Abstract
Several enteric clostridial diseases can affect humans and animals. Of these, the enteric infections caused by Clostridium perfringens and Clostridium difficile are amongst the most prevalent and they are reviewed here. C. perfringens type A strains encoding alpha toxin (CPA) are frequently associated with enteric disease of many animal mammalian species, but their role in these diseased mammals remains to be clarified. C. perfringens type B encoding CPA, beta (CPB) and epsilon (ETX) toxins causes necro-hemorrhagic enteritis, mostly in sheep, and these strains have been recently suggested to be involved in multiple sclerosis in humans, although evidence of this involvement is lacking. C. perfringens type C strains encode CPA and CPB and cause necrotizing enteritis in humans and animals, while CPA and ETX producing type D strains of C. perfringens produce enterotoxemia in sheep, goats and cattle, but are not known to cause spontaneous disease in humans. The role of C. perfringens type E in animal or human disease remains poorly defined. The newly revised toxinotype F encodes CPA and enterotoxin (CPE), the latter being responsible for food poisoning in humans, and the less prevalent antibiotic associated and sporadic diarrhea. The role of these strains in animal disease has not been fully described and remains controversial. Another newly created toxinotype, G, encodes CPA and necrotic enteritis toxin B-like (NetB), and is responsible for avian necrotic enteritis, but has not been associated with human disease. C. difficile produces colitis and/or enterocolitis in humans and multiple animal species. The main virulence factors of this microorganism are toxins A, B and an ADP-ribosyltransferase (CDT). Other clostridia causing enteric diseases in humans and/or animals are Clostridium spiroforme, Clostridium piliforme, Clostridium colinum, Clostridium sordellii, Clostridium chauvoei, Clostridium septicum, Clostridium botulinum, Clostridium butyricum and Clostridium neonatale. The zoonotic transmission of some, but not all these clostridsial species, has been demonstrated.
Keywords: animals, clostridial, Clostridium spp, humans, pathogenesis
Introduction
Diseases caused by clostridia are usually classified as enteric, neurotoxic or histotoxic. Within each of these three categories, there are diseases that affect both humans and animals, but also diseases that affect one group but not the other (Table 1). Of those affecting humans and animals, the enteric infections caused by Clostridium perfringens and Clostridium difficile are amongst the most prevalent. Because several clostridial species can be found in the environment and in the intestine of humans and animals as normal flora, enteric clostridial infections represent a constant threat to both human and animal health, and are challenging to diagnose utilizing culture-based methods alone (Uzal et al, 2016). Diagnostic criteria for most enteric clostridial diseases require, in addition to culture, evaluation of clinical, gross and microscopic findings, and detection of virulence factors in intestinal content and/or feces.
Table 1.
Clostridial enteric infections of humans and animals, main virulence factors and diseases.
| ORGANISM | Main virulence factors | Diseases | |
|---|---|---|---|
| Humans | Animals | ||
| Clostridium perfringens type A | CPA; others? | No major role in human enteric disease* | Sugggested to be involved in enterocolitis in pigs and horses, enterotoxemia in sheep and cattle and abomasitis of cattle, sheep and goats. Definitive proof lacking |
| Clostridium perfringens type B | CPB; ETX | No demonstrated role in humen enteric disease** | Hemorrhagic enteritis of sheep |
| Clostridium perfringens type C | CPB | Enteritis necroticans in children and adults with trypsin deficiency | Necrohemorrhagic enteritis of neonatal pigs, sheep, goats, horses, others |
| Clostridium perfringens type D | ETX | No demonstrated role in humen enteric disease | Enterotoxemia of sheep, goats and cattle |
| Clostridium perfringens type E | ITX | No demonstrated role in humen enteric disease | Suggested to be involved in enteritis of sheep, cattle and rabbits |
| Clostridium perfringens type F | CPE | Food poisoning, antibiotic associated diarrhea, sporadic diarrhea | Suggested to be involved in canine hemorrhagic gastroenteritis and colitis of horses |
| Clostridium perfringens type G | NetB | No demonstrated role in humen enteric disease | Necrotic enteritis of poultry |
| Clostridium difficile | TcdA, TcdB, CDT | Pseudomembranous and hemorrhagic colitis | Enterocolitis in horses, pigs, rabbits, hamsters, others |
| Clostridium piliforme | No virulence factor identified | No demonstrated role in humen enteric disease | Colitis, hepatitis, myocarditis in horses, cattle, cats, rabbits, others |
| Clostridium sordellii | TcsL and TcsH | No demonstrated role in humen enteric disease | Suggested to be involved in gastroenteritis of cattle and horses |
| Clostridium colinum | No virulence factor identified | No demonstrated role in humen enteric disease | Ulcerative enteritis of quail and other poultry species |
| Clostridium spiroforme | CST | No demonstrated role in humen enteric disease | Enterocolitis of rabbits |
| Clostridium botulinum | Toxins A and B (humans) Toxin C (animals) |
Infant botulism (toxicoinfection) | Possible toxicoinfection in horse, cattle and poultry |
| Clostridium neonatale | Gas, butyrate and other metabolismm products | Neonatal necrotic enteritis | No demonstrated role in humen enteric disease |
| Clostridium butyricum | Gas, butyrate and other metabolismm products | Neonatal necrotic enteritis | No demonstrated role in humen enteric disease |
Exception are sepsis cases involving one or more organs of the alimentary system
ETX produced in the intestine of human patients suggested to be involved in the pathogenesis of multiple sclerosis
We briefly review here the comparative pathogenesis of enteric clostridial diseases, with special emphasis on those caused by C. perfringens.
Clostridium perfringens
C. perfringens has been historically classified into five toxinotypes (A, B, C, D and E) based on its capacity to encode four so-called typing toxins, i.e. alpha (CPA), beta (CPB), epsilon (ETX) and iota (ITX) (Uzal, 2004). Two additional types have, however, been recently added to this typing system, including C. perfringens type F and G (Rood et al, 2018) (Table 2). In addition, individual strains of each toxinotype may also produce one or more of a variety of so-called non-typing toxins, such as perfringolysin O, beta2 toxin (CPB2) and others. The toxinotypes of C. perfringens cause several different enteric diseases in both humans and/or animals. These diseases are mediated by one or more toxins of C. perfringens (Uzal et al, 2014; Rood et al, 2018). Of the enteric infections associated with C. perfringens, those caused by type C strains have been confirmed to affect both humans and animals (Uzal et al, 2016; Sayeed et al, 2008), while the other toxinoitypes have been confirmed to cause disease in either humans or animals, but not in both (Table 1).
Table 2.
New proposed classification system of Clostridium perfringens based on the production of six major toxins (Rood et al, 2018).
| Type | Toxin produced
|
|||||
|---|---|---|---|---|---|---|
| α (CPA) | β (CPB) | ε (ETX) | ι (ITX) | CPE | NetB | |
| A | + | − | − | − | − | − |
| B | + | + | + | − | − | − |
| C | + | + | − | − | −/+ | − |
| D | + | − | + | − | −/+ | − |
| E | + | − | − | + | −/+ | − |
| F | + | − | − | − | + | − |
| G | + | − | − | − | − | + |
Type A infections
According to the newly revised toxinotyping of C. perfringens, type A strains produce CPA, but not CPB, ETX, ITX, enterotoxin (CPE) or NetB (Rood et al, 2018) (Table 2). C. perfringens type A has been associated with several enteric syndromes in mammals, including enterocolitis in pigs and horses, enterotoxemia in sheep and cattle (Uzal et al, 2016) and abomasitis of cattle, sheep and goats (Prescott et al, 2016). However, the role of C. perfringens type A in the gastro-enteric diseases of animals mentioned above is controversial and not fully confirmed. In part this is because type A is the toxinotype most commonly found in the environment and in the intestine of clinically healthy humans and animals (Uzal et al, 2016), which renders isolation of this microorganism of little, if any, diagnostic value.
The recently discovered NetF toxin has been suggested to be involved in canine hemorrhagic gastroenteritis and necrotizing enterocolitis of foals (Gohari et al, 2015, 2016). This suggestion was based on the fact that NetF-positive strains of C. perfringens type A were found with high prevalence in dogs and foals suffering the syndromes mentioned above, respectively (Gohari et al, 2015, 2016). Final evidence of the role of NetF in canine and equine enteric disease is however, lacking. Animal experiments, including fulfillment of molecular Koch’s postulates for NetF, are currently under way. So far, no evidence of NetF involvement in human enteric disease has been provided.
C. perfringens type A strains are not considered to be involved in enteric infections of humans, except for occasional cases of sepsis involving one or more organs of the alimentary system (Sarvari et al, 2016).
Type B infections
C. perfringens type B encodes CPB and ETX (Table 2) but, as is the case for other C. perfringens toxinotypes, individual strains can also encode one or more of several other non-typing toxins (Uzal 2004; Uzal and Songer, 2008; Uzal and Songer, 2016). CPB and ETX are, however, considered the two main virulence factors of type B strains (Table 1).
CPB is an oligomerizing, ~ 35 kDa pore forming toxin that causes cell death and lysis (Sayeed et al, 2008; Uzal and McClane, 2011) and is lethal for mice (Sakurai and Duncan, 1978; Shatursky et al, 2000; Fisher et al, 2006). The pores formed by CPB in the cell membrane allow the entry of Ca2+, Na+ and Cl− into the cells and the efflux of K+. The consequence of these ion exchanges is cell swelling, which leads to necrosis (Nagahama et al, 2003a and b; Nagahama et al, 2013), although it has been recently suggested that necropstosis may also be involved in CPB-associated cell death (Autheman et al, 2013).
