Abstract
The current World Health Organization (WHO) classification for endocervical adenocarcinoma (EA) is based on descriptive morphological characteristics; however, it does not fully reflect our current knowledge of the diverse pathogenesis of cervical glandular neoplasia. A novel classification system, the International Endocervical Adenocarcinoma Criteria and Classification (IECC), which incorporates etiology and biologic behavior into the morphologic scheme, has been recently proposed. We aimed to validate the IECC by assessing its interobserver reproducibility in comparison to the WHO system. A cohort of 75 EAs was reviewed independently by 7 gynecologic pathologists and categorized following IECC and WHO criteria based on hematoxylin and eosin (H&E) material alone and after immunohistochemistry results for p16, PR, p53, Napsin-A, vimentin, CDX2 and GATA3 were provided. HPV in situ hybridization (ISH) and polymerase chain reaction (PCR) results were compared with consensus diagnoses. IECC was superior to WHO in terms of interobserver agreement with K=0.46 vs 0.3 respectively on H&E review and K=0.51 vs 0.33 respectively with immunohistochemistry. Under the IECC, 73 (97%) of EAs had majority agreement (≥ 4 reviewers in agreement) while 42 (56%) had perfect agreement (7/7 reviewers in agreement). Conversely, WHO showed majority agreement in 56 (75%) and perfect agreement in only 7 (10%) EAs. Reproducibility was poor in HPV-related WHO types (usual K=0.36, mucinous NOS K=0.13, intestinal K=0.31, villoglandular K=0.21) and good in major HPV-unrelated categories (gastric type K=0.63, clear cell K=0.81, mesonephric K=0.5). Classification as per the IECC had excellent correlation with HPV status (by RNA ISH or PCR). We have shown that the IECC has superior interobserver agreement compared to the WHO classification system, and that distinction between HPV-related and HPV-unrelated EA can be made with good reproducibility and excellent prediction of HPV status. WHO morphologic variants of HPV-related EA are poorly reproducible. Conversely, agreement is high among important high-risk HPV-unrelated subtypes. Thus, our results further support replacing the current WHO classification with the IECC.
Keywords: Cervix, endocervical, adenocarcinoma, HPV, gastric type endocervical adenocarcinoma, clear cell carcinoma, mesonephric carcinoma, serous carcinoma, villoglandular carcinoma, stratified mucin producing carcinoma, mucinous adenocarcinoma, classification, IECC, reproducibility, interobserver agreement
INTRODUCTION
In contrast to cervical squamous cell carcinoma which shows a rate of high-risk HPV detection approaching 100%, endocervical adenocarcinoma (EA) shows a variable prevalence of HPV depending on several factors including geographic region, tumor subtype, and detection method1–5. Indeed, it is now known that several EA subtypes are unrelated to HPV infection6–8 and have distinct clinico-pathologic features and prognosis compared to HPV-related tumors8,9.
The current World Health Organization (WHO) classification of tumors of female genital organs classifies EA based on morphological characteristics, particularly cytoplasmic features10. The practicality of this classification system has been questioned, as the reproducibility of the terminology has not been thoroughly explored and tumor type definitions are vague. In addition, the lack of robust biological basis of the current classification has led to further questioning of its clinical value. Notably, classification of epithelial neoplasia of the vulva11–14 and oropharynx15,16 has shifted towards a system based on pathogenesis, as it has been found to be more clinically informative and reproducible.
The International Endocervical Adenocarcinoma Criteria and Classification (IECC), which categorizes malignant glandular endocervical lesions based on underlying etiology and biologic behavior, was recently proposed by an international group of experts in gynecologic pathology (Table 1)17. The system, applied to a well-annotated cohort of 409 invasive EAs, showed reliable segregation of HPV-related EA (HPVA) and HPV-unrelated EA (NHPVA) by morphology alone, and was further improved by the use of a selective immunohistochemical panel (p16, p53, PR, vimentin). Under the IECC, EA is classified as HPVA and NHPVA based on the presence or absence of HPV-related features (easily identifiable apical mitoses and apoptotic bodies). NHPVA was further subdivided into known categories with updated and improved definitions. Finally, the category of EA-not otherwise specified (EA-NOS) was assigned when a tumor could not be classified by IECC criteria. This classification is a promising step towards categorizing EA in a more consistent and clinically meaningful manner. While treatment algorithms for cervical cancer incorporating HPV status do not exist currently for EA18, a precedent for such approach has been set in other organ systems like head and neck15,16,19,20.
Table 1.
International Endocervical Adenocarcinoma Criteria and Classification (IECC) and World Health Organization (WHO) classification systems
| International Endocervical Adenocarcinoma Criteria and Classification (IECC) | World Health Organization (WHO) | |
|---|---|---|
|
| ||
| Main categories | Morphologic subcategories (optional) | |
|
| ||
| 1. HPV-related endocervical adenocarcinoma | HPV adenocarcinoma, usual type | 1. Usual type adenocarcinoma |
|
|
||
| HPV adenocarcinoma, villoglandular type | 2. Villoglandular adenocarcinoma | |
|
|
||
| HPV adenocarcinoma, mucinous NOS type | 3. Mucinous adenocarcinoma NOS | |
|
|
||
| HPV adenocarcinoma, mucinous intestinal type | 4. Mucinous adenocarcinoma, intestinal type | |
|
|
||
| HPV adenocarcinoma, signet-ring cell type | 5. Mucinous adenocarcinoma, signet-ring cell type | |
| HPV adenocarcinoma, stratified mucin producing type | ||
|
| ||
| 2. Non-HPV adenocarcinoma, gastric type | 6. Mucinous adenocarcinoma, gastric type | |
|
| ||
| 3. Non-HPV adenocarcinoma, endometrioid type | 7. Endometrioid carcinoma | |
|
| ||
| 4. Non-HPV adenocarcinoma, clear cell type | 8. Clear cell carcinoma | |
|
| ||
| 5. Non-HPV adenocarcinoma, mesonephric type | 9. Mesonephric carcinoma | |
|
| ||
| 6. Non-HPV adenocarcinoma, serous type | 10. Serous carcinoma | |
|
| ||
| 7. Invasive adenocarcinoma, NOS | 11. Invasive adenocarcinoma, NOS | |
Adoption of the IECC is justified by its more biologically congruent approach and simple pathologic definitions. However, its interobserver reproducibility has yet to be addressed. We therefore aimed to evaluate the IECC by documenting its interobserver agreement in comparison to the WHO system and its predictive value of the HPV status. In this context, we also intended to study the reproducibility of HPVA and NHPVA subcategories as defined by WHO and IECC and to compare the reproducibility of EA diagnoses in biopsy versus excision material.
