previously (Son et al., 2014). Cadmium-transformed cells and their passage-matched untransformed BEAS-2BR cells were maintained in DMEM supplemented with 10% fetal bovine serum and 1% antibiotic-antimycotic. When grown with serum, BEAS-2B cells become serum resistant and lose their ability to differentiate, thus we refer to them as BEAS-2BR cells when grow in a serum system to signify that they are serum resistant BEAS-2B cells (Ke et al., 1988).
Plasmids, shRNA, and transfection
Overexpression of catalase, SOD1 and SOD2 were performed as described previously (Son et al., 2012). Nrf2 and p62 shRNA were purchased from Origene (Rockville, MD). Transfections were performed using Lipofectamine™ 2000 (Invitrogen) according to the manufacturer’s protocol.
Western blot analysis
Western blot analysis was performed as described previously (Son et al., 2010a). Monoclonal antibodies specific for β-actin were purchased from Sigma-Aldrich (St. Louis, MO). Antibodies specific for NF-κB and Ref-1 were purchased from Santa Cruz, CA). Antibodies against COX-2, TNF-α, Bcl-2, PARP, C-Caspase 3, GAPDH, p62, and Nrf2 were purchased from Cell signaling Technology (Danvers, MA). Secondary antibodies and enhanced chemiluminescence substrate were purchased from Pierce Biotechnology (Waltham, MA). The blots were exposed to Hyperfilm (Amersham Bioscience, Piscataway, NJ). To generate cytoplasmic and nuclear protein, cadmium-treated cells were washed with cold PBS and scrape the cells in 1 ml PBS. Then the cells were suspended in 0.4ml lysis buffer (10 mM Hepes, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA) and lysed on ice for 15 min. Next 12.5 µl of 10% NF-40 was added, vortexed for 10 s, and spun for 1 min at max speed at 4°C. The supernatant is cytoplasm protein. 40 µl extraction buffer (20 mM Hepes (PH 7.9), 0.4M KCl, 1 mM EDTA and 1 mM EGTA) was added and then spun for 5 min at max speed at 4°C. The supernatant is nuclear protein. For lysis buffer and extraction buffer, protease inhibitor cocktail was added before use.
Intracellular ROS determination
2’,7’–dichlorofluorescin diacetate (DCFDA), also known as H2DCFDA, is specific dye used for ROS. BEAS-2BR cells were seeded into 60 mm culture dishes before treating the cells with different concentrations of cadmium and catalase (1000 U/ml) for 24 h. Finally, the cells were exposed to DCFDA at a final concentration of 5 µM for 30 min and processed for flow cytometric analysis. All measurements of electron spin resonance (ESR) were conducted using Bruker EMS spectrometer (Bruker Instruments) and a flat cell assembly as described previously (Son et al., 2010b).
GFP-LC3 and mCherry-EGFP-LC3 puncta formation assays
Briefly as previously described (Son et al., 2011), cadmium-transformed cells and non-transformed BEAS-2BR cells were transfected with GFP-LC3 or mCherry-EGFP-LC3 plasmids (catalog no. 24920 and 22418, Addgene, Cambridge, MA). The transfected cells were seeded onto coverslips placed in 6-well plates (0.2×106 cells/coverslip). Fluorescence-positive cells were counted using a fluorescence microscope (Carl Zeiss, Germany).
Statistical analysis
All the data was expressed as mean ± standard error (SE). One-way analysis of variance (ANOVA) using SPSS ver.10.0 software was used for multiple comparisons. A value of P<0.05 was considered statistically significant.
RESULTS
Cd(II) exposure induced ROS in BEAS-2BR cells and antioxidants exhibited inhibitory effects
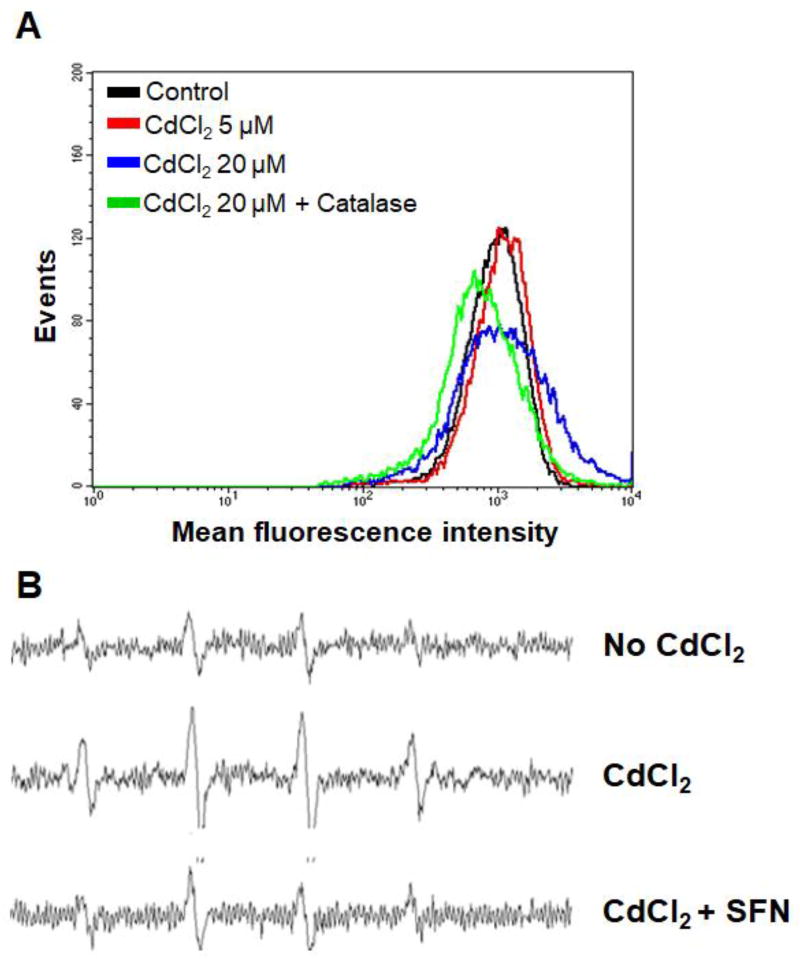
ROS production was measured by flow cytometry using the cell permeant reagent 2’,7’-dichlorofluorescin diacetate (DCFDA, also known as H2DCFDA) (Fig. 1A). DCFDA is a fluorogenic dye that measures ROS activity within the cell. The results showed that when BEAS-2BR cells were treated with cadmium the fluorescence peak was shifted to the right, indicating an elevated ROS generation (Fig. 1A). The enhanced ROS generation is cadmium concentration dependent (Fig. 1A). The ROS generation decreased when catalase, which is a specific antioxidant against H2O2, was added to cadmium-treated BEAS-2BR cells (Fig. 1A). We also measured the oxygen radical levels in cadmium-treated BEAS-2BR cells and the possible inhibition by an antioxidant using ESR spin trapping according to the method described previously (Shi et al., 1994a; Shi et al., 1994b). This method involves the addition-type reaction of a short-lived radical with a paramagnetic compound (spin trap) to form a relatively long-lived free radical product (spin adduct), which can then be studied using ESR. The intensity of ESR signal is used to measure the amount of short-lived radicals trapped and the hyperfine couplings of the spin adduct are generally characteristic of the original trapped radicals. As shown in Fig. 1B, ESR signal intensity generated in untreated BEAS-2BR cells is low. Cadmium treatment generated a typical 1:2:2:1 quartet ESR signal, an evidence of ROS generation. Addition of sulforaphane decreased the cadmium-generated oxygen radicals. These results revealed that cadmium exposure induces ROS generation in BEAS-2BR cells and that antioxidants, catalase and sulforaphane exhibit inhibitory effects.
