Abstract
Background
People living with HIV (PLWH) commonly have low bone mineral density (BMD) (low bone mass and osteoporosis) and are at high risk for fractures. Fractures and low BMD are significant causes of morbidity and mortality, increasingly relevant as PLWH age. Alcohol use is common among PLWH and known to affect bone health. The association between alcohol use and changes in BMD among PLWH is not well understood.
Methods
We conducted a 3.5-year prospective cohort study of 250 PLWH with substance use disorder or ever injection drug use. Annual alcohol consumption was measured as a mean of grams per day of alcohol, mean number of heavy drinking days per month, mean number of days abstinent per month, and any heavy drinking, using the 30-day Timeline Followback method twice each year. The primary outcome was annual change in BMD measured each year by dual energy x-ray absorptiometry in grams per square centimeter (g/cm2) at the femoral neck. Additional dependent variables included annual change in total hip and lumbar spine BMD, > 6% annual decrease in BMD at any site, and incident fractures in the past year. Regression models adjusted for relevant covariates.
Results
The median age of participants was 50 years old. The median duration of HIV infection was 16.5 years and the mean time since ART initiation was 12.3 years. At study entry, 67% of participants met criteria for low BMD (46% low bone mass, 21% osteoporosis). Median follow-up was 24 months. We found no significant associations between any measure of alcohol consumption and changes in BMD (g/cm2) at the femoral neck (adjusted β for grams/day of alcohol= −0.0032, p=0.7487), total hip, or lumbar spine. There was no significant association between any measure of alcohol consumption and >6% annual decrease in BMD at any site, or incident fractures.
Conclusion
In this sample of PLWH and substance use disorders or ever injection drug use, we detected no association between any of the alcohol measures used in the study and changes in BMD or incident fractures.
Keywords: HIV, alcohol, bone density, osteoporosis, fracture
1. INTRODUCTION
People living with HIV (PLWH) commonly have low bone mineral density (BMD) (low bone mass (formerly referred to as osteopenia) and osteoporosis), and are at high risk for fractures (Ventura et al., 2017; Brown and Qaqush, 2006). Fractures are substantial causes of morbidity and mortality in general and are becoming more relevant to PLWH as this population continues to age (Hansen et al., 2012; Womack et al., 2011; Arnsten et al., 2007). PLWH have some of the same risk factors for low BMD as do those without HIV infection, but they also have additional risks, such as HIV infection itself and its treatments, both shown to independently impact bone density (Cotter et al., 2014; Walker Harris and Brown, 2012). In addition, those with HIV infection are more likely to have other risk factors such as: comorbid conditions that impact bone health, such as hepatitis C infection, secondary hyperparathyroidism, smoking, low body mass index, weight loss, and chronic kidney disease (Mondy et al., 2003; Casado et al., 2014).
Substance use is common among PLWH and alcohol, cocaine and opioid use have all been associated with low BMD (McComsey et al., 2010; Sharma et al., 2015; Kim et al., 2006). Alcohol has been associated with decreased bone formation, particularly heavy drinking (Chakkalakal, 2005; Friday and Howard, 1991). Some studies have found that low amounts of alcohol have been associated with higher bone density but the results of these studies have been inconsistent and inconclusive (Cawthon et al., 2006; Tucker et al., 2009; Feskanich et al., 1999). Such studies have often had limited measurement of alcohol consumption (Berg et al., 2008). Thus, it has not been clear whether associations between alcohol use and bone density were directly due to alcohol consumption or to concomitant risk.
We reported results among PLWH from a cross-sectional study of the association between recent and lifetime alcohol use and bone mineral density; although there were no significant associations between lifetime use and BMD, recent alcohol use was associated with lower BMD (Ventura et al., 2017). However, neither that analysis, nor other extant studies have adequately evaluated the association between alcohol consumption and change in bone density and incident fractures among PLWH. Therefore, using validated measures of alcohol consumption, in a population of PLWH and current substance use disorder or ever injection drug use, we prospectively studied the associations between alcohol consumption and both change in BMD and incident fractures adjusting for potential confounders. We hypothesized that greater alcohol consumption would be associated with decreased BMD and more incident fractures. Such information is important for informing patients and clinicians regarding alcohol use and low BMD risks.
2. MATERIALS AND METHODS
2.1 Participants
Participants were enrolled in the Boston ARCH prospective cohort study examining the effect of alcohol on bone health in PLWH with substance use disorder or ever injection drug use. Research participants were recruited from an urban academic HIV primary care clinic based in a hospital and a community health center serving homeless patients from December 2012 to November 2014. Participants were recruited over a two-year period and had varying maximum planned observation periods given the fixed maximum of 3.5 years of involvement.
Inclusion criteria were: (1) confirmed documentation of HIV infection in any medical record (Massachusetts Department of Public Health algorithm) or HIV viral load >10,000 copies/mL; (2) past 12-month Diagnostic and Statistical Manual of Mental Disorders 4th edition drug or alcohol use dependence by Mini International Neuropsychiatric Interview (M.I.N.I. 6.0) (Sheehan et al., 2010) or ever injection drug use; (3) ability to speak English; (4) ≥18 years old; (5) willingness to provide contact information for 1 or more person likely to know the participant’s whereabouts. Exclusion criteria were: pregnancy, determined by urine test, and plans to leave Boston in the next year. This study population was described in a cross-sectional analysis reported previously that focused on the relationship between recent and lifetime drinking and bone mineral density based on data at study entry only (Ventura et al. 2017). Due to the longitudinal nature of the current study, an additional inclusion criterion specific to the analyses reported herein was all participants had to have BMD measured by DXA at the same bone site 2 or more times during the study period.
Participants provided written informed consent for the study and they were compensated for each visit. The Boston University Medical Campus Institutional Review Board approved the study. The National Institute on Alcohol Abuse and Alcoholism (NIAAA) further protected participants with a Certificate of Confidentiality. Additionally, the US Department of Health and Human Services approved the follow up of incarcerated participants.
2.2 Assessments of Bone Density, Fractures, and Alcohol Use
Bone density measurements were performed at study entry and at up to two annual follow up visits. As described in Ventura et al. (2017), all measurements were performed by bone densitometry technologists certified by the International Society for Clinical Densitometry. The DXA machines used were: (1) Hologic QDR 4500 Discovery W (software version 12.6.1) (Waltham, MA, USA); (2) Hologic QDR 4500 Discovery W (software version 13.4.2) (Waltham, MA, USA); and (3) General Electric (GE) Lunar iDXA (GE Healthcare, Madison, WI, USA). The two Hologic DXAs (hereafter referred to as Hologic 1 and Hologic 2) were cross-calibrated using a standard phantom. All BMD measurements taken from the GE Lunar iDXA (hereafter referred to as iDXA) were converted to Hologic-equivalent values by applying industry-accepted conversion formulas (Wilson, 2011). Fractures were assessed by self-report at study entry and at 12-months follow-up (“In the past 12 months, have you fractured or broken a bone?”).
We examined alcohol exposure over a one-year period to match the time period assessed by DXA. Daily alcohol use was assessed at study entry, 6, 12, 18 and 24 months, using the 30-day Timeline Followback (TLFB, a validated interview calendar method for measuring past month daily alcohol use) (Sobell and Sobell, 1992).
2.3 Additional Assessments
We assessed the following at study entry by interview: age, biological sex, race/ethnicity, duration of HIV infection, lifetime drinking (total volume) (Skinner and Sheu, 1982), and years of regular cocaine use (McLellan et al., 1980). Additionally, we assessed tenofovir use, menopause, ever injection drug use, current tobacco use (Heatherton, et al., 1991), daily calcium intake (Sebring et al., 2007), weight-bearing physical activity (Marshall et al. 2005), recent (30-day) cocaine use (McLellan et al., 1980), recent illicit opioid use (McLellan et al., 1980), and current ART use. Number of days of cocaine use in past 30 days, and number of days of illicit opioid use (including prescription opioids and non-prescription opioids) in past 30 days were assessed using the Addiction Severity Index (McLellan et al., 1980). Menopause was evaluated using the following question: “In the past year, have you had menopause (at least 12 months since your last period)?”
At the baseline visit, each study participant’s medical record was reviewed for the most recent HIV viral load (copies/mL), and the most recent CD4 cell count (cells/mm3). If unavailable within three months before baseline, CD4 and HIV viral load were tested. Blood was additionally tested for total 25-hydroxy-vitamin D [25(OH)D] by liquid chromatography-tandem mass spectrometry. Height and weight were recorded during the study visit, and used to calculate body mass index (BMI).
Medications and conditions that can affect bone mineral density (e.g. including secondary causes of osteoporosis) were collected from the electronic medical record. Medications that decrease bone mineral density include: glucocorticoids (oral and inhaled), antiasthma (only budesonide/formoterol, fluticasone/salmeterol), anti-inflammatory (inhaled, including beclomethasone dipropionate, budesonide, ciclesonide, flunisolide, fluticasone, mometasone), anticonvulsants (only divalproex, phenytoin, carbamazepine), chemotherapeutic agents, anticoagulants, and depo-provera (Van Staa et al. 2003; Chee et al. 2014; Drummond et al. 2008; Lee et al. 2010; Brufsky et al. 2008; Rey-Sanchez et al. 2011; Gbolade 2002). Medications that increase bone mineral density include: denosumab, progestin only, contraceptive (systemic, levonorgesterel), bisphosphonates, androgen/anabolics (testosterone, topical or transdermal), and estrogen (transdermal, oral) (Cummings et al. 2009; Caird et al. 1994; Munk-Jensen et al. 1988; Russell et al. 2011; Rodriguez-Tolra et al. 2013; Manolagas et al. 2002).