MATERIALS AND METHODS
Case selection and review
Material selected for independent review was obtained from consecutive invasive EA specimens collected between 2002 and 2017 at Sunnybrook Health Sciences Centre (Toronto, Canada). Specimens with all histologic slides and at least one suitable tissue block available were included; others were excluded from this study. Cases further selected included cervical biopsy and/or excision material (loop electrosurgical excision procedure (LEEP), cold knife cone, trachelectomy, hysterectomy). All original slides were reviewed by one gynecologic pathologist (CPH), and the most representative slides were selected for independent review by all the participants (1 to 4 slides per case).
Tissue microarray construction
A representative paraffin-embedded tissue block from each tumor was retrieved. Tissue microarrays (TMAs) were constructed using two 2-mm cores of representative tumor tissue per case. Normal tissue controls were included in each TMA block.
Immunohistochemistry
TMA sections were stained with p16, p53, PR, vimentin, CDX2, Napsin-A, and GATA3 (Table 2). Stains were interpreted by a gynecological pathologist (CPH) and results recorded using the following definitions: p16 staining was considered “overexpressed” if diffuse block-like nuclear and cytoplasmic staining was seen in ≥80% of tumor cells, “patchy” if positivity was patchy (<80% of tumor cells) or cytoplasmic only, or “absent” if no staining was seen; p53 was scored as “overexpressed” if ≥80% of tumor nuclei were strongly positive, “null” when no staining was seen in tumor cells in the presence of an intact internal control, or “wild type” when nuclear expression was heterogeneous in intensity. For p16 and p53, results on TMA slides were compared to those from available archival immunohistochemistry whole-section slides. Scores for PR (nuclear), vimentin (cytoplasmic or membranous), Napsin-A (cytoplasmic), CDX2 (nuclear), and GATA3 (nuclear) were based on the percentage of positive tumor cells (0–100%) and the average intensity (weak, moderate, or strong).
Table 2.
Immunohistochemical antibody information
| Antibody | Clone | Vendor | Protocol | Detection system |
|---|---|---|---|---|
| P16 | JC8 | Ventana | CC1-24,8 | Ultraview |
| P53 | DO-7 | Ventana | CC1-76,32,+AMP | Ultraview |
| PR | 16 | Vector | CC1-64,32 | Ultraview |
| Vimentin | V9 | Ventana | CC1-36,16 | Ultraview |
| CDX2 | EPR2764Y | Ventana | CC136,16 | Ultraview |
| GATA-3 | L50-423 | Ventana | CC1-32,32 | Optiview |
| Napsin A | MRQ-60 | Ventana | CC1-16,8 | Optiview |
HPV detection studies
In situ hybridization (ISH) for high-risk HPV detection was performed on TMA slides. In situ hybridization (ISH) with a chromogen was performed using the Advanced Cell Diagnostics (ACD) (Hayward, CA) RNAscope® system (catalogue no. 312598). The RNAscope® Probe “HPV HR18” contains probes targeting E6 and E7 mRNA for the following high risk subtypes: HPV16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73 and 82. Historic tumors known to contain high risk-HPV were used as positive controls, while those known to be high risk-HPV negative were used as negative controls during assay optimization. Subsequently, a negative control slide lacking application of the probes was prepared and examined, particularly when adjudicating tumors with rare or equivocal signals. A full range of cytoplasmic and nuclear signals were encountered, as has been previously described26.
HPV detection by polymerase chain reaction (PCR) was performed in all tumors with negative ISH results, and in tumors with undetermined ISH results and residual tissue available. We used the Roche Cobas® 4800 system (Pleasanton, California) which detects the presence of 14 high-risk HPV types. The HPV DNA is detected by real-time PCR with specific fluorescent-labelled DNA probes. Qualitative results are generated separately for HPV Types 16 and 18, and a combined results for 12 other high-risk types of 31,33,35,39,45,51,52,56,58,59,66,68. Each run included a no-template negative control and a positive control supplied by the system; both control results were required to be valid in each run prior to reporting. In addition, the β-globin gene in each human sample was amplified in the same reaction tube as internal control.
To determine the IECC predictive value of HPV status, the majority diagnosis from the 7 reviewers for each case was compared to the HPV results as determined by RNA ISH and / or PCR.
Pathologist independent review
Seven gynecologic pathologists from 6 major institutions in Canada, the United States and Romania were recruited to participate in the study. Four reviewers (KJP, EO, SS, RS) were part of the original group that developed the IECC17. Reviewers were blinded to all clinical features and HPV status of each case. Written instructions were distributed to each reviewer summarizing the WHO and IECC terminology and criteria in tabular and graphic formats. Slide review and diagnosis assignment were done independently. The review process was first based on evaluation of routine hematoxylin and eosin (H&E) stained slides only. Subsequently, the immunohistochemical profile for each case was provided and the reviewers rendered a revised diagnosis.