Fig. 1. Exposure of BEAS-2BR cells to cadmium generated ROS and antioxidants decreased the generation.
(A) ROS levels from BEAS-2BR cells exposed to cadmium at indicated concentrations of cadmium were measured using flow cytometry. (B) ESR spectra were recorded from BEAS-2BR cells exposed to cadmium at different experimental conditions. The concentrations of the reagents used are: 0 and 10 µM CdCl2, 10 µM CdCl2 with 10 µM sulforaphane (SFN). Spectrometer settings were: receiver gain, 1.25×105; modulation amplitude, 1.25 G; scan time 200 s; field 3480 + 50 G; and time constant, 1.5 seconds. 5,5-dimethyl-1-pyrroline N-oxide (DMPO) was used as a spin (radical) trapping agent.
Exposure of BEAS-2BR cells increased inflammation and ROS played an important role
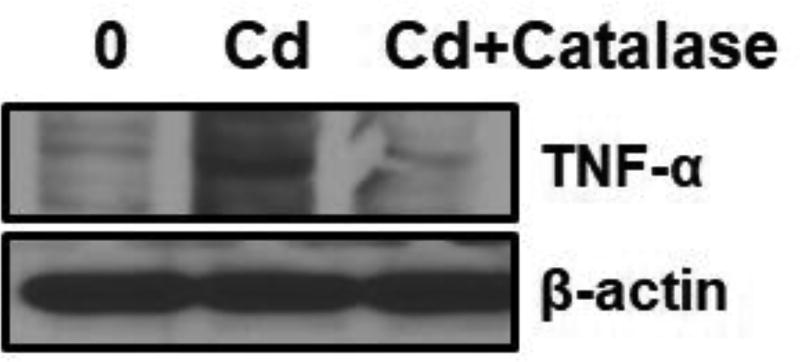
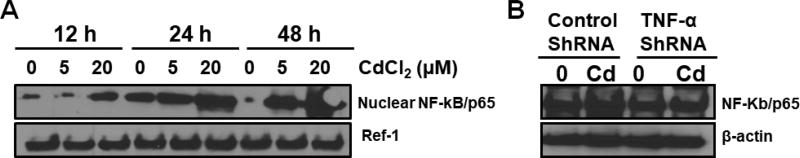
We treated BEAS-2BR cells with cadmium (10 µM) for 24 hours with and without antioxidants to investigate cadmium-induced inflammation and the role of ROS in this process. As expected, increased expression of TNF-α was observed in cadmium-treated BEAS-2BR cells (Fig. 2). When catalase was added as a co-treatment, TNF-α expression decreased (Fig. 2), showing that ROS play an essential role in cadmium-induced TNF-α induction. Since TNF-α has a property to upregulate NF-kB, we investigated the NF-κB activation by measuring the protein level of NF-κB/p65, a subunit of NF-κB transcription complex, which plays a crucial role in inflammatory and immune responses. BEAS-2BR cells were exposed to different concentrations of cadmium for different periods of times. Activation of NF-κB/P65 was observed (Fig. 3A). We investigated the positive role of TNF-α in cadmium-induced NF-κB activation using TNF-α shRNA to inhibit TNF-α. As shown in Fig. 3B, inhibition of TNF-α decreased NF-κB activation, showing that TNF-α is the upstream of NF-κB. Because COX-2 is the NF-κB downstream protein, we expected that exposure of cadmium to BEAS-2BR cells would increase this protein. BEAS-2BR cells were treated with different concentrations (0, 5, and 20 µM) of cadmium for different periods of times (12, 24, and 48 h). The results showed that COX-2 protein level was increased in a dose and time dependent manner (Fig. 4). Overall, the results show that exposure of BEAS-2BR cells to cadmium increased inflammatory proteins, TNF-a, COX-2, and NF-κB and that ROS play an important role in these processes.
Fig. 2. Exposure of BEAS-2BR cells to cadmium increased TNF-α expression and catalase inhibited it.
BEAS-2BR cells or BEAS-2BR cells with catalase expression were incubated with 5 µM CdCl2 for 24 hours. Whole cell lysates were isolated. Protein levels of TNF-α were analyzed using immunoblotting.
Fig. 3. Exposure of BEAS-2BR cells to cadmium caused TNFα-dependent NF-kB activation.
(A) BEAS-2BR cells were exposed to cadmium at different concentrations for different periods of time. The cells were harvested and nuclear protein was isolated. The expressions of nuclear NF-kB/p65 and Ref-1 were examined by immunoblotting. (B) BEAS-2BR cells were transfected with control scramble and TNFα shRNA and was exposed to 20 µM CdCl2 for 48 h. The whole cell lysates were collected and expression of NF-kB/p65 and β-actin were examined by immunoblotting.
Fig. 4. Exposure of BEAS-2BR cells to cadmium increased COX-2 expression.
BEAS-2BR cells were exposed to cadmium at different concentrations for different periods of time. The cells were harvested and whole protein was isolated. The expressions of COX-2 were examined by immunoblotting.
