Abstract
There is a global epidemic of chronic kidney disease (CKD) characterized by a progressive loss of nephrons, ascribed in large part to a rising incidence of hypertension, metabolic syndrome, and type 2 diabetes mellitus. There is a ten-fold variation in nephron number at birth in the general population, and a 50% overall decrease in nephron number in the last decades of life. The vicious cycle of nephron loss stimulating hypertrophy by remaining nephrons and resulting in glomerulosclerosis has been regarded as maladaptive, and only partially responsive to angiotensin inhibition. Advances over the past century in kidney physiology, genetics, and development have elucidated many aspects of nephron formation, structure and function. Parallel advances have been achieved in evolutionary biology, with the emergence of evolutionary medicine, a discipline that promises to provide new insight into the treatment of chronic disease.
This review provides a framework for understanding the origins of contemporary developmental nephrology, and recent progress in evolutionary biology. The establishment of evolutionary developmental biology (evo-devo), ecological developmental biology (eco-devo), and developmental origins of health and disease (DOHaD) followed the discovery of the hox gene family, the recognition of the contribution of cumulative environmental stressors to the changing phenotype over the life cycle, and mechanisms of epigenetic regulation. The maturation of evolutionary medicine has contributed to new investigative approaches to cardiovascular disease, cancer, and infectious disease, and promises the same for CKD. By incorporating these principles, developmental nephrology is ideally positioned to answer important questions regarding the fate of nephrons from embryo through senescence.
Keywords: chronic kidney disease, development, epigenetics, evolution, genetics, physiology
1 Introduction
Ten percent of the global population is affected by chronic kidney disease (CKD); for those aged 65 and older, the prevalence is over 20%, and is rapidly increasing [1]. While the growing epidemic of type 2 diabetes has figured as the leading proximate cause of this public health crisis, there is growing evidence for major contributing factors in early embryonic through postnatal development, generating questions that require explanation. 1) Why is there is a tenfold variation in nephron number at birth in the human population, with a 50% decrease during adult life? 2) What are the underlying factors (the ultimate causes) driving the progression of CKD, currently regarded as a maladaptive process? This review examines these questions from the perspective of the growth of four biologic disciplines over the past century (physiology, developmental biology, genetics, and evolution), and provides an evolutionary framework for future investigation in kidney development and its importance in the lifetime risk of CKD.
2 Origins of Developmental Nephrology
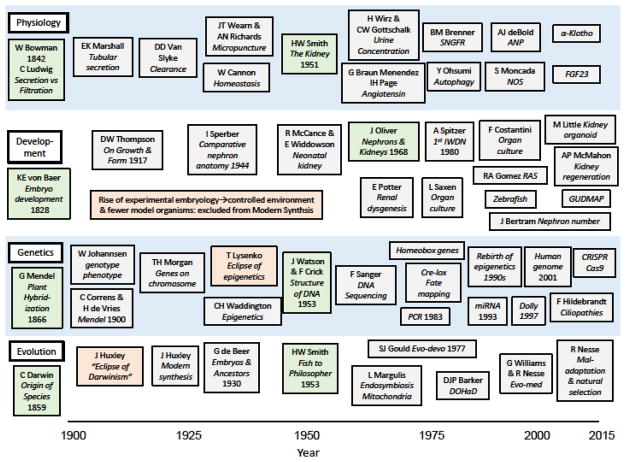
2.1 Renal physiology (Fig 1)
Figure 1.
Parallel timelines of significant advances and publications in physiology, developmental biology, and genetics contributing to the current status of developmental nephrology. Whereas progress in these disciplines has been interdependent, inclusion of evolutionary biology has been minimal, with the exception of studies by Homer Smith and Jean Oliver in the mid-20th century. A century after Charles Darwin’s publication of the Origin of Species in 1859, major advances in molecular genetics have led to a renaissance for evolutionary biology. The new disciplines of evolutionary developmental biology (evo-devo), developmental origins of health and disease (DOHaD) and evolutionary medicine (evo-med) are particularly relevant to future investigation of kidney development and the progression of chronic kidney disease. Green boxes, key publications; red boxes, factors delaying progress. Abbreviations: ANP, atrial natriuretic factor; CRISPR, Clustered Regularly Interspaced Short Palindromic Repeats; Cas9, CRISPR associated protein 9; DNA, deoxyribonucleic acid; DOHaD, developmental origins of health and disease; Dolly, first cloned mammal (sheep); Evo-devo, evolutionary developmental biology; Evo-med, evolutionary medicine; FGF23, fibroblast growth factor 23; GUDMAP, GenitoUrinary Development Molecular Anatomy Project; IWDN, International Workshop of Developmental Nephrology; miRNA, micro RNA; NOS, nitric oxide synthase; PCR, polymerase chain reaction; RAS, renin-angiotensin system; SNGFR, single nephron glomerular filtration rate.
Based on his anatomic study, Bowman in 1842 postulated that urine formation begins with glomerular secretion, whereas Carl Ludwig proposed glomerular filtration followed by tubular reabsorption [2]. The 20th century saw rapid advances in renal physiology, with the demonstration of tubular secretion by Marshall in 1923, and the confirmation of filtration/reabsorption by Wearn and Richards using micropuncture techniques in 1924 [2]. Equally important were the introduction of the concept of “clearance” by van Slyke in 1928 and of “homeostasis” by Walter Cannon in 1932. These set the stage for seminal contributions to renal physiology by Homer Smith in the 1930s and 1940s, culminating in his magisterial text, The Kidney [3]. In the postwar years, normal and abnormal nephron structure and function were elucidated using micropuncture and in vitro techniques [4–6]. These were enhanced by concomitant discoveries of endocrine, paracrine, and autocrine compounds and signaling molecules regulating nephron growth and function [7–9]. The elucidation of the renin-angiotensin-aldosterone system spans the entire 20th century, from the discovery of a renin by Tigerstedt and Bergman in 1898 to angiotensin by Braun Menendez and Page in 1958 and subsequent pharmacologic innovations [10]. The 21st century has brought remarkable insight into the protean role of this regulatory system in kidney morphogenesis, homeostasis, and regeneration [11].
2.2 Developmental nephrology (Fig 1)
In 1828, Karl E. von Baer described 4 Scholia (“laws”) characterizing similarities of early embryonic development across organisms, with later differentiation [12]. While a student at St. Bartholomew’s Hospital in 1875, surgeon Walter Pye published a report on nephron development in a human fetus (ca. 4 months gestation), illustrating centrifugal maturation of glomeruli [13]. In 1944, Swedish anatomist Ivar Sperber published a detailed phylogenetic study of nephron morphometry, including over 100 vertebrate species [14]. The definitive atlas of macroscopic human kidney development was published in 1968 by American anatomist Jean Oliver: Nephrons and Kidneys: A Quantitative Study of Developmental and Evolutionary Mammalian Renal Architectonics [15]. Recent reviews of mammalian nephrogenesis have elucidated the molecular genetics and cellular mechanisms underlying Oliver’s magnificent atlas [16–18].
In the mid-20th century, Robert McCance and Elsie Widdowson were pioneers in the study of human early postnatal renal physiology [19]. Building on the work of Jean Oliver, Edith Potter, a pediatric pathologist at the University of Chicago, quantitated the morphology of normal and abnormal nephron development [20]. Research in developmental nephrology intensified, leading to the First International Workshop on Developmental Renal Physiology in 1980, organized by Adrian Spitzer in New York [21]. Meeting triennially, the Workshop has incorporated many of the advances of the past 36 years, changing its name in 1995 to the International Workshop on Developmental Nephrology to reflect the increasing role of cell biology and molecular genetics [22,23]. Significant progress has resulted from pioneering studies of kidney organ culture by Saxen, elegant time-lapse studies of branching morphogenesis by Costantini, and human organoids by Little and her associates [24–26]. This was propelled also by the cloning of zebrafish by Streisinger and advances in kidney stem cell and regeneration biology by Drummond and others [27,28]. The current state of the art is represented by the GenitoUrinary Development Molecular Anatomy Project (GUDMAP), funded by the National Institutes of Health [29].
2.3 Genetics and epigenetics (Fig 1)
How did genetics arrive at its current central role in the life sciences? Whereas the foundation of modern genetics is rooted in Mendel’s landmark paper of 1866, because of its appearance in an obscure journal it remained unrecognized until its rediscovery by Correns and de Vries in 1900 [30]. Wilhelm Johannsen first coined the term “gene” and distinguished genotype and phenotype in 1909, and T. H. Morgan demonstrated the location of genes on chromosomes in 1910 [31,32]. The discipline of genetics experienced a dark era from the 1920s to 1960s as a result of Trofim Lysenko’s stronghold on Soviet biology, his rejection of the “gene” concept, and his promotion of “vernalization” of wheat crops, a pseudoscience based on heritability of acquired characteristics (Fig. 1) [33]. A British biologist, Conrad Waddington, coined the term “epigenetics” in 1942, referring to characteristics acquired during development [34]. Unfortunately, as a result of its association with Lysenko, Waddington’s early formulation of “developmental plasticity” was largely ignored until recently [35]. The elucidation of the structure of DNA by Watson and Crick in 1953 set the stage for the extraordinary expansion of molecular genetics in the years to come [36]. This includes DNA sequencing, polymerase chain reaction, Cre-lox fate-mapping, and most recently, CRISPR-Cas genome editing technology. Landmark discoveries include the cloning of a sheep from an adult somatic cell (“Dolly”) in 1997 and the initial publication of the human genome in 2001 [37,38].
In the past 25 years, elucidation of DNA modification by methylation, histone modification, and noncoding RNA has resulted in a rebirth of epigenetics, complementing continued advances in genomics [39]. In the embryonic kidney, progenitors in the cap mesenchyme are marked by a unique histone signature on lineage-specific genes, which become derepressed in early nephron development [40]. MicroRNAs activated by Dicer1 in the stroma of the developing kidney regulate nephron differentiation and ultimate nephron number [41]. The reversibility of epigenetic marks identified in acute kidney injury (AKI) and CKD have made them new potential therapeutic targets [42].
3 Ontogeny and Phylogeny
3.1 History of evolution (Fig 1)
Jean-Baptiste Lamarck is credited with the first modern conception of biologic evolution in the publication of his Philosophie Zoologique in 1809 [43]. He described transmutation of species through a process of inheritance of characteristics acquired in response to adaptation to the environment. Fifty years later, Charles Darwin published his seminal work, The Origin of Species, in which he accounted for the diversity of life based on 3 principles: 1) inter-individual variation within a population; 2) heritability of variations; and 3) selection by the environment for fittest organisms which then differentially reproduce [44]. While Darwin’s formulation of the evolutionary process has largely stood the test of time, it continues to be critiqued by his many opponents and supporters, and significantly modified by advances in biology. There continues to be disagreement regarding the source of variation, the contribution of genomic vs. epigenomic inheritance, and the target of natural selection (individuals vs. populations). However, natural selection remains the central concept of Darwinian evolution: the interaction of environmental factors and the organism that determine its reproductive fitness [45]. In On Growth and Form, published in 1917, Scottish biologist D’Arcy Thompson argued that major changes in the morphology of organisms could be explained by physical forces described mathematically [46]. Although beautifully written, the work failed to encompass evolution by natural selection. Significantly, the recent discovery of homeotic genes places Thompson’s work in a new light [47]. The period spanning the turn of the century (ca. 1880–1920) was described by Julian Huxley as the “eclipse of Darwinism,” before the establishment of the science of genetics (Fig. 1) [48]. The incorporation of Mendelian genetics and genetic variation into evolutionary theory was led by influential biologists Ernst Mayr and Theodosius Dobzhansky as described by Julian Huxley in Evolution: The Modern Synthesis [49].