The IECC classification system has been thoroughly described in the original study17. Briefly, the diagnosis of HPVA requires apical mitotic figures and apoptotic bodies appreciable at scanning magnification; if those features are not identified a cursory examination at 200× is performed to detect mitoses and/or apoptoses (Figure 1) and in their absence a diagnosis of NHPVA is warranted. NHPVAs are then classified based on established criteria as endometrioid21, gastric including minimal deviation adenocarcinoma5,9, serous, clear cell or mesonephric (Figure 2). Finally, a designation of EA-NOS is used when a tumor cannot be classified into HPVA or NHPVA categories. In addition to the above diagnoses, adenocarcinoma in situ (AIS) was available as a possible choice, as it has been noted that distinguishing between florid AIS and certain forms of invasive EA can be challenging22,23. Reviewers were asked to sub-classify HPVA following WHO cytoplasmic morphologic criteria as usual type, villoglandular, mucinous NOS, mucinous intestinal type, mucinous signet ring cell type, and invasive stratified mucin-producing carcinoma24,25. Within the HPVA category, the reviewers were also given the option to select “adenosquamous carcinoma” as a diagnosis.
Figure 1.
HPV-related endocervical adenocarcinoma (HPVA). A. Well differentiated HPVA with hyperchromatic nuclei and mucin depletion. B. Multiple hyperchromatic figures can be appreciated at scanning (10×) magnification (yellow arrows). C. At higher magnification (20×), these figures are confirm to be apoptoses (blue arrows) and apical mitoses (red arrows). D. HPVA, invasive stratified mucin producing variant, also with distinguishable apoptoses and mitoses at scanning (10×) magnification (yellow arrows).
Figure 2.
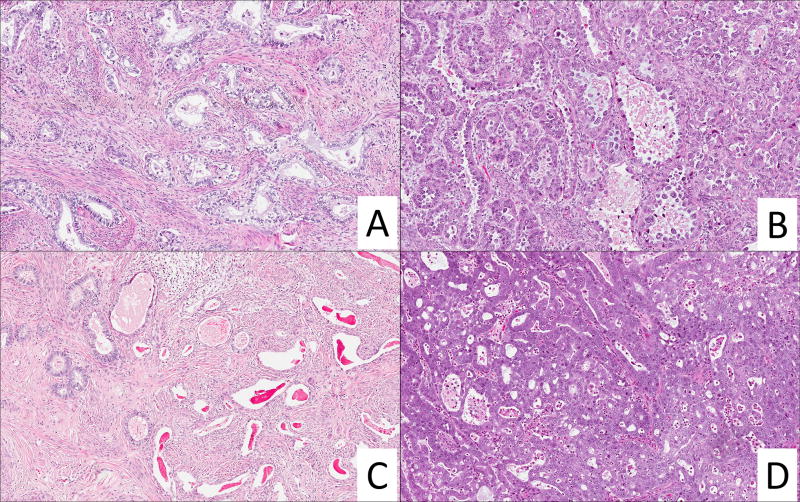
HPV-unrelated (NHPVA) and unclassifiable (EA-NOS) endocervical adenocarcinoma. A. Gastric-type NHPVA with infiltrative growth and clear mucinous cytoplasm. B. Clear cell NHPVA with high grade nuclear features and "hobnail" pattern. C. Mesonephric NHPVA with pseudo-endometrioid glands (left aspect) and dilated glands with eosinophilic luminal material (right aspect). D. High grade adenocarcinoma not amenable for classification into IECC categories by reviewers; HPV ISH was negative in this case.
Statistical analysis
All statistical analyses were performed using the SPSS software 24.0 (IBM Corporation, New York, NY, United States). The interobserver agreement of classification among individual pathologists, based on histology alone as well as based on histology and results of immunohistochemistry studies was assessed using Fleiss’ kappa (K) statistics. Kappa analysis was also applied to compare pathologist diagnoses as HPVA or NHPVA with HPV ISH results. Kappa values as well as the 95% confidence interval (CI) were calculated, and P values of less than 0.05 were considered to be statistically significant. Kappa was interpreted categorically following Fleiss guidelines for comparisons between >2 raters as follows: poor (K<0.4), fair/good (K=0.4–0.75) and perfect (K>0.75)27.
RESULTS
A total of 83 EA were identified and reviewed. Of these, 75 were successfully tested for HPV by ISH and/or PCR, and constitute the final study cohort. 47/75 cases (63%) had material from excision +/− biopsy specimens, while the remaining 28 cases in the cohort (37%) were composed of biopsy material only (including 13 from patients with advanced stage at presentation who underwent primary radiotherapy +/− chemotherapy without surgical treatment). A total of 73 tumors were successfully sampled in TMAs and were evaluable by immunohistochemistry (IHC). Of the 75 EAs, 64 (85%) were positive for high-risk HPV types by either ISH (63) or PCR (1). The remaining 11 (15%) were negative for high-risk HPV types either by PCR (3) or ISH (9 cases; PCR confirmed a negative result in 4 and resulted undetermined in 5).
Interobserver agreement
Individual tumor classification by each reviewer following the IECC based on H&E review only (Figure 3) showed superior interobserver reproducibility compared to classification as per the WHO system (Table 3). Interobserver agreement was higher for IECC with both H&E review (IECC K=0.46 vs WHO K=0.3) and after revision with IHC results (IECC K=0.51 vs. WHO K=0.33). Restricting the analysis to a three-tier classification (NHPVA vs. NHPVA vs. EA-NOS) also showed good reproducibility (K=0.45 on H&E and K=0.51 with IHC). Of note, reproducibility of the EA-NOS category was poor (K=0.2 and 0.19 by H&E and after IHC review, respectively).
Figure 3.
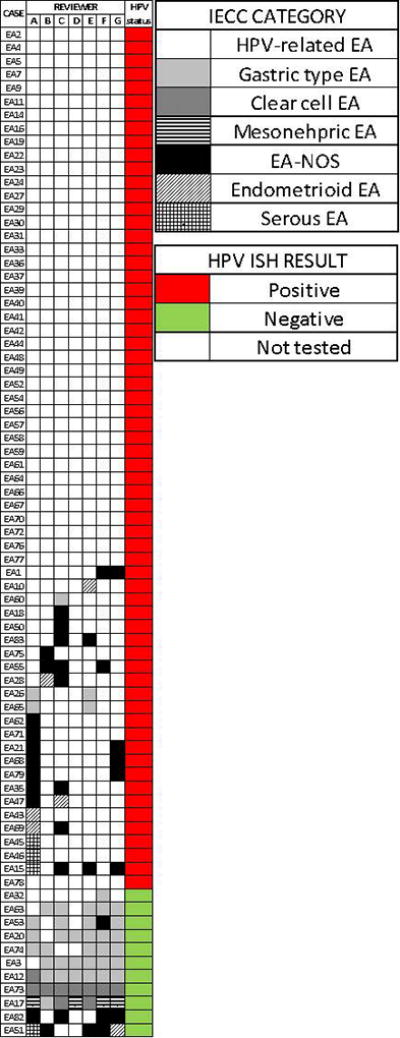
Diagram illustrating the IECC diagnosis provided by each reviewer.