Cadmium caused ROS-dependent malignant transformation of BEAS-2BR
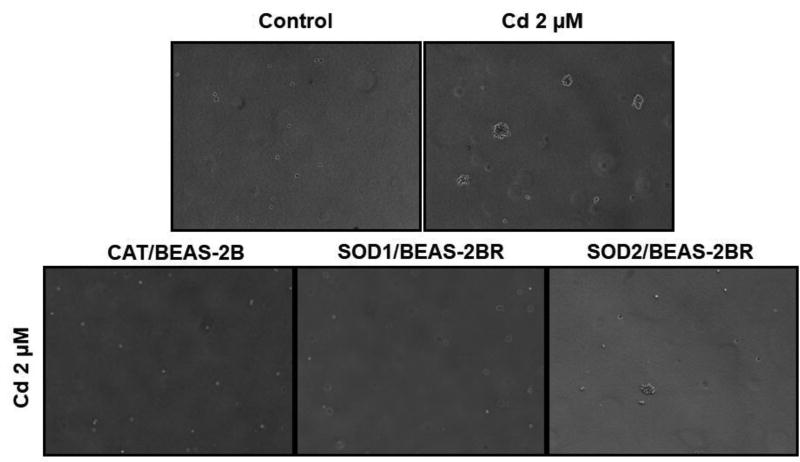
An anchorage-independent colony formation assay was performed 5 months after exposure of BEAS-2BR cells to 2 µM cadmium. Cadmium treated BEAS-2BR cells increased in size and number of colonies (Fig. 5). To investigate whether ROS are involved in cadmium-induced malignant transformation, untransformed BEAS-2BR cells with stable expression of catalase (CAT, antioxidant against H2O2), superoxide dismutase 1 (SOD1, antioxidant against O2•−) and superoxide dismutase 2 (SOD2, antioxidant against O2•−) were established. The soft agar assay shows that the colony formation was significantly inhibited in BEAS-2BR cells overexpressing antioxidant enzymes (CAT, SOD1 and SOD2) compared to BEAS-2BR cells treated with cadmium (2 µM) (Fig. 5). Overexpression of these antioxidants inhibited cadmium-induced anchorage-independent colony formation, showing that ROS are important in cadmium-induced cell transformation.
Fig. 5. Inhibition of cadmium-induced cell transformation by overexpression of antioxidant enzymes.
BEAS-2BR cells or BEAS-2BR cells with overexpression of catalase (CAT), superoxide dismutase 1 (SOD1), or superoxide dismutase (SOD2) were exposed to 2 µM CdCl2. Fresh medium was added every 3 days. Cell transformation (soft agar) assay was performed as described in in our previous studies (Son et al., 2012). The anchorage-independent formation of colonies was stained with 1 mg/mL iodonitrotetrazolium violet overnight.
Cadmium-transformed cells have the property of autophagy deficiency and sulforaphane was able to enhance autophagy in untransformed cells and restore apoptosis in cadmium-transformed BEAS-2BR cells
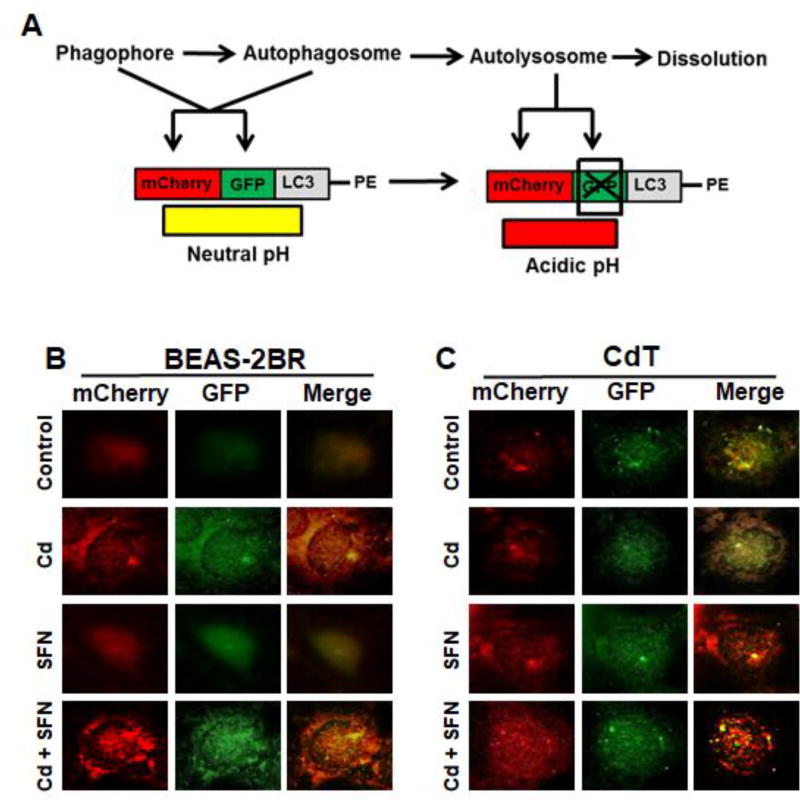
To test the effect of sulforaphane on cadmium-induced autophagy in untransformed and cadmium-transformed BEAS-2BR cells, tandem fluorescence protein mCherry-GFP-LC3 construct was transfected into untransformed BEAS-2BR cells and cadmium-transformed cells. As shown in Fig. 6A, when autophagosome are accumulated, both red and green LC-3 puncta were observed. The merging of these two images generates a yellow color, which indicates that autophagosomes were not fused with lysosomes to form autolysosomes and that the autophagy was not completed. When the fusion occurs and autolysosomes are generated, and green fluorescence dissolves and red color is maintained and then merged image color would a red shade and not yellow.
Fig. 6. Sulforaphane (SFN) increased Cd(II)-induced autophagy in untransformed and Cd(II)-transformed cells.
(A) Schematic illustration of detection of autophagosomes and autolysosomes. The mCherry-GFP-LC3-PE reporter is red and green when it associates with phagophores (also called isolation membranes) and autophagosomes (PE denotes phosphatidylethanolamine). After fusion with lysosomes to form autolysosomes, GFP signal is quenched by the acidic condition. (B) Sulforaphane increases Cd(II)-induced autophagy in untransformed BEAS-2BR cells and Cd(II)-transformed BEAS-2BR cells. Passage-matched untransformed cells (BEAS-2BR) (left) and Cd(II)-transformed cells (BEAS-2B-Cd) (right) were transfected with the mCherry-EGFP-LC3 construct. The cells were treated with Cd(II), sulforaphane (SFN), or Cd(II) together with SFN for 24 h. Images were captured using fluorescence microscope.