Not only did Homer Smith make seminal contributions to renal physiology, but his scholarship also led to a deeper understanding of kidney evolution. He explained the counterintuitive architecture and function of the nephron in terms of the high glomerular filtration rate that was developed by our ancestors who migrated from a marine to a freshwater environment, and the differentiated tubule segments that evolved to reabsorb most of the filtrate necessary for transition to a terrestrial environment [50,51]. These evolutionary adaptations resulting from natural selection driven by environmental pressure are distinguished from physiologic adaptation to short-term homeostatic responses to environmental stimuli [52]. The difference between the proximate cause of an adaptation (physiologic response) and its ultimate cause (evolutionary history) becomes important in accounting for the apparent maladaptation characteristic of progressive nephron loss in CKD (discussed below).
3.2 Evolutionary developmental biology (evo-devo)
Whereas the Modern Synthesis incorporated genetics in evolutionary theory, embryology was largely excluded until the late 20th century. This may have resulted in part from Johannsen’s genotype-phenotype distinction, which has been bridged by the rise of evolutionary developmental biology (evo-devo). British embryologist Gavin de Beer was an exception to this divide: in his book Embryology and Evolution (1930) (expanded to Embryos and Ancestors in 1940) he was one of the first to postulate a role for selection of developmental processes in evolution [53,54]. The split between developmental biology and evolution followed a transition from descriptive embryology in the 19th century to experimental embryology in the 20th century. The former was based on the study of a broad range of species in relation to their environment, whereas experimental embryology became limited to a few model organisms in strictly controlled environments (Fig. 1).
The foundation of evo-devo has been ascribed to Stephen Jay Gould, a Harvard evolutionary biologist whose book Ontogeny and Phylogeny (1977) championed de Beer’s controversial concept of heterochrony (evolutionary shifts in developmental timing) as a driver of evolution [55]. The discovery of homeobox genes in 1983 led to the recognition of transcription factors responsible for morphogenetic programs common to animals, plants, fungi, and unicellular eukaryotes. Such “deep homologies” across phyla have opened many new avenues of research in kidney development, including the inclusion of a broader range of animal models and the elucidation of evolutionary history [56]. A prime example of the value of deep homology in renal disorders, evolutionary conservation of ciliopathy genes has been shown across phyla with common ancestry dating back a billion years [57]. Importantly, selection for adult traits may drive upstream as well as downstream regulators of Hox genes in the developing embryo [58]. There remains considerable debate regarding the relative contribution of mutations in cis-regulatory sequences of pleiotropic developmental regulatory loci vs. structural mutations [59,60].
3.3 Ecological developmental biology (eco-devo)
While the reductionist approach has led directly to remarkable advances in contemporary cell and molecular biology, the exclusion of environmental factors from most studies removed a key component of the evolutionary process. This component has now been restored by the concept of developmental plasticity and programming, whereby the phenotype is instructed by environmental stimuli interacting with the genotype [61]. Until recently, implementation of a research program that connects genotype, phenotype and fitness has been limited by the difficulty in testing the function of candidate genes using genetic manipulations in nonmodel organisms. By pairing multiple single-guide RNAs with Cas9, the CRISPR/Cas9 gene editing technology has already been applied to a variety of vertebrates and invertebrates, and can simultaneously target different loci, even targeting individual single nucleotide polymorphisms [62,63].
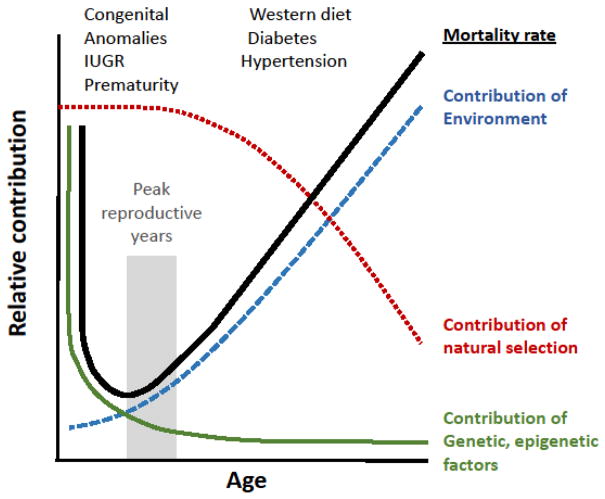
The variation in phenotype in response to environmental stimuli, defined as the reaction norm, is greater in humans than most animals, and accounts for the extraordinary variety of B-cell lymphocytes responding to individual pathogens and neuronal plasticity in which synaptic connections are determined by sensory stimuli [61]. The demonstration of a 10-fold variation in the number of pancreatic islets and in the number of nephrons at birth are additional examples of the large reaction norm in man [64,65]. Some of the determinants of nephron number were revealed in an elegant study comparing branching morphogenesis in the fetal mouse kidney: pregnant mice were subjected to vitamin A deficiency or protein deficiency and compared to FGF7 knockout mice [66]. The authors concluded that kidney organogenesis is governed by growth, patterning, branching rate, and nephron induction, each of which is modulated by genetic and environmental factors determining nephron number. Developmental plasticity regulated by environmental factors is balanced by most genetic regulatory programs, which are characterized by robustness (constraint) [67,68]. In addition to identified mutations underlying congenital anomalies of the kidney and urinary tract (CAKUT) [69], environmental stressors (including pathogens or toxins) that exceed the limits of developmental plasticity can result in teratogenic responses to intrauterine environmental exposure, many of which account for the high morbidity in early embryonic life (Fig. 2) [70].
Figure 2.
Factors contributing to mortality rate across the human lifespan. The V-shaped solid line represents overall mortality rate, which is high in early embryonic life, decreases to a nadir in early adolescence, then increases linearly in adulthood. The mortality curve is the summation of two intersecting curves: genetic and epigenetic factors predominate in early development (early embryonic mortality is primarily the result of lethal mutations), whereas cumulative environmental factors account for the increasing mortality in post reproductive life. Natural selection operates from conception through reproductive years, and decreases thereafter. Congenital anomalies of the kidneys and urinary tract, compounded by intrauterine growth restriction and prematurity, are the leading cause of CKD in childhood; diabetes and hypertension, promoted by the Western diet, account for most CKD in adulthood. However, prenatal and perinatal factors are now recognized as additional contributors to the progression of adult CKD. Adapted from Barton Childs, Acceptance of the Howland Award, Pediatr. Res. 26:392, 1989 [70].
As noted above, however, epigenetic factors appear to play an important role in organ development, and are now recognized as significant contributors to the evolutionary process: evo-devo must be complemented by ecological developmental biology (eco-devo) [61]. In this regard, micro RNA (miRNA)-defined tissues evolved over 500 million years ago, with a marked increase in the number of miRNAs in vertebrates [71,72]. The evolution of epigenetic control mechanisms may represent host defenses to parasitic nucleic acid sequences [73]. Notably, some miRNAs are encoded in the Hox gene locus, and unlike protein-coding genes, lineage-specific miRNA families were added throughout metazoan evolution, with little secondary loss [72]. The importance of miRNAs is underscored by their coevolution with their target genes [74].
3.4 Developmental origins of health and disease (DOHaD) (Fig. 3)
Figure 3.
Factors determining the developmental origins of health and disease (DOHaD). Environmental cues can affect the epigenome of gametes of both parents, the zygote, and the developing embryo, fetus, and infant. Of primary relevance to developmental nephrology, epigenetic factors can modulate nephron number at birth. Through cumulative effects on gene expression and metabolism, susceptibility to CKD is increased. K.M. Godfrey et al. The developmental environment, epigenetic biomakers and long-term health, J. Devel. Origins Health Dis. 6:403, 2015 [79].
The importance of developmental programming in evolutionary biology has been emphasized by Wallace Arthur, who places this epigenetic mechanism between mutation and natural selection, which acts on the phenotype rather than the genotype [75]. David Barker, a British epidemiologist, demonstrated in 1986 an association between infant mortality, childhood nutrition, and adult ischemic heart disease [76]. This was followed by the observation that low birth weight is a strong predictor of death from ischemic heart disease [77]. These studies prompted the birth of a new discipline, developmental origins of health and disease (DOHaD), addressing epigenetic programming during fetal and early postnatal life with effects on health and disease throughout the life cycle (Fig. 3) [78,79]. There is accumulating evidence that low birth weight resulting from either intrauterine growth restriction or prematurity is associated with reduced nephron number at birth, which in turn represents a risk factor for CKD [80–84]. Animal models have provided supporting evidence for the delayed effects of impaired nephrogenesis secondary to stress in early development. Growth restriction before or after birth in rats reduces nephron number and increases blood pressure in later life [85]. Proteomic analysis of kidneys from rats subjected to intrauterine growth restriction revealed abnormalities of metabolism, imbalance of redox and apoptosis, cell signaling, and proliferation, with similar findings in placentas from human fetuses with intrauterine growth restriction [86,87]. An ingenious study of podocin knockout mice with nephrotic syndrome and cross-fostering with animals of differing genetic background revealed complex interactions between maternal behavior and genetically determined factors on the progression of kidney disease [88]. There is increasing evidence for epigenetic modification by Pax2 in the developing kidney, and altered DNA methylation and histone modification in CKD [89].
In their thrifty phenotype hypothesis, Hales and Barker proposed that a fetus exposed to a nutritionally deficient maternal environment would respond by shunting nutrition from organs not essential to early fetal development (such as kidneys) to brain and heart, both needed to complete fetal development and to prepare it for a nutritionally deficient postnatal environment [90]. As reviewed below, evolutionary responses are constrained by limited nutritional (energy) availability, and the need to allocate energy to growth, maintenance, or reproduction, as natural selection acts on fitness rather than longevity [91]. However, exposure to an excessively rich postnatal environment (such as the current “Western” diet) could result in a mismatch of the phenotype (low nephron number) and increased risk for the development of metabolic syndrome, type 2 diabetes, and CKD [92]. Mitochondria, the drivers of energy throughout life, are also subject to fetal programming and may lead to increased susceptibility to CKD in adult life [93]. This hypothesis has been tested in a primate model of intrauterine growth restriction, in which pregnant female baboons were fed a diet reduced to 70% of the control group. Fetal kidney mRNA was analyzed for differential expression of transcripts encoding mitochondrial metabolite transport and dynamics proteins, which differed significantly between calorie-restricted and control groups [94]. The alteration in mRNA levels was accompanied by a decrease in mitochondrial protein cytochrome c oxidase subunit.
Patrick Bateson envisioned the predictive adaptive response, an epigenetic response during early development that improves fitness later in the life cycle. This was further refined to restrict the evolutionary fitness benefit to modest variation in fetal nutrition [95]. A fetus exposed to very severe intrauterine nutritional restriction would suffer developmental disruption and high intrauterine mortality, whereas excessive nutrition of the fetus would also be detrimental postnatally, placing both conditions outside the operation of the predictive adaptive response. There is disagreement regarding the evidence for predictive adaptive responses in the human, a species with a long life span, living in unstable environments. An alternative hypothesis is the operation of a dynamic relationship between mother and fetus, with environmental factors being buffered by the maternal phenotype [96]. There is evidence also for environmental cues acting on early embryos, and even on gametes prior to fertilization, leading to an altered postnatal phenotype and increased fitness or increased risk of disease, depending on the environment [97]. A dramatic demonstration of this process was revealed in bullfrog embryos bathed in water exposed to predatory fish (largemouth bass): exposure of embryos to fish cues resulted in longer embryos that also developed evasive behavior [98]. In mammals, placental function is a key mediator of fetal nutrition and growth, determined by interaction of both maternal and fetal signals [99]. From an evolutionary standpoint, lifetime reproductive fitness of the mother must be balanced with placental allocation of energy to the fetus. Fetal growth may be constrained to protect the mother’s future fertility or because optimal fetal survival weight was limited by nutrition in earlier evolutionary environments [99]. The complexities of the DOHaD paradigm lend themselves to a systems biology approach: such an analysis has revealed different pathways recruited in intrauterine growth restriction and maternal obesogenic environment [100].