ETX is an ~ 33 kDa pore forming toxin, which binds to endothelial cells, oligodendrocytes and renal tubular epithelial cells (Popoff, 2011; Uzal 2004). The receptor for ETX has not been definitively determined. However, in vitro, ETX binds to the Hepatitis A virus cellular receptor (HAVCR1) in MDCK cells and in ACHN cells (human kidney-derived cells), and the myelin and lymphocyte (MAL) protein seems to be required for ETX cytotoxicity in several cell types (Rumah et al, 2015; Navarro et al, 2018).
Following binding, ETX forms a prepore and then inserts itself into the plasma membrane to form an active pore (Robertson et al, 2011), which leads to entry of Cl− and Na+ into the cells and loss of intracellular K+. This is followed by intracytoplasmic increase of iCa2+ (Petit et al, 2001). These exchanges produce marked cell swelling, followed by disappearance of mitochondria, blebbing, membrane disruption, ATP depletion, reduction of nucleus size and increase propidium iodine uptake, all of which are compatible with necrosis (Petit et al, 2001; Chassin et al, 2007; Popoff, 2011).
Type B infection has been mostly described in sheep, in which it causes necro-hemorrhagic enteritis and, more rarely focal symmetrical necrosis (encephalomalacia) (Uzal 2004; Uzal and Songer, 2008; Uzal and Songer, 2016; Fernandez Miyakawa et al, 2007). Infections by this toxinotype have been described in the Middle East, Europe and South Africa, with no cases reported in other parts of the world (Uzal and Songer, 2008). The pathogenesis of these infections has not been fully elucidated, although it is believed that intestinal lesions are produced by CPB, while brain lesions are produced by ETX (Fernandez Miyakawa et al, 2007). ETX requires proteases to become activated, while CPB is exquisitely sensitive to the action of these enzymes. Because of this, it is assumed that in different clinical cases, either ETX or CPB action predominates and, depending upon which of these toxins exerts its major action, clinical signs and lesions could be different (Fernandez Miyakawa et al, 2007). This is, however, speculative and it has not been demonstrated in clinical cases of type B disease or in animal experiments.
Although traditionally C. perfringens type B was not considered a human pathogen, recently a type B strain was isolated from the stool of a young woman with multiple sclerosis (MS) (Rumah et al, 2013). In addition, antibodies against ETX were found at a prevalence higher in the serum of patients with MS than in healthy controls (Rumah et al, 2013; Wagley et al, 2018). Based on these results it has been postulated that ETX may be responsible for the initial lesions of MS (Rumah et al, 2013; Wagley et al, 2018). Final evidence for a role of C. perfringens type B in human cases of MS is, however, lacking. If type B strains are involved in the pathogenesis of MS, it is likely that ETX is at least in part responsible for the brain changes characteristic of the disease, as ETX produces serious brain changes in sheep and, ocassionally, goats. A role for CPB in the pathogenesis of MS cannot completely be ruled out either, as mice inoculated with CPB frequently display neurological signs, and several animal species spontaneously infected with CPB-producing C. perfringens type C also show neurological signs (Sayeed et al, 2008). Lesions in the brain of these animals have not been described and the pathogenesis of CPB-associated neurological diseases remains undetermined.
Type C infections
Type C strains must produce CPA and CPB, with some type C strains also producing CPE and/or CPB2 (Table 2) (Fisher et al, 2006; Sayeed et al, 2008). C. perfringens type C infections in humans and animals are characterized by necrotizing or necro-hemorrhagic enteritis and/or enterocolitis. The disease occurs most frequently in neonates of several animal species, including, but not limited to sheep, cattle, horses and pigs (Diab et al, 2012). Type C disease in humans is frequently referred to as enteritis necroticans (EN), pigbel or darmbrand (Sayeed et al, 2008).
It has been demonstrated that most clinical signs and lesions of type C disease are the consequence of CPB action, and this toxin is considered the main virulence factor of C. perfringens type C (Sayeed et al, 2008). Because CPB is extremely sensitive to trypsin and other proteolytic enzymes such as chymotrypsin, neonate animals in environments contaminated with C. perfringens type C are particularly at risk of type C disease. This predisposition is believed to be associated with the inhibitory action that the colostrum has on trypsin, an action aimed at preventing the proteolytic breakdown of immunoglobulins during the first days of life, which also protects CPB (Diab et al, 2012).
In humans, EN occurs sporadically in several Southeast Asian countries and, occasionally, in other parts of the world. This disease was endemic, with a high prevalence, in Papua New Guinea in the 1960s, where sporadic cases are still observed (Johnson and Gerding, 1997; Lawrence and Cooke, 1980; Kreft and Dalhoff, 2000, Li and McClane, 2014). EN also occurs, although less frequently, in diabetic patients elsewhere in the world. Persons suffering from EN often survive less than 48 h after the first appearance of symptoms (Matsuda et al, 2007; Petrillo et al, 2000). In Papua New Guinea most cases of EN were observed in malnourished children thought to be trypsin deficient, which is consistent with the occurrence of type C infection occurring in neonate animals in which most of the trypsin activity is inhibited by the action of colostrum (Johnson and Gerding, 1997; Lawrence and Cooke, 1980; Diab et al, 2012).
C. perfringens type C disease and the effect of CPB and other toxins has been experimentally studied in several animal models including mice, rabbits, guinea pigs, sheep, goats and pigs (Field and Goodwin, 1959; Kohler et al, 1979; Lawrence and Cooke, 1980; Sayeed et al, 2008; Uzal et al, 2009; Garcia et al, 2013; Schumacher et al, 2013). Molecular Koch’s postulates for CPB were fulfilled in rabbit (Sayeed et al, 2008) and mouse models (Uzal et al, 2009), which provided convincing evidence that CPB is required for type C disease to occur (Figs. 1 and 2). More recently, synergism between CPB and CPE was demonstrated in a rabbit ligated intestinal loop model (Ma et al, 2014), which provided for the first time, proof of synergistic activity of two toxins during intestinal C. perfringens infections (Fig. 3). Based on those experimental results, it was suggested that both CPB and CPE may act synergistically in some cases of EN (Ma et al, 2014), although this has not yet been proved.
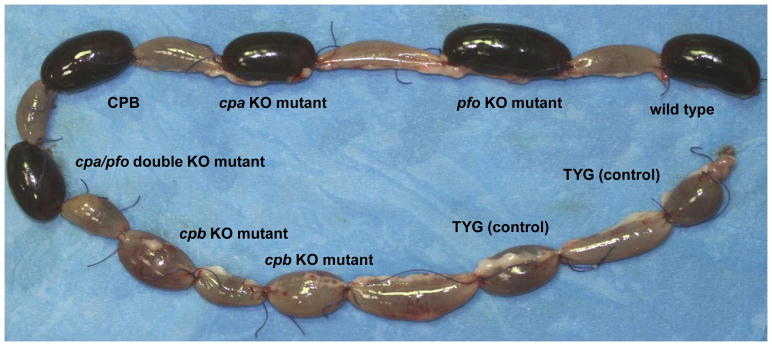
Figure 1. CPB is required for the virulence of Clostridium perfringens type C strains: Gross Pathology.
Gross changes in rabbit-ligated intestinal loops inoculated with C. perfringens type C, wild-type strain CN3685, isogenic single- and double-toxin mutants, purified CPB or sterile culture medium (negative control). These loops were incubated for 6 h after inoculation. Loops inoculated with the wild-type strain, BMC101 (plc null mutant), BMC102 (pfoA null mutant), BMC103 (double plc/pfoA null mutant) or purified CPB are distended with fluid and haemorrhagic. The loops inoculated with BMC100 (cpb null mutant) or sterile culture medium do not show significant gross abnormalities. Reproduced with permission from Sayeed et al, 2007.
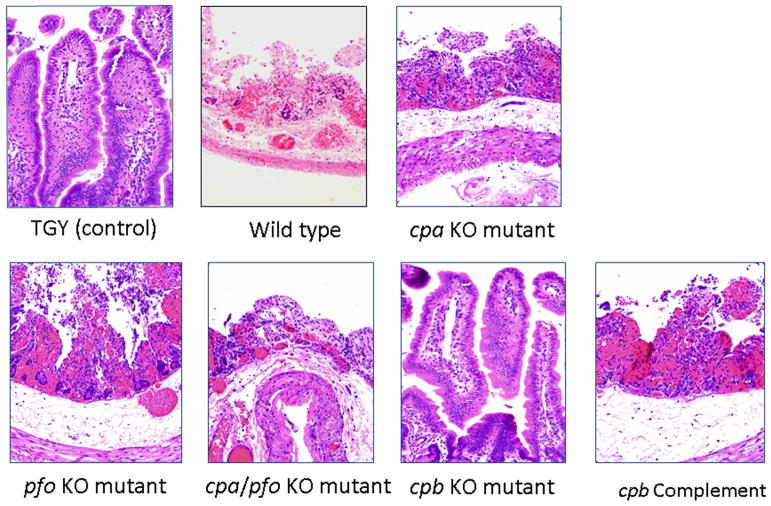
Figure 2. CPB is required for the virulence of Clostridium perfringens type C: Histology.
Microscopic changes in rabbit ligated small intestinal loops incubated for 6 h with C. perfringens type C wild type strain CN3685, its mutants, purified CPB or sterile culture medium (negative control). Loops inoculated with sterile culture medium or the cpb null mutant strain BMC100 showed no significant microscopic abnormalities, while loops inoculated with wild-type CN3685, BMC101 (plc null mutant), BMC102 (pfoA null mutant) or BMC103 (double plc/pfoA null mutant) showed severe damage including mucosal necrosis and hemorrhage, villus blunting, and mild neutrophilic infiltration of mucosa and sub-mucosa. H&E, 200×. Reproduced with permission from Sayeed et al, 2007.
