Abstract
In recent years, drug development costs have soared, primarily due to the failure of preclinical animal and cell culture models, which do not directly translate to human physiology. Organ-on-a-chip (OOC) is a burgeoning technology with the potential to revolutionize disease modeling, drug discovery, and toxicology research by strengthening the relevance of culture-based models while reducing costly animal studies. Although OOC models can incorporate a variety of tissue sources, the most robust and relevant OOC models going forward will include stem cells. In this review, we will highlight the benefits of stem cells as a tissue source while considering current limitations to their complete and effective implementation into OOC models.
Keywords: induced pluripotent stem cells, embryonic stem cells, organ-on-a-chip, differentiation, cell culture, microfluidics, 3D culture
Graphics Abstract
1. Introduction
In recent years, pharmaceutical research has become increasingly inefficient, with costs associated with preclinical work and clinical trials skyrocketing as the number of successful drug candidates plummets. In 2014, the cost of producing one new drug was estimated to be as high as $1.2 billion, with the combined research and approval processes taking an average of 10 years [1]. A sizeable portion of these expenses is associated with preclinical drug testing in cell culture and animal models. As evidence mounts that both types of preclinical models are limited in their ability to faithfully replicate human pathophysiology, it is becoming clearer that translational science is in dire need of a new model system to complement existing models that is more physiologically representative of human disease.
A variety of animal models, ranging from rats to pigs to non-human primates, have been utilized in studies of human diseases in nearly all organ systems, including the cardiovascular system and liver [2,3]. However, a number of investigational compounds that showed initial promise in preclinical trials in animals failed in human clinical trials [4–6]. The major assumption justifying the use of animals in drug discovery and toxicology research is that animal models are predictive of human response [7]. While some of the failures of animal models can be attributed to a lack of standardized experimental conditions, the majority are likely due to the failure of this assumption [8]. Animal models do currently have the advantage of allowing study of multi-organ interactions and system-wide drug effects. However, the inbreeding of many laboratory animals limits the genetic variability of these models, which is not representative of the diversity of the human population to which these results will be applied (Figure 1).
Figure 1. Genetic Heterogeneity of Induced Pluripotent Stem Cells (iPSCs) Compared to Laboratory Animals.
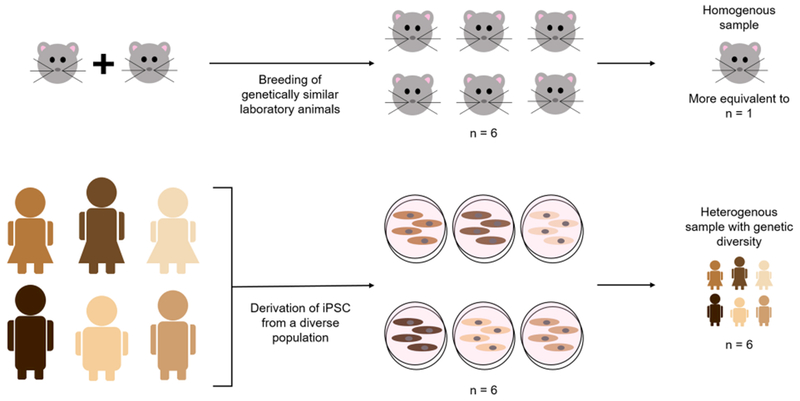
Laboratory animals, especially rodents, are often inbred and are thus very genetically similar. As a result, it is often difficult to reach population-level conclusions on drug safety and efficacy or disease mechanisms from animal studies. In contrast, iPSCs can be derived from a diverse patient population, producing data more representative of the relevant human population.
Similarly, most commonly used in vitro drug testing methods do not accurately recapitulate the pathophysiology of human disease, even in the cases where human cells are used. Dissociated cell culture of primary cells and immortalized cell lines in 2D dishes is the main cell culture model currently used to evaluate drug efficacy and toxicology due to its availability, ease of use, and relatively low cost [9]. However, typical 2D culture methods are limited in that they frequently only incorporate one cell type without providing the cell matrix and mechanical cues found in in vivo tissue [10]. These factors have significant implications for cell morphology and phenotype, which in turn can affect drug response [11]. Even current 3D models do not always include physiologically relevant scaffold architecture or extracellular matrix components [10,12,13]. Additionally, static 2D and 3D models also do not account for the interconnectivity of organs, which becomes important when drugs of interest are metabolized by one organ into compounds that produce differing effects in downstream organs [9].
Organ-on-a-chip (OOC) technology is a promising complement to current preclinical models that can potentially combine patient-specific cell models, 3D tissue culture, microfluidics, and high throughput analysis methods to create a powerful tool for disease modeling, drug screening, and toxicity testing. Generally, the term OOC encompasses any device that incorporates cells into a microfluidic system within an engineered architecture that attempts to replicate some or all aspects of the native tissue structure. By this definition, all OOCs include a microfluidic chip with cultured cells or tissue and either a pump-driven or pumpless supply of culture media or other nutrient sources, and some incorporate 3D cell culture techniques, although this is not yet common practice [13–17]. Many OOCs also incorporate co-culture to look at interactions among different cell types within an organ [18]. This principle can be extended to the integration of multiple different OOCs into one body-on-a-chip (BOC) device, which provides a physical mimic of physiologically-based pharmacokinetic models that can be combined with computational pharmacokinetic and systems biology models to produce a better understanding of drug metabolism, bioavailability, and distribution in human systems [19–22]. Additionally, comparing human and animal cell-based BOC systems could facilitate extrapolation of preclinical animal work to human clinical trials [23]. BOC and OOC technologies are currently limited in their adoption and application due to the technical knowledge required for device design and cell culture, as well as the lack of integration with available high-throughput screening methods. However, OOC research has significantly expanded in the past several years and ongoing research both in industry and academia has the potential to increase the adoption of these technologies in drug discovery (Figure 2) [10].
Figure 2. Organ-on-a-Chip Publications.
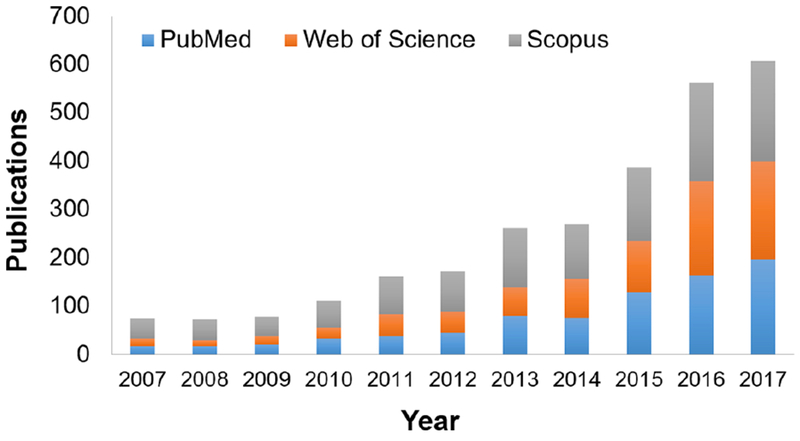
Publications related to organ-on-a-chip devices each year since 2007, defined by a search for “organ on chip” on PubMed, Web of Science, and Scopus. Publications may be duplicated across databases.
One of the most important parameters in OOC design is the biological tissue source. Stem cells will allow us to source cells from humans without requiring a tissue biopsy. By definition, a stem cell is any cell that can self-renew and has the potential to differentiate into one or more specialized cell types. The most common types of stem cells used in biological research are embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and adult stem cells (ASCs). These cells can be transformed into a variety of terminally-differentiated adult cell types, which can then be used for disease modeling or toxicity testing [24]. A variety of studies have already established the applicability of stem cells to drug testing and disease modeling as an alternative or additional preclinical model, demonstrating patient-specific drug responses and replicating clinical manifestations that were not observed in other preclinical models [25–28]. Stem cell technologies also allow investigation of diseases and drug effects in a more diverse population than for cell lines or animals (Figure 1). Stem cell technology and differentiation techniques have seen substantial improvements in recent years, and although there are still some obstacles to complete implementation, stem cells will likely be widely applicable to OOC models in the near future (Figure 3).
Figure 3. Tissue Sources for Organ-on-a-Chip (OOC) Devices.