While there is convincing evidence that paternal or maternal environmental factors can modify the epigenotype of the offspring, transmission to subsequent generations has been shown in Caenorhabditis elegans, but has not been established in mammals [101]. Studies of intergenerational epigenetic inheritance in adult metabolic disease reveal an association between epigenetic modulation and change in cell function, but a causative relationship between observed epigenetic changes and phenotype remains to be confirmed [101].
Climate change has had a profound effect on biologic evolution, and on human evolution in particular [102,103]. An increasing incidence of extreme climate is already exceeding human physiologic tolerance and is contributing to the epidemic of CKD [104,105]. The study of mammalian species better adapted to extreme water deprivation may lead to a better understanding of limitations in human physiology. Prolonged (72 hour) water deprivation in a desert rodent resulted in a 23% loss of body weight, accompanied by regulation of genes responsible for water and salt balance, but no increase in serum creatinine concentration or other biomarkers of kidney injury [106]. The mechanism of renal protection from severe dehydration in this species remains to be determined. The impact of climate change on maternal health and fetal environment is of particular concern, and will require large scale population studies [107].
4 Homeostasis and Evolution
4.1 Evolutionary medicine and chronic kidney disease
Nephron hypertrophy in response to renal ablation is a response that was described in the pronephros of the amphibian a century ago [108]. Jean Oliver not only mapped out the morphogenesis of nephron development, placing it in an evolutionary context, but also emphasized how nephron heterogeneity increases dramatically in CKD, with the formation of two populations: atrophic and hypertrophic nephrons [15,109]. In a series of landmark papers in the 1980s, Barry Brenner and associates demonstrated that experimental renal ablation results in hypertrophy of remaining glomeruli, leading to eventual glomerulosclerosis [110–113]. Although hyperfiltration initially partially compensates for the loss in kidney function resulting from reduced nephron number, the vicious cycle of nephron hypertrophy and atrophy has been regarded as maladaptive, secondary to the injurious effects of maximized hypertrophy leading to glomerulosclerosis and interstitial fibrosis [114]. This paradigm has driven research in CKD for over 30 years, primarily focused on understanding mechanisms underlying the fibrotic process, but yielding few therapeutic advances beyond inhibition of the renin-angiotensin system, which slows but does not stop the process [115]. Interpreted as a physiologic adaptation to maintain homeostasis (proximate cause), there are physical constraints on nephron size, limited by the effective surface area for reabsorption and tubule length that offers resistance to flow. The transition of hypertrophied nephrons to atrophy and fibrosis appears maladaptive if regarded as a deviation from an optimal homeostatic response.
A complementary approach to understanding apparent maladaptation is to consider the ultimate cause, explained by its evolutionary origin through natural selection. As noted above, Homer Smith described normal mammalian renal physiology in the context of our evolutionary history, which entailed sequential adaptation of our ancestors to life in freshwater then to land [50]. These evolutionary adaptations were acquired to enhance the fitness (reproductive success) of the organism in response to changing environmental pressures. Such adaptations, enabled by the action of natural selection on genetic mutation as well as on variation in noncoding RNA and other epigenetic mechanisms, are never optimized, but are the result of tradeoffs. An important corollary of evolutionary theory is the decreasing contribution of natural selection to longevity in the post-reproductive period, during which environmental factors increasingly contribute to mortality (Fig 2). The evolution of a wide variety of reproductive strategies reflects the penetration of almost every conceivable niche by each living organism. Life history theory, the study of the trajectory from zygote to death, encompasses the allocation of resources to early development (embryo, larva, infant), number of offspring, parental care, and senescence [116]. Human life history is characterized by long gestation, extended childhood (dependence on adult care), and a long post reproductive life which doubled in the West with the Industrial Revolution of the last two centuries.
Only recently recognized, the majority of surviving children with CAKUT who eventually require renal replacement therapy do not progress to kidney failure until adulthood [117]. The study of variation in genotype and phenotype and their temporal interaction with environmental factors in defined human populations may yield new insight into the susceptibility of nephrons to injury through the life cycle. Increasing evidence for the contribution of AKI to CKD has focused attention on the proximal tubule as the primary target of injury in the progression of CKD [118]. For example, endothelial nitric oxide synthase (eNOS) is expressed in proximal tubules of the mouse in early development, but not in adulthood, when its expression shifts to the vasculature [119]. Proximal tubules of immature mice with targeted deletion of eNOS undergo cell death, leading to the formation of cortical scars in adulthood [119]. The role of eNOS as a survival factor for nephrons is reinforced by the demonstration of increased susceptibility to the progression of CKD in patients with eNOS polymorphisms [120]. Understanding life history theory is important in the interpretation of animal models of kidney development, and of the pathogenesis of CKD. Evolutionary divergence of mouse and human is estimated to be 90 million years, with notable differences in adaptation to very different environmental niches. These factors underlie differences in gene expression and cell fate during early mouse and human nephrogenesis, currently being elucidated and compiled in GUDMAP [121].
Despite the recognition of the central importance of Darwinian evolution to the development of biology over the past 150 years, its application to medicine was largely ignored until George Williams and Randolph Nesse introduced the discipline of evolutionary medicine in the 1990s (see reference [122] for an expanded review of evolutionary medicine). Williams proposed that mutations in pleiotropic genes selected for fitness also increased susceptibility to disease in post-reproductive years, a phenomenon he termed “antagonistic pleiotropy,” regarded as the evolutionary origin for senescence in all animals [123,124]. A search for polygenic selection signals underlying coronary artery disease in global populations revealed that such loci are enriched for lifetime reproductive success, supporting the concept of antagonistic pleiotropy [125].
4.2 Energy conservation
Human kidneys comprise 1% of body weight, but account for 10% of total body oxygen consumption, required for reabsorption of 99% of glomerular filtrate [126]. This is a result of our evolutionary heritage: transition from a marine to freshwater environment, then to land, accounts for the high energy requirement for urine concentration; and a meat-rich diet consumed by our Pleistocene ancestors explains the maintenance of high glomerular filtration rate required for the excretion of accumulated urea [50,127]. Life history strategies are determined by adaptations that are constrained by allocation of energy, which can be diverted to growth, tissue maintenance/regeneration, or offspring [91]. Rapid growth characterizes the pre-reproductive period, after which energy is diverted to maintenance of nephron integrity, which decreases with senescence as a result of accumulating environmental stressors and the expression of pleiotropic genes selected for their essential role in early growth and development.
Hypertrophy following renal ablation initially results in a doubling of nephron oxygen consumption, and mathematical modeling predicts that oxygen consumption is increased in all nephron segments, the greatest in the proximal tubule [128,129]. Such energy expenditure cannot be maintained indefinitely, limited by the capacity of mitochondria to match increased demands. As proposed by Nick Lane, this may relate to the evolutionary history of mitochondria, originally representing bacteria engulfed by an archaeal cell resulting in a symbiotic relationship requiring the accommodation of nuclear and mitochondrial DNA [91,130]. Genes were transferred from mitochondria to the host nucleus at no energetic cost to the eukaryote, while the mitochondria retained genes necessary to regulate chemiosmotic coupling. In the process of cell division mutant mitochondria are randomly distributed among daughter cells: a mismatch of the two genomes can lead to activation of cell death pathways and progression of chronic disease [91]. This is a classic tradeoff between the benefit of mitochondrial variation as a substrate for natural selection to enhance fitness vs. the risk of mitochondrial-nuclear incompatibility that increases with age.
Combined angiotensin inhibition and renal artery stenosis in the rat leads to the development of tubular atrophy and 90% decrease in Na-K-ATPase activity which is reversible within 3 weeks of removal of the arterial clip and discontinuation of angiotensin inhibition [131,132]. By contrast, patients with severe chronic renal artery stenosis develop irreversible tubular atrophy with glomerulotubular disconnection and formation of atubular glomeruli [133]. The formation of atubular glomeruli is a final common pathway in virtually all forms of kidney disease (glomerular, tubular, and toxic disorders), and may represent an atavism expressed by a common ancestor with teleost fish that adapted from freshwater to a marine environment [134]. During maturation, nephrons of the sculpin, a marine teleost, undergo cell death at the glomerulotubular junction, resulting in aglomerular tubules that require less oxygen consumption than is otherwise expended on reclamation of filtered sodium [135].
The rodent model of unilateral ureteral obstruction (UUO) has become the most widely employed model of CKD, and results in a marked reduction in oxygen consumption with a reduction in mitochondrial number, tubular atrophy and formation of atubular glomeruli [136,137]. The reduction of tubular oxygen consumption in a nonfunctioning nephron may represent an evolutionary tradeoff that contributes to fitness. This can be demonstrated also in genetic models of human disease. Murine models of polycystic kidney disease reveal progressive obstruction of nephrons by expanding cysts, with the eventual accumulation of atubular glomeruli as also revealed in kidneys from patients with polycystic disease requiring renal replacement therapy [138]. In a mouse model of nephropathic cystinosis, cystine accumulation in proximal tubule cells results in their marked phenotypic transition, with flattening, loss of mitochondria, and thickening of the tubular basement membrane [139]. In this case, the juxtaglomerular segment of the proximal tubule (“swan neck lesion”) acts as a conduit for tubular fluid, excluding solute transport, which in compensated by downstream tubule cells. With progression of the lesion, the glomerulotubular junction eventually undergoes cell death with formation of atubular glomeruli, developing in both murine and human disease [139]. Thus, by conservation of energy and prolonging life through the reproductive period, apparent nephron maladaptation can increase fitness.
4.3 Evolution and aging
The lifetime risk for development of end-stage CKD increases from less than 1% for individuals under 40 years of age to 8% in those over 65 years, with significantly greater risk for black than white subjects, and for males than females [140]. Notably, following toxic AKI in mice, all young females survived, whereas aged females suffered significant mortality and young males had more severe renal injury than young females [141]. There is a 50% decrease in nephron number in cadaveric kidney transplant donors over 55 years of age compared to those under 45 years [142]. As with CKD, the loss of nephrons with aging is due to atrophy and reabsorption of globally sclerotic glomeruli with hypertrophy of remaining nephrons [143,144]. It is therefore not surprising that the urinary proteome for the healthy aging population is very similar to that of patients with CKD [145].