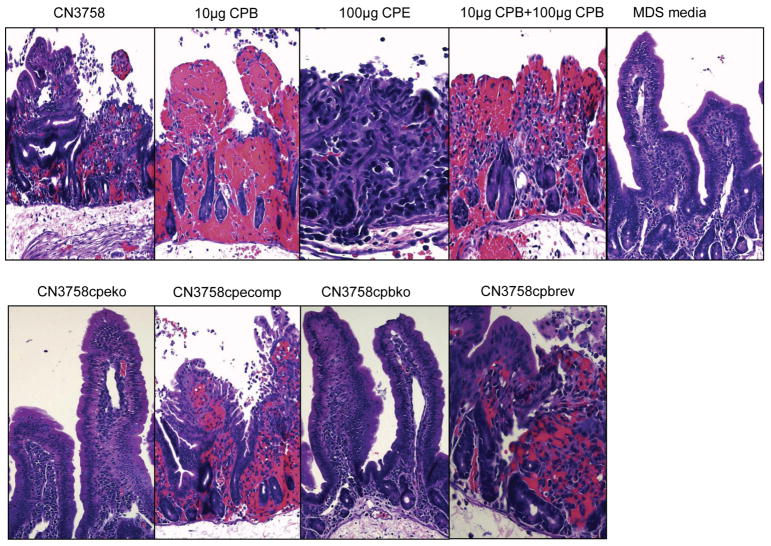
Figure 3. Clostridium perfringens CPB and CPE can act synergistically to produce necrosis in the small intestine of rabbits.
Microscopic changes in rabbit small intestinal loops challenged with supernatants from MDS sporulating culture lysates of C. perfringens type C wild type strain CN3685 or its mutants: CN3758cpbko, CN3758cpbrev, CN3758cpeko and CN3758cpecomp. The rabbit intestinal loops were incubated for 6 h after inoculation. 10 μg of purified CPB, 100 μg of purified CPE, and a mix of 10 μg of purified CPB and 100 μg of purified CPE were used as controls. Sterile, nontoxic MDS medium was used as negative control. H&E, 200× magnification. Reproduced with permission from Ma et al, 2014.
Type D infections
Type D isolates encode CPA and ETX, with individual isolates also encoding one or more other toxins such as CPE or perfringolysin (McClane et al, 2006). C. perfringens type D causes enterotoxemia in sheep, goats and cattle (Uzal and Songer, 2008; Uzal et al, 2016a and b). Most cases of type D enterotoxemia in sheep occur after sudden changes in diet involving access to feeds rich in highly fermentable carbohydrates (Uzal and Songer, 2008; Uzal et al, 2016). It is assumed that a consequence of these changes is passage into the intestine of large amounts of undigested carbohydrates (Uzal et al, 2016). In vitro, C. perfringens type D produces more ETX in low glucose media (Li et al, unpublished), resembling what is likely to occur when undigested complex carbohydrates bypass the gastric compartments of sheep. Other factors that are likely to upset the intestinal environment (i.e., heavy parasite infestation) may also predispose sheep to type D enterotoxemia (Uzal and Songer, 2008; Uzal et al, 2016). While it is possible that these factors also predispose type D infection in goats, little evidence is available in this regard, and cases of enterotoxemia have been described in goats fed hay diets for long periods of time (Uzal and Songer, 2008).
During type D infections, ETX is produced in the intestine as a prototoxin that, in contact with trypsin and/or other proteases, including chemotrypsin and carboxypeptidases, loses amino acids from the C-terminus and becomes activated (Minami et al, 1997; Freedman et al, 2014). Once ETX is activated in the intestine, the toxin facilitates its own absorption into the blood stream and it is distributed to the target organs, including brain, lungs, heart and kidneys (Goldstein et al, 2009; Uzal and Songer, 2008). After binding to endothelial cells in the brain, lungs and heart, the main effect of ETX is to increase vascular permeability, causing edema in those organs and fluid accumulation in body cavities. If the animals survive long enough, the edema leads to parenchymal necrosis, mostly in the brain (Buxton et al, 1976). In the brain, ETX also crosses the blood-brain barrier and has a direct effect on neurons and olygodendrocytes (Finnie et al, 1999; Finnie, 2003; Popoff, 2011).
The intravenous effects of ETX has been studied in several animal models including sheep (Buxton and Morgan, 1976; Uzal and Kelly, 1997), goats (Uzal and Kelly, 1997), cattle (Uzal et al, 2002), mice (Finnie, 1984a and 1984b; Sayeed et al, 2005; Dorca-Arevalo et al, 2014) and rats (Finnie et al, 1999), while the effects of intraduodenal inoculation of C. perfringens type D have also been studied in sheep, goats and cattle (Buxton and Morgan, 1976; Blackwell et al, 1991; Uzal and Kelly, 1997, 1998). Definitive evidence that ETX is the main virulence factor of type D infections was obtained when molecular Koch’s postulates were fulfilled in sheep, goats and mice (Garcia et al, 2013) (Fig. 4). No cases of enteric type D disease have been reported in humans.
Figure 4. ETX is required for the virulence of Clostridium perfringens type D.
Survival progression in sheep (A), goats (B), and mice (C) inoculated with C. perfringens type D CN1020 (WT) and its isogenic derivative mutants: strain JIR4081 (etx mutant -etx KO-), strain JIR12604 (complemented derivative -etx complemented-) or sterile culture medium (TGY). Each inoculum was administered to 6 sheep, 5 goats and 15 mice. Reproduced with permission from Garcia et al, 2013.
Type E infections
C. perfringens type E encodes CPA and ITX (Table 2), with individual strains also encoding one or more additional toxins such as CPE or CPB2. ITX is a binary toxin that includes an enzyme component (Ia) and a binding component (Ib) (Sakurai et al, 2009). The so-called lipolysis-stimulated lipoprotein receptor (LSR) is thought to be a receptor for Ib. After binding to this receptor, heptamers of Ib insert into the cell membrane and form functional channels, which allows endocytosis of Ia and movement of ions (Richard et al, 2002; Nagahama et al, 2002; Nagahama et al, 2012; Knapp et al, 2016). The internalized Ia also leads to ADP-ribosylation of actin to cause the depolymerization of actin filaments and increase in G-actin monomers, all of which produces disorganization of intercellular junctions and changes in cell morphology (Richard et al, 2002). The cytotoxic effects of ITX include several features consistent with necrosis, i.e. cell swelling, mitochondrial dysfunction, ATP depletion and increased IP intake.
In the past few years, some cases of type E-associated disease have been reported in several animal species (Kim et al, 2013; Songer and Miskimmins, 2004; Redondo et al, 2015). However, most of those reports were based on isolation of C. perfringens type E from the intestinal content of animals with enteric disease, which is not considered a diagnostic criterion for these infections, so the role of this microorganism in production of enteric disease remains uncertain (Songer, 2016). Molecular Koch postulates have not been fulfilled for C. perfringens type E.
Although the role of type E strains in human enteric disease remains undetermined, recently, type E strains with a plasmid that carries a variant cpe locus and a variant ITX genes have been identified (Miyamoto et al, 2011. Isolates with this plasmid were detected in environmental samples and in feces of healthy individuals. However, these findings are undermined by the fact these CPE carrying type E strains have only been found in healthy individuals and their role in the pathogenesis of type E human infections remains, therefore undetermined (Miyamoto et al, 2006; Li et al, 2007; Miyamoto et al, 2011; Freedman at al., 2015).
Type F infections
These strains, formerly called CPE-postive type A, are characterized by carrying the cpa and the cpe toxin genes; they do not carry the genes encoding CPB, ETX or ITX (Table 2). Type F strains produce CPE upon sporulation (Li et al, 2016; Rood et al, 2018). Diseases produced by these strains are generically known as enterotoxigenic infections.
Enterotoxigenic infections involving type F strains occur frequently in humans but, while a few reports of these infections in animals have been published (Bueschel et al, 1998; Busch et al, 2015), the role of CPE-producing C. perfringens strains in animal disease remains controversial and poorly characterized (Busch et al, 2015). C. perfringens type F food poisoning is considered the second most common food associated bacterial disease in the USA (Scallan et al, 2011; https://www.cdc.gov/foodsafety/diseases/clostridium-perfringens.html). CPE-positive type F strains are also responsible for 3 to 15% of non-food borne gastrointestinal diseases in humans (McClane et al, 2006).
CPE is an ~35 kDa, 319 amino acid single polypeptide (Czeczulin et al, 1993) that is released into the intestinal tract during sporulation. It binds to specific claudin-receptors in the intestinal epithelium (Katahira et al, 1997a and b; Fujita et al, 2000; Shresta et al, 2013), starting at villus tips in small intestinal loops (McDonel and Duncan, 1975, Sherman et al, 1994), where there is a higher density of claudin-4 (Smedley 3rd et al, 2008). When CPE binds to claudin receptors, it becomes part of an ~90 kDa small complex (Wieckowski et al, 1994; Robertson et al, 2007), which then oligomerizes to form a ~450 kDa SDS-resistant complex called CH-1. The latter contains 6 copies of CPE, plus receptor and non-receptor claudins (Robertson et al, 2007). Once the CH-1 complex is formed, it inserts into the cytoplasmic cell membrane and forms a pore (Smedley et al, 2007), which increases membrane permeability. This, in turn, is followed by Ca2+ influx, which then activates apoptotic or oncotic death pathways (Chakrabarti et al, 2003; Chakrabarti and McClane, 2005). Simultaneously, the cell damage originating with the initial pore formation exposes the basolateral cell surface of the cells, resulting in formation of a ~600 kDa CPE complex (CH-2) (Singh et al, 2000, 2001; Robertson et al, 2007). CPE-induced epithelial cell death promotes fluid and transport changes with the net result of fluid accumulation in the intestine leading to diarrhea (McClane et al, 2006). In vitro, low CPE doses induce apoptosis, while high doses induce oncosis (Chakrabarti et al, 2003). Recent investigations on the mechanism of CPE-induced cell death in vivo using a ligated small intestinal mouse model, showed that this toxin causes caspase-3 activation in a dose- and time-dependent manner in small intestinal epithelial cells (Freedman et al, 2018). However, intestinal damage occurred before this CPE-induced caspase-3 activation, and inhibition of intestinal caspase-3 activity did not prevent intestinal epithelium damage or CPE-induced lethality in mice (Freedman et al, 2018). These results indicate that, while caspase-3 activation occurs in CPE-treated intestine of mice, the activation of this enzyme is not required for those effects to occur (Freedman et al 2018)
Molecular Koch’s postulates have been fulfilled for CPE using rabbit intestinal loops (Fig. 4). Those experiments in rabbits confirmed that CPE is necessary for enterotoxigenic C. perfringens type F to cause enteric disease (Sarker et al, 1999). In addition, experiments in mice demonstrated that CPE can cause enterotoxemia and death. Elevated potassium has been found in the serum of mice inoculated into the small intestine with CPE, which led to the sugggestion that hyperpotassemia is at least partly responsible for the death of some CPE-intoxicated individuals (Caserta et al, 2011).