Conditions (ICD-9 codes) included primary hyperparathyroidism (252.01, 252.0), secondary, tertiary, unspecified, or ectopic hyperparathyroidism (252.00, 252.02, 252.08, 259.3, 252.0), Cushing’s syndrome (255), hyperthyroidism, Graves’ disease, toxic goiter (242.00, 242.0–242.4, 242.8, 242.90), male hypogonadism (257.1–257.2), premature menopause (256.2, 256.31, 256.39, 626.0, 627.4), hyperprolactinemia (253.1), acromegaly (253), panhypopituitarism (253.2), osteomalacia, hypovitaminosis D (268.0–268.9), celiac disease, sprue (579.0–579.1), malnutrition, malabsorption (263.0, 263.1, 263.9, 579.3, 579.8, 579.9), Crohn’s disease (555.0–555.9), ulcerative colitis (556.0–556.9), anorexia, bulimia nervosa (307.1, 307.51), primary biliary cirrhosis (571.6) chronic liver disease, cirrhosis (571.9), hemochromatosis (275), chronic kidney disease (585.0–585.6, 585.9), renal hyperparathyroidism (588.81, 588.8), renal osteodystrophy (588), hypercalciuria (275.4), renal tubular acidosis (588.88, 588.89), hypocalcemia (275.41), hypercalcemia (275.42), rheumatoid arthritis (714), systemic lupus erythematosus (273.1), monoclonal gammopathy of undetermined significance (273.1), multiple myeloma (203.00–203.01), mastocytosis (202.6, 757.33), leukemia (208.00–208.91), lymphoma (202.80–202.88), sickle cell anemia (282.60–282.69), thalassemia (282.4, 282.49), hemophilia (286), amyloidosis (277.3), hypophosphatasia (275.3), mastocytosis (757.33), gonadal dysgenesis, Turner’s, XO (758.6), Kleinfelter’s syndrome (758.7), pseudo/pseudohypoparathyroidism, nephrocalcinosis (275.49), cystic fibrosis (277.00–277.09), immobilization >3 months (780.72), previous organ transplant (V42.0–V42.9), hypervitaminosis A (278.2), aluminum toxicity (973.0 + E858.4), osteogenesis imperfect (756.51), Ehlers–Danlos syndrome (756.83), Marfan’s syndrome (759.82), Gaucher’s disease (272.7), homocystinuria (270.4), and hypophosphatasia (275.3) (McKiernan et al. 2011). Procedure codes for gastrectomy (43620–43634, 43638, 43639), small bowel resection (44120–44128, 44202, 44203), and bariatric surgery (43644, 43645, 43659, 43842–43848) were also included (McKiernan et al. 2011).
2.4 Primary Outcome Variables
The primary outcome variable was annual change in BMD (g/cm2) measured by dual energy x-ray absorptiometry (DXA) at the femoral neck.
2.5 Secondary Outcome Variable
Secondary outcomes were annual change in 1) total hip and 2) lumbar spine BMD 3) > 6% annual decrease in BMD at any site (Binkley et al., 2016; Stellbrink et al., 2010) and 4) any fracture in the past year. Among the 187 participants without osteoporosis at study entry, only 5 newly met criteria at a subsequent assessment, thus we did not analyze incident osteoporosis as a secondary outcome.
2.6 Main independent variable
The main independent variable was mean grams per day of alcohol consumption over the previous year. Mean alcohol consumption was calculated by averaging daily alcohol consumption reported at 3 study interviews—for year 1, baseline, 6 month and 12 month time points; and for year 2, 12 month, 18 month and 24 month time points. For year 1, 98% had 3 observations and 2 participants were missing one observation. Fewer participants were followed through year two because the cohort study period ended as planned. However, for year two among those with planned follow-up, 98% had 3 observations and 2 participants were missing one observation.
2.7 Additional independent variables
In addition to mean daily alcohol consumption, the following alcohol measures were calculated: (1) mean number of heavy drinking days per month, based on National Institute on Alcohol and Alcohol Abuse (NIAAA) heavy drinking day threshold: ≥5 drinks for males and ≥4 drinks for females) (2) mean number of days abstinent per month (3) any heavy drinking. Past 30-day drinking (assessed every 6 months) was assumed to be the exposure until the next time point for which data were available.
We also defined categories of alcohol use by drinks (0 – <0.7 drinks/day, 0.7 – 1.7 drinks/day, >1.7 drinks/day) (Berg et al., 2008). Categories based on NIAAA standard thresholds for risky drinking were also developed: (1) Abstinence: no drinking in past 30 days (2) Not heavy: drank, but did not exceed NIAAA daily (≥5 for males and ≥4 for females) or weekly (>14 for males and >7 for females) limits in past 30 days (3) Heavy or very heavy: exceeded NIAAA daily or weekly limits. Heavy was defined as 0–4 days per month exceeding the daily limit. Very heavy was defined as 5 or more days per month exceeding the daily limits.
2.8 Covariates
Covariates include all listed above under “additional assessments.” To account for the heterogeneity of the study population, we controlled for multiple potential confounders including age, sex, race/ethnicity, duration of HIV infection, NIAAA drinking group, DXA machine used, lifetime drinking volume, years of regular cocaine use, tenofovir use, menopause, ever injection drug use, body mass index, current smoker, CD4 cell count, calcium, weight bearing physical activity, total [25(OH)D] ng/mL, recent cocaine use, recent opioid use, and current ART use. Medications and conditions that can affect bone mineral density were controlled for in sensitivity analyses.
2.9 Statistical analysis
All analyses were conducted using SAS software, Version 9.3 (SAS Institute Inc., Cary, NC, USA). Initial analyses focused on testing the association between mean alcohol consumption (grams/day over the past year) and annual change in BMD. Adjusted analyses included all covariates above except those highly correlated (r>0.45). Number of years since initiation of ART was excluded due to high correlation with duration of HIV infection (r=0.67). HIV viral load suppression (<200/200+ cells/mm3) was excluded due to high correlation with current ART use (r=0.60). Two pairs of variables (i.e., age and duration of HIV infection; and tenofovir use and current ART) were moderately correlated (r=0.40 and r=0.45, respectively), and were included as covariates in the analyses. All other covariate correlations were <0.40.
Primary analyses were mixed effects linear regression models with random intercepts focused on associations between alcohol consumption (measured as grams per day as the primary independent variable) and annual change in femoral neck BMD (dependent variable). Covariates (see above) were selected for inclusion based on either clinical suspicion or statistical significance of their association with alcohol use or change in bone mineral density.
Secondary analyses also included mixed effects linear regression models focused on associations between alcohol use (measured as grams per day as the primary independent variable) and change in total hip BMD, change in lumbar spine BMD. Additional secondary analyses included mixed effects logistic regression models testing the association between alcohol use and the other secondary outcomes: >6% annual decrease in BMD at any site and any fractures in the past year. Additional measures of alcohol use including mean number of heavy drinking days, mean number of days abstinent, mean numbers of drinks per day categorized as 0 – <0.7, 0.7 – 1.7, and >1.7, and consumption over the past month categorized as abstinence, not heavy, heavy, and very heavy, were used as independent variables; secondary analyses explored the relationship between those alcohol measures and secondary bone outcomes.
Additionally, we conducted a number of sensitivity analyses to check the robustness of the primary analysis. Due to the use of multiple DXA machines, a sensitivity analysis was performed for the primary regression model stratified by the DXA used (both tests done on Hologic 1; both tests done on Hologic 2; first test done on Hologic 1 and second on Hologic 2; excluding iDXA tests) (Supplemental Table 1). We also tested an interaction between alcohol use and sex. We also ran the unadjusted model including a squared term for grams of alcohol per day, to determine if a quadratic model would be appropriate.
To understand the impact of protease inhibitors (PI), we expanded our original antiretroviral therapy variable, which previously included 1) tenofovir containing regimen, 2) protease inhibitor, and 3) all other ART classes. We developed another variable consisting of 1) no ART, 2) tenofovir and no PI, 3) tenofovir and PI, 4) PI and no tenofovir, 5) other ART (no PI or tenofovir), and fit the primary analysis model for average alcohol use (grams/day) and changes in femoral neck BMD adding this new ART variable. To further explore the role of secondary causes of osteoporosis in the relationship between alcohol use and bone mineral density, we created a dichotomous variable for any such condition at baseline and at follow-up to use as a covariate in the primary analysis. Additionally, we performed a sensitivity analysis adding medications with known bone impact as one of two covariates—medications that increase BMD and medications that decrease BMD.
Third, to understand the role of vitamin D (e.g. if is on causal pathway, or a covariate), we fit our primary model without total [25(OH)D]. Also, as there is evidence that Caucasian and Hispanic people, and post-menopausal women have a higher incidence of low bone mineral density we tested the interaction of alcohol and race/ethnicity, and alcohol and sex/menopausal status for the primary model (Wright et al. 2014; Hedlund et al. 1989).