There are many evolutionary theories to explain senescence [146]. An attractive hypothesis, the “double-agent theory” proposes that oxidative stress evolved as a critical signal against environmental threats (such as infection), but aging is a function of increasing intracellular oxidative stress associated with free-radical leakage from aging mitochondria which stimulates an inflammatory response similar to that summoned by the innate immune response to infection [147,148]. Thus, because selection pressure is greater in the reductive environment through the reproductive years, genes associated with age-related diseases are less effective in the senescent oxidizing environment. In this regard, the double-agent theory which considers epigenetic mechanisms mediated by environmental factors expands Williams’s antagonistic pleiotropy concept, which is driven purely by genetic mechanisms [123,149]. The importance of oxidative and inflammatory injury in the aging kidney has been emphasized in the current literature [150,151]. The central role of energy balance in this process has been demonstrated experimentally: calorie restriction can reduce podocyte hypertrophy and glomerular sclerosis as well as tubular atrophy in the aging rat [152,153]. Cytochrome c oxidase, the final complex of the mitochondrial electron transport system, is reduced in aging tubule epithelial cells that contain mitochondrial DNA deletion mutations [153]. When the capacity of podocytes to adequately cover the enlarging filtration surface is exceeded, glomerular sclerosis reflects a limited adaptive response to growth-induced podocyte stress [154]. This explains previous experiments demonstrating that glomerular sclerosis results from hypertrophy but not hyperfiltration of remaining nephrons [155]. The importance of the fetal environment in the rate of senescence has been demonstrated in kidneys of low-birth-weight rats subjected to intrauterine protein restriction fed high calorie diets to enhance catch-up growth: kidney RNA and protein markers of stress-induced senescence as well as reactive oxygen species generation were increased following catch-up growth [156].
4.4 Evolution and regeneration
As noted above, there are physical and energy constraints on the mechanisms for repair and regeneration of injured or aging kidneys: nephron hypertrophy can only maintain homeostasis for a limited period, after which podocyte and tubule cell death are followed by glomerulosclerosis, tubular atrophy, and interstitial fibrosis. Whereas nephrons can be formed de novo in adult kidneys of fish, amphibians and reptiles (ectotherms), this is not the case for adult birds and mammals (endotherms), who must rely on the presence of resident progenitor cells to regenerate components of damaged nephrons [157]. Niches of such cells have been localized to the vascular pole of Bowman’s capsule, which can differentiate into podocytes, and to the urinary pole of Bowman’s capsule, which can differentiate into tubular cells [157]. Ectotherms evolved a life history strategy incorporating the formation of blastema in injured tissue, which can generate new nephrons, an energy-consuming process [158]. The survival advantages of endothermic homeostasis reduced the vulnerability of mammals to kidney injury below the threshold needed to preserve regrowth, and they evolved mechanisms of wound healing that actually block the formation of blastema [157–159]. Activation of inflammatory and fibrotic responses following adult mammalian tissue injury is directed at reducing hemorrhage and infection, mechanisms that can lead to CKD if the injurious stimulus persists. This is not the case for a reduction in nephron number in mammalian fetal life, when risk of infection is low: unilateral nephrectomy in fetal lambs or pigs results in a 45–50% increase in nephron number of the remaining kidney [160–162]. The number of progenitor cells available for regeneration of injured nephrons is constrained by the tradeoff of their potential to result in malignant transformation [163].
4.5 Cell-cell signaling and complex disease
As described in this review, there are many threads in the history of biologic thought that have become intertwined in attempts to explain biodiversity, the development of organisms, aging and disease. Extraordinary advances in cell and molecular biology have elucidated many mechanisms of development and disease, but a unified conception is needed. John Torday has proposed a novel view of evolution, based on the transition from unicellular to multicellular organisms, which adapted to their environments through cell-cell communication and metabolic cooperativity [164]. Central to this argument is the symbiotic incorporation of bacteria by an Archaean cell 500 million years ago, leading to mitochondria providing the energy source for evolution of multicellular organisms. Thereafter, environmental factors that drove natural selection have continued to act on individual organisms from zygote to death, with aging reflecting the breakdown of cell-cell communication as the mirror image of development and homeostasis [164]. Elucidation of this history of cell physiology can be deduced from contemporary developmental, physiologic, and regenerative gene regulatory networks, which determine form and function through cell-cell signaling. Disruption of signaling pathways involving cytokines, mitochondrial biogenesis, and autophagy may explain the increased susceptibility to AKI in patients with CKD [165].
There are many parallels between lung and kidney development, structure, and function. Type IV collagen evolved about 300 million years ago when vertebrates transitioned from water to land, and contributes to the integrity of alveoli and the glomerular basement membrane. Both fetal alveolus and glomerulus contribute to amniotic fluid and have stretch receptors (fibroblast in lung, and mesangial cell in glomerulus) that mediate surfactant production or glomerular blood flow through release of parathyroid hormone-related protein (PTHrP) by epithelial cells (alveolar epithelial type II cells or podocytes) [164]. Following injury to alveolus or glomerulus, breakdown of PTHrP/PTHrP receptor signaling leads to enhanced Wnt/β catenin signaling, differentiation of fibroblasts to myofibroblasts, and initiation of fibrosis. Whereas Wnt signaling is essential to normal nephrogenesis, its activation in the adult maintains kidney cells in an immature form, contributing to the progression of glomerular injury in CKD [166]. However, activation of the Wnt pathway plays a salutary role in the repair and regeneration of acute tubular injury [166].
PTHrP is unusual in being expressed in the endoderm, binding to the mesoderm during morphogenesis, also playing a central role in homeostasis and repair. The PTHrP pathway was also critical for the evolution the skeleton and skin barrier, both important in the transition from water to land. Due to its hydrophobic and electrostatic properties, the evolved non-collagenous domain 1 (NC1) of the 3α chain of type IV [(IV)NC1] domain provides a barrier against exudation of serum and proteins into alveoli and glomeruli, conferring a selective advantage in the transition from water to land, which was attempted several times in vertebrate evolutionary history [167]. Parallels between evolution of lung and kidney become clinically relevant in the pathophysiology of Goodpasture syndrome, caused by autoantibodies targeted to the [3(IV)NC1] domain or Goodpasture autoantigen [167]. The functional homologies of PTHrP and Type IV collagen in lung and kidney provide an example of deep pleiotropic homologies that are revealed by molecular developmental and physiological study, but not predicted by comparative anatomy [168]. Cell biology can therefore identify the continuum between proximate and ultimate causes of evolution.
4.6 Selection of cells and nephrons for survival or death
Nobel laureate Frank McFarlane Burnet developed the clonal selection theory, whereby the vertebrate adaptive immune system generates multiple unique B-cell lymphocytes in response to exposure to specific antigens, a process comparable to natural selection [169]. Another Nobel recipient, Gerald Edelman, applied the same reasoning to the development of the nervous system, which undergoes a process of excess neural production, followed by selection for survival of some neurons establishing synaptic connections, and massive death of the remainder [170]. The underlying variation among lymphocytes and neurons results from pleomorphic gene expression and epigenetic factors regulated by environmental stimuli that result in phenotypic plasticity. Unlike redundancy (in which the same function is performed by identical elements), this process, defined by Edelman as degeneracy, is a property of complexity in biological systems and involves structurally different elements that may yield different functions depending on the environmental context [171]. Degeneracy is a prerequisite for natural selection in a complex system: multiple genes contribute to each phenotypic feature undergoing selection through interaction between functional specialization and functional integration [171]. Expressing extraordinary tissue-specific heterogeneity in development, this process has been shown to operate for endothelial cells, that are modeled by response to hemodynamic shear stress and extracellular milieu [172].
Analogous to lymphocytes and neurons, in early metanephric development, the metanephric blastema undergoes massive apoptosis, and contact with the ureteric bud induces nephron differentiation only in mesenchymal cells rescued from cell death [173]. It appears that the number of remaining mesenchymal cells constitutes an important determinant of nephron number: nephrogenesis in the mouse ceases abruptly with a burst of nephron differentiation that depletes remaining metanephric mesenchyme [174]. One approach to elucidating the gene regulatory networks responsible for the determination of nephron number is an analysis of data for the temporal sequence of global gene expression during kidney development. This has been done using a computational process to generate two-dimensional self-organizing maps reducing over 30,000 genes to 650 metagenes that identify potential stages of rat kidney development and points of stability and transition [175]. Mapping the metagenes reveals a transition in entropy at embryonic day 17 (e17) marking the beginning of nephrogenesis and delineation of the cortex and medulla, which continue through e22 (time of birth). Network analysis of genes demonstrating a high correlation with glomerular density reveals known genes implicated in nephrogenesis as well as additional genes [175]. The identified time points of transition may be particularly subject to natural selection, and may represent periods of vulnerability to environmental injury.
The postnatal development of the mammalian kidney involves the differentiation of nephrons that reflect their evolutionary history as well as a transition from an intrauterine aqueous maternal environment to a terrestrial environment requiring adaptation by lungs to air breathing and by kidneys to provide fluid/osmotic homeostasis and excretory function. This includes the elongation of the loops of Henle, as well as differentiation of short and long looped nephrons [176]. The increasingly hypoxic and hyperosmotic microenvironment of the maturing inner medulla and elongation of the long loops of Henle increase their susceptibility to hypoxic injury [177].
A major question arises from the life history of aging and CKD: in response to their genetic/epigenetic inheritance as well as their microenvironment within the renal capsule, what factors account for the selection of individual nephrons to survive, undergo hypertrophy, or die? The nature of the stressor can determine the response of pleomorphic genes such as TNF-related weak inducer of apoptosis (TWEAK), a cytokine that induces tubular cell proliferation following unilateral nephrectomy (a non-inflammatory microenvironment), but stimulates apoptosis in folic acid-induced AKI (an inflammatory microenvironment) [178]. There is a reciprocal relationship between cell volume and stiffness mediated through water efflux: the microenvironment can modulate the mechanical properties of cells, determining cell function and even altering stem cell differentiation [179]. Four weeks following renal ablation in rats, glomerular expression of platelet-derived growth factor-B (PDGF-B) is increased along with glomerulosclerosis, both of which are prevented by treatment with angiotensin receptor blocker. Notably, there is marked heterogeneity among glomeruli, with varying stages of sclerosis strongly correlated with individual glomerular expression of PDGF-B, but disappearance of the correlation in glomeruli with advanced sclerosis [180]. These findings suggest marked heterogeneity in single nephron activity of the renin-angiotensin system, with the microenvironment surrounding an individual nephron mediating the response to stress through activation of the angiotensin AT1 receptor. Evolution of components of the renin-angiotensin system originated in primitive chordates and tunicates over 500 million years ago, and were assembled in the bony fishes with the addition of the Mas receptor in amphibians about 100 million years later [181]. Recent advances in the study of this regulatory system reveal its truly pleiotropic (“degenerative”) functioning, including immune responses, hematopoietic development, morphogenesis, tissue repair and regeneration, as well as fluid and electrolyte homeostasis and control of blood pressure [11].
5 A New Synthesis
The 20th century provides a rich history of several biologic disciplines that have elucidated the development, structure and function of the kidney. The rise of molecular genetics has afforded new insight into the evolutionary origins of this complex organ. It is time for a new “postmodern synthesis” of evolutionary biology, one that encompasses evo-devo, eco-devo, and DOHaD as well as evolutionary medicine. Developmental biology would play a central role in such a multidisciplinary approach, and there is an urgent need for its application to address the global epidemic of CKD. This will lead to improved understanding of the variation in nephron number and its determinants, constraints on nephron hypertrophy and regeneration, and selection of nephrons for survival through environmental threats from conception to senescence. Cell-cell communication is essential not only during nephrogenesis, but throughout the life of the nephron: its disruption calls into play a variety of adaptive responses that have evolved to enhance reproductive fitness rather than longevity. These processes involve tradeoffs between plasticity (wide variation in nephron number at birth) and robustness (conservation of morphogenetic programs), and operate at the level of the single cell in the context of a microenvironment that is a product of interactions between the external environment and homeostatic responses. An investigative approach that spans the molecular/cellular, genetic, physiologic, and evolutionary disciplines will most likely lead to an understanding of the fate of nephrons in the life cycle, and the basis of CKD.