Type G infections
The newly defined type G strains are characterized by producing CPA and NetB, but they do not produce CPB, ETX or ITX (Table 2; Rood et al, 2018). Type G strains cause necrotic enteritis of poultry, a highly prevalent infection that produces severe losses to the poultry industry worldwide. The β-pore-forming NetB toxin has been shown to be the main virulent factors for type G strains (Keyburn et al, 2008). However, cases of necrotc enteritis have also been described in birds apparently free from NetB-positive C. perfringens strains (type A strains), which could suggest that other virulence factors, in addition to NetB, may also be responsible for necrotic enteritis (Smyth and Martin, 2010). However the fulfilment of molecular Koch’s postulates with netB-positive strains confirmed that netB-positive strains are sufficient to cause necrotic enteritis in poultry (Keyburn et al, 2008). Both spontaneous and experimental disease induction is complex, with one or more predisposing factor involved. Under natural conditions, the most frequent predisposing factor is intestinal infection by Eimeria spp., while experimentally the disease can be readily reproduced by feeding birds a high protein diet, followed by oral challenge with netB-positive strains (Cooper and Songer, 2010; Cooper et al, 2010). No cases of type G diseases have been reported in non-poultry animals or humans.
Clostridium difficile
Abundant information on C. difficile enteric infections in humans and animals is available in the literature (Burke and Lamont, 2014; Rineh et al, 2014; Diab at al, 2013, 2016; Leffler and Lamont, 2015; Pant at al, 2013; Kuiper et al, 2017), and a complete review of that information is beyond the scope of this paper. Therefore, only a brief mention of the comparative aspects of these infections between humans and animals will be presented here.
C. difficile causes enteric disease in humans and numerous animal species, including, but not limited to, gerbils, guinea pigs, hamsters, horses, rabbits and pigs (Diab et al, 2016). In humans, C. difficile associated disease (CDAD) was always assumed to affect individuals of any age, except during the neonatal period (Sammons et al, 2013; Kuiper et al, 2017) as it was thought that this specific group may lack specific C. difficile toxin receptors. Although between 25 and 70% of human neonates are colonized with C. difficile (Kuiper et al, 2017), these microorganisms have been largely considered part of the commensal microbiota. Recently, however, two 9 or 18 month-old children were diagnosed with CDAD (Kuiper et al, 2017), providing evidence that C. difficile is a a potential cause of bloody diarrhea in neonates and young infants. In most animal species, CDAD is not age-dependent (Keel and Songer, 2006). The exception to this are pigs, which are almost exclusively affected during the neonatal period, up to approximately one week of age (Smits et al, 2016).
The most important virulence factors of C. difficile are toxins A (TcdA) and B (TcdB) (Lyon et al, 2016), although in recent years a role for an actin-specific ADP-ribosyltransferase (CDT) produced by some strains of C. difficile has emerged (Cowardin et al, 2016). TcdA is sometimes considered as an enterotoxin and TcdB as a cytotoxin.
For many years it was thought that TcdB had little activity in vivo unless there was prior damage to mucosal epithelium. Althought it was later on proposed that both toxins can act together, with TcdA creating widespread damage to the mucosa that permits TcdB to affect epithelial cells (Awad et al., 2014), it was recently demonstrated that TcdB is the major virulence factor of C. difficile (Carter et al., 2015). This is supported by the fact that there are numerous clinical reports of CDAD in patients infected by TcdA-negative, TcdB-positive strains of C. difficile (Kim et al, 2012).
At a cellular level, the first step of toxin activity is binding of TcdA or TcdB to several cell receptors, which is followed by endocytosis of the toxins into a cellular endosome. The next step is acidification of the endosome, which is followed by insertion of the toxin into the endosomal membrane. This results in formation of a pore which facilitates release of the active glucosyltransferase domain into the cytosol. The net result of these events is glucosylation and inactivation of Rho family GTPases (Awad et al., 2014; Yuan et al, 2015). The net effect of this chain of events is compromise of signal transduction for molecules associated with maintenance and regulation of actin filaments, and apoptosis, all of which results in loss of cell-cell contact, increased paracellular permeability of the intestinal mucosa and cell death by apoptosis or necroptosis (Awad et al, 2014).
Some C. difficile strains produce CDT (Cowardin et al, 2016), an adenosine diphosphate-ribosyltransferase that causes actin cytoskeletal disruption. This toxin is produced by most of the hypervirulent strains of C. difficile, and it has been shown to enhance the virulence of ribotype 027 strains in mice. Most CDT-expressing strains show enhanced virulence (Cowardin et al, 2016)
Antibiotic treatment remains an important risk factor both for humans and animals, except for piglets and neonatal foals in which antibiotic treatment is seldom part of the clinical history (Keel and Songer, 2006; Diab et al, 2013). Hospitalization is also a major risk factor for horses, as it is for humans. Both in humans and animals, however, cases can occur in individuals that were neither hospitalized nor subjected to antibiotic treatment (Ogielska et al 2015). Transmission of C. difficile from animals and food to people is suspected, but so far this has not been definitively proved. This possibility is supported by the fact that some C. difficile ribotypes that are highly virulent for humans (e.g. ribotype 078) have been isolated from retail food of animal origin and from the intestinal tract of food animals (Rupnik and Songer, 2010; Warriner et al, 2017). Nevertheless, a direct link between C. difficile and food-borne illness outbreaks has not been demonstrated, and conclusive evidence that spores of this microorganism can germinate in food matrices is lacking (Warriner et al, 2017).
Other clostridia causing enteric diseases
Clostridium spiroforme causes typhlocolitis and enterotoxemia in rabbits. Little is known about the pathogenesis of this infection. C. spiroforme produces a binary toxin (CST) which is thought to be responsible for the virulence of this microorganism, although definitive evidence in this regards is lacking. No human infections by C. spiroforme have been reported (Songer and Uzal, 2016).
Clostridium piliforme, the only gram negative of the pathogenic clostridia, is responsible for Tyzzer’s disease, a highly lethal condition characterized by necrotizing hepatitis, colitis and/or myocarditis, that affects multiple laboratory animal species, and neonatal or juvenile horses, cattle, cats, birds and several other domestic animals. Very little is known about the pathogenesis of Tyzzer’s disease and no specific virulence factors have been identified for C. piliforme. No human infections by C. piliforme have been reported (Fresneda et al, 2016).
Clostridium colinum, a close relative of C. piliforme, causes ulcerative enteritis in young game birds, chickens, turkeys, and occasionally other avian species. Because the infection was first described in quail, the disease is usually referred to as quail disease. Very little is known about the pathogenesis of this infection; the genome of C. colinum has not been characterized and the basis of its remarkable virulence is unknown. No reports of C. colinum infection in non-avian species have been published (Cooper et al, 2013).
Several other clostridial species, including Clostridium sordellii, Clostridium chauvoei, Clostridium septicum and others have been implicated, albeit in individual cases and sporadically, in enteric disease of animals and/or humans (Uzal et al, 2016; Rimoldi et al, 2015; Jones and Wilson, 1993).
Although botulism is not usually classified as an enteric disease, it has been postulated that at least some cases of animal botulism progress from intestinal colonization by Clostridium botulinum with toxin produced in the intestine and being absorbed into the blood, similar to a classical enterotoxemia (toxicoinfection). However, ingestion of preformed toxins is considered the main route of exposure in cases of animal botulism (Le Marechal et al, 2016). Horses, cattle and poultry are the species in which cases of toxicoinfection are most likely to occur. It has been suggested that most cases of botulism in broiler chickens occur due to botulinum toxins (BoNT) production in the cecum, because a high level of type C toxin is necessary to induce botulism and those amounts of toxin have not been found in the environment (Le Marechal et al, 2016).
In humans toxicoinfection seems to be the mechanism by which most cases of human infant botulism occur (Rosow and Strober, 2015). The disease occurs after ingestion of C. botulinum types A or B spores which germinate within the gastrointestinal tract and produce toxins. Infant botulism is the most common form of of the disease in the United States (Brown and Desai, 2013).
Clostridium butyricum and Clostridium neonatale have been associated with neonatal necrotic enteritis in humans. No bacterial protein toxins produced by either microorganism have been identified, and it is therefore thought that intestinal lesions are produced by gas, butyrate and other metabolites, which vary with the food composition (Alpha et al, 2002; Schonherr-Hellec et al, 2017). No animal diseases associated with these microorganisms have been reported.