In addition to the sensitivity analyses, we conducted exploratory cross-sectional analyses in baseline data (i.e. at study entry) to understand the relationship between alcohol use and bone loss. We analyzed alcohol use (grams/day) and the following three outcomes of femoral neck bone mineral density (g/cm2) procollagen type 1 amino-terminal propeptide (P1NP, a bone remodeling marker for osteoblast activity), and Type I Collagen C-telopeptide (CTX-1, a bone remodeling marker for osteoclast activity). Analyses were unadjusted and adjusted for the same covariates as the primary analysis.
The study enrolled 250 adults, anticipating a 20% or lower loss to follow up. Assuming an evaluable sample of 200 participants with one observation per subject, the study had 80% power to detect a difference in means of −3.0% vs. −1.9% between those with heavy use and those abstinent or with less than heavy use. The study also had 80% power to detect a significant quadratic trend in the association between alcohol use and change in BMD, assuming uniformly distributed alcohol use (evaluated in simulations before study start which predicted 80 abstainers, 30 drinkers without heavy use (≥5 drinks for males and ≥4 for females) and 150 with heavy alcohol use. As aforementioned, a squared term for alcohol consumption (grams/day) was included in a model, to determine if a quadratic model was justified.
3. RESULTS
3.1 Study participants
Of 1,460 patients approached, 673 completed screening; 299 did not meet eligibility criteria; the most common reason for ineligibility was that the patient did not have past 12-month alcohol or drug dependence or had never injected drugs (82%). Of the 374 patients who met all eligibility criteria, 250 (67%) enrolled and 234 (94%) participants received ≥2 DXA scans at ≥1 bone sites. Sixteen did not receive ≥2 DXA scans at ≥1 site due to loss to follow-up. Of the 250 cohort participants, follow-up was 99% at 6 months, 96% at 18 months, 95% at 24 months, and 96% at 30 months. Characteristics of study participants are detailed in Table 1.
Table 1.
Baseline characteristics of PLWH with substance dependence or ever injection drug use (n=234)
| Characteristic | % (n) | |
|---|---|---|
|
| ||
| Sex and Menopause Status | ||
|
| ||
| Male | 64% (149) | |
| Female, post-menopausal | 19% (44) | |
| Female, pre-menopausal | 17% (41) | |
|
| ||
| Age (years, median [IQR]) | 50 [44–56] | |
|
| ||
| Male | 64% (149) | |
| Female | 36% (85) | |
|
| ||
| Race/Ethnicitya | ||
|
| ||
| White or other race, non-Hispanic | 24% (57) | |
| Black, non-Hispanic | 51% (120) | |
| Hispanic (regardless of race) | 24% (57) | |
|
| ||
| Current smokingb | 78% (182) | |
|
| ||
| Married or living with partner | 26% (62) | |
|
| ||
| BMI (kg/m2, median [IQR]) | 26.0 [22.8–29.7] | |
|
| ||
| Weight bearing physical activity | ||
|
| ||
| Minimal: 0–2 sessions/week | 26% (60) | |
| Low: 3–4 sessions/week | 22% (52) | |
| Adequate: 5–7 sessions/week | 38% (89) | |
| High: 8 sessions/week | 14% (33) | |
|
| ||
| Duration of HIV infection (years, median [IQR]) | 16.5 [0.10–23.6] | |
|
| ||
| Currently on ART medication | 89% (208) | |
|
| ||
| Years since initiation of ART (n=224) (years, median [IQR])c | 13 [5–18] | |
|
| ||
| CD-4 cell count <200 cells/μL | 10% (24) | |
|
| ||
| HIV Viral Load >500 copies/mL | 23% (53) | |
|
| ||
| Tenofovir use | 87% (202) | |
|
| ||
| No current ART | 11% (26) | |
|
| ||
| Tenofovir and no protease inhibitors | 39% (92) | |
|
| ||
| Tenofovir and protease inhibitors | 35% (82) | |
|
| ||
| Protease inhibitors and no tenofovir | 9% (20) | |
|
| ||
| All other | 6% (14) | |
|
| ||
| NIAAA drinking groupd | ||
|
| ||
| Abstinent | 31% (74) | |
| Did not exceed daily or weekly limits | 17% (39) | |
| Exceeded daily or weekly limits | 52% (121) | |
|
| ||
| Lifetime drinking, total volume (100 kg) (mean ± SD) | 7.2 ± 10.6 | |
|
| ||
| Ever IDU | 57% (134) | |
|
| ||
| DSM-IV alcohol dependence onlye | 10% (23) | |
|
| ||
| DSM-IV drug dependence onlye | 21% (50) | |
|
| ||
| Both DSM-IV alcohol and other drug dependencee | 50% (118) | |
|
| ||
| Years of regular cocaine use (mean ± SD)f | 9.5 ± 9.9 | |
|
| ||
| Days used cocaine in past 30 days (mean ± SD) | 2.6 ± 6.3 | |
|
| ||
| Days used illicit opioids in past 30 days (mean ± SD)g | 2.1 ± 6.3 | |
|
| ||
| Lumbar spine BMD (g/cm2, mean ± SD) | 1.02 ± 0.17 | |
|
| ||
| Total hip BMD (g/cm2, mean ± SD) | 0.94 ± 0.15 | |
|
| ||
| Femoral neck BMD (g/cm2, mean ± SD) | 0.83 ± 0.14 | |
|
| ||
| Low bone density h | 67% (154) | |
|
| ||
| Osteoporosis | 21% (47) | |
|
| ||
| Any fracture, past 12 mos. | 7% (17) | |
|
| ||
| Daily calcium intake from all sources (mgs, median [IQR])i | 1280.4 [781.6, 1948.4] | |
|
| ||
| Total 25-hydroxy-vitamin D [25(OH)D] (ng/mL, median [IQR]) | 24.5 [16.6, 35.6] | |
|
| ||
| Medications that increase BMDj | 14.6% (34) | |
|
| ||
| Medications that decrease BMDk | 36.1% (84) | |
|
| ||
| Secondary causes of low BMDl | 50.0% (117) | |
Abbreviations: IQR, Interquartile range; BMI, body mass index; ART, antiretroviral therapy; BMD, bone mineral density; SD, standard deviation; ASI, Addiction Severity Index
Race/ethnicity as recorded in DXA machine at time of scan
CDC definition: ≥100 cigarettes in lifetime and now smoking “every day” or “some days”
Period from first positive HIV test to initiation of first ART regimen
National Institute on Alcohol and Alcohol Abuse standard threshold for risky drinking: >14 standard drinks per week on average for males (>7 for females) or ≥1 heavy drinking day (>4 drinks in a day for males, >3 for females) in the past 30 days; assessed by 30-day timeline follow-back method
past 12-month Diagnostic and Statistical Manual of Mental Disorders 4th edition drug or alcohol use dependence by Mini International Neuropsychiatric Interview (M.I.N.I. 6.0) (Sheehan et al., 2010)
Regular cocaine use: ≥3x/week on average or 2 day binges/week, assessed by ASI
Includes both prescription and illicit opioids, assessed by ASI
Low bone mass (Osteopenia) or osteoporosis
Assessed by NIH Short Calcium Questionnaire
Denosumab, progestin only, contraceptive (systemic, levonorgesterel), bisphosphonates, androgen/anabolics (testosterone, topical or transdermal), and estrogen (transdermal, oral).
Glucocorticoids (oral and inhaled), antiasthma (only budesonide/formoterol, fluticasone/salmeterol), anti-inflammatory (inhaled, including beclomethasone dipropionate, budesonide, ciclesonide, flunisolide, fluticasone, mometasone), anticonvulsants (only divalproex, phenytoin, carbamazepine), chemotherapeutic agents, anticoagulants, and depo-provera.
Primary hyperparathyroidism (252.01, 252.0), secondary, tertiary, unspecified, or ectopic hyperparathyroidism (252.00, 252.02, 252.08, 259.3, 252.0), Cushing’s syndrome (255), hyperthyroidism, Graves’ disease, toxic goiter (242.00, 242.0–242.4, 242.8, 242.90), male hypogonadism (257.1–257.2), premature menopause (256.2, 256.31, 256.39, 626.0, 627.4), hyperprolactinemia (253.1), acromegaly (253), panhypopituitarism (253.2), osteomalacia, hypovitaminosis D (268.0–268.9), celiac disease, sprue (579.0–579.1), malnutrition, malabsorption (263.0, 263.1, 263.9, 579.3, 579.8, 579.9), Crohn’s disease (555.0–555.9), ulcerative colitis (556.0–556.9), anorexia, bulimia nervosa (307.1, 307.51), primary biliary cirrhosis (571.6) chronic liver disease, cirrhosis (571.9), hemochromatosis (275), chronic kidney disease (585.0–585.6, 585.9), renal hyperparathyroidism (588.81, 588.8), renal osteodystrophy (588), hypercalciuria (275.4), renal tubular acidosis (588.88, 588.89), hypocalcemia (275.41), hypercalcemia (275.42), rheumatoid arthritis (714), systemic lupus erythematosus (273.1), monoclonal gammopathy of undetermined significance (273.1), multiple myeloma (203.00–203.01), mastocytosis (202.6, 757.33), leukemia (208.00–208.91), lymphoma (202.80–202.88), sickle cell anemia (282.60–282.69), thalassemia (282.4, 282.49), hemophilia (286), amyloidosis (277.3), hypophosphatasia (275.3), mastocytosis (757.33), gonadal dysgenesis, Turner’s, XO (758.6), Kleinfelter’s syndrome (758.7), pseudo/pseudohypoparathyroidism, nephrocalcinosis (275.49), cystic fibrosis (277.00–277.09), immobilization >3 months (780.72), previous organ transplant (V42.0–V42.9), hypervitaminosis A (278.2), aluminum toxicity (973.0 + E858.4), osteogenesis imperfect (756.51), Ehlers–Danlos syndrome (756.83), Marfan’s syndrome (759.82), Gaucher’s disease (272.7), homocystinuria (270.4), hypophosphatasia (275.3), gastrectomy (43620–43634, 43638, 43639), small bowel resection (44120–44128, 44202, 44203), bariatric surgery (43644, 43645, 43659, 43842–43848).
