Abstract
We present the case of a 63-year-old woman presenting with a huge pelvic and retroperitoneal high flow arteriovenous malformation (AVM) causing high-output heart failure, who was treated with combined therapies, including transarterial embolization with n-butyl cyanoacrylate–iodized oil mixture (NBCA–lip) and coils for the right ovarian, both internal iliac, 3rd and 4th lumber arteries, venous sclerotherapy using coils and ethanolamine oleate (EO) for the right ovarian and both internal iliac veins with balloon-occluded retrograde transvenous obliteration technique, and direct percutaneous sclerotherapy using the NBCA–lip and EO for the large nidus of AVM under outflow control using occlusion balloon catheters.
<Learning objective: Huge arteriovenous fistulae or malformation (AVF/M) are potentially life threatening due to the potential for spontaneous hemorrhaging and high-output heart failure and are notoriously difficult to diagnose and treat. To improve the high-output heart failure, intensive and invasive combined treatments for huge AVF/M are needed including transarterial and transvenous embolization and sclerotherapy and percutaneous nidus sclerotherapy.>
Keywords: Heart failure, High-output, Arteriovenous malformation, Embolization
Introduction
Arteriovenous fistulae or malformation (AVF/M) that cause heart failure commonly occur due to congenital, post-traumatic, or iatrogenic disorders [1], [2], [3]. The etiology of AVF/M causing heart failure is usually congenital AVM, post-traumatic vascular injuries, or iatrogenic complications after catheterization or surgery [1], [2], [3]. The symptoms of AVF following trauma or surgical complications typically develop soon after the vascular injury. Several cases of surgical and interventional treatments of high flow AVMs in the liver have been reported. In the late 1970s, surgical arterial ligation was the sole method of treatment and ligation of the common hepatic artery was carried out for the patients with large hepatic AVM to reduce large left-to-right shunts [4]. In the patients initially treated with transarterial embolization (TAE) using coils for feeding arteries, developed collateral circulation did not improve the symptoms [5]; however, the effects have varied. In this case, we treated a high-output heart failure woman with the hybrid therapy of TAE, balloon-occluded retrograde transvenous obliteration (B-RTO), and direct percutaneous sclerotherapy under outflow control using large occlusion balloon catheters.
Case report
A 63-year-old woman suffered from high-output heart failure caused by huge left peritoneal and right-sided pelvic AVMs. The patient was 152 cm tall and weighed 51 kg; her blood pressure was 119/69 mmHg and heart rate was 92 beats/min. She had undergone hysterectomy 18 years prior due to uterine myoma; she had also undergone resection of right atrial and lung tumors 11 years previously due to intravenous leiomyomatosis (IVL) that extended into the right atrium and lung via the inferior vena cava (IVC). Hormone therapy was applied for two years after the resection of the atrial and lung tumors. However, stent grafts were placed to reduce the high-output heart failure caused by high flow pelvic AVM two years previously. A main body Powerlink stentgraft (25 × 55 mm; Endologix Inc., Irvine, CA, USA) was placed to reduce the inferior mesenteric arterial flow and a Zenith leg stentgraft (Cook Japan, Tokyo, Japan) was placed to reduce the right internal iliac arterial flow into pelvic AVM but was unsuccessful. The patient was admitted to our hospital, and echocardiography showed normal left ventricular (LV) function (ejection fraction: 67%, diastolic LV diameter: 55 mm) with pulmonary hypertension (estimated pulmonary arterial pressure: 60 mmHg). The pulse oximetry was 97% under room air and an electrocardiogram showed tachycardia (111 beats/min) and a complete right bundle branch block. A blood examination showed elevated initial B-type natriuretic peptide level (BNP: 587.2 pg/ml), total bilirubin (2.1 mg/dl), creatinine (1.06 mg/dl), blood urea nitrogen (30 mg/dl), which may have been associated with the high-output heart failure.
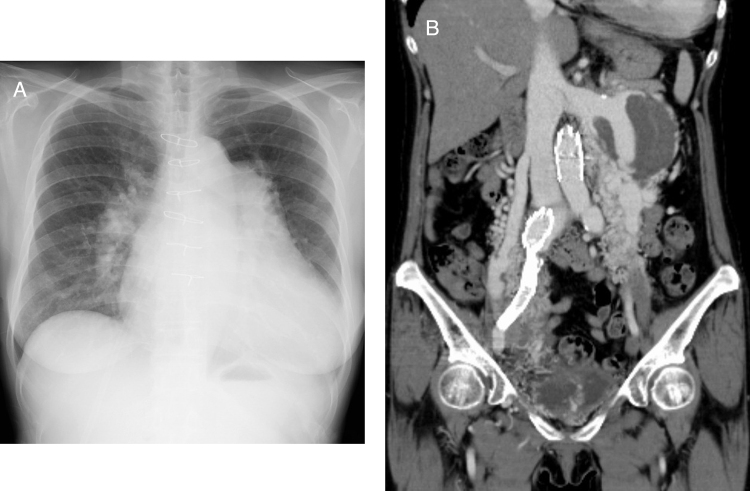
A chest X-ray showed cardiomegaly with dilated pulmonary arteries, suggesting congestive heart failure, and sternal wire was placed at the resection of the atrial tumor (Fig. 1A). On contrast-enhanced computed tomography (CT), numerous meandering vessels were noted at the right pelvis and left retroperitoneum with an aneurysmal-dilated left renal vein (Fig. 1B).
Fig. 1.