Conclusions
Although several clostridial species produce enteric disease in both humans and animals, there are some species that affect animals only, but the inverse does not seem to apply, i.e. all clostridial species causing human disease also seem to be associated with animal disease. While transmission from animals to humans has been speculated for several clostridial species (e.g. C. perfringens), this has not been fully proved and more work is needed in this regard. Diagnosis of enteric clostridial infections remains challenging as several clostridial species (e.g. C. perfringens and C. difficile) can be found in the intestine of healty individuals which renders culture-based procedures alone of little diagnostic value.
HIGHLIGHTS.
Enteric infections caused by Clostridium difficile and Clostridium perfringens are amongst the most prevalent enteric diseases affecting both humans and animals
Clostridium perfringens type A producing enterotoxin is responsible for food poisoning in humans, and the less prevalent antibiotic associated diarrhea and sporadic diarrhea
C. perfringens type A NetB positive strains are responsible for avian necrotic enteritis
The other types of C. perfringens are mostly associated with animal disease
C. difficile produces disease in animals and humans
Other clostridia causing enteric diseases in humans and/or animals are Clostridium spiroforme, Clostridium piliforme, Clostridium colinum, Clostridium sordellii, Clostridium chauvoei and Clostridium septicum.
Acknowledgments
This research was generously supported by grant AI0198844-35 from the National Institute of Allergy to Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thanks Ms S. Fitisemanu for her help formatting the references.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alpha MJ, Robson D, Davi M, Bernard K, Van Caeseele P, Harding GKM. An outbreak of necrotizing enterocolitis associated with a novel Clostridium Species in a neonatal intensive care unit. Clin Infect Dis. 2002;1:35. doi: 10.1086/341929. [DOI] [PubMed] [Google Scholar]
- 2.Autheman D, Wyder M, Popoff M, D’Herde K, Christen S, Posthaus H. Clostridium perfringens beta-toxin induces necrostatin-inhibitable, calpain-dependent necrosis in primary porcine endothelial cells. PLoS One. 2013;85:e64644. doi: 10.1371/journal.pone.0064644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awad MM, Johanesen PA, Carter GP, Rose E, Lyras D. Clostridium difficile virulence factors: Insights into an anaerobic spore-forming pathogen. Gut Microbes. 2014;55:579–93. doi: 10.4161/19490976.2014.969632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackwell TE, Butler DG, Prescott JF, Wilcock BP. Differences in signs and lesions in sheep and goats with enterotoxemia induced by intraduodenal infusion of Clostridium perfringens type D. Am J Vet Res. 1991;52:1147–1152. [PubMed] [Google Scholar]
- 5.Brown N, Desai S. Infantile botulism: a case report and review. J Emerg Med. 2013;456:842–5. doi: 10.1016/j.jemermed.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Bueschel D, Walker R, Woods L, Kokai-Kun J, McClane B, Songer JG. Enterotoxigenic Clostridium perfringens type A necrotic enteritis in a foal. J Am Vet Med Assoc. 1998;213:1305–1307. [PubMed] [Google Scholar]
- 7.Burke KE, Lamont JT. Clostridium difficile infection: a worldwide disease. Gut Liver. 2014;81:1–6. doi: 10.5009/gnl.2014.8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busch K, Suchodolski JS, Kühner KA, Minamoto Y, Steiner JM, Mueller RS, Hartmann K, Unterer S. Clostridium perfringens enterotoxin and Clostridium difficile toxin A/B do not play a role in acute haemorrhagic diarrhoea syndrome in dogs. Vet Rec. 2015;176:253. doi: 10.1136/vr.102738. [DOI] [PubMed] [Google Scholar]
- 9.Buxton D, Morgan KT. Studies of lesions produced in the brains of colostrum deprived lambs by Clostridium welchii (Clostridium perfringens) type D toxin. J Comp Path. 1976;86:435–447. doi: 10.1016/0021-9975(76)90012-8. [DOI] [PubMed] [Google Scholar]
- 10.Carter GP, Chakravorty A, Pham Nguyen TA, Mileto S, Schreiber F, Li L, Howarth P, Clare S, Cunningham S, Sambol SP, Cheknis A, Figueroa I, Johnson S, Gerding D, Rood JI, Dougan G, Lawley TD, Dena Lyras D. Defining the roles of TcdA and TcdB in localized gastrointestinal disease, systemic organ damage, and the host response during Clostridium difficile infections. mBio. 2015;6:e00551–15. doi: 10.1128/mBio.00551-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caserta JA, Robertson SL, Saputo J, Shrestha A, McClane BA, Uzal FA. Development and application of a mouse intestinal loop model to study the in vivo action of Clostridium perfringens enterotoxin. Infect Immun. 2011;79:3020–3027. doi: 10.1128/IAI.01342-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakrabarti G, Zhou X, McClane BA. Death pathways activated in CaCo-2 cells by Clostridium perfringens enterotoxin. Infect Immun. 2003;71:4260–4270. doi: 10.1128/IAI.71.8.4260-4270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakrabarti G, McClane BA. The importance of calcium influx, calpain, and calmodulin for the activation of CaCo-2 cell death pathways by Clostridium perfringens enterotoxin. Cell Microbiol. 2005;7:129–146. doi: 10.1111/j.1462-5822.2004.00442.x. [DOI] [PubMed] [Google Scholar]
- 14.Chassin C, Bens M, de Barry J, Courjaret R, Bossu JL, Cluzeaud F, Ben Mkaddem S, Gibert M, Poulain B, Popoff MR, Vandewalle A. Pore-forming epsilon toxin causes membrane permeabilization and rapid ATP depletion-mediated cell death in renal collecting duct cells. Am J Physiol Renal Physiol. 2007;293:927–937. doi: 10.1152/ajprenal.00199.2007. [DOI] [PubMed] [Google Scholar]
- 15.Cooper KK, Songer JG, Uzal FA. Diagnosing clostridial enteric disease in poultry. J Vet Diagn Invest. 2013;25:314–327. doi: 10.1177/1040638713483468. [DOI] [PubMed] [Google Scholar]
- 16.Cooper KK, Songer JG. Virulence of Clostridium perfringens in an experimental model of poultry necrotic enteritis. Vet Microbiol. 2010;142:323–328. doi: 10.1016/j.vetmic.2009.09.065. [DOI] [PubMed] [Google Scholar]
- 17.Cowardin CA, Buonomo EL, Saleh MM, Wilson MG, Burgess SL, Kuehne SA, Schwan C, Eichhoff AM, Koch-Nolte F, Lyras D, Aktories K, Minton NP, Petri WA., Jr The binary toxin CDT enhances Clostridium difficile virulence by suppressing protective colonic eosinophilia. Nat Microbiol. 2016;1:16108. doi: 10.1038/nmicrobiol.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czeczulin JR, Hanna PC, McClane BA. Cloning, nucleotide sequencing, and expression of the Clostridium perfringens enterotoxin gene in Escherichia coli. Infect Immun. 1993;61:3429–39. doi: 10.1128/iai.61.8.3429-3439.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diab SS, Uzal FA, Songer JG. Diseases produced by Clostridium difficile. In: Uzal FA, Songer JG, Prescott J, Popoff M, editors. Clostridial diseases of animals. Willey and Blackwell; Ames, IA: 2016. pp. 177–195. [Google Scholar]
- 20.Diab SS, Songer G, Uzal FA. Clostridium difficile infection in horses: a review. Vet Microbiol. 2013;29:42–49. doi: 10.1016/j.vetmic.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 21.Diab SS, Kinde H, Moore J, Shahriar MF, Odani J, Anthenill L, Songer JG, Uzal FA. Pathology of Clostridium perfringens type C enterotoxemia in horses. Vet Pathol. 2012;49:255–263. doi: 10.1177/0300985811404710. [DOI] [PubMed] [Google Scholar]
- 22.Dorca-Arévalo J, Pauillac S, Díaz-Hidalgo L, Martín-Satué M, Popoff MR, Blasi J. Correlation between in vitro cytotoxicity and in vivo lethal activity in mice of epsilon toxin mutants from Clostridium perfringens. PLoS One. 2014;11:e102417. doi: 10.1371/journal.pone.0102417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez-Miyakawa ME, Fisher DJ, Poon R, Sayeed S, Adams V, Rood JI, McClane BA, Uzal FA. Both epsilon-toxin and beta-toxin are important for the lethal properties of Clostridium perfringens type B isolates in the mouse intravenous injection model. Infect Immun. 2007;75:1443–1452. doi: 10.1128/IAI.01672-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Field HI, Goodwin RF. The experimental reproduction of enterotoxaemia in piglets. J Hyg. 1959;57:81–91. doi: 10.1017/s0022172400019914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finley A, Gohari IM, Parreira VR, Abrahams M, Staempfli HR, Prescott JF. Prevalence of netF-positive Clostridium perfringens in foals in southwestern Ontario. Can J Vet Res. 2016;80:242–244. [PMC free article] [PubMed] [Google Scholar]
- 26.Finnie JW, Blumbergs PC, Manavis J. Neuronal damage produced in rat brains by Clostridium perfringens type D epsilon toxin. J Comp Pathol. 1999;120:415–20. doi: 10.1053/jcpa.1998.0289. [DOI] [PubMed] [Google Scholar]
- 27.Finnie JW. Histopathological changes in the brain of mice given Clostridium perfringens type D epsilon toxin. J Comp Pathol. 1984a;94:363–370. doi: 10.1016/0021-9975(84)90024-0. [DOI] [PubMed] [Google Scholar]