When untransformed BEAS-2BR were treated with cadmium, significant increases in red and green LC-3 puncta were observed and merged image color was a red shade, indicating that cadmium induced autophagy in untransformed BEAS-2BR cells and the autophagy was complete (Fig. 6B). Sulforaphane itself induced weak but observable autophagy in BEAS-2BR cells. When untransformed BEAS-2BR cells were co-treated with cadmium and sulforaphane, both red and green LC-3 puncta had higher intensities and when the images were merged, there was a red color (Fig. 6B), demonstrating that sulforaphane increased cadmium-induced autophagy of BEAS-2BR cells. When cadmium-transformed BEAS-2BR cells were treated with cadmium, both red and green LC-3 puncta were observed. The merged image color a yellow shade rather than red, signifying that autophagosome were generated and were not fused with lysosome to generate autolysosomes, showing that cadmium-transformed cells have a property of autophagy deficiency (Fig. 6C). In addition, sulforaphane treatment alone increased the intensities in both red and green LC-3 puncta and the merged image color was red. These results demonstrate that sulforaphane was able to increase autophagy of cadmium-induced transformed cells (Fig. 6C). Co-treatment of cadmium-transformed cells with sulforaphane further increased the autophagy. In summation, the results show that sulforaphane was able to increase cadmium-induced autophagy and that cadmium-transformed BEAS-2BR cells had a property of autophagy deficiency, and sulforaphane could restore the autophagy deficiency of cadmium-transformed cells.
p62 and Nrf2 were constitutively upregulated in cadmium-transformed cells
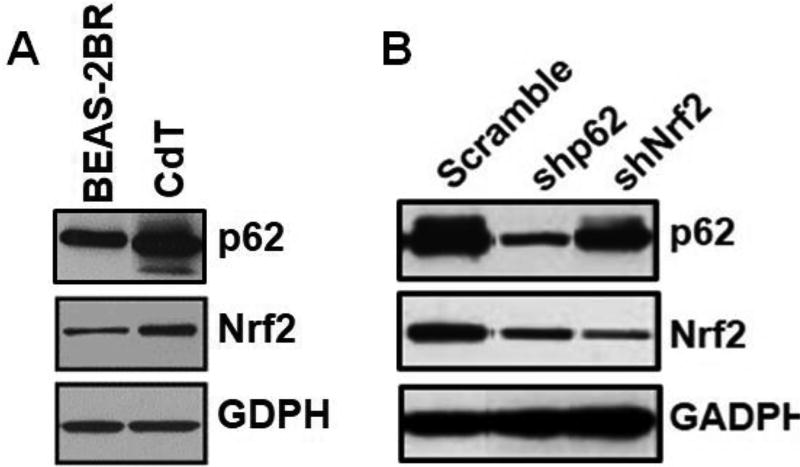
The results in the prior section indicated that cadmium-transformed BEAS-2BR cells had a property of autophagy deficiency with accumulation of autophagosomes. The high level of autophagosomes indicated an increased level of p62. The basal expression of p62 and Nrf2 were measured in untransformed BEAS-2BR cells and cadmium-transformed BEAS-2BR cells by western blot analysis. (Fig. 7A). The results showed that p62 and Nrf2 were constitutively activated in cadmium-transformed cells. For the relationship between p62 and Nrf2, we noted that recent studies linked p62 overexpression to Nrf2 activation through binding of p62 to Keap1, a negative regulator of Nrf2 activation. It is expected that autophagy deficiency in cadmium-transformed cells increased p62 (Fig. 7A) and p62 bound to Keap1 and thus activated Nrf2. Our results (Fig. 7B) showed that knockdown of p62 by shRNA reduced Nrf2 level in Cd(II)-transformed cells (Fig. 7B), indicating that p62 was a positive regulator of Nrf2. Inhibition of Nrf2 also decreased p62 levels, suggesting that p62 had a positive feedback relationship with Nrf2. In the p62 gene promotor, there is an antioxidant response element (ARE) site. It is likely that Nrf2 upregulated p62 via binding to the ARE site in the gene promotor of p62. Overall, the results above show that p62 was upregulated in cadmium-transformed cells, which upregulated Nrf2 and that upregulated Nrf2 also upregulated p62 and formed a positive feed-back loop.
Fig. 7. Constitutively high levels of p62 and Nrf2 in cadmium-transformed BEAS-2BR cells and p62 positively regulated Nrf2, which had a positive feedback relationship with p62.
(A) Constitutively high levels of p62 and Nrf2 in cadmium-transformed cells. Whole cell lysates from cadmium-transformed cells (CdT) and their passage-matched untransformed cells (BEAS-2BR) were isolated. Protein levels of p62 and Nrf2 were analyzed using immunoblotting. (B) p62 positively regulates Nrf2, which has a positive feedback relationship with p62 in cadmium-transformed cells. These cells with or without knockdown of p62 or Nrf2 were cultures in 100 mm dishes. Whole cell lysates were isolated. Protein levels of p62 and Nrf2 were analyzed using immunoblotting.
Cadmium-transformed cells were resistant to apoptosis
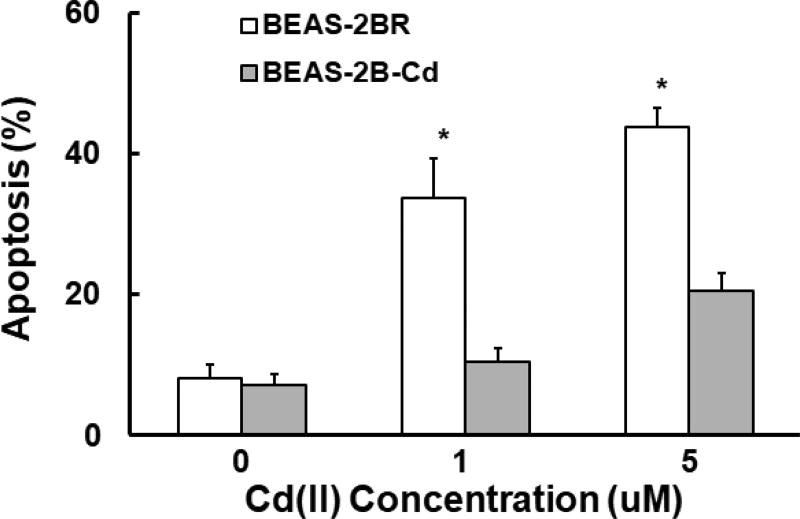
Our previous study shows that cadmium-transformed cells highly expressed antiapoptotic proteins Bcl-2 and Bcl-xl (Son et al., 2014). There are ARE sites on the gene promotors of these two proteins. Thus, Nrf2 upregulated Bcl-2 and Bcl-xl, leading to apoptosis resistance. The results showed that the cadmium-induced apoptotic cell deaths increased in a dose-dependent manner in untransformed BEAS-2BR cells, while the increase in apoptotic cell deaths in cadmium-transformed BEAS-2BR were small compared to untransformed BEAS-2BR cells (Fig. 8). The results show that in cadmium-transformed cells increase in Nrf2 upregulated Bcl-2 and Bcl-xl through binding of Nrf2 to the ARE sites in the gene promotors of these two anti-apoptotic proteins, leading to apoptosis resistance.
Fig. 8. Cadmium-transformed cells were resistant to apoptosis.
Passage-matched untransformed cells (BEAS-2BR) and Cd(II)-transformed cells (BEAS-2B-Cd) were exposed to CdCl2 (0–5 µM) for 24 h. The cells were analyzed by flow cytometer after staining with Annexin V-fluorescein isothiocyanate/propidium iodide. The results are shown as mean±SD in three independent experiments. *, p<0.05, compared to BEAS-2BR cells without treatment.