Acknowledgments
Funding
Portions of the author’s work were supported by National Institutes of Health Center of Excellence in Pediatric Nephrology DK096373.
The author is indebted to R. Ariel Gomez for his thoughtful review of the manuscript and recommendations. Portions of the work were presented at the annual meeting of the International Society for Evolution, Medicine, and Public Health, Groningen, Netherlands, August 18–21, 2017, and the 10th World Congress on Developmental Origins of Health and Disease, Rotterdam, Netherlands, October 16–19, 2017.
6 Glossary
- Antagonistic pleiotropy
a gene controls more than one trait, including one or more that diminish reproductive fitness as well as at least one that benefit fitness.
- Deep homology
developmental programs conserved across a wide range of species (e.g. insects and mammals).
- Developmental origins of health and disease (DOHaD)
fetal through early postnatal epigenetic responses to environmental cues that increase susceptibility to disease in adult life.
- Developmental plasticity
the ability of a genotype to alter phenotype through epigenetic mechanisms in response to environmental cues.
- Developmental programming
epigenetic modifications in response to early environmental events that result in modified gene expression and phenotype later in life.
- Double-agent theory
a tradeoff between oxidative stress as a critical redox signal that marshals genetic defenses against physiological stress (such as infection) and oxidative stress due to free radical leakage from mitochondria in age-related disease.
- Ecological developmental biology (Eco-devo)
the ability of a genotype to generate multiple phenotypes in response to environmental cues during development, providing a source of variation subject to natural selection.
- Evo-devo
based on the premise that natural selection acts during organogenesis, comparison of developmental processes of different organisms informs the ancestral relationships between them and how the developmental processes evolved.
- Evolutionary medicine
the application of modern evolutionary theory to understanding health and disease.
- Fitness
a measure of individual reproductive success, equal to the average contribution to the gene pool of the next generation that is made by individuals of the specified genotype or phenotype.
- Heterochrony
developmental change in the timing or rate of events, leading to changes in morphology.
- Homeotic gene
specifies the anterior-posterior axis, as well as segment identity during early embryonic development.
- Life history theory
explains organisms’ anatomy, physiology and behavior through study of their life histories (reproductive development, life span and post-reproductive behavior) that have been shaped by natural selection.
- Maladaptation and mismatch
apparent maladaptive deviation from an “optimal” physiologic adaptation differs from an evolutionary adaptation which can never be optimal, and may represent a mismatch with the present environment.
- Modern synthesis
consolidation of evolutionary theory from the 1920s through the 1950s that supported and reconciled the Darwinian theory of evolution (natural selection) and the Mendelian laws of inheritance (genetic variation), but excluded developmental biology.
- Predictive adaptive response
a form of developmental plasticity in which cues received in fetal life influence the development of a phenotype that is adapted to the anticipated extrauterine environmental conditions.
- Proximate vs. ultimate cause
the proximate cause of an adaptation represents an immediate homeostatic response, whereas its ultimate cause is an evolutionary adaptation to ancestral environments.
- Reaction norm
range of variation in phenotypic expression of a single genotype across a range of environments.
- Redundancy vs. degeneracy
redundancy results from two or more genes performing the same function, and inactivation of one gene does not alter phenotype; degeneracy results from structurally different elements that may yield different functions depending on the environmental context.
- Robustness (constraint)
persistence of a certain characteristic or phenotype under perturbations or conditions of uncertainty.
- Thrifty phenotype hypothesis
restricted fetal organ growth in response to limited maternal nutrient supply resulting in increased susceptibility to disease later in life.
- Tradeoff
an evolutionary adaptation to increase reproductive fitness that also increases risk of disease or reduced lifespan.
Footnotes
The author has no competing interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Global Facts About Kidney Disease. National Kidney Foundation; Oct 22, 2017. https://www.kidney.org/kidneydisease/global-facts-about-kidney-disease, 2015. [Google Scholar]
- 2.Jamison RL. Resolving an 80-yr-old controversy: the beginning of the modern era of renal physiology. Adv Physiol Educ. 2014;38:286–295. doi: 10.1152/advan.00105.2014. [DOI] [PubMed] [Google Scholar]
- 3.Smith HW. The Kidney. Oxford University Press; New York: 1951. [Google Scholar]
- 4.Wirz H, Hargitay B, Kuhn W. Lokalisation des konzentrierungsprozesses in der niere durch direkte kryoskopie. Helv Physiol Pharmcol Acta. 1951;9:196–207. [PubMed] [Google Scholar]
- 5.Gottschalk CW, Mylle M. Micropuncture study of the mammalian urinary concentrating mechanism: evidence for the countercurrent hypothesis. Am J Physiol. 1959;196:936. doi: 10.1152/ajplegacy.1959.196.4.927. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton KL, Moore AB. 50 years of renal physiology from one man and the perfused tubule: Maurice B. Burg. Am J Physiol Renal Physiol. 2016;311:F291–F304. doi: 10.1152/ajprenal.00198.2016. [DOI] [PubMed] [Google Scholar]
- 7.Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 8.de Bold AJ. A decade of atrial natriuretic factor research. Introduction. Can J Physiol Pharmacol. 1991;69:1477–1479. doi: 10.1139/y91-222. [DOI] [PubMed] [Google Scholar]
- 9.Nabeshima Y. The discovery of alpha-Klotho and FGF23 unveiled new insight into calcium and phosphate homeostasis. Cell Mol Life Sci. 2008;65:3218–3230. doi: 10.1007/s00018-008-8177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall JE. Historical perspective of the renin-angiotensin system. Mol Biotechnol. 2003;24:27–39. doi: 10.1385/MB:24:1:27. [DOI] [PubMed] [Google Scholar]
- 11.Gomez RA, Sequeira Lopez MLS. Renin cells in homeostasis, regeneration and immune defence mechanisms. Nat Rev Nephrol. 2018 doi: 10.1038/nrneph.2017.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Baer KE. On the Development of Animals, with observations and reflections in Fragments relating to philosophical zoology, 1828. In: Huxley TH, translator; Hall TS, editor. A Source Book in Animal Biology. Harvard University Press; Cambridge: 1951. pp. 392–399. [Google Scholar]
- 13.Pye W. Observations on the development and structure of the kidney. J Anat Physiol. 1875;9:271–279. [PMC free article] [PubMed] [Google Scholar]
- 14.Sperber I. Studies in the Mammalian Kidney. Almquist and Wiksells; Uppsala: 1944. [Google Scholar]
- 15.Oliver J. Nephrons and Kidneys: A Quantitative Study of Developmental and Evolutionary Mammalian Renal Architectonics. Hoeber Medical Division, Harper and Row; New York, Evanston, London: 1968. [Google Scholar]
- 16.Al-Awqati Q, Goldberg MR. Architectural patterns in branching morphogenesis in the kidney. Kidney Int. 1998;54:1832–1842. doi: 10.1046/j.1523-1755.1998.00196.x. [DOI] [PubMed] [Google Scholar]
- 17.Cebrian C, Borodo K, Charles N, Herzlinger DA. Morphometric index of the developing murine kidney. Devel Dynam. 2004;231:601–608. doi: 10.1002/dvdy.20143. [DOI] [PubMed] [Google Scholar]
- 18.Short KM, Smyth IM. The contribution of branching morphogenesis to kidney development and disease. Nat Rev Nephrol. 2016;12:754–767. doi: 10.1038/nrneph.2016.157. [DOI] [PubMed] [Google Scholar]
- 19.Ashwell M, Black D, Cassels B, Cowen T, Cowley J, Dauncey MJ, Dickerson J, Elkinton JR, Ellis JS, Elneil H, Fiorotto M, Glaser E, Gurr M, Hervey R, Huxley A, John P, Jonxis J, Lister D, McCance R, McCracken K, Morrison AB, Oftedal O, Parizkova J, Paul A, Pickles V, Robinson J, Robinson M, Rosenbaum D, Southgate D, Spray C, Strangeways L, Tabor D, Tayler E, Wharton B, Whitehead R, Whitteridge D, Widdowson E, Young A, Young M. A Scientific Partnership of 60 Years 1933 to 1993. British Nutrition Foundation; London: 1993. McCance & Widdowson. [Google Scholar]
- 20.Potter EL. Normal and Abnormal Development of the Kidney. Year Book; Chicago: 1972. [Google Scholar]
- 21.The Kidney During Development. Masson Publishing; New York: 1982. [Google Scholar]
- 22.Spitzer A. Twenty-one years of developmental nephrology: the kidney then and now. Pediatr Nephrol. 2003;18:165–173. doi: 10.1007/s00467-002-1059-z. [DOI] [PubMed] [Google Scholar]
- 23.Chesney RW, Chevalier RL. Thirty-three years of progress: the International Workshops on Developmental Nephrology and the role of IPNA. Pediatr Nephrol. 2014;29:499–504. doi: 10.1007/s00467-013-2750-y. [DOI] [PubMed] [Google Scholar]
- 24.Saxen L. Organogenesis of the Kidney. Cambridge University Press; Cambridge: 1987. [Google Scholar]
- 25.Costantini F, Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Devel Cell. 2010;18:698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, Little MH. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526:564–568. doi: 10.1038/nature15695. [DOI] [PubMed] [Google Scholar]
- 27.Streisinger G, Walker C, Dower N, Knauber D, Singer F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio) Nature. 1981;291:293–296. doi: 10.1038/291293a0. [DOI] [PubMed] [Google Scholar]
- 28.Oxburgh L, Carroll TJ, Cleaver O, Gossett DR, Hoshizaki DK, Hubbell JA, Humphreys BD, Jain S, Jensen J, Kaplan DL, Kesselman C, Ketchum CJ, Little MH, McMahon AP, Shankland SJ, Spence JR, Valerius MT, Wertheim JA, Wessely O, Zheng Y, Drummond IA. (Re)Building a kidney. J Am Soc Nephrol. 2017;28:1370–1378. doi: 10.1681/ASN.2016101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harding SD, Armit C, Armstrong J, Brennan J, Cheng Y, Haggarty B, Boughton D, Lloyd-MacGilp S, Pi X, Roochun Y, Sharghi M, Tindal C, McMahon AP, Gottesman B, Little MH, Georgas K, Aronow BJ, Potter SS, Burnskill EW, Southard-Smirth EM, Mendelsohn C, Baldock RA, Davies JA, Davidson D. The GUDMAP database - an online resource for genitourinary research. Development. 2011;138:2845–2853. doi: 10.1242/dev.063594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dodson EO. Mendel and the rediscovery of his work. Sci Monthly. 1955;81:187–195. [Google Scholar]