- 28.Finnie JW. Ultrastructural changes in the brain of mice given Clostridium perfringens type D epsilon toxin. J Comp Pathol. 1984b;94:445–452. doi: 10.1016/0021-9975(84)90031-8. [DOI] [PubMed] [Google Scholar]
- 29.Finnie JW. Pathogenesis of brain damage produced in sheep by Clostridium perfringens type D epsilon toxin: a review. Aust Vet J. 2003;81:219–221. doi: 10.1111/j.1751-0813.2003.tb11474.x. [DOI] [PubMed] [Google Scholar]
- 30.Finnie JW, Blumbergs PC, Manavis J. Neuronal damage produced in rat brains by Clostridium perfringens type D epsilon-toxin. J Comp Path. 1999;120:415–420. doi: 10.1053/jcpa.1998.0289. [DOI] [PubMed] [Google Scholar]
- 31.Fisher DJ, Fernandez-Miyakawa ME, Sayeed S, Poon R, Adams V, Rood JI, Uzal FA, McClane BA. Dissecting the contributions of Clostridium perfringens type C toxins to lethality in the mouse intravenous injection model. Infect Immun. 2006;74:5200–5210. doi: 10.1128/IAI.00534-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freedman JC, Theoret JR, Wisniewski JA, Uzal FA, Rood JI, McClane BA. Clostridium perfringens type A–E toxin plasmids. Res Microbiol. 2015;166:264–279. doi: 10.1016/j.resmic.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freedman JC, Li J, Uzal FA, McClane BA. Proteolytic processing and activation of Clostridium perfringens epsilon toxin by caprine small intestinal contents. MBio. 2014;21:e01994–2014. doi: 10.1128/mBio.01994-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freedman JC, Navarro MA, Morrell E, Beingesser J, Shrestha A, McClane BA, Uzal FA. Evidence that Clostridium perfringens Enterotoxin-Induced Intestinal Damage and Enterotoxemic Death in Mice Can Occur Independently of Intestinal Caspase-3 Activation. Inf Immun. 2018;10:1128. doi: 10.1128/IAI.00931-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fresneda KC, Carvallo Chaigneau FR. Tyzzer’s disease. In: Uzal FA, Songer JG, Prescott J, Popoff M, editors. Clostridial diseases of animals. Willey and Blackwell; Ames IA: 2016. pp. 281–291. [Google Scholar]
- 36.Fujita K, Katahira J, Horiguchi Y, Sonoda N, Furuse M, Tskuita S. Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction membrane protein. FEBS Letters. 2000;476:258–261. doi: 10.1016/s0014-5793(00)01744-0. [DOI] [PubMed] [Google Scholar]
- 37.Garcia JP, Beingesser J, Fisher DJ, Sayeed S, McClane BA, Posthaus H, Uzal FA. The effect of Clostridium perfringens type C strain CN3685 and its isogenic beta toxin null mutant in goats. Vet Microbiol. 2012;157:412–419. doi: 10.1016/j.vetmic.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia JP, Adams V, Beingesser J, Hughes ML, Poon R, Lyras D, Hill A, McClane BA, Rood JI, Uzal FA. Epsilon toxin is essential for the virulence of Clostridium perfringens type D infection in sheep, goats, and mice. Infect Immun. 2013;81:2405–2414. doi: 10.1128/IAI.00238-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldstein J, Morris WE, Loidl CF, Tironi-Farinati C, McClane BA, Uzal FA, Fernandez Miyakawa ME. Clostridium perfringens epsilon toxin increases the small intestinal permeability in mice and rats. PLoS One. 1993;18:e7065. doi: 10.1371/journal.pone.0007065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson S, Gerding DN. Enterotoxemic infections. In: Rood JI, McClaine BA, Songer JG, Titball RW, editors. The Clostridia: Molecular Biology and Pathogenesis. San Diego: Academic Press; 1997. pp. 117–140. [Google Scholar]
- 41.Jones SL, Wilson WD. Clostridium septicum septicemia in a neonatal foal with hemorrhagic enteritis. Cornell Vet. 1993;83:143–51. [PubMed] [Google Scholar]
- 42.Katahira J, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Molecular cloning and functional characterization of the receptor for Clostridium perfringens enterotoxin. J Cell Biol. 1997a;136:1239–1247. doi: 10.1083/jcb.136.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katahira J, Sugiyama H, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Clostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. J Biol Chem. 1997b;272:26652–26658. doi: 10.1074/jbc.272.42.26652. [DOI] [PubMed] [Google Scholar]
- 44.Keel MK, Songer JG. The comparative pathology of Clostridium difficile-associated disease. Vet Pathol. 2006;43:225–40. doi: 10.1354/vp.43-3-225. [DOI] [PubMed] [Google Scholar]
- 45.Keyburn AL, Boyce JD, Vaz P, Bannam TL, Ford ME, Parker D, Di Rubbo A, Rood JI, Moore RJ. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008;4:e26. doi: 10.1371/journal.ppat.0040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim HY, Byun JW, Roh IS, Bae YC, Lee MH, Kim B, Songer JG, Jung BY. First isolation of Clostridium perfringens type E from a goat with diarrhea. Anaerobe. 2013;22:141–3. doi: 10.1016/j.anaerobe.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J, Pai H, Seo MR, Kang JO. Clinical and microbiologic characteristics of tcdA-negative variant Clostridium difficile infections. BMC Infect Dis. 2012;12:109. doi: 10.1186/1471-2334-12-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kohler B, Rosch B, Haase H, Baumann G. Studies of necrotizing enteritis of suckling piglets (Cl. perfringens type C enterotoxemia) in industrialized sow breeding units. 3. Experimental reproduction of the disease. Archiv fur experimentelle Veterinarmedizin. 1979;33:313–333. [PubMed] [Google Scholar]
- 49.Kreft B, Dalhoff K, Sack K. Necrotizing enterocolitis: a historical and current review. Med Klin (Munich) 2000;95:435–441. doi: 10.1007/s000630050003. [DOI] [PubMed] [Google Scholar]
- 50.Kuiper GA, van Prehn J, Ang W, Kneepkens F, van der Schoor S, de Meij T. Clostridium difficile infections in young infants: Case presentations and literature review. IDCases. 2017;10:7–11. doi: 10.1016/j.idcr.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lawrence G, Cooke R. Experimental pigbel: The production and pathology of necrotizing enteritis due to Clostridium welchii Type C in the guinea pig. Br J Exp Path. 1980;61:261–271. [PMC free article] [PubMed] [Google Scholar]
- 52.Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372:1539–48. doi: 10.1056/NEJMra1403772. [DOI] [PubMed] [Google Scholar]
- 53.Le Marechal C, Woudstra C, Fach P. In: Clostridial diseases of animals. Uzal FA, Songer JG, Prescott J, Popoff M, editors. Willey and Blackwell; Ames IA: 2016. pp. 303–330. [Google Scholar]
- 54.Leipig-Rudolph M, Busch K, Prescott JF, Mehdizadeh Gohari I, Leutenegger CM, Hermanns W, Wolf G, Hartmann K, Verspohl J, Unterer S. Intestinal lesions in dogs with acute hemorrhagic diarrhea syndrome associated with netF-positive Clostridium perfringens type A. J Vet Diagn Invest. 2018 doi: 10.1177/1040638718766983. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J, Miyamoto K, McClane BA. Comparison of virulence plasmids among Clostridium perfringens type E isolates. Infect Immun. 2007;75:1811–19. doi: 10.1128/IAI.01981-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li J, et al. Microbiology Spectrum (on type F strains; former type A 2016 [Google Scholar]
- 57.Li J, McClane BA. Contributions of NanI sialidase to Caco-2 cell adherence by Clostridium perfringens type A and C strains causing human intestinal disease. Infect Immun. 2014;82:4620–30. doi: 10.1128/IAI.02322-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li J, Paredes-Sabja D, Sarker MR, McClane BA. Clostridium perfringens Sporulation and Sporulation-Associated Toxin Production. Microbiol Spectr. 2016;4:10. doi: 10.1128/microbiolspec.TBS-0022-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lyon SA, Hutton ML, Rood JI, Cheung JK, Lyras D. CdtR Regulates TcdA and TcdB Production in Clostridium difficile. PLoS Pathog. 2016;4:e1005758. doi: 10.1371/journal.ppat.1005758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma M, Gurjar A, Theoret JR, Garcia JP, Beingesser J, Freedman JC, Fisher DJ, McClane BA, Uzal FA. Synergistic effects of Clostridium perfringens enterotoxin and beta toxin in rabbit small intestinal loops. Infect Immun. 2014;82:2958–70. doi: 10.1128/IAI.01848-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsuda T, Okada Y, Inagi E, Tanabe Y, Shimizu Y, Nagashima K, Sakurai J, Nagahama M, Tanaka S. Enteritis necroticans ‘pigbel’ in a Japanese diabetic adult. Pathol Int. 2007;57:622–626. doi: 10.1111/j.1440-1827.2007.02149.x. [DOI] [PubMed] [Google Scholar]
- 62.McClane BA, Uzal FA, Miyakawa ME, Lyerly MF, Wilkins D. In: The Enterotoxic Clostridia. Dworkin M, Falkow S, Rosenburg E, Schleifer H, Stackebrandt E, editors. The Prokaryotes; New York: 2006. pp. 688–752. [Google Scholar]