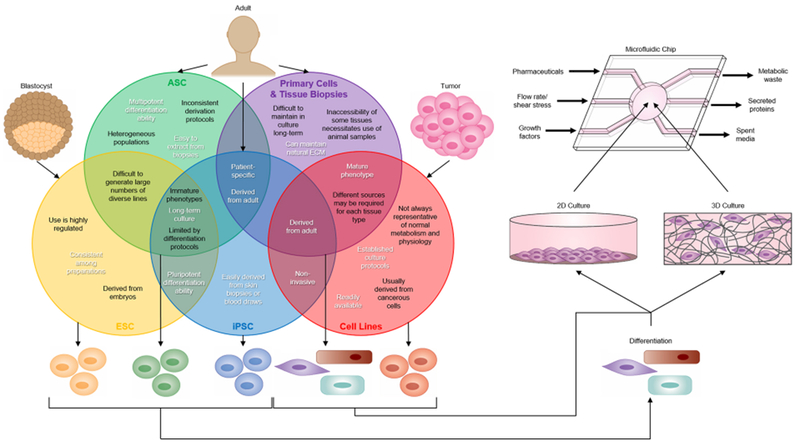
Embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and adult stem cells (ASCs) can be differentiated and incorporated into microfluidic chips in the same way as cell lines and primary cells. The Venn diagram illustrates advantages (in white) and limitations (in black) for the use of ESCs, ASCs, iPSCs, primary cells and tissue biopsies, and cell lines in OOC devices. Cell lines and primary cells have been more commonly used in OOCs because they generally have well-characterized biological responses. However, cell lines are not representative of normal physiology, and primary cells have limited culture time and inconsistent quality. In contrast, stem cells are easy to obtain and represent an essentially unlimited cell source. Even with current limitations in differentiation and maturation protocols, stem cells are a promising technology for incorporation into OOC devices.
2. Current Stem Cell Technologies
2.1. Adult Stem Cells (ASCs)
Human ASCs, the most common of which are mesenchymal stem cells (MSCs), are multipotent stem cells extracted from adult tissues. MSCs are typically derived from either the bone marrow or adipose tissue, making them an attractive option because they are relatively easy to extract from tissue biopsies [29]. They are multipotent stem cells, meaning that they can only differentiate into a limited number of cell types. MSCs are most commonly differentiated into mesodermal cells of bone, muscle, cartilage, and fat lineages [29]. In addition to being isolated from multiple different starting tissues, MSCs are isolated and cultured with a diverse range of protocols, leading to heterogeneous phenotypes in populations of MSCs differing from lab to lab and even from preparation to preparation [30]. Due to both their limited differentiation ability and the lack of consistent derivation protocols and defined biological responses, MSCs are less applicable to OOC models than their pluripotent counterparts.
2.2. Embryonic Stem Cells (ESCs)
Human ESCs are derived either from the blastocyst or from the inner cell mass of embryos. Depending on their source, they are either totipotent or pluripotent cells, both of which can differentiate into any type of adult human cell from any of the three germ layers [31]. An estimated 200 to 300 different ESC lines have been derived from different laboratories around the world, but unlike MSCs, comparative studies of these lines have demonstrated similar levels of pluripotency and surfaces marker gene expression among the different lines [32]. Although ESCs do have an unlimited differentiation potential and a more consistent phenotype than MSCs, the fact that human ESCs must be derived from human embryos makes their use ethically controversial, which has in turn led to increased regulation of their use in research. Due to the ethical debate surrounding ESCs and the technical difficulties generating large numbers of genetically diverse cell lines, it is harder to apply human ESCs than alternatives to precision medicine for disease modeling and therapeutic drug evaluations.
2.3. Induced Pluripotent Stem Cells (iPSCs)
Like ESCs, iPSCs are pluripotent and can differentiate into cell types from all three germ layers [33]. Human iPSCs were first derived in 2007 by introducing four defined factors (Oct4, Nanog, Sox2, and c-Myc) into human adult fibroblast cells [34]. Protocols now exist to derive iPSCs using a wide selection of transfection methods [35] and adult somatic cell types including skin fibroblasts, adipose stromal cells, and blood samples [36–38]. Because iPSCs are derived from adult tissue instead of embryonic tissue, they avoid the ethical concerns associated with ESCs. ESCs and iPSCs derived from the same genetic background have no significant differences in gene expression levels, surface marker expression, or morphology [39,40]. In addition to sidestepping ethical controversies, another advantage of iPSCs over ESCs is that they can be derived from donors with known disease phenotypes, whose cells can then be used in patient-specific disease models and drug screens.
2.4. Stem Cell Differentiation
The principal cells of interest for toxicity testing are cardiomyocytes, neurons, and hepatocytes, as their respective organs are the most likely to exhibit a drug-induced toxicity [24,33]. However, primary cells and tissue samples from these organs are difficult to obtain. As a result, the development of iPSC and ESC differentiation protocols have primarily focused on these three cell types [41]. Cardiomyocytes can be derived from iPSCs and ESCs with a chemically defined medium for the evaluation of drug response and cardiotoxicity using transcriptome profiling and functional analyses [25,42]. Studies have shown a predictive response of patient-specific iPSCs, including those derived from patients with known cardiac conditions, to cardiotoxic therapeutics such as tyrosine kinase inhibitors, doxorubicin, roglitazone, nicorandil, and cisapride [25,26,28,43]. Patient-specific neurons, neural stem cells, and motor neurons have been derived from iPSCs and used in drug screens for Alzheimer’s disease, Neimann-Pick disease type C, and amyotrophic lateral sclerosis [44–46]. Protocols to produce hepatocyte-like cells from iPSCs have been implemented in studies of patient-specific hepatotoxicity and drug-induced liver injury [47,48]. These cell types are also relevant in disease modeling and mechanism studies, including for neurological diseases such as schizophrenia [49,50] and Alzheimer’s [51] and cardiovascular diseases such as familial dilated cardiomyopathy [52].
In addition to these cell types, iPSC and ESC differentiation protocols have been expanded to include a range of cell types from retinal cells to skeletal muscle [53,54]. Although these studies all illustrate the promise of iPSC- and ESC-derived cells in disease modeling, drug screening, and toxicity testing, many of them were limited to traditional 2D culture techniques, restricting their clinical applicability due to their lower throughput, unrealistic cell microenvironment, and limited number of co-cultured cell types [10]. By incorporating iPSC- and ESC-derived cells into OOCs, researchers can take advantage of the unique ability to create a wide variety of cell types combined with the potential to create high-throughput systems that have a complete 3D cell microenvironment as well as the ability to provide multiple stimuli and asses multiple functional readouts [12,13,55].
3. Biological Tissue Sources for OOCs
Although there is a clear potential for incorporating stem cells into OOC devices, this is far from common practice. The most common types of cell used in OOC devices to date are primary cells and tissue biopsies and cell lines, with stem cell sources only occasionally used (Figure 4) [56,57]. Cell lines have been incorporated into OOC models for organs ranging from the liver to the placenta [58,59]. They have also been used to study organ interactions in multi-organ chip systems, including combinations of the lung, liver, gastrointestinal tract, kidneys, skin, bone marrow, and adipose tissue [15,60]. Primary cells have been used in OOC simulations of a variety of tissues, including heart valves, lung, kidney proximal tubule, liver, and neurovascular units [14,61–64]. However, some of these studies were limited to using animal primary cells due to the difficulty of extracting human primary tissue and, in other cases, co-cultures with established cell lines were required to more accurately model cell- or organ-level interactions [14,61,62]. Ex vivo tissue culture has been used in several OOC models, including co-cultures of intestinal and liver slices and culture of endocrine tissues [65,66]. A combination of all three tissue sources was used in at least one study that modeled multi-organ interactions among a skin biopsy, an intestinal barrier model containing primary cells, and a liver cell line, demonstrating that the appropriate tissue source may vary among organs [67].
Figure 4. Prevalence of Varied Tissue Sources in Organ-on-a-chip Research.

Online search was completed for the listed keywords plus “lab chip”. Original research articles produced by the search were then manually screened to determine the cell types used. Review articles, editorials, and commentary papers were excluded from analysis. Primary cells and tissues were the most common tissue source used in organ-on-a-chip research publications.
Each of these sources has a unique set of advantages, but each is also limited in comparison to stem cells (Figure 3). Tissue biopsies and primary cells are similar in that they are derived directly from adult tissue, and thus provide potentially more accurate information on the biological properties of mature tissue [57]. Tissue biopsies have the added benefit of maintaining some of the natural extracellular matrices and three-dimensional tissue structures that are not obtainable with 2D cell cultures. As discussed previously, dissociated cells can be incorporated into a 3D architecture, but current methods often lack the organ-specific structures and matrix components found in tissue biopsies. One specific limitation of tissue-based devices is that tissue samples often do not survive more than 48 hours in ex vivo culture, which restricts their ability to be studied long-term [57].