Sulforaphane increased inducible Nrf2 expression in untransformed BEAS-2BR cells and decreased constitutive Nrf2 expression in cadmium-transformed BEAS-2BR cells
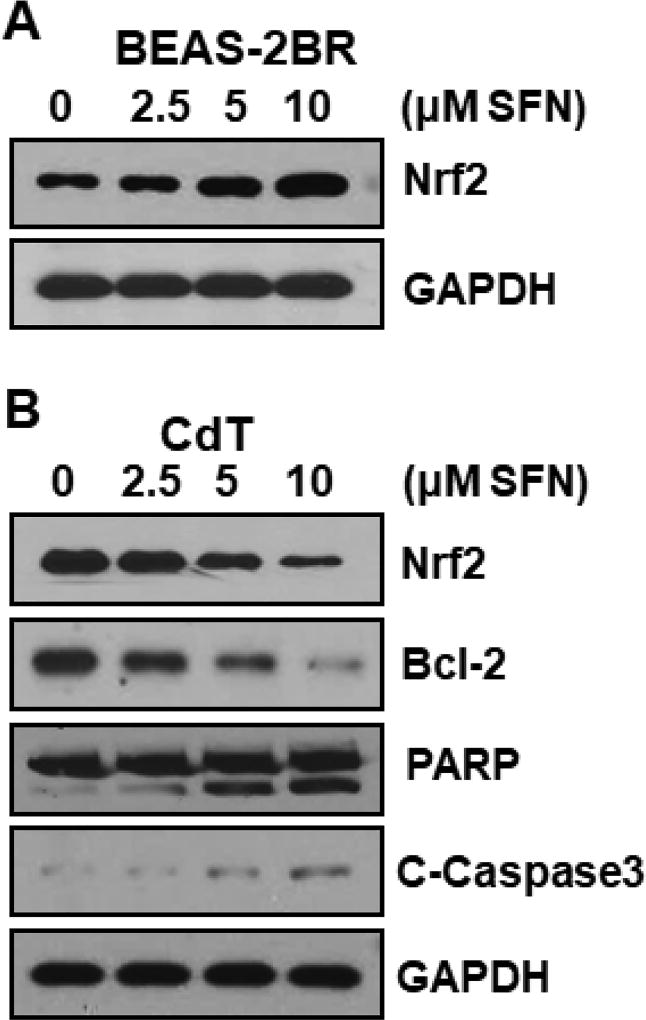
Fig. 1–8 results demonstrate that ROS were important in cadmium-induced malignant cell transformation. Thus, decreasing the ROS by increasing inducible Nrf2 inhibited cadmium-induced malignant cell transformation. In contrast, in cadmium-transformed cells, constitutive Nrf2 activation through p62 upregulation caused apoptosis resistance. Sulforaphane is a well-recognized antioxidant. It can also restore autophagy deficiency and decrease p62 in cadmium-transformed cells. We speculated that sulforaphane activated inducible Nrf2 in untransformed BEAS-2BR cells and decreased constitutive Nrf2 in cadmium-transformed cells. Our results demonstrate that sulforaphane indeed upregulated inducible Nrf2 expression in untransformed BEAS-2BR cells (Fig. 9A). In cadmium-transformed cells sulforaphane decreased constitutive Nrf2 and its target anti-apoptotic gene Bcl-2 (Fig. 9B). The elevated expression of both cleaved poly ADP ribose polymerase (C-PARP) and cleaved caspase-3 (C-caspase 3) further demonstrated that sulforaphane restored apoptosis capability of cadmium-transformed cells (Fig. 9B).
Fig. 9. Sulforaphane activated inducible Nrf2 in untransformed cells and decreased constitutive Nrf2 and Bcl-2 in cadmium-transformed cells.
(A) Sulforaphane increased Nrf2 expression in untransformed cells. BEAS-2BR cells were treated with sulforaphane at different concentrations (0, 2.5, 5, and 10 µM) for 24 hours. Cells were harvested and whole protein lysates were extracted. Nrf2 expression was examined by immunoblotting. (B) Sulforaphane inhibited Nrf2 and Bcl-2 and restored apoptosis in cadmium-transformed cells. These transformed cells (BEAS-2B-Cd) were treated with sulforaphane (SFN) at different concentrations (0, 2.5, 5, and 10 µM) for 24 h. The whole protein lysates were extracted. Protein levels were examined by immunoblotting.
DISCUSSION
The goal of present study was to understand the mechanism of cadmium-induced carcinogenesis and develop mechanism-based prevention strategy by investigating whether sulforaphane can activate inducible Nrf2 in untransformed cells and decrease constitutive Nrf2 in cadmium-transformed cells. Carcinogenesis is a multistage process, but for the purpose of discussing the mechanisms of metal-induced carcinogenesis, we conceptually divide metal-induced carcinogenesis into two stages (early and late) (Son et al., 2012; Xu et al., 2017). Cadmium carcinogenesis is divided into two stages. In the first stage, untransformed cells undergo transformation to cadmium-transformed cells. Then In the second stage, transformed cells can progress into tumorigenesis. Using flow cytometry and ESR spin trapping, the present study demonstrates that exposure of BEAS-2BR cells to cadmium generated ROS and catalase and sulforaphane inhibited the cadmium-induced ROS generation. The results obtained from soft agar assay shows overexpression of oxidant enzymes (catalase, SOD1, or SOD2) inhibited soft agar colony formation induced by cadmium. These results indicate cadmium-induced ROS generation is an important mechanism in cadmium-induced malignant cells transformation. Considering the importance of ROS in cadmium-induced malignant cell transformation, ROS can be a target to prevent cadmium-induced carcinogenesis. Our study also shows in BEAS-2BR cells, sulforaphane decreased cadmium-induced ROS generation and sulforaphane activated inducible Nrf2 in untransformed BEAS-2BR cell. Sulforaphane increased inducible Nrf2 in untransformed BEAS-2BR cells and upregulated the Nrf2 target antioxidant (catalase and SOD) through binding to the ARE site in the promotor region of these antioxidant proteins. Sulforaphane reduced Cd-induced ROS and inhibited Cd-induced cellular transformation. These results indicate that sulforaphane can be used to induce Nrf2 and to prevent cadmium-induced cell transformation in the first stage of cadmium carcinogenesis.