3.2 Demographic characteristics
At baseline, the median age of participants was 50 years old. The study population was predominantly comprised of racial and ethnic minorities: 51% (120) identified as Non-Hispanic Black, and 24% (57) identified as Hispanic. About 36% (85) of participants were female and 19% (44) were postmenopausal, defined as the absence of menstruation for greater than a year. The majority (78% (182)) of participants were current smokers. The median BMI was 26.0 kg/m2. The median duration of HIV infection was 16.5 years and the median duration since ART initiation was 13 years. The study population largely consisted of participants on ART (89% (208)).
3.3 Substance use characteristics
Approximately half (52%, (121)) of the study population exceeded NIAAA daily (≥5 for males and ≥4 for females) or weekly (>14 for males and >7 for females) alcohol limits at study entry; 31% (74) reported past 30-day abstinence. The mean total lifetime alcohol consumption was 720 kg. Over half of the study population (57%, (134)) had ever injected drugs. The proportions of participants reporting past 30-day injection, cocaine use and opioid use were 10% (24), 30% (70), and 23% (54), respectively.
3.4 Bone characteristics
At study entry, 67% (154) of participants met criteria for low BMD (low bone mass 46% (107) [at least one T-score at any site between -1 and -2.5] or osteoporosis 21% (47) [T-score ≤ -2.5]). T-score represents standard deviation compared with average peak bone mass in young adult men. Among women (82), 55% (45) had low bone mass and 19% (15) had osteoporosis at baseline. Among men (146), low bone mass and osteoporosis were more common (75% (109) and 22% (32), respectively). Seven percent (17) of participants had reported any fracture in the past 12 months. The median daily calcium intake was 1280.4 mgs. The mean scores for lumbar spine, total hip, and femoral neck BMD were 1.02 g/cm2, 0.94 g/cm2, and 0.83 g/cm2, respectively.
3.5 Primary analyses: mean alcohol consumption and changes in femoral neck BMD
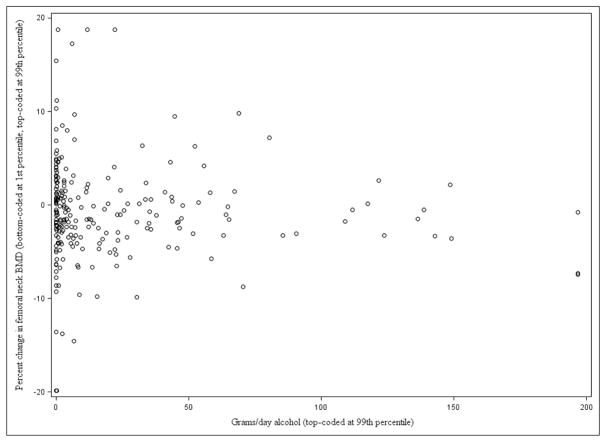
In unadjusted and adjusted multivariable regression analyses, mean alcohol consumption (g/day) was not associated with changes in femoral neck BMD (β= −0.0097, p=0.2417, adjusted β= −0.0032, p=0.7487) (Table 3) (Figure 1).
Table 3.
Unadjusted and adjusted* regression of association between alcohol consumption and changes in bone mineral density among PLWH and substance dependence or ever injection drug use (n=234)
| Independent Variable | % change in femoral neck BMD | % change in total hip BMD | % change in total spine BMD | > 6% annual decrease any site | Any fracture past year | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| β (p) | adj. β (p) | β (p) | adj. β (p) | β (p) | adj. β (p) | OR (95% CI) | adj. OR (95% CI) | OR (95% CI) | adj. OR (95% CI) | |
|
| ||||||||||
| Mean alcohol consumption (g/day) | −0.0097 (0.24) | −0.0032 (0.75) | −0.0028 (0.57) | −0.0009 (0.87) | −0.0002 (0.97) | −0.0060 (0.41) | 1.01 (1.00, 1.01) | 1.00 (0.99, 1.01) | 1.00 (0.98, 1.01) | 0.99 (0.98, 1.01) |
|
| ||||||||||
| Mean no. of heavy drinking days | −0.0200 (0.67) | 0.0339 (0.53) | −0.0058 (0.84) | −0.0010 (0.98) | −0.0147 (0.68) | −0.0237 (0.55) | 1.02 (0.97, 1.07) | 1.00 (0.93, 1.07) | 0.95 (0.86, 1.05) | 0.95 (0.84, 1.07) |
|
| ||||||||||
| Mean no. of days abstinent | 0.0268 (0.50) | −0.0057 (0.90) | 0.0054 (0.83) | 0.0053 (0.84) | 0.0221 (0.46) | 0.0380 (0.25) | 1.00 (0.96, 1.05) | 1.02 (0.96, 1.08) | 1.06 (0.98, 1.16) | 1.07 (0.96, 1.18) |
|
| ||||||||||
| Mean alcohol consumption (drinks/day) | ||||||||||
| 0.7–1.7 vs. 0–<0.7 | 0.0116 | 0.2678 | −0.4746 | −0.7609 | 0.0128 | −0.3538 | 0.80 (0.29, 2.22) | 0.86 (0.25, 2.89) | 0.61 (0.13, 2.85) | 0.62 (0.11, 3.54) |
| >1.7 vs. 0–<0.7 | −0.4151 (0.82) | 0.2980 (0.91) | −0.0822 (0.68) | 0.1016 (0.32) | −0.3326 (0.81) | −0.8043 (0.36) | 1.07 (0.51, 2.22) | 1.10 (0.40, 3.03) | 0.65 (0.20, 2.05) | 0.54 (0.13, 2.32) |
|
| ||||||||||
| Heavy Drinking | ||||||||||
| Not heavy vs. abstinent | 0.5186 | 0.3658 | 0.4461 | 0.3419 | −0.2611 | −0.7427 | 0.48 (0.17, 1.36) | 0.71 (0.20, 2.49) | 0.39 (0.10, 1.60) | 0.38 (0.07, 2.04) |
| Heavy vs. abstinent | −0.8159 | −0.8463 | −0.3422 | −0.4459 | 0.2175 | −0.0576 | 0.96 (0.41, 2.24) | 1.54 (0.50, 4.78) | 0.68 (0.22, 2.12) | 0.68 (0.15, 3.17) |
| Very heavy vs. abstinent | −0.1452 (0.49) | −0.4597 (0.41) | −0.1433 (0.51) | −0.1322 (0.55) | −0.3109 (0.81) | −0.7380 (0.48) | 0.78 (0.35, 1.77) | 1.05 (0.34, 3.21) | 0.41 (0.13, 1.35) | 0.36 (0.08, 1.70) |
Adj.=adjusted
OR= odds ratio
BMD=bone mineral density
Covariates adjusted for included: age, sex, race/ethnicity, duration of HIV infection, NIAAA drinking group, DXA machine used, lifetime drinking volume, years of regular cocaine use, tenofovir use, menopause, ever injection drug use, body mass index, current smoker, CD4 cell count, calcium, weight bearing physical activity, total 25(OH)D ng/mL, recent cocaine use, recent opioid use, and current ART use.
Figure 1.
Scatter plot of alcohol consumption (grams/day) and percent change in femoral neck bone mineral density (g/cm2) (n=234) over Year 1*
BMD=bone mineral density
Each circle represents one participant’s change in bone mineral density and mean grams per day of alcohol consumption over the first year of observation calculated by averaging daily alcohol consumption reported at 3 study interviews: baseline, 6 month and 12 month time points.
Year 1: n (%) Baseline to 12 month: 222 (95%), Baseline to 18 month: 8 (3%), Baseline to 24 month: 2 (1%), 6 to 12 month: 2 (1%)
3.6 Secondary analyses (Table 3)
In unadjusted and adjusted regression analyses, there were no associations between mean alcohol consumption (g/d) and the following secondary outcomes: change in total hip BMD (Adjusted (adj.) β= −0.0009, p=0.8719), change in lumbar spine BMD (adj. β= −0.0060, p= 0.4067), >6% annual decrease in BMD at any site (adj. OR=1.00, 95% CI=0.99, 1.01), and any fractures in the past year (adj. OR 0.99, 95% CI=0.98, 1.01). Additional measures of alcohol consumption, including mean number of heavy drinking days, mean number of days abstinent, mean numbers of drinks per day categorized as 0 – <0.7, 0.7 – 1.7, and >1.7, and consumption over the past month categorized as abstinence, not heavy, heavy, and very heavy, were not associated with changes in femoral neck, total hip, and lumbar spine, BMD. The aforementioned additional measures were not associated with >6% annual decrease in BMD at any site, or any fractures in the past year.