Cell lines are similar to primary cells in that they are a source of more mature cells, with the same limitation of lack of natural extracellular matrix. Additionally, cell lines are more likely to be a homogenous population and to produce reproducible results compared to primary cells, but, as a tradeoff, they lack the patient-specificity found in primary cells, tissue biopsies, and stem cells. The lack of patient-specificity limits the use of cell lines for disease modeling studies. For toxicity testing, the cell lines used often have induced overexpression of proteins known to be involved in specific toxicity-related pathways, and as such are limited to assessing known mechanisms of toxicity [9]. One study evaluating cell lines for organ-specific drug responses observed that the majority of compounds had similar effects in all three of the cell lines studied, with little correlation to in vivo organ-specific toxicities [68]. Additionally, there is growing evidence that cell lines may not accurately recapitulate tissue function. One comparison of protein expression in a hepatoma cell line and primary hepatocytes indicated that the cell line had downregulated drug-metabolizing enzymes and some normal metabolic pathways to favor cell-cycle-associated proteins [69]. Thus, while cell lines may currently be one of the more commonly used biological tissue source for OOCs, they are far from being the most applicable.
Despite the clear advantages to using primary cells, biopsies, and cell lines, stem cell-based cultures provide advantages in that they are often easier to obtain, last longer in culture, and have the potential to differentiate into many cell types. Stem cells provide the potential to recreate multiple organ-like structures with the same genetic background, which is useful in disease modeling and drug testing for genetic diseases. However, there are limitations to current stem cell technologies that will need to be resolved before they become widely applicable to OOC models for all organ types.
4. Current Limitations of Stem Cells in OOCs
One of the biggest obstacles to the use of stem cells is the variability in efficiency and robustness of differentiation protocols. The differentiation of stem cells into distinct, mature cell phenotypes is also limited. For example, the heart is composed of a mixture of cardiomyocytes, cardiac fibroblasts, and endothelial cells. Well-defined differentiation protocols exist for cardiomyocytes and endothelial cells, but not for cardiac fibroblasts. Some studies have succeeded in producing fibroblast-like cells that recapitulate the morphology of cardiac fibroblasts [26]. However, the lack of specific cell type markers for cardiac fibroblasts, beyond myofibroblast and mesenchymal genes common to all fibroblast cell lineages, makes it difficult to determine the efficiency of differentiation protocols.
Even in cells for which clear markers exist, such as cardiomyocytes and endothelial cells, there is variability in the differentiation methods used across different research groups, including in the small molecules used to induce differentiation and the environment in which the cells are cultured [42,70–74]. Early protocols [74,75] have continually been modified to make differentiation more efficient [76,77]. This is not limited to cells of cardiovascular lineage; differentiation protocols for other cell types, such as skeletal muscle, also vary among research groups [78,79]. In part, this is because the field is constantly improving protocols and adoption of new protocols across different laboratories can be slow. Nevertheless, the constant improvement in existing protocols and the development of protocols for differentiating into new cell types, such as a recently published method which illustrated differentiation into podocytes [80], will increase the applicability of stem cells to OOC models.
Once the stem cells differentiate into the desired cell type, the next obstacle is ensuring that the cells achieve a mature adult phenotype that is representative of the adult organ [33]. For example, ESC- and iPSC-cardiomyocytes derived using current protocols are more similar to fetal than adult heart tissue on the transcriptome level, and have immature electrophysiological behaviors, metabolism, calcium-handling, and sarcomere structural features [81–83]. While this is a concern for incorporation of these cells into an OOC model, numerous research groups are developing methods for pushing the cardiomyocytes towards a more mature phenotype, such as electrical or mechanical stimulation, geometrical confinement, overexpressing maturation-related microRNAs, introducing growth hormone, and increasing culture time [84–86]. Recent publications have demonstrated that advanced maturation can be achieved in 3D cardiac tissues by electrically stimulating tissue formed from early stage iPSC-cardiomyocytes [87] and that progressively changing the afterload experienced by cardiac tissues can improve maturation or induce a disease-like state [88].
In addition to the relative immaturity of most iPSC-cardiomyocytes, another issue is separating out individual cell subtypes. Cardiomyocytes can be atrial, ventricular, or nodal, and differentiation protocols typically result in a mixture of all three subtypes. While protocols exist to derive each of these cell subtypes independently [89–91], protocols which result in a heterogeneous population are much more common. Even with differentiation into individual subtypes, it is unclear which of these subtypes would be relevant to include in OOC models and the relative proportions they would need for incorporation.
Like iPSC- and ESC-derived cardiomyocytes, stem cell-derived endothelial cells, neurons, and hepatocytes display an immature phenotype and a lack of distinctly separable cell subtypes. Endothelial cells can be consistently derived with high purity, but protocols to separately differentiate arterial, venous, valvular, and lymphatic endothelial cells are still lacking [71–73]. ESC- and iPSC-derived neuronal cells also usually contain a heterogeneous population of neural subtypes. Although several protocols for specific neuronal subtypes exist, these have low efficiency [33,49,92]. Similar to the cardiomyocytes, stem cell-derived hepatocytes are often called hepatocyte-like cells, as they express more fetal markers than adult markers and have lower metabolic activity than primary cultures [93–95].
Another limitation of iPSC- and ESC-derived cells in OOCs beyond the cell phenotype is the lack of native 3D tissue structure. Unlike tissue biopsies, which contain the native extracellular matrix, stem cell-based cultures have derivation protocols that usually utilize 2D culture conditions. A number of laboratories have approached this issue by creating microtissues, usually containing iPSC-derived cells and an extracellular matrix. Some of these microtissues have been developed for heart OOC models, thus heightening the relevance of iPSC- and ESC-derived cardiomyocytes by incorporating a 3D scaffold and improving their maturation [96–98]. Although this is a promising improvement, it is not yet common practice, and some parameters of these types of models will need to be optimized before they are fully representative of human physiology. These may involve the use of matrix components found in native heart tissue and the incorporation of the multiple other cell types, including fibroblasts and endothelial cells, at the same ratios found in vivo.
Another consideration for using stem cells in OOCs is whether differentiation will be conducted on or off the chip. On-chip differentiation would permit precise control of the cell microenvironment and culture conditions, including relevant chemical, electrical, and mechanical cues for differentiation, while simultaneously measuring a variety of functional outputs [99–102]. Yoshimitsu et al. described the expansion of human iPSCs in a microfluidic chip after optimizing both surface coating and media flow rate. They demonstrated differentiation of the iPSCs into extra-embryonic trophoblast lineage cells and observed that iPSCs in the microfluidic chip exhibited the expected response to anti-tumor drugs [101]. Giobbe et al. investigated the effect of perfusion frequency on iPSCs and ESCs, and found that controlling the frequency affected pluripotency gene expression of the stem cells and the extent of homogenous differentiation of the cells into ectodermal, mesodermal, and endodermal lineages once the cells were cultured in differentiation media. They also demonstrated successful differentiation into functional cardiomyocytes with approximately 65% efficiency, as well as on-chip differentiation of eight human iPSC lines into hepatocyte-like cells [103]. Similarly, Hesari et al. successfully differentiated human iPSCs into neurons on a hybrid microfluidic chip containing aligned poly(lactic-co-glycolic) acid nanofibers. The differentiation of neural cells on the hybrid device increased the expression of neural marker genes compared to 2D cultures and scaffolds alone, demonstrating the potential for these devices to help overcome some of the issues associated with stem cell-based cultures [104].
Taken together, these studies show the promise of microfluidics in stem cell culture and differentiation. However, they also point to some of the potential design problems for microfluidic OOC systems, especially in the context of multi-organ devices or individual OOCs incorporating more than one cell type. With different flow rates, scaffolding, culture time, and biophysical and chemical stimuli required for differentiation of different cell types, device design will become much more complex, making widespread adoption of the technology challenging. One potential solution would be to culture individual OOCs and combine them into one multiorgan system once all of the components completed their differentiation, as suggested by Loskill et al. in their μOrgano chip design [105]. However, this type of design would still be limited by the difficulty of timing the differentiation of the multiple components and by the types of connections that are possible among the organs. It also does not address the issues of how to derive multiple cell types for use within a single organ model and how to optimize the cell culture media to promote survival of all cells once the organs are connected.