- 31.Sturtevant AH. A History of Genetics. Harper and Row; New York: 1965. [Google Scholar]
- 32.Dunn LC. The Development of Some of the Main Lines of Thought 1864–1939. McGraw Hill Book Co; New York: 1965. A Short History of Genetics. [Google Scholar]
- 33.Graham L. Epigenetics and Russia. Harvard University Press; Cambridge MA and London: 2016. Lysenko’s Ghost. [Google Scholar]
- 34.Waddington CH. Science and Ethics. George Allen and Unwin Ltd; London: 1942. [Google Scholar]
- 35.Hall BK. Waddington’s legacy in development and evolution. Amer Zool. 1992;32:113–122. [Google Scholar]
- 36.Chambers DA. DNA: The Double Helix. Perspective and Prospective at Forty Years; Proceedings of a Conference sponsored by the New York Academy of Sciences, the University of Illinois at Chcago, and Green College, University of Oxford; Chicago, Illinois. October 13–16, 1993; New York: The New York Academy of Sciences; 1995. [Google Scholar]
- 37.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KHS. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 38.International Human Genome Sequencing Consortium. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 39.Felsenfeld G. A brief history of epigenetics. Cold Spring Harb Persp Biol. 2014;6:a018200. doi: 10.1101/cshperspect.a018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hilliard SA, El-Dahr SS. Epigenetic mechanisms in renal development. Pediatr Nephrol. 2016;31:1055–1060. doi: 10.1007/s00467-015-3228-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakagawa N, Xin C, Roach AM, Naiman N, Shankland SJ, Ligresti G, Ren S, Szak S, Gomez IG, Duffield JS. Dicer1 activity in the stromal compartment regulates nephron differentiation and vascular patterning during mammalian kidney organogenesis. Kidney Int. 2015;87:1125–1140. doi: 10.1038/ki.2014.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reddy MA, Natajaran R. Recent developments in epigenetics of acute and chronic kidney diseases. Kidney Int. 2015;88:250–261. doi: 10.1038/ki.2015.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Honeywell R. Two Centuries of Genius and Jealousy. Murdoch Books; Millers Point NSW and Putney London: 2008. Lamarck’s Evolution. [Google Scholar]
- 44.Ruse M, Largent MA, Olby R, Richards RJ, Sloan PR, Kohn D, Lustig AJ, Herbert S, Norman D, Bowler PJ, Richards RA, Nyhart LK, Smocovitis VB, Depew DJ, Brooke JH, Beer G, Beck N, Lewens T, Kohler M, Kohler C. The Cambridge Companion to the Origin of Species. Cambridge University Press; Cambridge, New York: 2009. [Google Scholar]
- 45.Ruse M. Darwinism and its Discontents. Cambridge University Press; Cambridge: 2006. [Google Scholar]
- 46.Ball P. On Growth and Form. Nature. 2013;494:32–33. [Google Scholar]
- 47.Arthur W. D’Arcy Thompson and the theory of transformations. Nat Rev Genet. 2006;7:401–406. doi: 10.1038/nrg1835. [DOI] [PubMed] [Google Scholar]
- 48.Bowler PJ. The Eclipse of Darwinism: Anti-Darwinian Evolution Theories in the Decades around 1900. Johns Hopkins Press; Baltimore: 1983. [Google Scholar]
- 49.Huxley J. Evolution The Modern Synthesis. George Allen & Unwin Ltd; London: 1942. [Google Scholar]
- 50.Smith HW. From Fish to Philosopher. Little, Brown and Co; Boston: 1953. [Google Scholar]
- 51.Kooman JP. Geology, paleoclimatology and the evolution of the kidney: some explorations into the legacy of Homer Smith. Blood Purif. 2012;33:263–274. doi: 10.1159/000337095. [DOI] [PubMed] [Google Scholar]
- 52.Bennett AF. Adaptation and the evolution of physiological characters. In: Dantzler WH, editor. Handbook of Physiology, Sect 13: Comparative Phsyiology. Oxford University Press; New York: 1997. pp. 3–16. [Google Scholar]
- 53.de Beer G. Embryology and Evolution. Clarendon Press; Oxford: 1930. [Google Scholar]
- 54.de Beer GR. Embryos and Ancestors. Clarendon Press; Oxford: 1940. [Google Scholar]
- 55.Gould SJ. Ontogeny and Phylogeny. Harvard University Press; Cambridge, London: 1977. [Google Scholar]
- 56.Schejter ED, Shilo BZ. Modular tubes: Common principles of renal development. Curr Biol. 2003;12:R511–R513. doi: 10.1016/s0960-9822(03)00442-1. [DOI] [PubMed] [Google Scholar]
- 57.Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. New Engl J Med. 2011;16:1533–1543. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Richardson MK. Vertebrate evolution: the developmental origins of adult variation. BioEssays. 1999;21:604–613. doi: 10.1002/(SICI)1521-1878(199907)21:7<604::AID-BIES9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 59.Carroll SB. Evo-devo and an expanding evolutonary synthesis: a genetic theory of morphological evolution. Cell. 2008;134:36. doi: 10.1016/j.cell.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 60.Hoekstra HE, Coyne JA. The locus of evolution: evo devo and the genetics of adaptation. Evolution. 2007;61–5:995–1016. doi: 10.1111/j.1558-5646.2007.00105.x. [DOI] [PubMed] [Google Scholar]
- 61.Gilbert SF. Ecological developmental biology: developmental biology meets the real world. Devel Biol. 2001;233:1–12. doi: 10.1006/dbio.2001.0210. [DOI] [PubMed] [Google Scholar]
- 62.Bono JM, Olesnicky EC, Matzkin LM. Connecting genotypes, phenotypes and fitness: harnessing the power of CRISPR/Cas9 genome editing. Mol Ecol. 2015;24:3810–3822. doi: 10.1111/mec.13252. [DOI] [PubMed] [Google Scholar]
- 63.Harrison MM, Jenkins BV, O’Connor-Giles KM, Wildonger J. A CRISPR view of development. Genes Devel. 2014;28:1859–1872. doi: 10.1101/gad.248252.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gomori G. The histology of the normal and diseased pancreas. Bull N Y Acad Med. 1945;21:99–111. [PMC free article] [PubMed] [Google Scholar]
- 65.Hughson MD, Farris AB, Douglas-Denton R, Hoy WE, Bertram JF. Glomerular number and size in autopsy kidneys: The relationship to birth weight. Kidney Int. 2003;63:2113–2122. doi: 10.1046/j.1523-1755.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 66.Sampogna RV, Schneider L, Al-Awqati Q. Developmental programming of branching morphogenesis in the kidney. J Am Soc Nephrol. 2015;26:2414–2422. doi: 10.1681/ASN.2014090886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chipman AD. Developmental exaptation and evolutionary change. Evol Devel. 2001;3:299–301. doi: 10.1046/j.1525-142x.2001.01033.x. [DOI] [PubMed] [Google Scholar]
- 68.Bateson P, Gluckman P. Plasticity, Robustness, Development and Evolution. Cambridge University Press; Cambridge: 2011. [Google Scholar]
- 69.Vivante A, Kohl S, Hwang D-Y, Dworschak GC, Hildebrandt F. Single-gene causes of congenital anomalies of the kidney and urinary tract (CAKUT) in humans. Pediatr Nephrol. 2014;29:695–704. doi: 10.1007/s00467-013-2684-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Childs B. Acceptance of the Howland Award. Pediatr Res. 1989;26:390–393. doi: 10.1203/00006450-198910000-00023. [DOI] [PubMed] [Google Scholar]
- 71.Christodoulou F, Raible F, Tomer R, Simakov O, Trachana K, Klaus S, Snyman H, Hannon GJ, Bork P, Arendt D. Ancient animal microRNAs and the evolution of tissue identity. Nature. 2010;463:1084–1088. doi: 10.1038/nature08744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kosik KS. MicroRNAs tell an evo-devo story. Nature Rev Neuro. 2009;10:754–759. doi: 10.1038/nrn2713. [DOI] [PubMed] [Google Scholar]
- 73.Matzke MA, Mette MF, Aufsatz W, Jakowitsch J, Matzke AJM. Host defenses to parasitic sequences and the evolution of epigenetic control mechanisms. Genetica. 1999;107:271–287. [PubMed] [Google Scholar]
- 74.Barbash S, Shifman S, Soreq H. Global coevolution of human microRNAs and their target genes. Mol Biol Evol. 2014;31:1237–1247. doi: 10.1093/molbev/msu090. [DOI] [PubMed] [Google Scholar]
- 75.Arthur W. The concept of developmental reprogramming and the quest for an inclusive theory of evolutionary mechanisms. Evol Devel. 2000;2:49–57. doi: 10.1046/j.1525-142x.2000.00028.x. [DOI] [PubMed] [Google Scholar]
- 76.Barker DJP, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 77.Barker DJP, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:578–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 78.Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27:358–368. doi: 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Godfrey KM, Costello PM, Lillycrop KA. The developmental environment, epigenetic biomakers and long-term health. J Devel Origins Health Dis. 2015;6:399–406. doi: 10.1017/S204017441500121X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luyckx VA, Brenner BM. Low birth weight, nephron number, and kidney disease. Kidney Int. 2005;68:S68–S77. doi: 10.1111/j.1523-1755.2005.09712.x. [DOI] [PubMed] [Google Scholar]
- 81.Vikse BE, Irgens LM, Leivestad T, Hallan S, Iversen BM. Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol. 2008;19:151–157. doi: 10.1681/ASN.2007020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.White SL, Perkovic V, Cass A, Chang CL, Poulter NR, Spector T, Haysom L, Craig JC, Al Salmi I, Chadban SJ, Huxley RR. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kid Dis. 2009;54:248–261. doi: 10.1053/j.ajkd.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 83.Remuzzi G, Luyckx V. The impact of kidney development on the life course: a consensus document for action. Nephron. 2017;136:3–49. doi: 10.1159/000457967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eriksson JG, Salonen MK, Kajantie E, Osmond C. Prenatal growth and CKD in older adults: longitudinal findings from the Helsinki Birth Cohort Study, 1924–1944. Am J Kidney Dis. 2017 doi: 10.1053/j.ajkd.2017.06.030. [DOI] [PubMed]
- 85.Wlodek ME, Westcott K, Siebel AL, Owens JA, Moritz KM. Growth restriction before or after birth reduces nephron number and increases blood pressure in male rats. Kidney Int. 2008;74:187–195. doi: 10.1038/ki.2008.153. [DOI] [PubMed] [Google Scholar]
- 86.Shen Q, Xu H, Wei L-M, Chen J, Liu H-M, Guo W. A comparative proteomic study of nephrogenesis in intrauterine growth restriction. Pediatr Nephrol. 2010;25:1063–1072. doi: 10.1007/s00467-009-1437-x. [DOI] [PubMed] [Google Scholar]
- 87.Buffat C, Mondon F, Rigourd V, Boubred F, Bessieres B, Fayol L, Feuerstein J-M, Gamerre M, Jammes H, Rebourcet R, Miralles F, Courbieres B, Basine A, Dignat-Georges F, Carbonne B, Simeoni U, Vaiman D. A hierarchical analysis of transcriptome alterations in intrauterine growth restriction (IUGR) reveals common pathophysiological pathways. J Pathol. 2007;213:337–346. doi: 10.1002/path.2233. [DOI] [PubMed] [Google Scholar]