3.7 Sensitivity analyses
We conducted a number of sensitivity analyses to check the robustness of the primary analysis. A sensitivity analysis addressing type of machine and software version yielded results similar to the result of the primary analysis (Supplemental Table 2). In the unadjusted quadratic model, a squared term for alcohol consumption (grams/day) was not significant (β = −0.0001, p=0.4504). There was no significant interaction between alcohol consumption and sex (p=0.4097). There was no significant interaction between alcohol, race, and menopausal status (p=0.2060), and alcohol and sex/menopausal status (p=0.3538).
To understand the impact of protease inhibitors, we expanded the antiretroviral therapy variable and fit the primary analysis model for average alcohol use (grams/day) and changes in femoral neck BMD. The results were similar to those with the original art variable (Adj. β= −0.0032, p=0.7491).
To further explore the role of secondary causes of osteoporosis in the relationship between alcohol use and bone mineral density, we included a dichotomous variable for any secondary cause of osteoporosis at baseline and at follow-up to use as a covariate in the primary analysis, which did not affect the primary finding (Adj. β= −0.0032, p=0.7436). Similarly, to account for other concomitant treatments with known bone impact, we performed a sensitivity analysis adding the two variables representing medications (that increase, or that decrease BMD) from data obtained from the electronic medical record. We fit the primary analysis model with these new variables, which did not affect the primary finding (Adj. β= −0.0027, p=0.7820). Removing vitamin D status as covariate did not impact the primary analysis finding (Adj. β= −0.0036, p=0.7112).
3.8 Exploratory analyses
In addition to the sensitivity analyses, we repeated the primary analysis of alcohol use cross-sectionally at baseline and did not find any significant association between average alcohol exposure (g/day) and femoral neck bone mineral density (β= −0.00004, p=0.8475, Adj. β= −0.0001, p=0.5050). We repeated the same cross-sectional analysis at baseline assessing the association between alcohol use and two bone marker outcomes: (P1N1 and CTX-1). There was a significant association between alcohol use and P1NP (β= −0.1130, p=0.0043, Adj. β= −0.1033, p=0.0304), but no significant association between alcohol use and CTX-1 (β= −0.0018, p=0.00852, Adj. β= −0.0013, p=0.2604).
4. DISCUSSION
4.1 Discussion
The main aim of this study was to assess the association between alcohol consumption and annual changes in BMD among people living with HIV (PLWH) and substance use disorders. We expected to find that higher alcohol consumption would be associated with decreased BMD. However, unexpectedly, although two-thirds of PLWH with substance use disorders had low BMD, and alcohol use may increase risk for low BMD, we did not detect any effects of drinking on change in BMD or incident fractures. A number of sensitivity analyses yielded similar findings. There was no significant finding in exploratory cross-sectional analyses. There was a significant association between alcohol use and P1NP (more alcohol, less bone formation) but no significant association between alcohol use and CTX-1, which is consistent with findings in the literature (Maurel et al. 2012; Gonzalez-Calvin JL et al. 1993; Alvisa-Negrin et al. 2009; Nyquist et al. 1998). However, the clinical significance of this latter finding is unclear and the association between alcohol and bone formation and resorption has been mixed and inconclusive in other studies, possibly due to other co-existing factors such as smoking, vitamin D deficiency and gastrointestinal diseases (Maurel et al. 2012; Turner 2000).
Although the cohort had a broad range of alcohol use, change in alcohol use was small. Relatively static alcohol consumption may have limited our ability to detect changes in BMD. Multiple competing risks may partially explain the null results; the prevalence of secondary causes of low BMD (e.g. smoking, opioid use), was high among the study population, and similar to that reported in other studies (Bonjoch et al., 2010; Brown and Qaqish, 2008). Consequently, it is possible that an effect of alcohol use was not detectable in the setting of concomitant risks.
These findings should be placed in the context of prior literature assessing alcohol consumption and bone outcomes in PLWH. In previous work, we did not detect an association between lifetime alcohol consumption and BMD though there was an association between current alcohol consumption and BMD; these analyses, however, were cross-sectional (Ventura et al., 2017). In cohort studies, high alcohol consumption has been associated with an increased risk of fracture in PLWH (Collin et al., 2009; Womack et al., 2011). In a 10-year cohort study of 1,281 PLWH, the incidence rate of fractures was 2.9-fold higher among those with excessive alcohol consumption (Collin et al., 2009). However, studies have not consistently found alcohol to increase risk for low BMD in PLWH (Brown et al., 2009; Escota et al., 2015; Mondy et al., 2003; Yin, 2012; Sharma et al., 2010; Cazanave et al., 2008; Kooij et al., 2015; Gill et al., 2015; Kasonde et al., 2014; Grund et al., 2009; Carr et al., 2015; Bedimo et al., 2007). Many of these studies used measures of alcohol consumption that may not be very accurate or specific (e.g. medical record reviews, or self-report measures that have not been validated), did not adjust for covariates related to bone health outcomes, and had short follow up periods. In the present study, we used a validated measure of alcohol use (Sobell and Sobell, 1992), adjusted for numerous potential confounding factors (e.g., smoking, opioid use, antiretroviral medication, BMI and CD4 count) and followed PLWH for up to three and a half years.
Our findings are subject to several study limitations. Study participants had current substance use disorder, or ever injection drug use, and were largely well-connected to clinical HIV care. Therefore, our findings may be most generalizable to other care-engaged PLWH with substance use disorders. Although adjusted for numerous confounders, it remains possible that there was unadjusted confounding. One possibility is that ongoing inflammation related to alcohol use and to HIV infection (Hunt et al., 2016; Kuller et al, 2008; Carrico et al., 2015) even among those virally suppressed might play a role as a confounder. We did not collect information about the first exposure to medications that could affect bone mineral density, when the risk of bone loss is most pronounced (Canalis et al. 2007). We did collect information about years since initiation of ART but did not include it as a covariate in regression analyses because it was highly correlated with duration of HIV infection. Additionally, our analyses included time-varying covariates representing ART exposure and other medications that can affect bone mineral density. Therefore, we do not believe that this limitation is likely to have a substantial impact on the findings. Alcohol consumption was assessed by self-report, which may be influenced by recall and social desirability biases. However, we used the 30-day Timeline Followback method (Sobell and Sobell, 1992), which is widely accepted as the best-validated method for assessing self-reported consumption, and we do not have reason to suspect that the aforementioned biases would be related to bone outcomes. Fractures were assessed by self-report, which may be influenced by recall bias. However, fractures are memorable events; people may not recall precise details of timing but they do likely remember fracture occurrence. Self-reported fractures have a sensitivity of 78%, and a specificity of 96% when compared to medical record documented fractures (Honkanen et al. 1999; Nevitt et al. 1992; Ismail et al. 2000). Another important limitation is that the alcohol measures obtained at 6 month intervals did not assess drinking beyond the past month. However, these repeated assessments captured and summarized daily drinking in a number of ways, and these detailed validated assessments of recent consumption likely reflect interim periods.
Although we measured BMD in a standardized fashion using DXA, we used 3 different machines, which could introduce measurement error; measurements can vary as much as 11% (Fan et al., 2010). To minimize this measurement error, we used an industry-accepted conversion formula to account for between-machine differences, controlled for DXA machine and software in regression models, and performed a sensitivity analysis by DXA machine. Finally, it is possible that the observation time (2 years) for the cohort was too short to detect any effects of alcohol on bone mineral density. However, other studies have detected changes in bone mineral density and incidence in osteoporotic fractures due to alcohol and other risk factors (e.g. ART) in shorter or comparable periods ranging from 6 months to two years (Kasonde et al., 2014; Sharma et al., 2010; Mondy et al., 2003; Duvivier et al. 2009; Siris et al., 2001; Arnsten et al. 2007; Avihingsanon et al. 2017; Rey et al. 2015; Young et al. 2011). Given the observed effect sizes, pre-planned study power to detect fairly small effects, and loss to follow-up much lower than planned, we do not believe power limitations are a likely explanation for the null findings.
At least among PLWH with multiple risk factors, alcohol use may not have a very large effect on BMD. Examining this question in a sample with greater change in alcohol use and fewer competing risks is worthy of consideration. But given the high prevalence of both low BMD and risk factors for it, understanding and addressing it in PLWH and the role that reversible causes play should remain a priority. The population of PLWH is now aging, thanks to efficacious treatments for HIV infection itself, and common comorbidities associated with aging and their treatments are emerging as the main contributors to overall health for PLWH. Low BMD and its fracture consequences remain of great importance along with other comorbidities such as substance use, cardiovascular disease, and neurologic diseases. Furthermore, given that PLWH and substance use disorder are also at high risk for injury and fracture, concerns regarding low BMD go beyond immune dysfunction, inflammation, and direct medication and substance effects on bone.
4.2 Conclusions
In this sample of PLWH with substance use disorder or ever injection drug use, we detected no effects of alcohol consumption on annual change in BMD or incident fractures. Larger diverse samples with longer follow-up may be able to discern the specific effects of alcohol as well as effects of multiple risk factors (e.g. alcohol combined with other exposures). Thus although we did not find effects, we cannot conclude that alcohol is safe even from the perspective of bone health for PLWH and substance use disorders.
Supplementary Material
Table 2.