Additionally, a question that is applicable to all OOC models, with special relevance for stem cell-based models, is if we care about modeling the general population, whose cells do we test? Regardless of whether primary cells or stem cells are used, there is an inherent variability in genotype from patient to patient, which can potentially translate to major differences in phenotype, including in drug metabolism and response [106]. Drug screening for efficacy can use patient-derived cells as a model, but identifying representative cell sources for toxicity testing will prove more challenging and may require the use of population-based genomics and computational modeling [107]. Disease modeling and mechanism studies using OOC models will also need to incorporate cells from multiple patients to demonstrate consistency in mechanism across patients of different genetic backgrounds.
Finally, it is important to take into consideration the greater time and cost currently associated with designing stem cell-based OOC devices. In contrast to cell lines, which are widely available, and primary cells, which can be isolated relatively quickly when tissue samples are available, stem cell-based models take a long time and a great deal of resources to develop (Figure 5). The process of deriving iPSC from blood or skin samples can take weeks to months, and differentiation protocols often take multiple additional weeks. Traditional differentiation protocols also generally yield a limited number of cells, especially for quiescent cell types such as cardiomyocytes. These factors limit the feasibility of commercial production of stem cells and their derivatives and make it difficult for research groups new to stem cell technology to begin using it. Large scale production of stem cell-derived cells, such as recent protocols developed for iPSC-cardiomyocytes, will improve accessibility [108,109]. However, cryopreservation of differentiated stem cells is often trickier and less successful than cryopreservation of cell lines or primary cells, which increases the difficulty of widespread distribution. For these reasons, research groups interested in using stem cells in OOC development should be prepared for the slower progress rate associated with their use. Despite these challenges, incorporation of stem cells into OOC devices is not impossible, and will ultimately lead to increased applicability of these devices as clinical models.
Figure 5. Timeline of Incorporation of iPSC into Organ-on-a-Chip Devices.

A representative timeline for incorporation of an iPSC line into an organ-on-a-chip device, from reprogramming of the line through drug testing or characterization studies.
5. Conclusions
OOC systems are a promising technology for drug screening, disease modeling, and toxicity testing applications. By incorporating multiple cell types and multiple functional analyses, they have the potential to model human pathophysiology and measure drug response more accurately than current preclinical studies, which typically employ 2D cell culture and animal models. Stem cell differentiation and maturation protocols require improvement before stem cells can be considered accurate models of human physiology. However, because stem cells are easier to obtain than many primary cell types and tissue biopsies, and because they are more physiologically representative than cell lines, it is likely that stem cells will be the primary tissue source for OOCs going forward. Continued research on methods for on-chip differentiation of stem cells into functional organ models will contribute to both improvement in stem cell methods and advancement of OOC technologies.
Acknowledgements
This publication was supported in part by the Department of Defense (DoD) through the National Defense Science & Engineering Graduate Fellowship (NDSEG) Program (A.W.), the Stanford Cardiovascular Institute (CVI) (H.Y.), the Gooter Foundation seed grant (H.Y.), American Heart Association (AHA) Postdoctoral Fellowship Award 18POST34030106 (H.Y.) and by research grants from the National Institutes of Health (NIH) R01 HL1130020, R01 HL128170, R01 HL113006, and California Institute of Regenerative Medicine (CIRM) DR2A-05394 (J.C.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- [1].Ciociola AA, Cohen LB, Kulkami P, Kefalas C, Buchman A, Burke C, Cain T, Connor J, Ehrenpreis ED, Fang J, Fass R, Karlstadt R, Pambianco D, Phillips J, Pochapin M, Pockros P, Schoenfeld P, Vuppalanchi R, How drugs are developed and approved by the FDA: Current process and future directions, Am. J. Gastroenterol 109 (2014) 620–623. doi: 10.1038/ajg.2013.407. [DOI] [PubMed] [Google Scholar]
- [2].Hasenfuss G, Animal models of human cardiovascular disease, heart failure and hypertrophy., Cardiovasc. Res 39 (1998) 60–76. doi: 10.1016/S0008-6363(98)00110-2. [DOI] [PubMed] [Google Scholar]
- [3].Liu Y, Meyer C, Xu C, Weng H, Hellerbrand C, ten Dijke P, Dooley S, Animal models of chronic liver diseases, AJP Gastrointest. Liver Physiol 304 (2013) G449–G468. doi: 10.1152/ajpgi.00199.2012. [DOI] [PubMed] [Google Scholar]
- [4].Eastwood D, Findlay L, Poole S, Bird C, Wadhwa M, Moore M, Burns C, Thorpe R, Stebbings R, Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4 + effector memory T-cells, Br. J. Pharmacol 161 (2010) 512–526. doi: 10.1111/j.l476-5381.2010.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N, Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412, N. Engl. J. Med 355 (2006) 1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- [6].Coussens LM, Fingleton B, Matrisian LM, Matrix metalloproteinase inhibitors and cancer: Trials and tribulations, Science (80-. ). 295 (2002) 2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- [7].Shi Y, Inoue H, Wu JC, Yamanaka S, Induced pluripotent stem cell technology: A decade of progress, Nat. Rev. Drug Discov 16 (2017)115–130. doi: 10.1038/nrd.2016.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Greek R, Menache A, Systematic reviews of animal models: Methodology versus epistemology, Int. J. Med. Sci 10 (2013) 206–221. doi: 10.7150/ijms.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Astashkina A, Mann B, Grainger DW, A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity, Pharmacol. Ther 134 (2012) 82–106. doi: 10.1016/j.pharmthera.2012.01.001. [DOI] [PubMed] [Google Scholar]
- [10].Horvath P, Aulner N, Bickle M, Davies AM, Del Nery E, Ebner D, Montoya MC, Östling P, Pietiäinen V, Price LS, Shorte SL, Turcatti G, von Schantz C, Carragher NO, Screening out irrelevant cell-based models of disease, Nat. Rev. Drug Discov 15 (2016) 751–769. doi: 10.1038/nrd.2016.175. [DOI] [PubMed] [Google Scholar]
- [11].Breslin S, O’Driscoll L, Three-dimensional cell culture: The missing link in drug discovery, Drug Discov. Today. 18 (2013)240–249. doi: 10.1016/j.drudis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- [12].Picollet-D’hahan N, Dolega ME, Liguori L, Marquette C, Le Gac S, Gidrol X, Martin DK, A 3D toolbox to enhance physiological relevance of human tissue models, Trends Biotechnol 34 (2016) 757–769. doi: 10.1016/j.tibtech.2016.06.012. [DOI] [PubMed] [Google Scholar]
- [13].Huh D, Hamilton GA, Ingber DE, From 3D cell culture to organs-on-chips, Trends Cell Biol 21 (2011) 745–754. doi: 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Esch MB, Ueno H, Applegate DR, Shuler ML, Modular, pumpless body-on-a-chip platform for the co-culture of GI tract epithelium and 3D primary liver tissue, Lab Chip. 16 (2016) 2719–2729. doi: 10.1039/C6LC00461J. [DOI] [PubMed] [Google Scholar]