Chronic inflammation promotes malignant cell transformation and carcinogenesis (Landskron et al., 2014). In untransformed cells, inflammatory proteins, such as COX-2, TNF-α, and NF-κB generated through metal-induced exposures are a considered important piece in the metal-induced carcinogenesis mechanism (Wang et al., 2016). The western blot results obtained in the present study showed that the levels of TNF-α, NF-κB, and COX-2 were elevated in cadmium-treated BEAS-2BR cells. Several studies suggest that TNF-α is a candidate, linking molecules between inflammation and cancer (Balkwill, 2002; Huang et al., 1999; Micheau and Tschopp, 2003). TNF-α may initiate an inflammatory cascade consisting of other inflammatory cytokines, chemokines, growth factors, and endothelial adhesion factors recruiting a variety of activated cells at the site of tissue damage (Balkwill, 2002). TNF-α is required in chemical carcinogen-elicited carcinogenesis and is also a major inducer for NF-κB activation, which shows anti-apoptotic activity (Philip et al., 2004). The present study shows that ROS generated by cadmium up-regulates TNF-α, which activates NF-κB. Another role of TNF-α in linking inflammation to cancer might be its regulation of a network of chemokines and COX-2 expression (Balkwill, 2002). The present study shows that the cadmium exposure increased COX-2. COX-2 expression can be induced by a wide range of stimuli including LPS, proinflammatory cytokines such as TNF-α and growth factors such as epidermal growth factor (Williams et al., 1999). The products of COX-2 enzyme are prostaglandins, which are key mediators of inflammation (Nathan, 2002; Steele et al., 2003). COX-2 is overexpressed in various types of cancer and involved in cellular proliferation, anti-apoptotic activity, angiogenesis, and an increase of metastasis (Eberhart et al., 1994; Hida et al., 1998; Hwang et al., 1998; Okami et al., 1999; Prescott and Fitzpatrick, 2000; Ristimaki et al., 1997; Tsujii et al., 1997). COX-2 is induced by compounds from cigarette smoke, indicating a possible mechanism for the cigarette smoking-triggered, cancer-prone, inflammatory lung diseases (Martey et al., 2004; Ouyang et al., 2007). The upregulation of COX-2 by cadmium indicates that COX-2 might play a role in cadmium-induced inflammation and lung carcinogenesis. Chronic inflammation has been implicated in the development of a wide array of human cancers, including lung cancer (Coussens and Werb, 2002). Inflammation is associated with enhanced survival signaling and epithelial cell proliferation. The increase of inflammation by cadmium exposure may create an inflammatory microenvironment that may participate in the initiation and promotion of malignant cell transformation and contribute over time to cadmium carcinogenesis.
Autophagy is an evolutionarily conserved catabolic process. Autophagy is important in maintenance of cellular homeostasis (Choi 2012). Our previous study shows that autophagy is a cell self-protective mechanism against arsenic-induced cell transformation by inhibiting ROS generation and facilitate mitochondrial turnover in arsenic-treated BEAS-2BR cells (Zhang et al., 2012). Many studies previously reported that autophagy can be a tumor-suppressive mechanism (Jiang et al., 2015). The present study shows that sulforaphane can enhance autophagy in cadmium-treated BEAS-2BR cells. It is very possible that the increase of autophagy by sulforaphane facilitates mitochondrial turnover in untransformed cells and inhibits ROS generation and cadmium-induced malignant transformation. The present study shows that cadmium-transformed BEAS-2BR cells exhibit the property of autophagy deficiency. In these autophagy deficient cells, autophagosomes do not fuse with lysosomes, leading to the accumulation of p62 (Mathew et al., 2009). Sulforaphane increased cadmium-induced autophagy in untransformed BEAS-2BR cells and autophagy flux by increasing the fusion of autophagosomes with lysosomes to generates autolysosomes in cadmium-transformed cells. Restoration of autophagy competence of cadmium-transformed cells by sulforaphane eliminated p62 accumulation in cadmium-transformed cells, because p62 is able to bind to Keap1, which is an inhibitor of Nrf2, the increase of p62 increases Nrf2. Sulforaphane restored autophagy competence of cadmium-transformed cells, inhibited p62, and decreased Nrf2 in cadmium-transformed cells.
Constitutive overexpression of Nrf2 increases the binding activities to the ARE regions of Bcl-2 and Bcl-xl in cadmium-transformed cells (Son et al., 2014). The increase of apoptosis resistance observed in the present study is likely due to the upregulation of Bcl-2 and Bcl-xl by Nrf2 through binding of this transcription factor to the ARE sites of these two anti-apoptotic proteins. The present study shows that sulforaphane inhibited constitutively Nrf2 expression in cadmium-transformed cells and decreased the expression of the anti-apoptotic protein, Bcl-2. It is likely that decrease of constitutive expression of Nrf2 by sulforaphane attenuates the binding activity of both anti-apoptosis and anti-oxidant genes, leading to increased apoptosis and elevated ROS expression.
In summary, the results obtained in the present study generated the following conclusions. (a) In untransformed cells, exposure of BEAS-2BR cells to cadmium generated ROS. Through ROS generation, cadmium created an inflammatory microenvironment, which may contribute to cadmium-induced malignant cell transformation via ROS reaction. (b) Cadmium-transformed cells have a property of autophagy deficiency, resulting in accumulation of autophagosomes and increase of p62. This protein upregulated Nrf2, which also upregulated p62 through positive feed-back mechanism. Constitutive Nrf2 increased its downstream antiapoptotic proteins, Bcl-2 and Bcl-xl, resulting in apoptosis resistance. (c) In untransformed BEAS-2BR cells, sulforaphane increased autophagy, activated Nrf2 and decreased ROS. In cadmium-transformed BEAS-2BR cells, sulforaphane restored autophagy competence, decreased Nrf2, and decreased apoptosis resistance. Thus, sulforaphane has dual roles. In untransformed BEAS-2BR cells, this natural compound induced inducible Nrf2 to decrease ROS and malignant cell transformation. In cadmium-transformed cells, it decreased constitutive Nrf2 and reduced apoptosis resistance. The dual roles of sulforaphane make this natural compound an ideal agent for prevention against cadmium-induced carcinogenesis.
Highlights.
Sulforaphane treatment of cadmium-transformed cells restores autophagy
Sulforaphane treatment of cadmium-transformed cells decreases constitutive Nrf2
Sulforaphane protects against cadmium-induced lung carcinogenesis
Sulforaphane reduces cadmium-induced ROS
Sulforaphane can activate inducible Nrf2 and decrease constitutive Nrf2
Acknowledgments
We would like to thank Hong Lin from the University of Kentucky technical and administrative support. This research was supported by National Institutes of Health [R01ES021771, R01ES020870, R01ES025515, R01ES28984, R01ES21771, and P30ES026529].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflicts of interest.