Alcohol use and change in bone mineral density in PLWH with substance dependence or ever injection drug use over each year of observation
| Characteristic | Year 1* (n=234) | Year 2** (n=127) | |
|---|---|---|---|
| Independent Variable: Alcohol Consumption | |||
| Grams of alcohol per day (g/d, mean ± SD) | 21.49 ± 38.27 | 16.24 ± 31.08 | |
| Number of heavy drinking daysa (mean ± SD) | 3.81 ± 6.39 | 3.07 ± 5.74 | |
| Number of days abstinent (mean ± SD) | 24.31 ± 7.39 | 25.25 ± 6.90 | |
| Mean alcohol exposure per day | |||
| 0 – <0.7 drinks/day | 61% (144) | 66% (84) | |
| 0.7 – 1.7 drinks/day | 13% (30) | 13% (16) | |
| >1.7 drinks/day | 26% (60) | 21% (27) | |
| Drinking | |||
| Abstinenceb | 21% (50) | 26% (33) | |
| Not-heavyc | 18% (43) | 24% (30) | |
| Heavyd | 27% (63) | 18% (23) | |
| Very heavye | 33% (78) | 32% (41) | |
| Dependent Variable | |||
| % change in femoral neck (mean ± SD) | −0.68 ± 5.29 | 0.51 ± 5.54 | |
| % change in total hip (mean ± SD) | 0.71 ± 3.51 | 0.71 ± 2.94 | |
| % change in total spine (mean ± SD) | 1.14 ± 4.06 | −0.15 ± 4.10 | |
| >6% decrease any site | 13% (30) | 13% (17) | |
| Any fracture | 7% (17) | 4% (5) | |
| Other Variables | |||
| No current ART | 11.1% (26) | 9.5% (12) | |
| Tenofovir and no protease inhibitors | 39.3% (92) | 33.9% (43) | |
| Tenofovir and protease inhibitors | 35.0% (82) | 33.9% (43) | |
| Protease inhibitors and no tenofovir | 8.6% (20) | 10.2% (13) | |
| All other | 6.0% (15) | 12.6% (16) | |
| Medications that increase BMDf | 17.1% (40) | 18.1% (23) | |
| Medications that decrease BMDg | 43.6% (102) | 43.3% (55) | |
| Secondary causes of low BMDh | 41.0% (96) | 39.4% (50) | |
Abbreviations: IQR, Interquartile range; BMI, body mass index; ART, antiretroviral therapy; BMD, bone mineral density; SD, standard deviation;
Year 1: n (%) Baseline to 12 month: 222 (95%), Baseline to 18 month: 8 (3%), Baseline to 24 month: 2 (1%), 6 to 12 month: 2 (1%)
Year 2: n (%) 12 month to 24 month: 124 (98%), 18 month to 24 month: 3 (2%)
Heavy drinking day: ≥5 drinks for males and ≥4 drinks for females
Abstinence: no drinking in past 30 days
Not heavy: drank, but did not exceed NIAAA daily (≥5 for males and ≥4 for females) or weekly ( >14 for males and >7 for females) limits in past 30 days
Heavy: exceeded NIAAA daily limits for ≤4 days/week in past 30 days
Very heavy: ≥5 days/week of heavy drinking (exceeding NIAAA daily limits) in past 30 days
Denosumab, progestin only, contraceptive (systemic, levonorgesterel), bisphosphonates, androgen/anabolics (testosterone, topical or transdermal), and estrogen (transdermal, oral).
Glucocorticoids (oral and inhaled), antiasthma (only budesonide/formoterol, fluticasone/salmeterol), anti-inflammatory (inhaled, including beclomethasone dipropionate, budesonide, ciclesonide, flunisolide, fluticasone, mometasone), anticonvulsants (only divalproex, phenytoin, carbamazepine), chemotherapeutic agents, anticoagulants, and depo-provera.
Primary hyperparathyroidism (252.01, 252.0), secondary, tertiary, unspecified, or ectopic hyperparathyroidism (252.00, 252.02, 252.08, 259.3, 252.0), Cushing’s syndrome (255), hyperthyroidism, Graves’ disease, toxic goiter (242.00, 242.0–242.4, 242.8, 242.90), male hypogonadism (257.1–257.2), premature menopause (256.2, 256.31, 256.39, 626.0, 627.4), hyperprolactinemia (253.1), acromegaly (253), panhypopituitarism (253.2), osteomalacia, hypovitaminosis D (268.0–268.9), celiac disease, sprue (579.0–579.1), malnutrition, malabsorption (263.0, 263.1, 263.9, 579.3, 579.8, 579.9), Crohn’s disease (555.0–555.9), ulcerative colitis (556.0–556.9), anorexia, bulimia nervosa (307.1, 307.51), primary biliary cirrhosis (571.6) chronic liver disease, cirrhosis (571.9), hemochromatosis (275), chronic kidney disease (585.0–585.6, 585.9), renal hyperparathyroidism (588.81, 588.8), renal osteodystrophy (588), hypercalciuria (275.4), renal tubular acidosis (588.88, 588.89), hypocalcemia (275.41), hypercalcemia (275.42), rheumatoid arthritis (714), systemic lupus erythematosus (273.1), monoclonal gammopathy of undetermined significance (273.1), multiple myeloma (203.00–203.01), mastocytosis (202.6, 757.33), leukemia (208.00–208.91), lymphoma (202.80–202.88), sickle cell anemia (282.60–282.69), thalassemia (282.4, 282.49), hemophilia (286), amyloidosis (277.3), hypophosphatasia (275.3), mastocytosis (757.33), gonadal dysgenesis, Turner’s, XO (758.6), Kleinfelter’s syndrome (758.7), pseudo/pseudohypoparathyroidism, nephrocalcinosis (275.49), cystic fibrosis (277.00–277.09), immobilization >3 months (780.72), previous organ transplant (V42.0–V42.9), hypervitaminosis A (278.2), aluminum toxicity (973.0 + E858.4), osteogenesis imperfect (756.51), Ehlers–Danlos syndrome (756.83), Marfan’s syndrome (759.82), Gaucher’s disease (272.7), homocystinuria (270.4), hypophosphatasia (275.3), gastrectomy (43620–43634, 43638, 43639), small bowel resection (44120–44128, 44202, 44203), bariatric surgery (43644, 43645, 43659, 43842–43848).
Acknowledgments
5. ACKNOWLEDGEMENTS
5.1 Acknowledgements
This publication is dedicated to the memory of Adrian K. Turner, a colleague who was instrumental in collecting and interpreting the bone scan data. We would like to thank the Boston ARCH cohort study participants; this work would not be possible without the time and effort they dedicated to the research. We also would like to thank all of the staff at Boston University and Boston Medical Center. Specifically, we want to acknowledge Margo Godersky, Kate Haworth, Keshia Toussaint, and Laura Vercammen for their hard work in recruiting, collecting data, and tracking study participants. We would also like to thank Tai Chen and Anqi Zhang for graciously providing their expertise for this work. Lastly, we also want to acknowledge the support from the staff of the General Clinical Research Unit at Boston University, Center for Infectious Diseases at Boston Medical Center, and Boston Healthcare for the Homeless Program.
5.3 Sources of Funding
This work was supported by grants the National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism (U01AA020784, U24AA020779 and U24AA020778) and National Center for Advancing Translational Science (UL1TR001430).
Footnotes
Data were presented, in part, at the 2017 Research Society of Alcoholism Scientific Meeting in Denver, Colorado on June 25, 2017.
Conflicts of Interest and Sources of Funding: Supported by grants from the National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism (U01AA020784, U24AA020779 and U24AA020778) and National Center for Advancing Translational Science (UL1TR001430). The authors report that the first author is the principal investigator of an NIH/NIAAA supported (to Boston University (BU)) study of people with alcohol use disorder; BU receives injectable naltrexone for that study from Alkermes.
5.2 Authorship Contributions
RS conceived the study question. AM wrote the first draft of the manuscript. MRW and GJP conducted statistical analysis with TCH’s support and guidance. MS and AYW provided clinical expertise. SMM and ASV assisted with procedures and oversight of data collection. MFH provided expertise on interpretation of bone mineral density. KJB was the National Institutes of Health collaborator involved providing input and feedback regarding the study question and design. RS was the Principal Investigator of the Boston ARCH Cohort study and JHS was the Principal Investigator of the URBAN ARCH Consortium. All authors met criteria for authorship and read, provided critical editing, and approved the final draft of the manuscript.