- [15].Miller PG, Shuler ML, Design and demonstration of a pumpless 14 compartment microphysiological system, Biotechnol. Bioeng 113 (2016) 2213–2227. doi: 10.1002/bit.25989. [DOI] [PubMed] [Google Scholar]
- [16].Sung JH, Shuler ML, A micro cell culture analog (μCCA) with 3-D hydrogel culture of multiple cell lines to assess metabolism-dependent cytotoxicity of anti-cancer drugs, Lab Chip. 9 (2009) 1385. doi: 10.1039/b901377f. [DOI] [PubMed] [Google Scholar]
- [17].Toh Y-C, Zhang C, Zhang J, Khong YM, Chang S, Samper VD, van Noort D, Hutmacher DW, Yu H, A novel 3D mammalian cell perfusion-culture system in microfluidic channels, Lab Chip. 7 (2007) 302. doi: 10.1039/b614872g. [DOI] [PubMed] [Google Scholar]
- [18].Guo X, Gonzalez M, Stancescu M, Vandenburgh HH, Hickman JJ, Neuromuscular junction formation between human stem cell-derived motoneurons and human skeletal muscle in a defined system, Biomaterials. 32 (2011) 9602–9611. doi: 10.1016/j.biomaterials.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sung JH, Esch MB, Shuler ML, Integration of in silico and in vitro platforms for pharmacokinetic–pharmacodynamic modeling, Expert Opin. Drug Metab. Toxicol 6 (2010) 1063–1081. doi: 10.1517/17425255.2010.496251. [DOI] [PubMed] [Google Scholar]
- [20].Sung JH, Srinivasan B, Esch MB, McLamb WT, Bemabini C, Shuler ML, Hickman JJ, Using physiologically-based pharmacokinetic-guided “body-on-a-chip” systems to predict mammalian response to drug and chemical exposure, Exp. Biol. Med 239 (2014) 1225–1239. doi: 10.1177/1535370214529397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cosgrove BD, Griffith LG, Lauffenburger DA, Fusing tissue engineering and systems biology toward fulfilling their promise, Cell. Mol. Bioeng 1 (2008) 33–41. doi: 10.1007/sl2195-008-0007-9. [DOI] [Google Scholar]
- [22].Esch MB, Smith AST, Prot JM, Oleaga C, Hickman JJ, Shuler ML, How multiorgan microdevices can help foster drug development, Adv. Drug Deliv. Rev 69–70 (2014) 158–169. doi: 10.1016/j.addr.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Esch MB, King TL, Shuler ML, The role of body-on-a-chip devices in drug and toxicity studies, Annu. Rev. Biomed. Eng 13 (2011) 55–72. doi: 10.1146/annurev-bioeng-071910-124629. [DOI] [PubMed] [Google Scholar]
- [24].Csöbönyeiová M, Polák Š, Danišovič L, Toxicity testing and drug screening using iPSC-derived hepatocytes, cardiomyocytes, and neural cells, Can. J. Physiol. Pharmacol 94 (2016) 687–694. doi: 10.1139/cjpp-2015-0459. [DOI] [PubMed] [Google Scholar]
- [25].Matsa E, Burridge PW, Yu KH, Ahrens JH, Termglinchan V, Wu H, Liu C, Shukla P, Sayed N, Churko JM, Shao N, Woo NA, Chao AS, Gold JD, Karakikes I, Snyder MP, Wu JC, Transcriptome profiling of patient-specific human iPSC-cardiomyocytes predicts individual drug safety and efficacy responses in vitro, Cell Stem Cell. 19 (2016) 311–325. doi: 10.1016/j.stem.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sharma A, Burridge PW, McKeithan WL, Serrano R, Shukla P, Sayed N, Churko JM, Kitani T, Wu H, Holmström A, Matsa E, Zhang Y, Kumar A, Fan AC, del Álamo JC, Wu SM, Moslehi JJ, Mercola M, Wu JC, High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells, Sci. Transl. Med 9 (2017) eaaf2584. doi: 10.1126/scitranslmed.aaf2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mathur A, Loskill P, Shao K, Huebsch N, Hong SG, Marcus SG, Marks N, Mandegar M, Conklin BR, Lee LP, Healy KE, Human iPSC-based cardiac microphysiological system for drug screening applications, Sci. Rep 5 (2015) 1–7. doi: 10.1038/srep08883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liang P, Lan F, Lee AS, Gong T, Sanchez-Freire V, Wang Y, Diecke S, Sallam K, Knowles JW, Wang PJ, Nguyen PK, Bers DM, Robbins RC, Wu JC, Drug screening using a library of human induced pluripotent stem cell-derived cardiomyocytes reveals disease-specific patterns of cardiotoxicity, Circulation. 127 (2013) 1677–1691. doi: 10.1161/CIRCULATIONAHA.113.001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR, Multilineage potential of adult human mesenchymal stem cells., Science. 284 (1999) 143–7. http://www.ncbi.nlm.nih.gov/pubmed/10102814. [DOI] [PubMed] [Google Scholar]
- [30].Wagner W, Ho AD, Mesenchymal stem cell preparations-comparing apples and oranges, Stem Cell Rev 3 (2007) 239–248. doi: 10.1007/sl2015-007-9001-l. [DOI] [PubMed] [Google Scholar]
- [31].Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM, Embryonic stem cell lines derived from human blastocysts, Science (80-. ). 282 (1998) 1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- [32].Adewumi O, Aflatoonian B, Ahrlund-Richter L, Amit M, Andrews PW, Beighton G, Bello PA, Benvenisty N, Berry LS, Bevan S, Blum B, Brooking J, Chen KG, Choo ABH, Churchill GA, Corbel M, Damjanov I, Draper JS, Dvorak P, Emanuelsson K, Fleck RA, Ford A, Gertow K, Gertsenstein M, Gokhale PJ, Hamilton RS, Hampl A, Healy LE, Hovatta O, Hyllner J, Imreh MP, Itskovitz-Eldor J, Jackson J, Johnson JL, Jones M, Kee K, King BL, Knowles BB, Lako M, Lebrin F, Mallon BS, Manning D, Mayshar Y, McKay RDG, Michalska AE, Mikkola M, Mileikovsky M, Minger SL, Moore HD, Mummery CL, Nagy A, Nakatsuji N, O’Brien CM, Oh SKW, Olsson C, Otonkoski T, Park KY, Passier R, Patel H, Patel M, Pedersen R, Pera MF, Piekarczyk MS, Pera RAR, Reubinoff BE, Robins AJ, Rossant J, Rugg-Gunn P, Schulz TC, Semb H, Sherrer ES, Siemen H, Stacey GN, Stojkovic M, Suemori H, Szatkiewicz J, Turetsky T, Tuuri T, Van Den Brink S, Vintersten K, Vuoristo S, Ward D, Weaver TA, Young LA, Zhang W, Characterization of human embryonic stem cell lines by the International Stem Cell Initiative, Nat. Biotechnol 25 (2007) 803–816. doi: 10.1038/nbtl318. [DOI] [PubMed] [Google Scholar]
- [33].Scott CW, Peters MF, Dragan YP, Human induced pluripotent stem cells and their use in drug discovery for toxicity testing, Toxicol. Lett 219 (2013) 49–58. doi: 10.1016/j.toxlet.2013.02.020. [DOI] [PubMed] [Google Scholar]
- [34].Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S, Induction of pluripotent stem cells from adult human fibroblasts by defined factors, Cell. 131 (2007) 861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- [35].Churko JM, Lee J, Ameen M, Gu M, Venkatasubramanian M, Diecke S, Sallam K, Im H, Wang G, Gold JD, Salomonis N, Snyder MP, Wu JC, Transcriptomic and epigenomic differences in human induced pluripotent stem cells generated from six reprogramming methods, Nat. Biomed. Eng 1 (2017). doi: 10.1038/s41551-017-0141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sanchez-Freire V, Lee AS, Hu S, Abilez OJ, Liang P, Lan F, Huber BC, Ong SG, Hong WX, Huang M, Wu JC, Effect of human donor cell source on differentiation and function of cardiac induced pluripotent stem cells, J. Am. Coll. Cardiol 64 (2014) 436–448. doi: 10.1016/j.jacc.2014.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sun N, Panetta NJ, Gupta DM, Wilson KD, Lee A, Jia F, Hu S, Cherry AM, Robbins RC, Longaker MT, Wu JC, Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells., Proc. Natl. Acad. Sci. U. S. A 106 (2009) 15720–5. doi: 10.1073/pnas.0908450106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Churko JM, Burridge PW, Wu JC, Generation of human iPSCs from human peripheral blood mononuclear cells using non-integrative Sendai virus in chemically defined conditions, in: Cell. Cardiomyoplasty Methods Mol. Biol. (Methods Protoc, 2013: pp. 81–88. doi: 10.1007/978-1-62703-511-8_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhao M-T, Chen H, Liu Q, Shao N-Y, Sayed N, Wo H-T, Zhang JZ, Ong S-G, Liu C, Kim Y, Yang H, Chour T, Ma H, Gutierrez NM, Karakikes I, Mitalipov S, Snyder MP, Wu JC, Molecular and functional resemblance of differentiated cells derived from isogenic human iPSCs and SCNT-derived ESCs, Proc. Natl. Acad. Sci (2017) 201708991. doi: 10.1073/pnas.l708991114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Narsinh KH, Plews J, Wu JC, Comparison of human induced pluripotent and embryonic stem cells: Fraternal or identical twins?, Mol. Ther 19 (2011) 635–638. doi: 10.1038/mt.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Engle SJ, Puppala D, Integrating human pluripotent stem cells into drug development, Cell Stem Cell. 12 (2013) 669–677. doi: 10.1016/j.stem.2013.05.011. [DOI] [PubMed] [Google Scholar]