- 88.Ratelade J, Lavin TA, Muda AO, Morisset L, Mollet G, Boyer O, Chen DS, Henger A, Kretzler M, Hubner N, Thery C, Gubler MC, Montagutelli X, Antignac C, Esquivel EL. Maternal environment interacts with modifier genes to influence progression of nephrotic syndrome. J Am Soc Nephrol. 2008;19:1491–1499. doi: 10.1681/ASN.2007111268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Woroniecki R, Gaikwad AB, Susztak K. Fetal environment, epigenetics, and pediatric renal disease. Pediatr Nephrol. 2011;26:705–711. doi: 10.1007/s00467-010-1714-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hales CN, Barker DJP. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 91.Lane N. Energy, Evolution, and the Origins of Complex Life. W W Norton & Co; New York and London: 2016. The Vital Question. [Google Scholar]
- 92.Odermatt A. The Western-style diet: a major risk factor for impaired kidney function and chronic kidney disease. Am J Physiol Renal Physiol. 2011;301:F919–F931. doi: 10.1152/ajprenal.00068.2011. [DOI] [PubMed] [Google Scholar]
- 93.Stangenberg S, Chen H, Wong MG, Pollock CA, Saad S. Fetal programming of chronic kidney disease: the role of maternal smoking, mitochondrial dysfunction, and epigenetic modification. Am J Physiol Renal Physiol. 2015;308:F1189–F1196. doi: 10.1152/ajprenal.00638.2014. [DOI] [PubMed] [Google Scholar]
- 94.Pereira SP, Oliveira PJ, Tavares LC, Moreno AJ, Cox LA, Nathanielsz PW, Nijland MJ. Effects of moderate global maternal nutrient reduction on fetal baboon renal mitochondrial gene expression at 0.9 gestation. Am J Physiol Renal Physiol. 2015;308:F1217–F1228. doi: 10.1152/ajprenal.00419.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bateson P, Gluckman P, Hanson M. The biology of developmental plasticity and the predictive adaptive response hypothesis. J Physiol. 2014;592:2357–2368. doi: 10.1113/jphysiol.2014.271460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wells JCK. A critical appraisal of the predictive adaptive response hypothesis. Int J Epid. 2012;41:229–235. doi: 10.1093/ije/dyr239. [DOI] [PubMed] [Google Scholar]
- 97.Fleming TP, Velazquez MA, Eckert JJ. Embryos, DOHaD and David Barker. J Devel Origins Health Dis. 2015;6:377–383. doi: 10.1017/S2040174415001105. [DOI] [PubMed] [Google Scholar]
- 98.Garcia TS, Urbina JC, Bredeweg EM, Ferrari MCO. Embryonic learning and developmental carry-over effects in an invasive anuran. Oecologia. 2017;184:623–631. doi: 10.1007/s00442-017-3905-5. [DOI] [PubMed] [Google Scholar]
- 99.Lewis RM, Hanson MA. Review: placenta, evolution and lifelong health, Placenta 33, Supplement A. Trophoblast Research. 2012;26:S28–S32. doi: 10.1016/j.placenta.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 100.Sookoian S, Gianotti TF, Burgueno AL, Pirola CJ. Fetal metabolic programming and epigentic modifications: a systems biology approach. Pediatr Res. 2013;73:531–542. doi: 10.1038/pr.2013.2. [DOI] [PubMed] [Google Scholar]
- 101.Fernandez-Twinn DS, Constancia M, Ozanne SE. Intergenerational epigenetic inheritance in models of developmental programming of adult disease. Sem Cell Devel Biol. 2015;43:85–95. doi: 10.1016/j.semcdb.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scheffers BR, De Meester L, Bridge TCL, Hoffmann AA, Pandolfi JM, Corlett RT, Butchart SHM, Pearce-Kelly P, Kovacs KM, Dudgeon D, Pacifici M, Rondinini C, Foden WB, Martin TG, Mora C, Bickford D, Watson JEM. The broad footprint of climate change from genes to biomes to people. Science. 2016;354:719. doi: 10.1126/science.aaf7671. [DOI] [PubMed] [Google Scholar]
- 103.Maslin MA, Christensen B. Tectonics, orbital forcing, global climate change, and human evolution in Africa: introduction to the paleoclimate special volume. J Hum Evol. 2007;53:443–464. doi: 10.1016/j.jhevol.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 104.Johnson RJ, Stenvinkel P, Jensen T, Lanaspa MA, Roncal C, Song Z, Bankir L, Sanchez-Lozada LG. Metabolic and kidney diseases in the setting of climate change, water shortage, and survival factors. J Am Soc Nephrol. 2016;27:2247–2256. doi: 10.1681/ASN.2015121314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barraclough KA, Blashki GA, Holt SG, Agar JWM. Climate change and kidney disease--threats and opportunities. Kidney Int. 2017;92:526–530. doi: 10.1016/j.kint.2017.03.047. [DOI] [PubMed] [Google Scholar]
- 106.MacManes MD. Severe acute dehydreation in a desert rodent elicits a transcriptional response that effectively prevents kidney injury. Am J Physiol Renal Physiol. 2017;313:F262–F272. doi: 10.1152/ajprenal.00067.2017. [DOI] [PubMed] [Google Scholar]
- 107.Rylander C, Odland JO, Sandanger TM. Climate change and the potential effects on maternal and pregnanacy outcomes: an assessment of the most vulnerable--the mother, fetus, and newborn child. Glob Health Action. 2013 doi: 10.3402/gha.v610.19538. [DOI] [PMC free article] [PubMed]
- 108.Howland RB. On the effect of removal of the pronephros of the amphibian embryo. PNAS. 1916;2:231–234. doi: 10.1073/pnas.2.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oliver J. Architecture of the Kidney in Chronic Bright’s Disease. Paul B. Hoeber Inc; New York: 1939. [Google Scholar]
- 110.Brenner BM. Chronic renal failure: A disorder of adaptation. Perspectives in Biology and Medicine, “reprinted for private circulation”. 1989;32:434–444. doi: 10.1353/pbm.1989.0049. [DOI] [PubMed] [Google Scholar]
- 111.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more of the other? Am J Hypertension. 1988;1:335–347. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 112.Brenner BM. Nephron adaptation to renal injury or ablation. American Journal of Physiology. 1985;1:F324–F337. doi: 10.1152/ajprenal.1985.249.3.F324. [DOI] [PubMed] [Google Scholar]
- 113.Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Hyperfiltration in remnant rephrons: a potentially adverse response to renal ablation. American Journal of Physiology. 1981;241:F85–F93. doi: 10.1152/ajprenal.1981.241.1.F85. [DOI] [PubMed] [Google Scholar]
- 114.Brenner BM. Chronic renal failure: A disorder of adaptation. Perspectives in Biology and Medicine, “reprinted for private circulation”. 1989;32:434–444. doi: 10.1353/pbm.1989.0049. [DOI] [PubMed] [Google Scholar]
- 115.Casas JP, Chua W, Loukogeorgakis S, Vallance P, Smeeth L, Hingorani AD, MacAllister RJ. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet. 2005;366:2026–2033. doi: 10.1016/S0140-6736(05)67814-2. [DOI] [PubMed] [Google Scholar]
- 116.Stearns SC. The Evolution of Life Histories. Oxford University Press; Oxford, New York, Toronto: 1992. [Google Scholar]
- 117.Wuhl E, van Stralen KJ, Verrina E, Bjerre A, Wanner C, Heaf JG, Zurriaga O, Hoitsma A, Niaudet P, Palsson R, Ravani P, Jager KJ, Schaefer F. Timing and outcome of renal replacement therapy in patients with congenital malformations of the kidney and urinary tract. Clin J Am Soc Nephrol. 2013;8:67–74. doi: 10.2215/CJN.03310412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chevalier RL. The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. Am J Physiol Renal Physiol. 2016;311:F145–F161. doi: 10.1152/ajprenal.00164.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Forbes MS, Thornhill BA, Park MH, Chevalier RL. Lack of endothelial nitric-oxide synthase leads to progressive focal renal injury. Am J Pathol. 2007;170:87–99. doi: 10.2353/ajpath.2007.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Noiri E, Satoh H, Taguchi J, Brodsky WV, Nakao A, Ogawa Y, Nishijima S, Yokomizo T, Tokunaga K, Fujita T. Association of eNOS Glu298Asp polymorphism with end-stage renal disease. Hypertension. 2002;40:535–540. doi: 10.1161/01.hyp.0000033974.57407.82. [DOI] [PubMed] [Google Scholar]
- 121.Chevalier RL. Evolution and kidney development: A Rosetta stone for nephrology. J Am Soc Nephrol. 2018;29:705–709. doi: 10.1681/ASN.2018010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chevalier RL. Evolutionary Nephrology. Kidney Int Rep. 2017;2:302–317. doi: 10.1016/j.ekir.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- 124.Gluckman P, Beedle A, Buklijas T, Low F, Hanson M. Principles of Evolutionary Medicine. Oxford University Press; Oxford: 2016. [Google Scholar]
- 125.Byars SG, Huang QQ, Gray L-A, Bakshi A, Ripatti S, Abraham G, Stearns SC, Inouye M. Genetic loci associated with coronary artery disease harbor evidence of selection and antagonistic pleiotropy. PLOS Genetics. 2017 doi: 10.1371/journal.pgen.1006328. [DOI] [PMC free article] [PubMed]
- 126.Cohen JJ. Relationship between energy requirements for Na+ reabsorption and other renal functions. Kidney Int. 1986;29:32–40. doi: 10.1038/ki.1986.5. [DOI] [PubMed] [Google Scholar]
- 127.Brenner BM, Meyer TW, Hostetter TH. Dietary protein intake and the progressive nature of kidney disease: The role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med. 1982;307:652–659. doi: 10.1056/NEJM198209093071104. [DOI] [PubMed] [Google Scholar]
- 128.Nath KA, Croatt AJ, Hostetter TH. Oxygen consumption and oxidant stress in surviving nephrons. Am J Physiol Renal Physiol. 1990;258:F1354–F1362. doi: 10.1152/ajprenal.1990.258.5.F1354. [DOI] [PubMed] [Google Scholar]
- 129.Layton AT, Edwards A, Vallon V. Adaptive change in GFR, tubular morphology, and transport in subtotal nephrectomized kidneys: modeling and analysis. Am J Physiol Renal Physiol. 2017;313:F199–F209. doi: 10.1152/ajprenal.00018.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Margulis L. Genetic and evolutionary consequences of symbiosis. Exp Parasitol. 1976;39:277–349. doi: 10.1016/0014-4894(76)90127-2. [DOI] [PubMed] [Google Scholar]
- 131.Grone H-J, Helmchen U. Impairment and recovery of the clipped kidney in two kidney, one clip hypertensive rats during and after antihypertensive therapy. Lab Invest. 1986;54:645–655. [PubMed] [Google Scholar]
- 132.Grone H-J, Warnecke E, Olbricht CJ. Characteristics of renal tubular atrophy in experimental renovascular hypertension: A model of kidney hibernation. Nephron. 1996;72:243–252. doi: 10.1159/000188849. [DOI] [PubMed] [Google Scholar]
- 133.Marcussen N. Atubular glomeruli in renal artery stenosis. Lab Invest. 1991;65:558–565. [PubMed] [Google Scholar]
- 134.Chevalier RL, Forbes MS. Generation and evolution of atubular glomeruli in the progression of renal disorders. J Am Soc Nephrol. 2008;19:197–206. doi: 10.1681/ASN.2007080862. [DOI] [PubMed] [Google Scholar]
- 135.Grafflin AL. Glomerular degeneration in the kidney of the daddy sculpin (Myoxocephalus scorpius) Anat Rec. 1933;57:59–78. [Google Scholar]