References
- Alvisa-Negrin J, Gonzalez-Reimers E, Santolaria-Fernandez F, Garcia-Valdecasas-Campelo E, Valls MR, Pelazas-Gonzalez R, Duran-Castellon MC, de Los Angeles Gomez-Rodriguez M. Osteopenia in alcoholics: effect of alcohol abstinence. Alcohol Alcohol. 2009;44:468–475. doi: 10.1093/alcalc/agp038. [DOI] [PubMed] [Google Scholar]
- Arnsten JH, Freeman R, Howard AA, Floris-Moore M, Lo Y, Klein RS. Decreased bone mineral density and increased fracture risk in aging men with or at risk for HIV infection. AIDS. 2007;21(5):617. doi: 10.1097/QAD.0b013e3280148c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avihingsanon A, Badal-Faesen S, De Wit S, Jacoby S, La Rosa A, Pujari S. Immediate initiation of antiretroviral therapy for HIV infection accelerates bone loss relative to deferring therapy: findings from the START bone mineral density substudy, a randomized trial. J Bone Miner Res. 2017;32(9):1945–1955. doi: 10.1002/jbmr.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedimo RJ, Adams-Huet B, Poindexter J, Brown G, Farukhi I, Castanon R, Turner D, Moore T, Tebas P, Maalouf NM. The differential effects of HIV and HCV on bone micro-architecture and fracture risk. Clinical Infect Dis. 2017 doi: 10.1093/cid/cix1011. [DOI] [PubMed] [Google Scholar]
- Berg KM, Kunins HV, Jackson JL, Nahvi S, Chaudhry A, Harris KA, Malik R, Arnsten JH. Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med. 2008;121(5):406–18. doi: 10.1016/j.amjmed.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkley N, Bilezikian JP, Kendler DL, et al. Official positions of the international society for clinical densitometry and executive summary of the 2005 position development conference. J Clin Densitom. 2006;9(1):4–14. doi: 10.1016/j.jocd.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Bonjoch A, Figueras M, Estany C, Perez-Alvarez N, Rosales J, del Rio L, di Gregorio S, Puig J, Gómez G, Clotet B, Negredo E. High prevalence of and progression to low bone mineral density in HIV-infected patients: a longitudinal cohort study. AIDS. 2010;24(18):2827–2833. doi: 10.1097/QAD.0b013e328340a28d. [DOI] [PubMed] [Google Scholar]
- Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20(17):2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- Brufsky AM. Cancer treatment-induced bone loss: pathophysiology and clinical perspectives. Oncologist. 2008;13(2):187–95. doi: 10.1634/theoncologist.2007-0152. [DOI] [PubMed] [Google Scholar]
- Caird LE, Reid-Thomas V, Hannan WJ, Gow S, Glasier AF. Oral progestogen-only contraception may protect against loss of bone mass in breast-feeding women. Clin Endocrinol. 1994;41(6):739–45. doi: 10.1111/j.1365-2265.1994.tb02788.x. [DOI] [PubMed] [Google Scholar]
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18(10):1319–1328. doi: 10.1007/s00198-007-0394-0. [DOI] [PubMed] [Google Scholar]
- Carr A, Grund B, Neuhaus J, Schwartz A, Bernardino JI, White D, Badel-Faesen S, Avihingsanon A, Ensrud K, Hoy J. Prevalence of and risk factors for low bone mineral density in untreated HIV infection: a substudy of the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med. 2015;16(S1):137–146. doi: 10.1111/hiv.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Hunt PW, Emenyonu NI, Muyindike W, Ngabirano C, Cheng DM, Winter MR, Samet JH, Hahn JA. Unhealthy alcohol use is associated with monocyte activation prior to starting antiretroviral therapy. Alcohol Clin Exp Res. 2015;39(12):2422–6. doi: 10.1111/acer.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casado JL, Bañon S, Andrés R, Perez-Elías MJ, Moreno A, Moreno S. Prevalence of causes of secondary osteoporosis and contribution to lower bone mineral density in HIV-infected patients. Osteoporosis Int. 2014;25(3):1071–1079. doi: 10.1007/s00198-013-2506-3. [DOI] [PubMed] [Google Scholar]
- Cawthon PM, Harrison SL, Barrett-Connor E, Fink HA, Cauley JA, Lewis CE, Orwoll ES, Cummings SR. Alcohol intake and its relationship with bone mineral density, falls, and fracture risk in older men. J Am Geriatr Soc. 2006;54(11):1649–1657. doi: 10.1111/j.1532-5415.2006.00912.x. [DOI] [PubMed] [Google Scholar]
- Cazanave C, Dupon M, Lavignolle-Aurillac V, Barthe N, Lawson-Ayayi S, Mehsen N, Mercié P, Morlat P, Thiébaut R, Dabis F Groupe d’Epidemiologie Clinique du SIDA en Aquitaine. Reduced bone mineral density in HIV-infected patients: prevalence and associated factors. AIDS. 2008;22(3):395–402. doi: 10.1097/QAD.0b013e3282f423dd. [DOI] [PubMed] [Google Scholar]
- Chakkalakal DA. Alcohol-induced bone loss and deficient bone repair. Alcohol Clin Exp Res. 2005;29(12):2077–2090. doi: 10.1097/01.alc.0000192039.21305.55. [DOI] [PubMed] [Google Scholar]
- Chee C, Sellahewa L, Pappachan JM. Inhaled corticosteroids and bone health. Open Respir Med J. 2014;8:85. doi: 10.2174/1874306401408010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin F, Duval X, Lemoing V, Piroth L, Al Kaied F, Massip P, Villes V, Chêne G, Raffi F. Ten-year incidence and risk factors of bone fractures in a cohort of treated HIV1-infected adults. AIDS. 2009;23(8):1021. doi: 10.1097/QAD.0b013e3283292195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter AG, Sabin CA, Simelane S, Macken A, Kavanagh E, Brady JJ HIV UPBEAT Study Group. Relative contribution of HIV infection, demographics and body mass index to bone mineral density. AIDS. 2014;28(14):2051–2060. doi: 10.1097/QAD.0000000000000353. [DOI] [PubMed] [Google Scholar]
- Cummings SR, Martin JS, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–65. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- Drummond MB, Dasenbrook EC, Pitz MW, Murphy DJ, Fan E. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300(20):2407–2416. doi: 10.1001/jama.2008.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvivier C, Kolta S, Assoumou L, Ghosn J, Rozenberg S, Murphy RL, Katlama C, Costagliola D ANRS 121 Hippocampe Study Group. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. Aids. 2009;23(7):817–24. doi: 10.1097/QAD.0b013e328328f789. [DOI] [PubMed] [Google Scholar]
- Escota GV, Mondy K, Bush T, Conley L, Brooks JT, Önen N, Patel P, Kojic EM, Henry K, Hammer J, Wood KC. High prevalence of low bone mineral density and substantial bone loss over 4 years among HIV-infected persons in the era of modern antiretroviral therapy. AIDS Res Hum Retrov. 2016;32(1):59–67. doi: 10.1089/aid.2015.0158. [DOI] [PubMed] [Google Scholar]
- Fan B, Lu Y, Genant H, Fuerst T, Shepherd J. Does standardized BMD still remove differences between Hologic and GE-Lunar state-of-the-art DXA systems? Osteoporosis Int. 2010;21(7):1227–1236. doi: 10.1007/s00198-009-1062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feskanich D, Korrick SA, Greenspan SL, Rosen HN, Colditz GA. Moderate alcohol consumption and bone density among postmenopausal women. J Womens Health. 1999;8(1):65–73. doi: 10.1089/jwh.1999.8.65. [DOI] [PubMed] [Google Scholar]
- Friday KE, Howard GA. Ethanol inhibits human bone cell proliferation and functionin vitro. Metabolis. 1991;40(6):562–565. doi: 10.1016/0026-0495(91)90044-w. [DOI] [PubMed] [Google Scholar]
- Gbolade BA. Depo-Provera and bone density. Review. J Fam Plann Reprod Health Care. 2002;28(1):7–11. doi: 10.1783/147118902101195910. [DOI] [PubMed] [Google Scholar]
- Gill US, Zissimopoulos A, Al-Shamma S, Burke K, McPhail MJ, Barr DA, Kallis YN, Marley RT, Kooner P, Foster GR, Kennedy PT. Assessment of bone mineral density in tenofovir-treated patients with chronic hepatitis B: can the fracture risk assessment tool identify those at greatest risk? J Infect Dis. 2014;211(3):374–382. doi: 10.1093/infdis/jiu471. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Calvin JL, Garcia-Sanchez A, Bellot B, Munoz-Torres M, Raya-Alvarez E, Salvatierra-Rios D. Mineral metabolism, osteoblastic function and bone mass in chronic alcoholism. Alcohol and Alcoholism. 1993;28(5):571–579. [PubMed] [Google Scholar]
- Grund B, Peng G, Gibert CL, Hoy JF, Isaksson RL, Shlay JC, Martinez E, Reiss P, Visnegarwala F, Carr AD. Continuous antiretroviral therapy decreases bone mineral density. AIDS. 2009;23(12):1519. doi: 10.1097/QAD.0b013e32832c1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen ABE, Gerstoft J, Kronborg G, Larsen CS, Pedersen G, Obel N. Incidence of low and high-energy fractures in persons with and without HIV infection: a Danish population-based cohort study. AIDS. 2012;26(3):285–293. doi: 10.1097/QAD.0b013e32834ed8a7. [DOI] [PubMed] [Google Scholar]
- Hedlund LR, Gallagher JC. The effect of age and menopause on bone mineral density of the proximal femur. J Bone Miner Res. 1989;4(4):639–42. doi: 10.1002/jbmr.5650040423. [DOI] [PubMed] [Google Scholar]
- Honkanen K, Honkanen R, Heikkinen L, Kröger H, Saarikoski S. Validity of self-reports of fractures in perimenopausal women. Am J Epidemiol. 1999;150(5):511–516. doi: 10.1093/oxfordjournals.aje.a010040. [DOI] [PubMed] [Google Scholar]