- [42].Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, Plews JR, Abilez OJ, Cui B, Gold JD, Wu JC, Chemically defined generation of human cardiomyocytes., Nat. Methods. 11 (2014) 855–60. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Burridge PW, Li YF, Matsa E, Wu H, Ong S-G, Sharma A, Holmström A, Chang AC, Coronado MJ, Ebert AD, Knowles JW, Telli ML, Witteles RM, Blau HM, Bernstein D, Altman RB, Wu JC, Human induced pluripotent stem cell–derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity, Nat. Med 22 (2016) 547–556. doi: 10.1038/nm.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Egawa N, Kitaoka S, Tsukita K, Naitoh M, Takahashi K, Yamamoto T, Adachi F, Kondo T, Okita K, Asaka I, Aoi T, Watanabe A, Yamada Y, Morizane A, Takahashi J, Ayaki T, Ito H, Yoshikawa K, Yamawaki S, Suzuki S, Watanabe D, Hioki H, Kaneko T, Makioka K, Okamoto K, Takuma H, Tamaoka A, Hasegawa K, Nonaka T, Hasegawa M, Kawata A, Yoshida M, Nakahata T, Takahashi R, Marchetto MCN, Gage FH, Yamanaka S, Inoue H, Drug screening for ALS using patient-specific induced pluripotent stem cells, Sci. Transl. Med 4 (2012) 145ral04-145ral04. doi: 10.1126/scitranslmed.3004052. [DOI] [PubMed] [Google Scholar]
- [45].Yu D, Swaroop M, Wang M, Baxa U, Yang R, Yan Y, Coksaygan T, DeTolla L, Marugan JJ, Austin CP, McKew JC, Gong D-W, Zheng W, Niemann–Pick disease type C: Induced pluripotent stem cell–derived neuronal cells for modeling neural disease and evaluating drug efficacy, J. Biomol. Screen. 19 (2014) 1164–1173. doi: 10.1177/1087057114537378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yahata N, Asai M, Kitaoka S, Takahashi K, Asaka I, Hioki H, Kaneko T, Maruyama K, Saido TC, Nakahata T, Asada T, Yamanaka S, Iwata N, Inoue H, Anti-Aβ drug screening platform using human iPS cell-derived neurons for the treatment of Alzheimer’s disease, PLoS One. 6 (2011). doi: 10.1371/journal.pone.0025788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Choudhury Y, Toh YC, Xing J, Qu Y, Poh J, Huan L, Tan HS, Kanesvaran R, Yu H, Tan M-H, Patient-specific hepatocyte-like cells derived from induced pluripotent stem cells model pazopanib-mediated hepatotoxicity, Sci. Rep 7 (2017) 41238. doi: 10.1038/srep41238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ware BR, Berger DR, Khetani SR, Prediction of drug-induced liver injury in micropatterned co-cultures containing iPSC-derived human hepatocytes, Toxicol. Sci 145 (2015) 252–262. doi: 10.1093/toxsci/kfv048. [DOI] [PubMed] [Google Scholar]
- [49].Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, McCarthy S, Sebat J, Gage FH, Modeling schizophrenia using hiPSC neurons, Nature. 473 (2011) 221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sarkar A, Mei A, Paquola ACM, Stem S, Bardy C, Klug JR, Kim S, Neshat N, Kim HJ, Ku M, Shokhirev MN, Adamowicz DH, Marchetto MC, Jappelli R, Erwin JA, Padmanabhan K, Shtrahman M, Jin X, Gage FH, Efficient generation of CA3 neurons from human pluripotent stem cells enables modeling of hippocampal connectivity in vitro, Cell Stem Cell. 22 (2018) 684–697. e9. doi: 10.1016/j.stem.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kondo T, Asai M, Tsukita K, Kutoku Y, Ohsawa Y, Sunada Y, Imamura K, Egawa N, Yahata N, Okita K, Takahashi K, Asaka I, Aoi T, Watanabe A, Watanabe K, Kadoya C, Nakano R, Watanabe D, Maruyama K, Hori O, Hibino S, Choshi T, Nakahata T, Hioki H, Kaneko T, Naitoh M, Yoshikawa K, Yamawaki S, Suzuki S, Hata R, Ueno SI, Seki T, Kobayashi K, Toda T, Murakami K, Irie K, Klein WL, Mori H, Asada T, Takahashi R, Iwata N, Yamanaka S, Inoue H, Modeling Alzheimer’s disease with iPSCs reveals stress phenotypes associated with intracellular Aβ and differential drug responsiveness, Cell Stem Cell. 12 (2013) 487–496. doi: 10.1016/j.stem.2013.01.009. [DOI] [PubMed] [Google Scholar]
- [52].Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ, Navarrete EG, Hu S, Wang, Lee A, Pavlovic A, Lin S, Chen R, Hajjar RJ, Snyder MP, Dolmetsch RE, Butte MJ, Ashley E. a., Longaker MT, Robbins RC, Wu JC, Patient-Specific Induced Pluripotent Stem Cells as a Model for Familial Dilated Cardiomyopathy, Sci. Transl. Med 4(2012) 130ra47-130ra47. doi: 10.1126/scitranslmed.3003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ramsden CM, Nommiste B, Lane AR, Carr A-JF, Powner MB, Smart MJK, Chen LL, Muthiah MN, Webster AR, Moore AT, Cheetham ME, da Cruz L, Coffey PJ, Rescue of the MERTK phagocytic defect in a human iPSC disease model using translational read-through inducing drugs, Sci. Rep 7 (2017) 51. doi: 10.1038/s41598-017-00142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Abujarour R, Bennett M, Valamehr B, Lee TT, Robinson M, Robbins D, Le T, Lai K, Flynn P, Myogenic differentiation of muscular dystrophy-specific induced pluripotent stem cells for use in drug discovery, Stem Cells Transl. Med 3 (2014) 149–160. doi: 10.5966/sctm.2013-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Pavesi A, Adriani G, Rasponi M, Zervantonakis IK, Fiore GB, Kamm RD, Controlled electromechanical cell stimulation on-a-chip, Sci. Rep 5 (2015) 1–12. doi: 10.1038/srepl1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hughes DJ, Kostrzewski T, Sceats EL, Opportunities and challenges in the wider adoption of liver and interconnected microphysiological systems, Exp. Biol. Med (2017) 153537021770897. doi: 10.1177/1535370217708976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Luni C, Serena E, Elvassore N, Human-on-chip for therapy development and fundamental science, Curr. Opin. Biotechnol 25 (2014) 45–50. doi: 10.1016/j.copbio.2013.08.015. [DOI] [PubMed] [Google Scholar]
- [58].Bavli D, Prill S, Ezra E, Levy G, Cohen M, Vinken M, Vanfleteren J, Jaeger M, Nahmias Y, Real-time monitoring of metabolic function in liver-on-chip microdevices tracks the dynamics of mitochondrial dysfunction, Proc. Natl. Acad. Sci 113 (2016) E2231–E2240. doi: 10.1073/pnas.1522556113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lee JS, Romero R, Han YM, Kim HC, Kim CJ, Hong J-S, Huh D, Placenta-on-a-chip: a novel platform to study the biology of the human placenta, J. Matern. Neonatal Med 29 (2016) 1046–1054. doi: 10.3109/14767058.2015.1038518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhang C, Zhao Z, Abdul Rahim NA, van Noort D, Yu H, Towards a human-on-chip: Culturing multiple cell types on a chip with compartmentalized microenvironments, Lab Chip. 9 (2009) 3185. doi: 10.1039/b915147h. [DOI] [PubMed] [Google Scholar]