Table 3.
Interobserver agreement (Kappa) in the classification of Endocervical Adenocarcinoma as per IECC and WHO systems
| All EA categories | H&E only* | H&E + IHC* |
|---|---|---|
| WHO | 0.3 (0.28–0.33) | 0.33 (0.31–0.35) |
| IECC | 0.46 (0.42–0.49) | 0.51 (0.47–0.55) |
| HPVA vs NHPVA | 0.45 (0.42–0.48)) | 0.5 (0.47–0.53) |
| HPVA | 0.46 (0.41–0.51) | 0.54 (0.49–0.59) |
| NHPVA | 0.53 (0.48–0.58) | 0.64 (0.59–0.69) |
| EA–NOS | 0.2 (0.16–0.26) | 0.19 (0.14–0.24) |
Mean (95% confidence interval)
Table 4 shows the different levels of agreement among the classification systems. Level of agreement was determined by the number of reviewers who gave the same diagnosis in each case. Overall, when using WHO criteria, a majority diagnosis was not achieved in a significant number of tumors (19, 25%) and among these, only 3 or fewer reviewers concurred with any given diagnosis. Similarly, under the WHO system, perfect agreement (7 out of 7 reviewers) was only reached only 7 (10%) cases. In contrast, levels of concordance in diagnosis as per IECC were significantly higher, with perfect agreement in 42 (56%) cases and lack of majority diagnosis in only 2 (3%) tumors. A simplified approach looking at HPVA vs. NHPVA vs EA-NOS showed similarly high levels of agreement.
Table 4.
Levels of agreement in the diagnosis of endocervical adenocarcinoma
| Level of agreement (# reviewers) | WHO | IECC | HPVA vs NHPVA vs EA-NOS |
|---|---|---|---|
| None (3 or less) | 19 (25%) | 2 (3%) | 2 (3%) |
| 4 | 18 (24%) | 4 (5%) | 4 (5%) |
| 5 | 18 (24%) | 12 (16%) | 12 (16%) |
| 6 | 13 (17%) | 15 (20%) | 14 (19%) |
| Perfect (7) | 7 (10%) | 42 (56%) | 43 (57%) |
WHO=World health organization; IECC=International endocervical adenocarcinoma criteria and classification; HPVA= HPV-related adenocarcinoma; NHPVA=HPV-unrelated adenocarcinoma; EA-NOS=Endocervical adenocarcinoma, no otherwise specified
Table 5 shows interobserver agreement among HPVA morphologic categories as per the WHO classification. Reproducibility among HPVA subtypes was poor and did not improve significantly with IHC. Poor reproducibility was also observed in exceedingly rare NHPVA categories (endometrioid and serous carcinoma) as shown in Table 6. Conversely, gastric, clear cell and mesonephric types showed good reproducibility both on H&E and after IHC review.
Table 5.
Interobserver agreement (Kappa) among WHO HPVA subcategories
| H&E only* | H&E + IHC* | |
|---|---|---|
| Usual | 0.36 (0.31–0.41) | 0.38 (0.33–0.43) |
| Villoglandular | 0.22 (0.17–0.27) | 0.22 (0.17–0.27) |
| Mucinous NOS | 0.13 (0.1–0.18) | 0.14 (0.09–0.19) |
| Intestinal | 0.32 (0.27–0.37) | 0.29 (0.24–0.34) |
| Signet ring cell | 0.08 (0.03–0.13) | 0.08 (0.03–0.13) |
| Stratified mucin producing | 0.28 (0.23–0.33) | 0.26 (0.21–0.31) |
Mean (95% confidence interval)
Table 6.
Interobserver agreement (Kappa) among NHPVA categories
| H&E only* | H&E + IHC* | |
|---|---|---|
| Gastric | 0.63 (0.58–0.68) | 0.76 (0.71–0.81) |
| Clear cell | 0.81 (0.76–0.86) | 0.5 (0.45–0.56) |
| Mesonephric | 0.5 (0.45–0.55) | 0.5 (0.45–0.55) |
| Serous | −0.008 (−0.06–0.04) | 0.06 (0.007–0.11) |
| Endometrioid | 0.012 (−0.06–0.04) | −0.012 (−0.06–0.04) |
Mean (95% confidence interval)
Interobserver agreement for both classification systems was evaluated in biopsy and resection specimens separately. In biopsy material, the IECC showed higher concordance than the WHO (K= 0.41 versus K=0.23, respectively). However, agreement in biopsies was lower compared to that of excision specimens for both systems (K=0.43 for IECC, K=0.34 for WHO).
IECC / HPV status correlation and immunohistochemical data
In order to determine the value of IECC criteria in predicting the HPV status, the majority diagnosis (≥4 reviewers) for each tumor was correlated with HPV ISH and PCR results. There was near perfect correlation between IECC diagnosis and HPV status: all 64 HPV-positive tumors were classified as HPVA, whereas the 9 of the 11 EAs negative for HPV testing were classified into one of the HPV-negative categories. The two remaining cases negative for HPV testing were classified as EA-NOS by consensus. p16 was overexpressed in 58 (92%) of the 63 HPVAs with p16 IHC available, while all 8 NHPVAs that were tested for p16 showed either negative or patchy p16 staining.