- 63.McDonel JL, Duncan CL. Histopathological effect of Clostridium perfringens enterotoxin in the rabbit ileum. Infect Immun. 1975;12:1214–1218. doi: 10.1128/iai.12.5.1214-1218.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gohari MI, Parreira VR, Nowell VJ, Nicholson VM, Oliphant K, Prescott JF. A novel pore-forming toxin in type A Clostridium perfringens is associated with both fatal canine hemorrhagic gastroenteritis and fatal foal necrotizing enterocolitis. PLoS One. 2015;10:e0122684. doi: 10.1371/journal.pone.0122684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gohari MI, Valeria R, Parreira, Prescott JF. NetF-associated necrotizing enteritis of foals and canine hemorrhagic gastroenteritis. In: Uzal FA, Songer JG, Prescott J, Popoff M, editors. Clostridial diseases of animals. Willey and Blackwell; Ames IA: 2016. pp. 117–122. [Google Scholar]
- 66.Minami J, Katayama S, Matsushita O, Matsushita C, Okabe A. Lambda-toxin of Clostridium perfringens activates the precursor of epsilon-toxin by releasing its N- and C-terminal peptides. Microbiol Immunol. 1997;41:527–535. doi: 10.1111/j.1348-0421.1997.tb01888.x. [DOI] [PubMed] [Google Scholar]
- 67.Miyamoto K, Fisher DJ, Li J, Sayeed S, Akimoto S, McClane BA. Complete sequencing and diversity analysis of the enterotoxin-encoding plasmids in Clostridium perfringens type A non-food-borne human gastrointestinal disease isolates. J Bacteriol. 2006;188:1585–98. doi: 10.1128/JB.188.4.1585-1598.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miyamoto K, Yumine N, Mimura K, Nagahama M, Li J, McClane BA. Identification of novel Clostridium perfringens type E strains that carry an iota toxin plasmid with a functional enterotoxin gene. PLoS One. 2011;6:e20376. doi: 10.1371/journal.pone.0020376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nagahama M, Hayashi S, Morimitsu S, Sakurai J. Biological activities and pore formation of Clostridium perfringens beta-toxin in HL 60 cells. J Biol Chem. 2003a;278:36934–36941. doi: 10.1074/jbc.M306562200. [DOI] [PubMed] [Google Scholar]
- 70.Nagahama M, Morimitsu S, Kihara A, Akita M, Setsu K, Sakurai J. Involvement of tachykinin receptors in Clostridium perfringens beta-toxin-induced plasma extravasation. Br J Pharmacol. 2003b;138:23–30. doi: 10.1038/sj.bjp.0705022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nagahama M, Shibutani M, Seike S, Yonezaki M, Takagishi T, Oda M, Kobayashi K, Sakurai J. The p38 MAPK and JNK pathways protect host cells against Clostridium perfringens beta-toxin. Infect Immun. 2013;81:3703–3708. doi: 10.1128/IAI.00579-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ogielska M, Lanotte P, Le Brun C, Valentin AS, Garot D, Tellier AC, Halimi JM, Colombat P, Guilleminault L, Lioger B, Vegas H, De Toffol B, Constans T, Bernard L. Emergence of community-acquired Clostridium difficile infection: the experience of a French hospital and review of the literature. Int J Infect Dis. 2015;37:36–41. doi: 10.1016/j.ijid.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 73.Petrillo TM, Beck-Sagué CM, Songer JG, Abramowsky C, Fortenberry JD, Meacham L, Dean AG, Lee H, Bueschel DM, Nesheim SR. Enteritis necroticans (pigbel) in a diabetic child. N Engl J Med. 2000;342:1250–1253. doi: 10.1056/NEJM200004273421704. [DOI] [PubMed] [Google Scholar]
- 74.Popoff MR. Epsilon toxin: a fascinating pore-forming toxin. FEBS J. 2011;278:4602–4515. doi: 10.1111/j.1742-4658.2011.08145.x. [DOI] [PubMed] [Google Scholar]
- 75.Pant C, Deshpande A, Altaf MA, Minocha A, Sferra TJ. Clostridium difficile infection in children: a comprehensive review. Curr Med Res Opin. 2013;29:967–84. doi: 10.1185/03007995.2013.803058. [DOI] [PubMed] [Google Scholar]
- 76.Prescott JF, Menzies PI, Fraser RS. Clostridial abomasitis. In: Uzal FA, Songer JG, Prescott J, Popoff M, editors. Clostridial diseases of animals. Willey and Blackwell; Ames IA: 2016. pp. 205–242. [Google Scholar]
- 77.Petit L, Maier E, Gibert M, Popoff MR, Benz R. Clostridium perfringens epsilon-toxin induces a rapid change in cell membrane permeability to ions and forms channels in artificial lipid bilayers. J Biol Chem. 2001;276:15736–40. doi: 10.1074/jbc.M010412200. [DOI] [PubMed] [Google Scholar]
- 78.Redondo LM, Carrasco JM, Redondo EA, Delgado F, Miyakawa ME. Clostridium perfringens type E virulence traits involved in gut colonization. PLoS One. 2015;10:e0121305. doi: 10.1371/journal.pone.0121305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Richard JF, Mainguy G, Gibert M, Marvaud JC, Stiles BG, Popoff MR. Transcytosis of iota-toxin across polarized CaCo-2 cells. Mol Microbiol. 2002;43:907–17. doi: 10.1046/j.1365-2958.2002.02806.x. [DOI] [PubMed] [Google Scholar]
- 80.Rimoldi G, Uzal F, Chin RP, Palombo EA, Awad M, Lyras D, Shivaprasad HL. Necrotic enteritis in chickens associated with Clostridium sordellii. Avian Dis. 2015;59:447–451. doi: 10.1637/11077-033115-Case.1. [DOI] [PubMed] [Google Scholar]
- 81.Rineh A, Kelso MJ, Vatansever F, Tegos GP, Hamblin MR. Clostridium difficile infection: molecular pathogenesis and novel therapeutics. Expert Rev Anti Infect Ther. 2014;12:131–50. doi: 10.1586/14787210.2014.866515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robertson SL, Smedley JG, 3rd, Singh U, Chakrabarti G, Van Itallie CM, Anderson JM, McClane BA. Compositional and stoichiometric analysis of Clostridium perfringens enterotoxin complexes in Caco-2 cells and claudin 4 fibroblast transfectants. Cell Microbiol. 2007;9:2734–2755. doi: 10.1111/j.1462-5822.2007.00994.x. [DOI] [PubMed] [Google Scholar]
- 83.Robertson SL, Li J, Uzal FA, McClane BA. Evidence for a prepore stage in the action of Clostridium perfringens epsilon toxin. PLoS One. 2011;6:e22053. doi: 10.1371/journal.pone.0022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rood JI, Adams Adams V, Lacey J, Lyras D, Mc Clane BA, Melville SB, Moore RJ, Popoff MR, Sarker MR, Songer JG, Uzal FA, Van Immerseel F. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe. 2018 doi: 10.1016/j.anaerobe.2018.04.011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rosow LK, Strober JB. Infant botulism: review and clinical update. Pediatr Neurol. 2015;52:487–92. doi: 10.1016/j.pediatrneurol.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 86.Rumah KR, Linden J, Fischetti VA, Vartanian T. Isolation of Clostridium perfringens type B in an individual at first clinical presentation of multiple sclerosis provides clues for environmental triggers of the disease. PLoS One. 2013;8:e76359. doi: 10.1371/journal.pone.0076359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rumah KR, Ma Y, Linden JR, Oo ML, Anrather J, Schaeren-Wiemers N, Alonso MA, Fischetti VA, McClain MS, Vartanian T. The myelin and lymphocyte protein MAL is required for binding and activity of Clostridium perfringens epsilon-toxin. PLoS Pathog. 2015;11:e1004896. doi: 10.1371/journal.ppat.1004896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rupnik M, Songer JG. Clostridium difficile: its potential as a source of foodborne disease. Adv Food Nutr Res. 2010;60:53–66. doi: 10.1016/S1043-4526(10)60003-4. [DOI] [PubMed] [Google Scholar]
- 89.Sakurai J, Duncan CL. Some properties of beta-toxin produced by Clostridium perfringens type C. Infect Immun. 1978;21:678–680. doi: 10.1128/iai.21.2.678-680.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sakurai J, Nagahama M, Oda M, Tsuge H, Kobayashi K. Clostridium perfringens iota-toxin: structure and function. Toxins. 2009;1:208–228. doi: 10.3390/toxins1020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sammons JS, Toltzis P, Zaoutis TE. Clostridium difficile Infection in children. JAMA Pediatr. 2013;167:567–73. doi: 10.1001/jamapediatrics.2013.441. [DOI] [PubMed] [Google Scholar]
- 92.Sarker MR, Carman RJ, McClane BA. Inactivation of the gene (cpe) encoding Clostridium perfringens enterotoxin eliminates the ability of two cpe-positive C. perfringens type A human gastrointestinal disease isolates to affect rabbit ileal loops. Molec Microbiol. 1999;33:946–958. doi: 10.1046/j.1365-2958.1999.01534.x. [DOI] [PubMed] [Google Scholar]
- 93.Sarvari KP, Vasas B, Kiss I, Lazar A, Horvath I, Simon M, Peto Z, Urban E. Fatal Clostridium perfringens sepsis due to emphysematous gastritis and literature review. Anaerobe. 2016;40:31–4. doi: 10.1016/j.anaerobe.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 94.Sayeed S, Fernandez-Miyakawa ME, Fisher DJ, Adams V, Poon R, Rood JI, Uzal FA, McClane BA. Epsilon-toxin is required for most Clostridium perfringens type D vegetative culture supernatants to cause lethality in the mouse intravenous injection model. Infect Immun. 2005;73:7413–21. doi: 10.1128/IAI.73.11.7413-7421.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sayeed S, Uzal FA, Fisher DJ, Saputo J, Vidal JE, Chen Y, Gupta P, Rood JI, McClane BA. Beta toxin is essential for the intestinal virulence of Clostridium perfringens type C disease isolate CN3685 in a rabbit ileal loop model. Mol Microbiol. 2008;67:15–30. doi: 10.1111/j.1365-2958.2007.06007.x. [DOI] [PubMed] [Google Scholar]