References
- Alkharashi NAO, Periasamy VS, Athinarayanan J, Alshatwi AA. Sulforaphane mitigates cadmium-induced toxicity pattern in human peripheral blood lymphocytes and monocytes. Environ Toxicol. Pharmacol. 2017;55:223–239. doi: 10.1016/j.etap.2017.08.026. [DOI] [PubMed] [Google Scholar]
- Alkharashi NAO, Periasamy VS, Athinarayanan J, Alshatwi AA. Assessment of sulforaphane-induced protective mechanisms against cadmium toxicity in human mesenchymal stem cells. Environ Sci. Pollut. Res. Int. 2018;25:10080–10089. doi: 10.1007/s11356-018-1228-7. [DOI] [PubMed] [Google Scholar]
- Balkwill F. Tumor necrosis factor or tumor promoting factor? Cytokine Growth Factor Rev. 2002;13:135–141. doi: 10.1016/s1359-6101(01)00020-x. [DOI] [PubMed] [Google Scholar]
- Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25:409–16. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- Brandenburg LO, Kipp M, Lucius R, Pufe T, Wruck CJ. Sulforaphane suppresses LPS-induced inflammation in primary rat microglia. Inflamm. Res. 2010;59:443–450. doi: 10.1007/s00011-009-0116-5. [DOI] [PubMed] [Google Scholar]
- Chen N, Karantza V. Autophagy as a therapeutic target in cancer. Cancer Biol. Ther. 2011;11:157–168. doi: 10.4161/cbt.11.2.14622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Xun P, Nishijo M, Sekikawa A, He K. Cadmium exposure and risk of pancreatic cancer: a meta-analysis of prospective cohort studies and case-control studies among individuals without occupational exposure history. Environ. Sci. Pollut. Res. Int. 2015;22:17465–17474. doi: 10.1007/s11356-015-5464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Xun P, Nishijo M, Carter S, He K. Cadmium exposure and risk of prostate cancer: a meta-analysis of cohort and case-control studies among the general and occupational populations. Sci. Rep. 2016a;6:25814. doi: 10.1038/srep25814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Xun P, Nishijo M, He K. Cadmium exposure and risk of lung cancer: a meta-analysis of cohort and case-control studies among general and occupational populations. J Expo Sci. Environ. Epidemiol. 2016b;26:437–444. doi: 10.1038/jes.2016.6. [DOI] [PubMed] [Google Scholar]
- Choi KS. Autophagy and cancer. Exp. Mol. Med. 2012;44:109–120. doi: 10.3858/emm.2012.44.2.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart C, Coffey R, Radhika A, Giardiello F, Ferrenbach S, Dubois R. Upregulation of cyclooxygenase-2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Echizen K, Oshima H, Nakayama M, Oshima M. The inflammatory microenvironment that promotes gastrointestinal cancer development and invasion. Adv Biol Regul. 2018 doi: 10.1016/j.jbior.2018.02.001. S2212-4926(18)30072-1. [DOI] [PubMed] [Google Scholar]
- Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J. Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman-Antosiewicz A, Johnson DE, Singh SV. Sulforaphane causes autophagy to inhibit release of cytochrome C and apoptosis in human prostate cancer cells. Cancer Res. 2006;66:5828–5835. doi: 10.1158/0008-5472.CAN-06-0139. [DOI] [PubMed] [Google Scholar]
- Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Nakamura S, Ogawa M, Mitsudomi T, Sugiura T, Takahashi T. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998;58:3761–3764. [PubMed] [Google Scholar]
- Huang C, Li J, Ma WY, Dong Z. JNK activation is required for JB6 cell transformation induced by tumor necrosis factor-alpha but not by 12-O-Tetradecanoylphorbol-13-Acetate. J. Biol. Chem. 1999;274:29672–29676. doi: 10.1074/jbc.274.42.29672. [DOI] [PubMed] [Google Scholar]
- Hwang D, Scollard D, Byrne J, Levine E. Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cancer. J. Natl. Cancer Inst. 1998;90:455–460. doi: 10.1093/jnci/90.6.455. [DOI] [PubMed] [Google Scholar]
- IARC. Beryllium, cadmium, mercury, and exposures in the glass manufacturing industry. Working Group views and expert opinions, Lyon, 9–16 February 1993. IARC Monogr Eval Carcinog Risks Hum. 1993;58:1–415. [PMC free article] [PubMed] [Google Scholar]
- Jain A, Lamark T, Sjøttem E, Larsen KB, Awuh JA, Øvervatn A, McMahon M, Hayes JD, Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Overholtzer M, Thompson CB. Autophagy in cellular metabolism and cancer. J. Clin. Invest. 2015;125:47–54. doi: 10.1172/JCI73942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y, Reddel RR, Gerwi BI, Miyashita M, McMenamin M, Lechner JF, Harris CC. Human bronchial epithelial cells with integrated SV40 virus T antigen genes retain the ability to undergo squamous differentiation. Differentiation. 1988;38:60–66. doi: 10.1111/j.1432-0436.1988.tb00592.x. [DOI] [PubMed] [Google Scholar]
- Kim J, Song H, Heo HR, Kim JW, Kim HR, Hong Y, Yang SR, Han SS, Lee SJ, Kim WJ, Hong SH. Cadmium-induced ER stress and inflammation are mediated through C/EBP-DDIT3 signaling in human bronchial epithelial cells. Exp. Mol. Med. 2017;49:e372. doi: 10.1038/emm.2017.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson SC, Orsini N, Wolk A. Urinary cadmium concentration and risk of breast cancer: a systematic review and dose-response meta-analysis. Am. J. Epidemiol. 2015;182:375–380. doi: 10.1093/aje/kwv085. [DOI] [PubMed] [Google Scholar]
- Lee JH, Jeong JK, Park SY. Sulforaphane-induced autophagy flux prevents prion protein-mediated neurotoxicity through AMPK pathway. Neuroscience. 2014;278:31–39. doi: 10.1016/j.neuroscience.2014.07.072. [DOI] [PubMed] [Google Scholar]
- Levine B, Klionsky DJ. Developmental of self-digestion: molecular mechanisms and biological functions of autophagy. Dev. Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- Martey CA, Pollock SJ, Turner CK, O'ReillyM KMA, Baglole CJ, Phipps RP, Sime PJ. Cigarette smoke induces cyclooxygenase-2 and microsomal prostaglandin E2 synthase in human lung fibroblasts: implications for lung inflammation and cancer. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2004;287:L981–L991. doi: 10.1152/ajplung.00239.2003. [DOI] [PubMed] [Google Scholar]
- Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, Dipaola RS, Karantza-Wadsworth V, White E. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated spoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- Nakahira K, Cloonan SM, Mizumura K, Choi AM, Ryter SW. Autophagy: a crucial moderator of redox balance, inflammation, and apoptosis in lung disease. Antioxid Redox Signal. 2014;20:474–494. doi: 10.1089/ars.2013.5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- Okami J, Yamamoto H, Fujiwara Y, Tsujie M, Kondo M, Noura S, Oshima S, Nagano H, Dono K, Umeshita K, Ishikawa O, Sakon M, Matsuura N, Nakamori S, Monden M. Overexpression of Cyclooxygenase-2 in Carcinoma of the Pancreas. Clin. Cancer Res. 1999;5:2018–2024. [PubMed] [Google Scholar]