Immunohistochemical results are displayed graphically in Figure 4. p53 was overexpressed in 4% of tested EA (3/73, 2 of which had majority agreement for NHPVA). PR staining of any intensity was detected in 13% of tested tumors (9/72), ranging from 5–90% positively stained nuclei. Vimentin expression (5–100%) was detected in 14% of tested EA (10/71). Staining was membranous in all positive cases; a basolateral staining pattern was not observed. CDX2 staining (5 to 100%) was seen across the majority of tested tumors (59%, 40/68), while GATA3 expression (5 to 100%) was detected in 43% tumors (30/69). Napsin-A (any staining) was seen in 6% of tested tumors (4/70). Tissue microarray p16 and p53 results were concordant with archival whole tissue section immunohistochemical stains in all 12 cases with archival stains available. The addition of IHC data resulted in a change of diagnosis by at least one reviewer in 18 out of 73 cases (25%); but only in 22% (4/18) of them the diagnosis was changed by ≥ 2 reviewers. In the remaining 55 EAs (75%), review of IHC data resulted in no change of H&E diagnosis.
Figure 4.
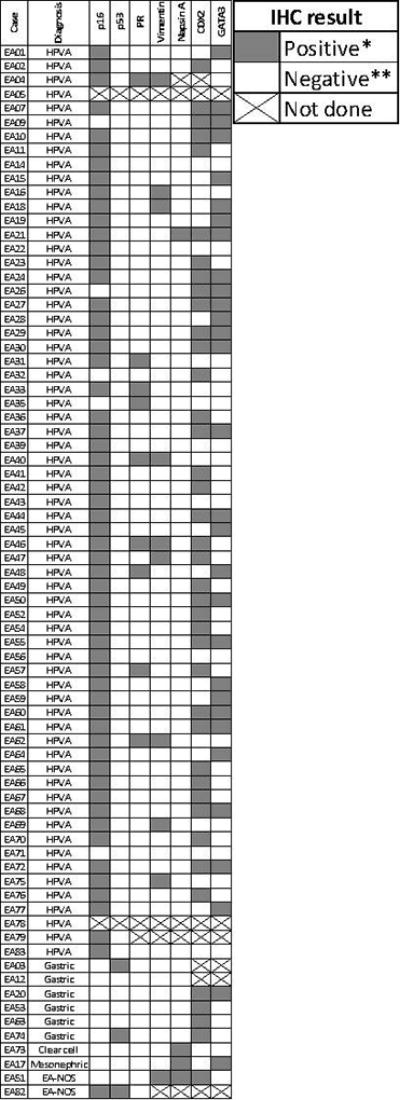
Diagram illustrating consensus IECC diagnosis and immunohistochemical results. *For p16 and p53, positive means overexpressed as defined in text. **For p16, negative means patchy or absent expression; for p53, negative means wild type expression as defined in text.
DISCUSSION
To the best of our knowledge, this is the first study to explore interobserver reproducibility of the traditional WHO and novel IECC systems in the classification of EA. Despite the broad spectrum of histologic subtypes within EA, classification by the IECC demonstrated higher levels of concordance. Our results validate the IECC as a more reproducible approach to classify EA compared to WHO, showing better Fleiss’ kappa values and higher proportion of cases with perfect or near perfect agreement among reviewers. Importantly, agreement improved when a selected panel of IHC was used to add in interpretation, as previously demonstrated in the seminal study describing the IECC17.
Distinction between HPVA and NHPVA is important given the differences in their pathogenesis and clinical behavior9,28–32. The latter in particular applies to gastric-type EA, which has significantly higher rates of adverse clinical features and outcome compared to HPVA30. Moreover, different treatment algorithms have been developed in other organ systems where tumors are dichotomized based on HPV status15,16,33 and it is conceivable that such an approach could also be implemented in EA for treatment purposes. We demonstrate that distinction between HPVA and NHPVA, the first step in the IECC categorization, has good levels of interobserver agreement among pathologists. Furthermore, the majority HPVA or NHPVA IECC diagnosis in each case had perfect correlation with HPV detection results, which were not provided to the reviewers. This observation further validates the IECC as an accurate estimator of HPV status and EA subtype.
The current WHO classification contains several morphologic categories within the spectrum of HPV-related endocervical glandular neoplasia. These categories include usual type (mucin depleted, intracellular mucin in <50% of tumor cells), mucinous NOS (intracellular mucin in ≥50% of tumor cells), intestinal type and villoglandular carcinoma. All these tumor types display the distinctive features of HPVA defined in the IECC, namely easily identifiable apical mitoses and apoptotic bodies. An additional HPVA variant not included in WHO is the recently described invasive stratified mucin-producing carcinoma24. As initially conceived, the IECC allows for the subdivision of HPVA into the above WHO categories, mostly to provide consistency with the pre-existing WHO classification system10. However, the present study shows that such sub-categorization of HPVA suffers from poor reproducibility. In fact, the high interobserver variability in the diagnosis of HPVA morphologic subtypes largely explains the overall lower reproducibility of the WHO classification. Our findings question the value of sub-categorization of HPVA, which should be addressed in future large clinical studies.
Among NHPVA, clinically important types have overall good reproducibility (gastric, clear cell and mesonephric). These types are rare, but can behave aggressively if presenting at advanced stage34,35. Our study shows that the diagnosis of these biologically and clinically relevant entities is reproducible. Endometrioid and serous EA, on the other hand, showed poor interobserver agreement. This can be explained by the rarity and arguable non-existence of these tumor types in the cervix. As per IECC, the term endometrioid carcinoma is reserved for tumors with low-grade endometrioid glands and confirmatory endometrioid features (squamous metaplasia or endometriosis). Under this definition, endometrioid carcinoma is rare, representing ~1% of cervical adenocarcinomas17. Primary cervical serous carcinoma has been rarely described in the literature36,37; nonetheless, it has been postulated that serous carcinoma, when strictly defined, does not occur in the cervix38,39. When considering this diagnosis, endometrial and/or tubo-ovarian primary tumors must be rigorously excluded. Further exploration of the arguable existence of primary cervical serous carcinoma requires additional patient cohort studies.