- [61].Chen MB, Srigunapalan S, Wheeler AR, Simmons CA, A 3D microfluidic platform incorporating methacrylated gelatin hydrogels to study physiological cardiovascular cell–cell interactions, Lab Chip. 13 (2013) 2591. doi: 10.1039/c31c00051f. [DOI] [PubMed] [Google Scholar]
- [62].Achyuta AKH, Conway AJ, Crouse RB, Bannister EC, Lee RN, Katnik CP, Behensky AA, Cuevas J, Sundaram SS, A modular approach to create a neurovascular unit-on-a-chip, Lab Chip. 13 (2013) 542–553. doi: 10.1039/C2LC41033H. [DOI] [PubMed] [Google Scholar]
- [63].Benam KH, Villenave R, Lucchesi C, Varone A, Hubeau C, Lee H-H, Alves SE, Salmon M, Ferrante TC, Weaver JC, Bahinski A, Hamilton GA, Ingber DE, Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro, Nat. Methods. 13 (2015) 151–157. doi: 10.1038/nmeth.3697. [DOI] [PubMed] [Google Scholar]
- [64].Jang K-J, Mehr AP, Hamilton GA, McPartlin LA, Chung S, Suh K-Y, Ingber DE, Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment, Integr. Biol 5 (2013) 1119. doi: 10.1039/c3ib40049b. [DOI] [PubMed] [Google Scholar]
- [65].van Midwoud PM, Merema MT, Verpoorte E, Groothuis GMM, A microfluidic approach for in vitro assessment of interorgan interactions in drug metabolism using intestinal and liver slices, Lab Chip. 10 (2010) 2778. doi: 10.1039/c01c00043d. [DOI] [PubMed] [Google Scholar]
- [66].Li X, Brooks JC, Hu J, Ford KI, Easley CJ, 3D-templated, fully automated microfluidic input/output multiplexer for endocrine tissue culture and secretion sampling, Lab Chip. 17 (2017) 341–349. doi: 10.1039/C6LC01201A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Maschmeyer I, Hasenberg T, Jaenicke A, Lindner M, Lorenz AK, Zech J, Garbe LA, Sonntag F, Hayden P, Ayehunie S, Lauster R, Marx U, Materne EM, Chip-based human liver-intestine and liver-skin co-cultures - A first step toward systemic repeated dose substance testing in vitro, Eur. J. Pharm. Biopharm 95 (2015) 77–87. doi: 10.1016/j.ejpb.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lin Z, Will Y, Evaluation of drugs with specific organ toxicities in organ-specific cell lines, Toxicol. Sci 126 (2012) 114–127. doi: 10.1093/toxsci/kfr339. [DOI] [PubMed] [Google Scholar]
- [69].Pan C, Kumar C, Bohl S, Klingmueller U, Mann M, Comparative proteomic phenotyping of cell lines and primary cells to assess preservation of cell type-specific functions, Mol. Cell. Proteomics. 8 (2009) 443–450. doi: 10.1074/mcp.M800258-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ, Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: A methods overview, Circ. Res 111 (2012) 344–358. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lian X, Bao X, Al-Ahmad A, Liu J, Wu Y, Dong W, Dunn KK, Shusta EV, Palecek SP, Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling, Stem Cell Reports. 3 (2014) 804–816. doi: 10.1016/j.stemcr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Patsch C, Challet-Meylan L, Thoma EC, Urich E, Heckel T, O’Sullivan JF, Grainger SJ, Kapp FG, Sun L, Christensen K, Xia Y, Florido MHC, He W, Pan W, Prummer M, Warren CR, Jakob-Roetne R, Certa U, Jagasia R, Freskgard PO, Adatto I, Kling D, Huang P, Zon LI, Chaikof EL, Gerszten RE, Graf M, Iacone R, Cowan CA, Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells, Nat. Cell Biol 17 (2015) 994–1003. doi: 10.1038/ncb3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Tan KS, Tamura K, Lai MI, Veerakumarasivam A, Nakanishi Y, Ogawa M, Sugiyama D, Molecular pathways governing development of vascular endothelial cells from ES/iPS cells, Stem Cell Rev. Reports. 9 (2013) 586–598. doi: 10.1007/sl2015-013-9450-7. [DOI] [PubMed] [Google Scholar]
- [74].Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G, Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines, Cell Stem Cell. 8 (2011) 228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- [75].Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ, Functional cardiomyocytes derived from human induced pluripotent stem cells, Circ. Res 104 (2009) e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP, Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling, Proc. Natl. Acad. Sci 109 (2012) E1848–E1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, Hsiao C, Kamp TJ, Palecek SP, Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions, Nat. Protoc 8 (2013) 162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Uchimura T, Otomo J, Sato M, Sakurai H, A human iPS cell myogenic differentiation system permitting high-throughput drug screening, Stem Cell Res 25 (2017) 98–106. doi: 10.1016/j.scr.2017.10.023. [DOI] [PubMed] [Google Scholar]
- [79].Mizuno Y, Chang H, Umeda K, Niwa A, Iwasa T, Awaya T, Fukada S, Yamamoto H, Yamanaka S, Nakahata T, Heike T, Generation of skeletal muscle stem/progenitor cells from murine induced pluripotent stem cells., FASEB J 24 (2010) 2245–53. doi: 10.1096/fj.09-137174. [DOI] [PubMed] [Google Scholar]
- [80].Musah S, Mammoto A, Ferrante TC, Jeanty SSF, Hirano-Kobayashi M, Mammoto T, Roberts K, Chung S, Novak R, Ingram M, Fatanat-Didar T, Koshy S, Weaver JC, Church GM, Ingber DE, Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip, Nat. Biomed. Eng 1 (2017). doi: 10.1038/s41551-017-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Karakikes I, Ameen M, Termglinchan V, Wu JC, Human induced pluripotent stem cell-derived cardiomyocytes: Insights into molecular, cellular, and functional phenotypes, Circ. Res 117(2015) 80–88. doi: 10.1161/CIRCRESAHA.117.305365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Sayed N, Liu C, Wu JC, Translation of human iPSCs: From clinical trial in a dish to precision medicine, J. Am. Coll. Cardiol 67 (2016) 2161–2176. doi: 10.1016/j.jacc.2016.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Synnergren J, Ameen C, Jansson A, Sartipy P, Global transcriptional profiling reveals similarities and differences between human stem cell-derived cardiomyocyte clusters and heart tissue, Physiol. Genomics. 44 (2012) 245–258. doi: 10.1152/physiolgenomics.00118.2011. [DOI] [PubMed] [Google Scholar]
- [84].Kuppusamy KT, Jones DC, Sperber H, Madan A, Fischer KA, Rodriguez ML, Pabon L, Zhu W-Z, Tulloch NL, Yang X, Sniadecki NJ, Laflamme MA, Ruzzo WL, Murry E, Ruohola-Baker H, Let-7 family of microRNA is required for maturation and adult-like metabolism in stem cell-derived cardiomyocytes, Proc. Natl. Acad. Sci 112 (2015) E2785–E2794. doi: 10.1073/pnas.1424042112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Yang X, Rodriguez M, Pabon L, Fischer KA, Reinecke H, Regnier M, Sniadecki NJ, Ruohola-Baker C.E. Murry, Tri-iodo-l-thyronine promotes the maturation of human cardiomyocytes-derived from induced pluripotent stem cells, J. Mol. Cell. Cardiol 72 (2014) 296–304. doi: 10.1016/j.yjmcc.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Piccini I, Rao J, Seebohm G, Greber B, Human pluripotent stem cell-derived cardiomyocytes: Genome-wide expression profiling of long-term in vitro maturation in comparison to human heart tissue, Genomics Data. 4 (2015) 69–72. doi: 10.1016/j.gdata.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song LJ, Sirabella D, Morikawa K, Teles D, Yazawa M, Vunjak-Novakovic G, Advanced maturation of human cardiac tissue grown from pluripotent stem cells, Nature. 556 (2018) 239–243. doi: 10.1038/s41586-018-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Leonard A, Bertero A, Powers JD, Beussman KM, Bhandari S, Regnier M, Murry CE, Sniadecki NJ, Afterload promotes maturation of human induced pluripotent stem cell derived cardiomyocytes in engineered heart tissues, J. Mol. Cell. Cardiol 118 (2018) 147–158. doi: 10.1016/j.yjmcc.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Lee JH, Protze SI, Laksman Z, Backx PH, Keller GM, Human pluripotent stem cell-derived atrial and ventricular cardiomyocytes develop from distinct mesoderm populations, Cell Stem Cell. 21 (2017) 179–194. e4. doi: 10.1016/j.stem.2017.07.003. [DOI] [PubMed] [Google Scholar]