- Hoy JF, Grund B, Roediger M, Schwartz AV, Shepherd J, Avihingsanon A, Badal-Faesen S, De Wit S, Jacoby S, La Rosa A, Pujari S. Immediate initiation of antiretroviral therapy for HIV infection accelerates bone loss relative to deferring therapy: findings from the START bone mineral density substudy, a randomized trial. J Bone Miner Res. 2017;32(9):1945–1955. doi: 10.1002/jbmr.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PW, Lee SA, Siedner MJ. Immunologic biomarkers, morbidity, and mortality in treated HIV infection. J Infect Dis. 2016;214(suppl_2):S44–50. doi: 10.1093/infdis/jiw275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail A, O’neill TW, Cockerill W, Finn JD, Cannata JB, Hoszowski K, Johnell O, Matthis C, Raspe H, Raspe A, Reeve J. Validity of self-report of fractures: results from a prospective study in men and women across Europe. Osteoporos Int. 2000;11(3):248–254. doi: 10.1007/s001980050288. [DOI] [PubMed] [Google Scholar]
- Kasonde M, Niska RW, Rose C, Henderson FL, Segolodi TM, Turner K, Smith DK, Thigpen MC, Paxton LA. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS One. 2014;9(3):e90111. doi: 10.1371/journal.pone.0090111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TW, Alford DP, Malabanan A, Holick MF, Samet JH. Low bone density in patients receiving methadone maintenance treatment. Drug Alcohol Depen. 2006;85(3):258–262. doi: 10.1016/j.drugalcdep.2006.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooij KW, Wit FW, Bisschop PH, Schouten J, Stolte IG, Prins M, Van der Valk M, Prins JM, van Eck-Smit BL, Lips P, Reiss P. Low bone mineral density in patients with well-suppressed HIV infection: association with body weight, smoking, and prior advanced HIV disease. J Infect Dis. 2015;211(4):539–548. doi: 10.1093/infdis/jiu499. [DOI] [PubMed] [Google Scholar]
- Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, Paton NI. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Lyles KW, Colón-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother. 2010;8(1):34–46. doi: 10.1016/j.amjopharm.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas SC, Kousteni S, Jilka RL. Sex steroids and bone. Recent Prog Horm Res 1. 2002;57:385–410. doi: 10.1210/rp.57.1.385. [DOI] [PubMed] [Google Scholar]
- Marshall AL, Smith BJ, Bauman AE, Kaur S. Reliability and validity of a brief physical activity assessment for use by family doctors. Br J Sports Med. 2005;39(5):294–7. doi: 10.1136/bjsm.2004.013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel DB, Boisseau N, Benhamou CL, Jaffre C. Alcohol and bone: review of dose effects and mechanisms. Osteoporos Int. 2012;23(1):1–16. doi: 10.1007/s00198-011-1787-7. [DOI] [PubMed] [Google Scholar]
- McComsey GA, Tebas P, Shane E, Yin MT, Overton ET, Huang JS, Aldrovandi GM, Cardoso SW, Santana JL, Brown TT. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis. 2010;51(8):937–946. doi: 10.1086/656412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan FE, Berg RL, Linneman JG. The utility of BMD Z-score diagnostic thresholds for secondary causes of osteoporosis. Osteoporos Int. 2011;22(4):1069–1077. doi: 10.1007/s00198-010-1307-1. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O’brien CP. An improved diagnostic evaluation instrument for substance abuse patients: the Addiction Severity Index. The J Nerv Ment Dis. 1980;168(1):26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Mondy K, Yarasheski K, Powderly WG, Whyte M, Claxton S, DeMarco D, Hoffmann M, Tebas P. Longitudinal Evolution of Bone Mineral Density and Bone Markers in Human Immunodeficiency Virus—Infected Individuals. Clin Infect Dis. 2003;36(4):482–490. doi: 10.1086/367569. [DOI] [PubMed] [Google Scholar]
- Munk-Jensen N, Nielsen SP, Obel EB, Eriksen PB. Reversal of postmenopausal vertebral bone loss by estrogen and progestogen: a double blind placebo controlled study. Br Med J (Clin Res Ed) 1988;296(6630):1150. doi: 10.1136/bmj.296.6630.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevitt MC, Cummings SR, Browner WS, Seeley DG, Cauley JA, Vogt SR, Black DM. The accuracy of self-report of fractures in elderly women: evidence from a prospective study. Am J Epidemiol. 1992;135(5):490–499. doi: 10.1093/oxfordjournals.aje.a116315. [DOI] [PubMed] [Google Scholar]
- Nyquist F, Ljunghall S, Berglund M, Obrant K. Biochemical markers of bone Metabolism after short and long time ethanol withdrawal in alcoholics. Bone. 1996;19:51–54. doi: 10.1016/8756-3282(96)00110-x. [DOI] [PubMed] [Google Scholar]
- Rey D, Treger M, Sibilia J, Priester M, Bernard-Henry C, Cheneau C, Javier RM. Bone mineral density changes after 2 years of ARV treatment, compared to naive HIV-1-infected patients not on HAART. Infect Dis (Lond) 2015;47(2):88–95. doi: 10.3109/00365548.2014.968610. [DOI] [PubMed] [Google Scholar]
- Rey-Sanchez P, Lavado-Garcia JM, Canal-Macias ML, Rodriguez-Dominguez MT, Bote-Mohedano JL, Pedrera-Zamorano JD. Ultrasound bone mass in patients Undergoing chronic therapy with oral anticoagulants. J Bone Miner Metab. 2011;29(5):546–51. doi: 10.1007/s00774-010-0250-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Tolrà J, Torremadé J, Di Gregorio S, Del Rio L, Franco E. Effects of testosterone treatment on bone mineral density in men with testosterone deficiency syndrome. Andrology. 2013;1(4):570–5. doi: 10.1111/j.2047-2927.2013.00090.x. [DOI] [PubMed] [Google Scholar]
- Russell RG. Bisphosphonates: the first 40 years. Bone. 2011;49(1):2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Sebring NG, Denkinger BI, Menzie CM, et al. Validation of three food frequency questionnaires to assess dietary calcium intake in adults. J Am Diet Assoc. 2007;107:752–9. doi: 10.1016/j.jada.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Flom PL, Weedon J, Klein RS. Prospective study of bone mineral density changes in aging men with or at risk for HIV infection. AIDS. 2010;24(15):2337. doi: 10.1097/QAD.0b013e32833d7da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Shi Q, Hoover DR, Anastos K, Tien PC, Young MA, Cohen MH, Golub ET, Gustafson D, Yin MT. Increased Fracture Incidence in Middle-Aged HIV-Infected and HIV-Uninfected Women: Updated Results From the Women’s Interagency HIV Study. J Acq Immun Def Synd. 2015;70(1):54–61. doi: 10.1097/QAI.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Janavs J, Sheehan KH, Sheehan M, Gray C. Mini international neuropsychiatric interview 6.0: High Prevalence Disorders, English version. Tampa, FL: 2010. [Google Scholar]
- Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, Berger ML, Santora AC, Sherwood LM. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA. 2001;286:2815–2822. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol. 1982;43(11):1157–70. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Sobell C, Sobell MB. Measuring alcohol consumption. Humana Press; Totowa, NJ: 1992. Timeline follow-back; pp. 41–72. [Google Scholar]
- Stellbrink HJ, Orkin C, Arribas JR, Compston J, Gerstoft J, Van Wijngaerden E, Lazzarin A, Rizzardini G, Sprenger HG, Lambert J, Sture G. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010;51(8):963–72. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- Tucker KL, Jugdaohsingh R, Powell JJ, Qiao N, Hannan MT, Sripanyakorn S, Cupples LA, Kiel DP. Effects of beer, wine, and liquor intakes on bone mineral density in older men and women. Am J Clin Nutr. 2009;89(4):1188–1196. doi: 10.3945/ajcn.2008.26765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RT. Skeletal response to alcohol. Alcohol Clin Exp Res. 2000;24:1693–1701. [PubMed] [Google Scholar]
- Van Staa TP, Leufkens HGM, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13(10):777–787. doi: 10.1007/s001980200108. [DOI] [PubMed] [Google Scholar]
- Ventura AS, Winter MR, Heeren TC, Sullivan MM, Walley AY, Holick MF, Patts GJ, Meli SM, Samet JH, Saitz R. Lifetime and recent alcohol use and bone mineral density in adults with HIV infection and substance dependence. Medicine. 2017;96(17) doi: 10.1097/MD.0000000000006759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker Harris V, Brown TT. Bone loss in the HIV-infected patient: evidence, clinical implications, and treatment strategies. J Infect Dis. 2012;205(suppl_3):S391–S398. doi: 10.1093/infdis/jis199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KE. Practical Considerations When Replacing a DXA System. Spine. 2011;(1):1–2. [Google Scholar]
- Womack JA, Goulet JL, Gibert C, Brandt C, Chang CC, Gulanski B, Fraenkel L, Mattocks K, Rimland D, Rodriguez-Barradas MC, Tate J. Increased risk of fragility fractures among HIV infected compared to uninfected male veterans. PloS One. 2011;6(2):e17217. doi: 10.1371/journal.pone.0017217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–2526. doi: 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin M. Vitamin D, bone, and HIV infection. Top Antivir Med. 2012;20(5):168. [PMC free article] [PubMed] [Google Scholar]
- Young B, Dao CN, Buchacz K, Baker R, Brooks JT. Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000–2006. Clin Infect Dis. 2011;52(8):1061–1068. doi: 10.1093/cid/ciq242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.