The use of a targeted immunohistochemical panel improved interobserver agreement in diagnosis. However, it is important to note that closer observation to rates of change in diagnosis based on IHC data showed that IHC more frequently confirmed, rather than changed, the initial interpretation made on morphologic grounds. The immunohistochemical markers used in this study are commonly used in the workup of lesions of the gynecologic tract including of the uterine cervix40,41, and are likely to be available in most pathology laboratories.
p16 immunohistochemistry showed high correlation with HPV results in our study. p16 overexpression in EA is mostly due to the oncogenic effects of HPV42,43; of note, loss of heterozygosity and methylation have been shown to result in loss of p16 staining even in HPV positive tumors44,45. None of the tumors classified as NHPVA showed p16 overexpression. We also explored the role of GATA3, CDX2 and Napsin-A in improving interobserver agreement, in addition to the initial panel of p16, p53, PR and vimentin suggested by Stolnicu et al17. We found significant rates of expression for CDX2 and GATA3 irrespective of EA type in our cohort, indicating the limited value of these markers. For example, positivity for GATA3, a sensitive and specific marker of both benign and malignant mesonephric lesions in the lower female genital tract46, was detected in over 40% of our cohort, but only 1 case resulted in a majority diagnosis (4 reviewers) of mesonephric carcinoma.
In the original IECC study, 2.4% of EA were unclassifiable, i.e. EA-NOS17. A similar proportion was found in our cohort (2 EAs, 3%). It was reported that this group of tumors contain both HPVA and NHPVA, based on immunophenotype and HPV ISH status17. In our study, the 2 EAs with majority classification as EA-NOS were HPV negative by ISH. Interestingly, of the 18 tumors where at least one reviewer changed the original diagnosis after IHC evaluation, 4 (22%) were changed from a HPVA or NHPVA diagnosis to EA-NOS. This finding is in keeping with previous observations recommending caution in the use of immunohistochemistry, in particular p16 which can be overexpressed in NHPVA and occasionally be patchy or negative in HPVA, as seen in our cohort. As previously recommended, a tumor classified as EA-NOS may benefit from HPV ISH testing in the clinical setting17.
Biopsy specimens are often poorly represented in reproducibility studies. The standard treatment of locally advanced cervical cancer, commonly defined as stage IB2 to IVA, is upfront chemoradiation, i.e. primary surgery is usually not indicated47–51. For this reason, biopsy material is often the only tissue available from these patients. We included a subset of EAs represented in biopsy material only to assess reviewer agreement in this subgroup. As might be expected, overall agreement based on biopsy evaluation is lower compared to that achieved upon examination of excision material. This finding highlights the inherent limitation of accurate diagnosis in biopsy specimens and the need for reliable biomarkers for different EA subtypes that can be used in the evaluation of scant tumor material.
A potential limitation of our study is that all of the cases originated from a single institution; however, this institution is a tertiary care center for gynecologic oncology that provides care to a wide spectrum of referral patients in the largest metropolitan area in Canada. We also recognize that our reviewers are all experts in gynecologic pathology, and thus the generalization of our results in the wider pathology community needs to be explored.
Future directions in this field include the search for reliable biomarkers for major EA histologic types and eventually, the development of a combined histologic-molecular-based classification for EA. While the reproducibility of IECC is superior to the current WHO, none of the comparisons made in our study reached perfect Kappa values, indicating a clear need for better criteria and ancillary tools to further optimize the reproducibility of the IECC. In this regard, molecular testing is a promising tool. Recent insights into the genomic landscape of HPVA show that prevalent mutations (KRAS, PIK3CA) correlate with morphology52 and have prognostic as well as therapeutic implications, suggesting a potential genomic role in the ultimate classification of EA. Finally, the IECC may benefit from the incorporation of other clinically useful systems such as the pattern-based classification for HPVA53. This classification provides useful prognostic information and can complement or even replace the current morphologic subtypes of HPVA outlined in the WHO.
In summary, the recently described IECC is a more reproducible system to categorize EA compared to the current WHO classification. As defined, the morphologic definitions provided by IECC allow for higher interobserver agreement and have excellent correlation with tumor HPV status. Our results support previous recommendations to replace the WHO classification with the IECC. The need remains to improve the value and reproducibility of the classification by identifying sensitive, specific and biologically significant biomarkers of endocervical glandular neoplasia.
Acknowledgments
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748 (KJ Park and RA Soslow).
Footnotes
Conflicts of interest and sources of funding: None to declare.