- 96.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson M, Roy S, Jones JL, Griffin PM. Foodborne illness acquired in the United States-major pathogens. Emer Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schonherr-Hellec S, Klein G, Delannoy J, Ferraris L, Friedel I, Roz JC, Butel MJ, Aires J. Comparative phenotypic analysis of “Clostridium neonatale” and Clostridium butyricum isolates from neonates. Anaerobe. 2017;48:76–82. doi: 10.1016/j.anaerobe.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 98.Schumacher VL, Martel A, Pasmans F, Van Immerseel F, Posthaus H. Endothelial binding of beta toxin to small intestinal mucosal endothelial cells in early stages of experimentally induced Clostridium perfringens type C enteritis in pigs. Vet Pathol. 2013;50:626–9. doi: 10.1177/0300985812461362. [DOI] [PubMed] [Google Scholar]
- 99.Shatursky O, Bayles R, Rogers M, Jost BH, Songer JG, Tweten RK. Clostridium perfringens beta-toxin forms potential-dependent cation-selective channels in lipid bilayers. Infect Immun. 2000;68:5546–5551. doi: 10.1128/iai.68.10.5546-5551.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sherman S, Klein E, McClane BA. Clostridium perfringens type A enterotoxin induces concurrent development of tissue damage and fluid accumulation in the rabbit ileum. J Diar Dis Res. 1994;12:200–207. [PubMed] [Google Scholar]
- 101.Shrestha A, Hendricks MR, Bomberger JM, McClane BA. Bystander Host Cell Killing Effects of Clostridium perfringens Enterotoxin. MBio. 2016;13:7. doi: 10.1128/mBio.02015-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Singh U, Van Itallie CM, Mitic L, Anderson JM, McClane BA. CaCo-2 cells treated with Clostridium perfringens enterotoxin form multiple large complex species one of which contains the tight junction protein occluding. J Biol Chem. 2000;275:18407–18417. doi: 10.1074/jbc.M001530200. [DOI] [PubMed] [Google Scholar]
- 103.Singh U, Mitic LL, Wieckowski EU, Anderson JM, McClane BA. Comparative biochemical and immunocytochemical studies reveal differences in the effects of Clostridium perfringens enterotoxin on polarized CaCo-2 cells versus Vero cells. J Biol Chem. 2001;276:33402–12. doi: 10.1074/jbc.M104200200. [DOI] [PubMed] [Google Scholar]
- 104.Smedley JG, 3rd, Saputo J, Parker JC, Fernandez-Miyakawa ME, Robertson SL, McClane BA, Uzal FA. Noncytotoxic Clostridium perfringens enterotoxin (CPE) variants localize CPE intestinal binding and demonstrate a relationship between CPE-induced cytotoxicity and enterotoxicity. Infec Immun. 2008;76:3793–3800. doi: 10.1128/IAI.00460-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Smedley JG, 3rd, Uzal FA, McClane BA. Identification of a prepore large-complex stage in the mechanism of action of Clostridium perfringens enterotoxin. Infect Immun. 2007;75:2381–2390. doi: 10.1128/IAI.01737-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smits WK, Lyras D, Lacy B, Wilcox MH, Kuijper EJ. Clostridium difficile infection. Nat Rev Dis Primers. 2016;2:16020. doi: 10.1038/nrdp.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Smyth JA, Martin TG. Disease producing capability of netB positive isolates of C. perfringens recovered from normal chickens and a cow and netB positive and negative isolates from chickens with necrotic enteritis. Vet Microbiol. 2010;146:76–84. doi: 10.1016/j.vetmic.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 108.Sonnabend WF, Sonnabend OA, Gründler P, Ketz E. Intestinal toxicoinfection by Clostridium botulinum type F in an adult. Case associated with Guillain-Barré syndrome. Lancet. 1987;14:357–61. doi: 10.1016/s0140-6736(87)91729-6. [DOI] [PubMed] [Google Scholar]
- 109.Songer JG, Miskimmins DW. Clostridium perfringens type E enteritis in calves: two cases and a brief review of the literature. Anaerobe. 2004;10:239–42. doi: 10.1016/j.anaerobe.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Songer JG. Infections by Clostridium perfringens type E. In: Uzal FA, Songer JG, Prescott J, Popoff M, editors. Clostridial diseases of animals. Willey and Blackwell; Ames IA: 2016. pp. 174–176. [Google Scholar]
- 111.Songer JG, Uzal FA. Diseases produced by Clostridium spiroforme. In: Uzal FA, Songer JG, Prescott J, Popoff M, editors. Clostridial diseases of animals. Willey and Blackwell; Ames IA: 2016. pp. 221–227. [Google Scholar]
- 112.Uzal Diagnosis of Clostridium perfringens intestinal infections in sheep and goats. Uzal FA. Anaerobe. 2004;10:135–43. doi: 10.1016/j.anaerobe.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 113.Uzal FA. Diseases produced by Clostridium perfringens type A in mammalian species. In: Uzal FA, Songer JG, Prescott J, Popoff M, editors. Clostridial diseases of animals. Willey and Blackwell; Ames IA: 2016. pp. 109–116. [Google Scholar]
- 114.Uzal FA, Giannitti F, Finnie JW, García JP. Diseases produced by Clostridium perfringens type D. In: Uzal FA, Songer JG, Prescott J, Popoff M, editors. Clostridial diseases of animals. Willey and Blackwell; Ames IA: 2016. pp. 157–176. [Google Scholar]
- 115.Uzal FA, Hostetter J, Plattner B. The alimentary system. In: Maxie MG, editor. Jubb Kennedy, and Palmer’s Pathology of domestic snimals. 6. Vol. 2. St. Louis MO: Elsevier; 2016. pp. 1–260. [Google Scholar]
- 116.Uzal FA, Songer JG. Diagnosis of Clostridium perfringens intestinal infections in sheep and goats. J Vet Diagn Invest. 2008;20:253–65. doi: 10.1177/104063870802000301. [DOI] [PubMed] [Google Scholar]
- 117.Uzal FA, Songer JG. Infections by Clostridium perfringens type B. In: Uzal FA, Songer JG, Prescott J, Popoff M, editors. Clostridial diseases of animals. Willey and Blackwell; Ames IA: 2016. pp. 139–142. [Google Scholar]
- 118.Uzal FA. Diagnosis of Clostridium perfringens intestinal infections in sheep and goats. Anaerobe. 2004;10:135–143. doi: 10.1016/j.anaerobe.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 119.Uzal FA, Freedman JC, Shrestha A, Theoret JR, Garcia J, Awad MM, Adams V, Moore RJ, Rood JI, McClane BA. Towards an understanding of the role of Clostridium perfringens toxins in human and animal disease. Future Microbiol. 2014;9:361–377. doi: 10.2217/fmb.13.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Uzal FA, Kelly WR. The effects of intravenous administration of Clostridium perfringens type D epsilon toxin on young goats and lambs. J Comp Path. 1997;116:63–71. doi: 10.1016/s0021-9975(97)80044-8. [DOI] [PubMed] [Google Scholar]
- 121.Uzal FA, Kelly WR. Experimental Clostridium perfringens type D enterotoxaemia in goats. Vet Path. 1998;35:132–140. doi: 10.1177/030098589803500207. [DOI] [PubMed] [Google Scholar]
- 122.Uzal FA, Kelly WR, Morris WE, Assis RA. Effects of intravenous injection of Clostridium perfringens type D epsilon toxin in calves. J Comp Path. 2002;126:71–75. doi: 10.1053/jcpa.2001.0514. [DOI] [PubMed] [Google Scholar]
- 123.Uzal FA, McClane BA. Recent progress in understanding the pathogenesis of Clostridium perfringens type C infections. Vet Microbiol. 2011;153:37–43. doi: 10.1016/j.vetmic.2011.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Uzal FA, Saputo J, Sayeed S, Vidal JE, Fisher DJ, Poon R, Adams V, Fernandez Miyakawa ME, Rood JI, McClane BA. Development and application of new mouse models to study the pathogenesis of Clostridium perfringens type C enterotoxemias. Infect Immun. 2009;77:5291–5299. doi: 10.1128/IAI.00825-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wagley S, Bokori-Brown M, Morcrette H, Malaspina A, D’Arcy C, Gnanapavan S, Lewis N, Popoff MR, Raciborska D, Nicholas R, Turner B, Titball RW. Evidence of Clostridium perfringens epsilon toxin associated with multiple sclerosis. Mult Scler. 2018;1:1352458518767327. doi: 10.1177/1352458518767327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Warriner K, Xu C, Habash M, Sultan S, Weese SJ. Dissemination of Clostridium difficile in food and the environment: Significant sources of C. difficile community-acquired infection? J Appl Microbiol. 2017;122:542–553. doi: 10.1111/jam.13338. [DOI] [PubMed] [Google Scholar]
- 127.Wieckowski EU, Wnek AP, McClane BA. Evidence that an ~50 kDa mammalian plasma membrane protein with receptor-like properties mediates the amphiphilicity of specifically-bound Clostridium perfringens enterotoxin. J Biol Chem. 1994;269:10838–10848. [PubMed] [Google Scholar]
- 128.Na Xi, Kim Ho, Moyer MP, Charalabos Thomas JT. gp96 Is a Human Colonocyte Plasma Membrane Binding Protein for Clostridium difficile Toxin A. Infect Immun. 2008;76:2862–2871. doi: 10.1128/IAI.00326-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yuan P, Zhang H, Cai C, Zhu S, Zhou Y, Yang X, He R, Li C, Guo S, Li S, Huang T, Perez-Cordon G, Feng H, Wei W. Chondroitin sulfate proteoglycan 4 functions as the cellular receptor for Clostridium difficile toxin B. Cell Res. 2015;25:157–68. doi: 10.1038/cr.2014.169. [DOI] [PMC free article] [PubMed] [Google Scholar]