- Olszowski T, Baranowska-Bosiacka I, Gutowska I, Chlubek D. Pro-inflammatory properties of cadmium. Acta Biochim Pol. 2012;59:475–482. [PubMed] [Google Scholar]
- Ouyang W, Ma Q, Li J, Zhang D, Ding J, Huang Y, Xing M, Huang C. Benzo[a]pyrene diol-epoxide (B[a]PDE) upregulates COX-2 expression through MAPKs/AP-1 and IKKβ/NF-κB in mouse epidermal Cl41 cells. Mol. Carcinog. 2007;46:32–41. doi: 10.1002/mc.20260. [DOI] [PubMed] [Google Scholar]
- Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Øvervatn A, Bjørkøy G, Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- Philip M, Rowley DA, Schreiber H. Inflammation as a tumor promoter in cancer induction. Semin. Cancer Biol. 2004;14:433–439. doi: 10.1016/j.semcancer.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Phuagkhaopong S, Ospondpant D, Kasemsuk T, Sibmooh N, Soodvilai S, Power C, Vivithanaporn P. Cadmium-induced IL-6 and IL-8 expression and release from astrocytes are mediated by MAPK and NF-κB pathways. Neurotoxicology. 2017;60:82–91. doi: 10.1016/j.neuro.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Prescott SM, Fitzpatrick FA. Cyclooxygenase-2 and carcinogenesis. Biochim. Biophys. Acta. 2000;1470:M69–M78. doi: 10.1016/s0304-419x(00)00006-8. [DOI] [PubMed] [Google Scholar]
- Rafati Rahimzadeh M, Rafati Rahimzadeh M, Kazemi S, Moghadamnia AA. Cadmium toxicity and treatment: An update. Caspian J. Intern. Med. 2017;8:135–145. doi: 10.22088/cjim.8.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristimaki A, Honkanen N, Jankala H, Sipponen P, Harkonen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997;57:1276–1280. [PubMed] [Google Scholar]
- Sestili P, Fimognari C. Cytotoxic and Antitumor Activity of Sulforaphane: The Role of Reactive Oxygen Species. Biomed Res Int. 2015;2015:402386. doi: 10.1155/2015/402386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Lenhart A, Mao Y. ESR spin trapping investigation on peroxynitrite decomposition: no evidence for hydroxyl radical production. Biochem. and Biophys. Res. Commun. 1994a;203:1515–1521. doi: 10.1006/bbrc.1994.2357. [DOI] [PubMed] [Google Scholar]
- Shi X, Dalal N, Kasprzak KS. Enhanced generation of hydroxyl radical and sulfur trioxide anion radical from oxidation of sodium sulfite, nickel(II) sulfite, and nickel subsulfide in the presence of nickel(II) complexes. Environ. Health Perspect. 1994b;102:91–96. doi: 10.1289/ehp.94102s391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son YO, Lee JC, Hitron JA, Pan J, Zhang Z, Shi X. Cadmium induces intracellular Ca2+- and H2O2-dependent apoptosis through JNK- and p53-mediated pathways in skin epidermal cell line. Toxicol. Sci. 2010a;113:127–137. doi: 10.1093/toxsci/kfp259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son YO, Hitron JA, Wang X, Chang Q, Pan J, Zhang Z, Liu J, Wang S, Lee JC, Shi X. Cr(VI) induces mitochondrial-mediated and caspase-dependent apoptosis through reactive oxygen species-mediated p53 activation in JB6 Cl41 cells. Toxicol. Appl. Pharmacol. 2010b;245:226–235. doi: 10.1016/j.taap.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son YO, Wang X, Hitron JA, Zhang Z, Cheng S, Budhraja A, Ding S, Lee JC, Shi X. Cadmium induces autophagy through ROS-dependent activation of the LKB1-AMPK signaling in skin epidermal cells. Toxicol. Appl. Pharmacol. 2011;255:287–296. doi: 10.1016/j.taap.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son YO, Wang L, Poyil P, Budhraja A, Hitron JA, Zhang Z, Lee JC, Shi X. Cadmium induces carcinogenesis in BEAS-2B cells through ROS-dependent activation of PI3K/AKT/GSK-3β/β-catenin signaling. Toxicol Appl Pharmacol. 2012;264:153–160. doi: 10.1016/j.taap.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son YO, Pratheeshkumar P, Roy RV, Hitron JA, Wang L, Zhang Z, Shi X. Nrf2/p62 signaling in apoptosis resistance and its role in cadmium-induced carcinogenesis. J. Biol. Chem. 2014;289:28660–28675. doi: 10.1074/jbc.M114.595496. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Steele VE, Hawk ET, Viner JL, Lubet RA. Mechanisms and applications of non-steroidal anti-inflammatory drugs in the chemoprevention of cancer. Mutat. Res. 2003;523–524:137–144. doi: 10.1016/s0027-5107(02)00329-9. [DOI] [PubMed] [Google Scholar]
- Sui X, Lei L, Chen L, Xie T, Li X. Inflammatory microenvironment in the initiation and progression of bladder cancer. Oncotarget. 2017;8:93279–93294. doi: 10.18632/oncotarget.21565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc. Natl. Acad. Sci. U. S. A. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wise JT, Zhang Z, Shi X. Progress and prospects of reactive oxygen species in metal carcinogenesis. Curr. Pharmacol. Rep. 2016;2:178–186. doi: 10.1007/s40495-016-0061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C, Mann M, DuBois R. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhou BP. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br J Cancer. 2010;102:639–644. doi: 10.1038/sj.bjc.6605530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wise JTF, Wang L, Schumann K, Zhang Z, Shi X. Dual Roles of Oxidative Stress in Metal Carcinogenesis. J Environ Pathol Toxicol Oncol. 2017;36:345–376. doi: 10.1615/JEnvironPatholToxicolOncol.2017025229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Palliyaguru DL, Kensler TW. Frugal Chemoprevention: Targeting Nrf2 with Foods Rich in Sulforaphane. Semin Oncol. 2016a;43:146–153. doi: 10.1053/j.seminoncol.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Long M, Yu LH, Li L, Li P, Zhang Y, Guo Y, Gao F, Liu MD, He JB. Sulforaphane Prevents Testicular Damage in Kunming Mice Exposed to Cadmium via Activation of Nrf2/ARE Signaling Pathways. Int. J. Mol. Sci. 2016b;17:E1703. doi: 10.3390/ijms17101703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Qi Y, Liao M, Xu M, Bower KA, Frank JA, Shen HM, Luo J, Shi X, Chen G. Autophagy is a cell self-protective mechanism against arsenic-induced cell transformation. Toxicol. Sci. 2012;130:298–308. doi: 10.1093/toxsci/kfs240. [DOI] [PubMed] [Google Scholar]