References
- 1.Pirog EC, Kleter B, Olgac S, et al. Prevalence of Human Papillomavirus DNA in Different Histological Subtypes of Cervical Adenocarcinoma. Am J Pathol. 2000;157:1055–1062. doi: 10.1016/S0002-9440(10)64619-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pirog EC, Lloveras B, Molijn A, et al. HPV prevalence and genotypes in different histological subtypes of cervical adenocarcinoma, a worldwide analysis of 760 cases. Mod Pathol. 2014;27:1559–1567. doi: 10.1038/modpathol.2014.55. [DOI] [PubMed] [Google Scholar]
- 3.Holl K, Nowakowski AM, Powell N, et al. Human papillomavirus prevalence and type-distribution in cervical glandular neoplasias: Results from a European multinational epidemiological study. Int J Cancer. 2015;137:2858–2868. doi: 10.1002/ijc.29651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An HJ, Kim KR, Kim IS, et al. Prevalence of human papillomavirus DNA in various histological subtypes of cervical adenocarcinoma: a population-based study. Mod Pathol. 2005;18:528–534. doi: 10.1038/modpathol.3800316. [DOI] [PubMed] [Google Scholar]
- 5.Park KJ, Kiyokawa T, Soslow RA, et al. Unusual endocervical adenocarcinomas: an immunohistochemical analysis with molecular detection of human papillomavirus. Am J Surg Pathol. 2011;35:633–646. doi: 10.1097/PAS.0b013e31821534b9. [DOI] [PubMed] [Google Scholar]
- 6.Houghton O, Jamison J, Wilson R, et al. p16 Immunoreactivity in unusual types of cervical adenocarcinoma does not reflect human papillomavirus infection. Histopathology. 2010;57:342–350. doi: 10.1111/j.1365-2559.2010.03632.x. [DOI] [PubMed] [Google Scholar]
- 7.Kusanagi Y, Kojima A, Mikami Y, et al. Absence of high-risk human papillomavirus (HPV) detection in endocervical adenocarcinoma with gastric morphology and phenotype. Am J Pathol. 2010;177:2169–2175. doi: 10.2353/ajpath.2010.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molijn A, Jenkins D, Chen W, et al. The complex relationship between human papillomavirus and cervical adenocarcinoma. Int J Cancer. 2016;138:409–416. doi: 10.1002/ijc.29722. [DOI] [PubMed] [Google Scholar]
- 9.Kojima A, Mikami Y, Sudo T, et al. Gastric morphology and immunophenotype predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am J Surg Pathol. 2007;31:664–672. doi: 10.1097/01.pas.0000213434.91868.b0. [DOI] [PubMed] [Google Scholar]
- 10.Kurman R, Carcangiu M, Herrington C, et al. WHO Classification of Tumours of Female Reproductive Organs. 4. WHO Press; 2014. [Google Scholar]
- 11.McAlpine JN, Leung SCY, Cheng A, et al. Human papillomavirus (HPV)-independent vulvar squamous cell carcinoma has a worse prognosis than HPV-associated disease: a retrospective cohort study. Histopathology. 2017;71:238–246. doi: 10.1111/his.13205. [DOI] [PubMed] [Google Scholar]
- 12.Dong F, Kojiro S, Borger DR, et al. Squamous Cell Carcinoma of the Vulva: A Subclassification of 97 Cases by Clinicopathologic, Immunohistochemical, and Molecular Features (p16, p53, and EGFR) Am J Surg Pathol. 2015;39:1045–1053. doi: 10.1097/PAS.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 13.McAlpine JN, Kim SY, Akbari A, et al. HPV-independent Differentiated Vulvar Intraepithelial Neoplasia (dVIN) is Associated With an Aggressive Clinical Course. Int J Gynecol Pathol. 2017;36:507–516. doi: 10.1097/PGP.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 14.Hoang LN, Park KJ, Soslow RA, et al. Squamous precursor lesions of the vulva: current classification and diagnostic challenges. Pathology. 2016;48:291–302. doi: 10.1016/j.pathol.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ajila V, Shetty H, Babu S, et al. Human Papilloma Virus Associated Squamous Cell Carcinoma of the Head and Neck. J Sex Transm Dis. 2015;2015 doi: 10.1155/2015/791024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sedghizadeh PP, Billington WD, Paxton D, et al. Is p16-positive oropharyngeal squamous cell carcinoma associated with favorable prognosis? A systematic review and meta-analysis. Oral Oncol. 2016;54:15–27. doi: 10.1016/j.oraloncology.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Stolnicu S, Barsan I, Hoang L, et al. International Endocervical Adenocarcinoma Criteria and Classification (IECC): A New Pathogenetic Classification for Invasive Adenocarcinomas of the Endocervix. Am J Surg Pathol. 2018;42:214–226. doi: 10.1097/PAS.0000000000000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh N, Gilks CB. The changing landscape of gynaecological cancer diagnosis: implications for histopathological practice in the 21st century. Histopathology. 2017;70:56–69. doi: 10.1111/his.13080. [DOI] [PubMed] [Google Scholar]
- 19.Nelson HH, Pawlita M, Michaud DS, et al. Immune Response to HPV16 E6 and E7 Proteins and Patient Outcomes in Head and Neck Cancer. JAMA Oncol. 2017;3:178–185. doi: 10.1001/jamaoncol.2016.4500. [DOI] [PubMed] [Google Scholar]
- 20.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 21.Lim D, Murali R, Murray MP, et al. Morphological and Immunohistochemical Reevaluation of Tumors Initially Diagnosed as Ovarian Endometrioid Carcinoma With Emphasis on High-grade Tumors. Am J Surg Pathol. 2016;40:302–312. doi: 10.1097/PAS.0000000000000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parra-Herran C, Taljaard M, Djordjevic B, et al. Pattern-based classification of invasive endocervical adenocarcinoma, depth of invasion measurement and distinction from adenocarcinoma in situ: interobserver variation among gynecologic pathologists. Mod Pathol. 2016;29:879–892. doi: 10.1038/modpathol.2016.86. [DOI] [PubMed] [Google Scholar]
- 23.Douglas G, Howitt BE, Schoolmeester JK, et al. Architectural overlap between benign endocervix and pattern-A endocervical adenocarcinoma: Are all pattern-A tumors invasive? Pathol Res Pract. 2017;213:799–803. doi: 10.1016/j.prp.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Lastra RR, Park KJ, Schoolmeester JK. Invasive Stratified Mucin-producing Carcinoma and Stratified Mucin-producing Intraepithelial Lesion (SMILE): 15 Cases Presenting a Spectrum of Cervical Neoplasia With Description of a Distinctive Variant of Invasive Adenocarcinoma. Am J Surg Pathol. 2016;40:262–269. doi: 10.1097/PAS.0000000000000543. [DOI] [PubMed] [Google Scholar]