- 136.Stecker JF, Jr, Vaughan ED, Jr, Gillenwater JY. Alteration in renal metabolism occurring in ureteral obstruction in vivo. Surg Gynecol Obstet. 1971;133:846–848. [PubMed] [Google Scholar]
- 137.Forbes MS, Thornhill BA, Minor JJ, Gordon KA, Galarreta CI, Chevalier RL. Fight-or-flight: murine unilateral ureteral obstruction causes extensive proximal tubular degeneration, collecting duct dilatation, and minimal fibrosis. Am J Physiol Renal Physiol. 2012;303:F120–F129. doi: 10.1152/ajprenal.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Galarreta CI, Grantham JJ, Forbes MS, Maser RL, Wallace DP, Chevalier RL. Tubular obstruction leads to progressive proximal tubular injury and atubular glomeruli in polycystic kidney disease. Am J Pathol. 2014;184:1957–1966. doi: 10.1016/j.ajpath.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Galarreta CI, Forbes MS, Thornhill BA, Antignac C, Gubler M-C, Nevo N, Murphy MP, Chevalier RL. The swan-neck lesion: proximal tubular adaptation to oxidative stress in nephropathic cystinosis. Am J Physiol Renal Physiol. 2015;308:F1155–F1166. doi: 10.1152/ajprenal.00591.2014. [DOI] [PubMed] [Google Scholar]
- 140.Grams ME, Chow EKH, Segev DL, Coresh J. Lifetime incidence of CKD Stages 3–5 in the United States. Am J Kidney Dis. 2013;62:245–252. doi: 10.1053/j.ajkd.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Boddu R, Fan C, Rangarajan S, Sunil B, Bolisetty S, Curtis L. Unique ses- and age-dependent effects in protective pathways in acute kidney injury. Am J Physiol Renal Physiol. 2017;313:F740–F755. doi: 10.1152/ajprenal.00049.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tan JC, Workeneh B, Busque S, Blouch K, Derby G, Myers BD. Glomerular function, structure, and number in renal allografts from older deceased donors. J Am Soc Nephol. 2009;20:181–188. doi: 10.1681/ASN.2008030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kaplan C, Pasternack B, Shsh H, Gallo G. Age-related incidence of sclerotic glomeruli in human kidneys. Am J Pathol. 1975;80:227–234. [PMC free article] [PubMed] [Google Scholar]
- 144.Denic A, Lieske JC, Chakkera HA, Poggio ED, Alexander MP, SIng P, Kremers WK, Lerman LO, Rule AD. The substantial loss of nephrons in healthy human kidneys with aging. J Am Soc Nephrol. 2016;28:313–320. doi: 10.1681/ASN.2016020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zurbig P, Decramer S, Dakna M, Jantos J, Good DM, Coon JJ, Bandin F, Mischak H, Bascands JL, Schanstra JP. The human urinary proteome reveals high similarity between kidney aging and chronic kidney disease. Proteomics. 2009;9:2108–2117. doi: 10.1002/pmic.200800560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Weinert BT, Timiras PS. Invited review: theories of aging. J Appl Physiiol. 2003;95:1706–1716. doi: 10.1152/japplphysiol.00288.2003. [DOI] [PubMed] [Google Scholar]
- 147.Lane N. A unifying view of ageing and disease: the double-agent theory. J Theor Biol. 2003;225:531–540. doi: 10.1016/s0022-5193(03)00304-7. [DOI] [PubMed] [Google Scholar]
- 148.Kooman JP, Dekker MJ, Usvyat LA, Kotanko P, van der Sande F, Schalkwijk CG, Shiels PG, Stenvinkel P. Inflammation and premature aging in advanced chronic kidney disease. Am J Physiol Renal Physiol. 2017;313:F938–F950. doi: 10.1152/ajprenal.00256.2017. [DOI] [PubMed] [Google Scholar]
- 149.Shiels PG, McGuinness D, Eriksson M, Kooman JP, Stenvinkel P. The role of epigenetics in renal ageing. Nat Rev Nephrol. 2017;13:471–482. doi: 10.1038/nrneph.2017.78. [DOI] [PubMed] [Google Scholar]
- 150.O’Sullivan ED, Hughes J, Ferenbach DA. Renal aging: causes and consequences. J Am Soc Nephrol. 2017;28:407–420. doi: 10.1681/ASN.2015121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schmitt R, Melk A. Molecular mechanisms of renal aging. Kidney Int. 2017;92:569–579. doi: 10.1016/j.kint.2017.02.036. [DOI] [PubMed] [Google Scholar]
- 152.Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, Kuick RD, Wiggins RC. Podocyte hypertrophy, “adaptation,” and decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol. 2005;16:2953–2966. doi: 10.1681/ASN.2005050488. [DOI] [PubMed] [Google Scholar]
- 153.McKiernan SH, Tuen VC, Baldwin K, Wanagat J, Djamali A, Aiken JM. Adult-onset calorie restriction delays the accumulation of mitochondrial enzyme abnormalities in aging rat kidney tubular epithelial cells. Am J Physiol Renal Physiol. 2007;292:F1751–F1760. doi: 10.1152/ajprenal.00307.2006. [DOI] [PubMed] [Google Scholar]
- 154.Nishizono R, Kikuchi M, Wang SQ, Chowdhury M, Nair V, Hartman J, Fukuda A, Wickman L, Hodgin JB, Bitzer M, Naik A, Wiggins J, Kretzler M, Wiggins RC. FSGS as an adaptive response to growth-induced podocyte stress. J Am Soc Nephrol. 2017;28:2931–2945. doi: 10.1681/ASN.2017020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yoshida Y, Fogo A, Ichikawa I. Glomerular hemodynamic changes vs. hypertrophy in experimental glomerular sclerosis. Kidney Int. 1989;35:654–660. doi: 10.1038/ki.1989.35. [DOI] [PubMed] [Google Scholar]
- 156.Luyckx VA, Compston CA, Simmen T, Mueller TF. Accelerated senescence in kidneys of low-birth-weight rats after catch-up growth. Am J Physiol Renal Physiol. 2009;297:F1697–F1705. doi: 10.1152/ajprenal.00462.2009. [DOI] [PubMed] [Google Scholar]
- 157.Romagnani P, Remuzzi G. Renal progenitors: an evolutionary conserved strategy for kidney regeneration. Nat Rev Nephrol. 2013;9:137–146. doi: 10.1038/nrneph.2012.290. [DOI] [PubMed] [Google Scholar]
- 158.Reichman OJ. Evolution of regeneration capabilities. Am Nat. 1984;123:752–763. [Google Scholar]
- 159.Goss RJ. The evolution of regeneration: adaptive or inherent? Journal of Theoretical Biology. 1992;159:241–260. doi: 10.1016/s0022-5193(05)80704-0. [DOI] [PubMed] [Google Scholar]
- 160.Rodriguez MM, Gomez AH, Abitbol CL, Chandar JJ, Duara S, Zilleruelo GE. Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Devel Pathol. 2004;7:17–25. doi: 10.1007/s10024-003-3029-2. [DOI] [PubMed] [Google Scholar]
- 161.Gubhaju L, Sutherland MR, Yoder BA, Zulli A, Bertram JF, Black MJ. Is nephrogenesis affected by preterm birth? Studies in a non-human primate model. Am J Physiol Renal Physiol. 2009;297:F1668–F1677. doi: 10.1152/ajprenal.00163.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Douglas-Denton R, Moritz KM, Bertram JF, Wintour EM. Compensatory renal growth after unilateral nephrectomy in the ovine fetus. J Am Soc Nephrol. 2002;13:406–410. doi: 10.1681/ASN.V132406. [DOI] [PubMed] [Google Scholar]
- 163.Alvarado AS. Cellular hyperproliferation and cancer as evolutionary variables. Curr Biol. 2012;22:R772–R778. doi: 10.1016/j.cub.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Torday JS, Rehan VK. Evolutionary Biology, Cell-Cell Communication, and Complex Disease. Wiley-Blackwell; Hoboken, NJ: 2012. [Google Scholar]
- 165.He L, Wei Q, Liu J, Yi M, Liu Y, Liu H, Sun L, Peng Y, Liu F, Venkatachalam MA, Dong Z. AKI on CKD: heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 2017;92:1071–1083. doi: 10.1016/j.kint.2017.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kawakami T, Ren S, Duffield JS. Wnt signalling in kidney diseases: dual roles in renal injury and repair. [Review] J Pathol. 2013;229:221–231. doi: 10.1002/path.4121. [DOI] [PubMed] [Google Scholar]
- 167.Torday JS. On the evolution of development. Trends Dev Biol. 2014;8:17–37. [PMC free article] [PubMed] [Google Scholar]
- 168.Torday JS. Pleiotropy as the mechanism for evolving novelty: same signal, different result. Biology. 2015;4:443–459. doi: 10.3390/biology4020443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Burnet M. The Clonal Selection Theory of Acquired Immunity. Vanderbilt University Press; Nashville: 1959. [Google Scholar]
- 170.Edelman GM. Neural Darwinism: The Theory of Neuronal Group Selection. Basic Books; 1987. [DOI] [PubMed] [Google Scholar]
- 171.Edelman GM, Gally JA. Degeneracy and complexity in biological systems. PNAS. 2001;98:13763–12768. doi: 10.1073/pnas.231499798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Aird WC. Endothelial cell heterogeneity. Cold Spring Harb Persp Med. 2012;2:a006429. doi: 10.1101/cshperspect.a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Koseki C, Herzlinger D, Al-Awqati Q. Apoptosis in metanephric development. J Cell Biol. 1992;119:1327–1333. doi: 10.1083/jcb.119.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hartman HA, Lai HL, Patterson P. Cessation of renal morphogenesis in mice. Devel Biol. 2007;310:379–387. doi: 10.1016/j.ydbio.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tsigeiny IF, Kouznetsova VL, Sweeney DE, Wu W, Bush KT, Nigam SK. Analysis of metagene portraits reveals distinct transitions during kidney organogenesis. Science Signaling. 2008;1:1–9. doi: 10.1126/scisignal.1163630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kondo Y, Morimoto T, Nishio T, Aslanova UF, Nishino M, Farajov EI, Sugawara N, Kumagai N, Ohsaga A, Maruyama Y, Takahashi S. Phylogenetic, ontogenetic, and pathological aspects of the urine-concentrating mechansm. Clin Exp Nephrol. 2006;10:165–174. doi: 10.1007/s10157-006-0429-4. [DOI] [PubMed] [Google Scholar]
- 177.Kopolovic J, Brezis M, Spokes K, Silva P, Epstein F, Rosen S. The vulnerability of the thin descending limbs of Henle’s loop in the isolated perfused rat kidney. Am J Kidney Dis. 1989;14:25–30. doi: 10.1016/s0272-6386(89)80089-7. [DOI] [PubMed] [Google Scholar]
- 178.Sanz AB, Sanchez-Nino MD, Izquierdo MC, Jakubowski A, Justo P, Blanco-Colio LM, Ruiz-Ortega M, Egido J, Ortiz A. Tweak induces proliferation in renal tubular epithelium: a role in uninephrectomy induced renal hyperplasia. J Cell Mol Med. 2009;13:3329–3342. doi: 10.1111/j.1582-4934.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Guo M, Pegoraro AF, Mao A, Zhou E, Arany PR, Han Y, Burnette DT, Jensen MH, Kasza KE, Moore JR, Mackintosh FC, Fredberg JJ, Mooney DJ, Lippincott-Schwartz J, Weitz DA. cell volume change through water efflux impacts cell stiffness and stem cell fate. PNAS. 2017 doi: 10.1073/pnas.1705179114. www.pnas.org/cgi/doi/10.1073/pnas.1705179114. [DOI] [PMC free article] [PubMed]
- 180.Tanaka R, Sugihara K, Tatematsu A, Fogo A. Internephron heterogeneity of growth factors and sclerosis-- Modulation of platelet-derived growth factor by angiotensin II. Kidney Int. 1995;47:131–139. doi: 10.1038/ki.1995.15. [DOI] [PubMed] [Google Scholar]
- 181.Fournier D, Luft FC, Bader M, Ganten D, Andrade-Navarro MA. Emergence and evolution of the renin-angiotensin-aldosterone system. J Mol Med. 2012;90:495–508. doi: 10.1007/s00109-012-0894-z. [DOI] [PMC free article] [PubMed] [Google Scholar]