- [90].Protze SI, Liu J, Nussinovitch U, Ohana L, Backx PH, Gepstein L, Keller GM, Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker, Nat. Biotechnol 35 (2016) 56–68. doi: 10.1038/nbt.3745. [DOI] [PubMed] [Google Scholar]
- [91].Zhang Q, Jiang J, Han P, Yuan Q, Zhang J, Zhang X, Xu Y, Cao H, Meng Q, Chen L, Tian T, Wang X, Li P, Hescheler J, Ji G, Ma Y, Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals, Cell Res 21 (2011) 579–587. doi: 10.1038/cr.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].D’Aiuto L, Zhi Y, Das DK, Wilcox MR, Johnson JW, Viggiano L, Sweet R, Kinchington PR, Bhattacharjee AG, Large-scale generation of human iPSC-derived neural stem cells / early neural progenitor cells and their neuronal differentiation, Organogenesis. 10 (2015) 365–377. doi: 10.1080/15476278.2015.1011921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Schwartz RE, Fleming HE, Khetani SR, Bhatia SN, Pluripotent stem cell-derived hepatocyte-like cells, Biotechnol. Adv 32 (2014) 504–513. doi: 10.1016/j.biotechadv.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Jozefczuk J, Prigione A, Chavez L, Adjaye J, Comparative analysis of human embryonic stem cell and induced pluripotent stem cell-derived hepatocyte-like cells reveals current drawbacks and possible strategies for improved differentiation, Stem Cells Dev 20 (2011) 1259–1275. doi: 10.1089/scd.2010.0361. [DOI] [PubMed] [Google Scholar]
- [95].Kratochwil NA, Meille C, Fowler S, Klammers F, Ekiciler A, Molitor B, Simon S, Walter I, McGinnis C, Walther J, Leonard B, Triyatni M, Javanbakht H, Funk C, Schuler F, Lavé T, Parrott NJ, Metabolic profiling of human long-term liver models and hepatic clearance predictions from in vitro data using nonlinear mixed-effects modeling, A APS J 19 (2017) 534–550. doi: 10.1208/sl2248-016-0019-7. [DOI] [PubMed] [Google Scholar]
- [96].Zhang YS, Arneri A, Bersini S, Shin SR, Zhu K, Goli-Malekabadi Z, Aleman J, Colosi C, Busignani F, Dell’Erba V, Bishop C, Shupe T, Demarchi D, Moretti M, Rasponi M, Dokmeci MR, Atala A, Khademhosseini A, Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip, Biomaterials. 110(2016) 45–59. doi: 10.1016/j.biomaterials.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Huebsch N, Loskill P, Deveshwar N, Spencer CI, Judge LM, Mandegar MA, Fox CB, Mohamed TMA, Ma Z, Mathur A, Sheehan AM, Truong A, Saxton M, Yoo J, Srivastava D, Desai TA, So P-L, Healy KE, Conklin BR, Miniaturized iPS-cell-derived cardiac muscles for physiologically relevant drug response analyses, Sci. Rep 6 (2016) 24726. doi: 10.1038/srep24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Agarwal A, Goss JA, Cho A, McCain ML, Parker KK, Microfluidic heart on a chip for higher throughput pharmacological studies, Lab Chip. 13 (2013) 3599. doi: 10.1039/c31c50350j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Ertl P, Sticker D, Charwat V, Kasper C, Lepperdinger G, Lab-on-a-chip technologies for stem cell analysis, Trends Biotechnol 32 (2014) 245–253. doi: 10.1016/j.tibtech.2014.03.004. [DOI] [PubMed] [Google Scholar]
- [100].Geraili A, Jafari P, Hassani MS, Araghi BH, Mohammadi MH, Ghafari AM, Tamrin SH, Modarres HP, Kolahchi AR, Ahadian S, Sanati-Nezhad A, Controlling differentiation of stem cells for developing personalized organ-on-chip platforms, Adv. Healthc. Mater 1700426 (2017) 1–26. doi: 10.1002/adhm.201700426. [DOI] [PubMed] [Google Scholar]
- [101].Yoshimitsu R, Hattori K, Sugiura S, Kondo Y, Yamada R, Tachikawa S, Satoh T, Kurisaki A, Ohnuma K, Asashima M, Kanamori T, Microfluidic perfusion culture of human induced pluripotent stem cells under fully defined culture conditions, Biotechnol. Bioeng 111 (2014) 937–947. doi: 10.1002/bit.25150. [DOI] [PubMed] [Google Scholar]
- [102].Zhang J, Wei X, Zeng R, Xu F, Li X, Stem cell culture and differentiation in microfluidic devices toward organ-on-a-chip, Futur. Sci. OA. 3 (2017) FS0187. doi: 10.4155/fsoa-2016-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Giobbe GG, Michielin F, Luni C, Giulitti S, Martewicz S, Dupont S, Floreani A, Elvassore N, Functional differentiation of human pluripotent stem cells on a chip, Nat. Methods. 12 (2015) 637–640. doi: 10.1038/nmeth.3411. [DOI] [PubMed] [Google Scholar]
- [104].Hesari Z, Soleimani M, Atyabi F, Sharifdini M, Nadri S, Warkiani ME, Zare M, Dinarvand R, A hybrid microfluidic system for regulation of neural differentiation in induced pluripotent stem cells, J. Biomed. Mater. Res. - Part A. 104 (2016) 1534–1543. doi: 10.1002/jbm.a.35689. [DOI] [PubMed] [Google Scholar]
- [105].Loskill P, Marcus SG, Mathur A, Reese WM, Healy KE, μorgano: A Lego®-like plug & play system for modular multi-organ-chips, PLoS One. 10 (2015) 1–13. doi: 10.1371/journal.pone.0139587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Tsamandouras N, Kostrzewski T, Stokes CL, Griffith LG, Hughes DJ, Cirit M, Quantitative assessment of population variability in hepatic drug metabolism using a perfused 3D human liver microphysiological system., J. Pharmacol. Exp. Ther 360 (2016) jpet.116.237495-. doi: 10.1124/jpet.116.237495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Eduati F, Mangravite LM, Wang T, Tang H, Bare JC, Huang R, Norman T, Kellen M, Menden MP, Yang J, Zhan X, Zhong R, Xiao G, Xia M, Abdo N, Kosyk O, Friend S, Dearry A, Simeonov A, Tice RR, Rusyn I, Wright FA, Stolovitzky G, Xie Y, Saez-Rodriguez J, Aittokallio T, Alaimo S, Amadoz A, Ammad-ud-din M, Azencott CA, Bacardit J, Barron P, Bernard E, Beyer A, Bin S, van Bömmel A, Borgwardt K, Brys AM, Caffrey B, Chang J, Chang J, Chheda H, Christodoulou EG, Clément-Ziza M, Cohen T, Cowherd M, Demeyer S, Dopazo J, Elhard JD, Falcao AO, Ferro A, Friedenberg DA, Giugno R, Gong Y, Gorospe JW, Granville CA, Grimm D, Heinig M, Hernansaiz RD, Hintsanen P, Hochreiter S, Huang LC, Huska M, Jaiswal A, Jiao Y, Kaski S, Kaur I, Ali Khan S, Klambauer G, Krasnogor N, Kuhn M, Bartosz Kursa M, Kutum R, Lazzarini N, Lee I, Leung MKK, Khong Lim W, Liu C, Llinares López F, Mammana A, Mayr A, Michoel T, Mongiovì M, Moore JD, Mpindi JP, Narasimhan R, Opiyo SO, Pandey G, Peabody AL, Perner J, Poso A, Pulvirenti A, Rawlik K, Reinhardt S, Riffle CG, Ruderfer D, Sander AJ, Savage RS, Scomet E, Sebastian-Leon P, Sharan R, Johann Simon-Gabriel C, Stoven V, Sun J, Tang J, Teixeira AL, Tenesa A, Vert JP, Vingron M, Walter T, Wennerberg K, Whalen S, Wisniewska Z, Wu Y, Xu H, Zhang S, Zhao J, Jim Zheng W, Ziwei D, Prediction of human population responses to toxic compounds by a collaborative competition, Nat. Biotechnol 33 (2015) 933–940. doi: 10.1038/nbt.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Chen VC, Ye J, Shukla P, Hua G, Chen D, Lin Z, Liu J, Chai J, Gold J, Wu J, Hsu D, Couture LA, Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells, Stem Cell Res 15 (2015) 365–375. doi: 10.1016/j.scr.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kempf H, Kropp C, Olmer R, Martin U, Zweigerdt R, Cardiac differentiation of human pluripotent stem cells in scalable suspension culture, Nat. Protoc 10 (2015) 1345–1361. doi: 10.1038/nprot.2015.089. [DOI] [PubMed] [Google Scholar]