- 25.Park JJ, Sun D, Quade BJ, et al. Stratified mucin-producing intraepithelial lesions of the cervix: adenosquamous or columnar cell neoplasia? Am J Surg Pathol. 2000;24:1414–1419. doi: 10.1097/00000478-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Evans MF, Peng Z, Clark KM, et al. HPV E6/E7 RNA in situ hybridization signal patterns as biomarkers of three-tier cervical intraepithelial neoplasia grade. PloS One. 2014;9:e91142. doi: 10.1371/journal.pone.0091142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleiss JL. Statistical methods for rates and proportions. 2. New York: John Wiley; 1981. [Google Scholar]
- 28.Higgins GD, Davy M, Roder D, et al. Increased age and mortality associated with cervical carcinomas negative for human papillomavirus RNA. Lancet. 1991;338:910–913. doi: 10.1016/0140-6736(91)91773-n. [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez-Carunchio L, Soveral I, Steenbergen RDM, et al. HPV-negative carcinoma of the uterine cervix: a distinct type of cervical cancer with poor prognosis. BJOG. 2015;122:119–127. doi: 10.1111/1471-0528.13071. [DOI] [PubMed] [Google Scholar]
- 30.Karamurzin YS, Kiyokawa T, Parkash V, et al. Gastric-type Endocervical Adenocarcinoma: An Aggressive Tumor With Unusual Metastatic Patterns and Poor Prognosis. Am J Surg Pathol. 2015;39:1449–1457. doi: 10.1097/PAS.0000000000000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li P, Tan Y, Zhu L-X, et al. Prognostic value of HPV DNA status in cervical cancer before treatment: a systematic review and meta-analysis. Oncotarget. 2017;8:66352–66359. doi: 10.18632/oncotarget.18558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodgson A, Park KJ, Djordjevic B, et al. International Classification of Endocervical Adenocarcinoma: validation and interobserver reproducibility. Mod Pathol. 2018;31 doi: 10.1097/PAS.0000000000001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kofler B, Laban S, Busch CJ, et al. New treatment strategies for HPV-positive head and neck cancer. Eur Arch Oto-Rhino-Laryngol. 2014;271:1861–1867. doi: 10.1007/s00405-013-2603-0. [DOI] [PubMed] [Google Scholar]
- 34.Thomas MB, Wright JD, Leiser AL, et al. Clear cell carcinoma of the cervix: a multi-institutional review in the post-DES era. Gynecol Oncol. 2008;109:335–339. doi: 10.1016/j.ygyno.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silver SA, Devouassoux-Shisheboran M, Mezzetti TP, et al. Mesonephric adenocarcinomas of the uterine cervix: a study of 11 cases with immunohistochemical findings. Am J Surg Pathol. 2001;25:379–387. doi: 10.1097/00000478-200103000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Gilks CB, Clement PB. Papillary serous adenocarcinoma of the uterine cervix: a report of three cases. Mod Pathol. 1992;5:426–431. [PubMed] [Google Scholar]
- 37.Nofech-Mozes S, Rasty G, Ismiil N, et al. Immunohistochemical characterization of endocervical papillary serous carcinoma. Int J Gynecol Cancer. 2006;16(Suppl 1):286–292. doi: 10.1111/j.1525-1438.2006.00514.x. [DOI] [PubMed] [Google Scholar]
- 38.McCluggage WG, Hurrell DP, Kennedy K. Metastatic carcinomas in the cervix mimicking primary cervical adenocarcinoma and adenocarcinoma in situ: report of a series of cases. Am J Surg Pathol. 2010;34:735–741. doi: 10.1097/PAS.0b013e3181d6b8fd. [DOI] [PubMed] [Google Scholar]
- 39.Kos Z, Broaddus RR, Djordjevic B. Fallopian tube high-grade serous carcinoma with intramucosal spread and presenting as a malignancy on pap smear. Int J Gynecol Pathol. 2014;33:443–448. doi: 10.1097/PGP.0b013e31829c728b. [DOI] [PubMed] [Google Scholar]
- 40.Buza N, Hui P. Immunohistochemistry in Gynecologic Pathology: An Example-Based Practical Update. Arch Pathol Lab Med. 2017;141:1052–1071. doi: 10.5858/arpa.2016-0541-RA. [DOI] [PubMed] [Google Scholar]
- 41.Mittal K, Soslow R, McCluggage WG. Application of immunohistochemistry to gynecologic pathology. Arch Pathol Lab Med. 2008;132:402–423. doi: 10.5858/2008-132-402-AOITGP. [DOI] [PubMed] [Google Scholar]
- 42.Klaes R, Friedrich T, Spitkovsky D, et al. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92:276–284. doi: 10.1002/ijc.1174. [DOI] [PubMed] [Google Scholar]
- 43.Nam EJ, Kim JW, Kim SW, et al. The expressions of the Rb pathway in cervical intraepithelial neoplasia; predictive and prognostic significance. Gynecol Oncol. 2007;104:207–211. doi: 10.1016/j.ygyno.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 44.Nuovo GJ, Plaia TW, Belinsky SA, et al. In situ detection of the hypermethylation-induced inactivation of the p16 gene as an early event in oncogenesis. Proc Natl Acad Sci U S A. 1999;96:12754–12759. doi: 10.1073/pnas.96.22.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poetsch M, Hemmerich M, Kakies C, et al. Alterations in the tumor suppressor gene p16(INK4A) are associated with aggressive behavior of penile carcinomas. Virchows Arch. 2011;458:221–229. doi: 10.1007/s00428-010-1007-4. [DOI] [PubMed] [Google Scholar]
- 46.Howitt BE, Emori MM, Drapkin R, et al. GATA3 Is a Sensitive and Specific Marker of Benign and Malignant Mesonephric Lesions in the Lower Female Genital Tract. Am J Surg Pathol. 2015;39:1411–1419. doi: 10.1097/PAS.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 47.Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–1161. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 48.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 49.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 50.Whitney CW, Sause W, Bundy BN, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17:1339–1348. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 51.Peters WA, Liu PY, Barrett RJ, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 52.Hodgson A, Amemiya Y, Seth A, et al. Genomic abnormalities in invasive endocervical adenocarcinoma correlate with pattern of invasion: biologic and clinical implications. Mod Pathol. 2017;30:1633–1641. doi: 10.1038/modpathol.2017.80. [DOI] [PubMed] [Google Scholar]
- 53.Park KJ, Roma AA. Pattern based classification of endocervical adenocarcinoma: a review. Pathology. 2017 doi: 10.1016/j.pathol.2017.09.011. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]