Initial chest X-ray and computed tomography (CT). A chest X-ray showed cardiomegaly with dilated pulmonary arteries, suggesting congestive heart failure (A). The contrast-enhanced CT shows hypervascular lesions in the left retroperitoneum and right-sided pelvis with numerous meandering and dilated arteries, dilated left renal vein, right ovarian vein, and both bilateral iliac veins (B).
Doppler ultrasound showed the pulse wave in the infrarenal IVC and laminar flow along the left renal vein. The pelvic AVM was speculated to affect the high-output heart failure more seriously than the left retroperitoneal AVM. To reduce the high-output flow from the pelvic AVM, TAE and nidus sclerotherapy under outflow control were necessary. Celiac, superior mesenteric, both renal, left internal iliac, right ovarian, and lumbar arteriographies were performed to confirm the feeding vessels to the AVMs. The right ovarian artery and left internal iliac arteries were main feeders for the pelvic AVM. To protect the pulmonary emboli caused by high venous returns through the AVM canals, occlusion balloons were placed in the right ovarian using a Selecon MP catheter with a 13 mm balloon diameter (Terumo Clinical Supply Co., Ltd., Gifu, Japan) and bilateral internal iliac veins using two TMP lock-balloon catheters (Tokai Medical Products Inc., Aichi, Japan) via the transjugular approach (Fig. 2A). Two Selecon MP catheters with a 9 mm balloon diameter were also used for the inflow controls and n-butyl cyanoacrylate–iodized oil mixture (NBCA–lip, NBCA:lipiodol = 1:7–8) and 0.035 inch Tornado microcoils (Cook Japan) ranging from 3 to 8 mm in diameter were used as embolic agents. The pelvic AVM nidus was directly punctured under CT guidance and sclerotherapeutic agents of 5% monoethanolamine oleate (EO: Fuji Chemical Industry Co., Ltd., Toyama, Japan) and 0.035 inch Tornado coils with 10 mm diameter (Cook Japan) were percutaneously inserted through this puncture needle. After this first session, the patient's dyspnea improved and the BNP level also decreased from 587.2 to 94.9 pg/ml, so she was discharged from our hospital.
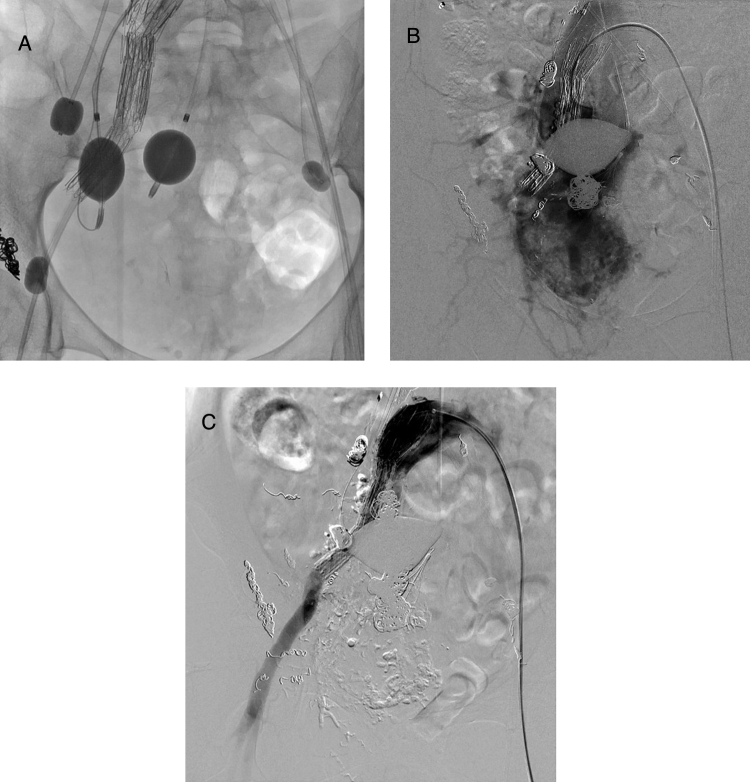
Fig. 2.
Embolization using occlusion-balloon catheters. Venous occlusion-balloon catheters were placed in the right ovarian and bilateral internal iliac veins and two arterial occlusion-balloon catheters were also placed in both internal iliac arteries to control the high-output flow from the pelvic arteriovenous malformation (A). Right common iliac arteriography shows the recanalized right internal iliac artery with the dilated and meandering branches (B), and these recanalized arteries were also embolized using metallic coil and n-butyl cyanoacrylate–iodized oil (lipiodol) mixture. After hybrid therapy, the right common iliac arteriography shows the complete embolization of right recanalized internal iliac artery (C).
Two months after the first session, congestive heart failure aggravated dyspnea again and she was rehospitalized. Contrast-enhanced CT showed the recanalization of the right internal iliac artery which had been occluded with stent grafts, and BNP also increased to 412.2 pg/ml. A second procedure was carried out in the same manner as the first session with outflow occlusion balloons in both internal iliac veins. The recanalized right internal iliac artery (Fig. 2B) was embolized with the NBCA–lip and 0.035 inch metallic coils ranging from 8 to 10 mm in diameter under flow-guidance using a Selecon MP catheter with a 9 mm balloon. The pelvic AVM nidus was also directly punctured through the right hip under CT guidance, and sclerotherapy was also performed using the NBCA–lip and 5% EO after additional coil deployment. After hybrid therapy, the right common iliac arteriography showed complete occlusion of the recanalized right internal iliac artery (Fig. 2C).
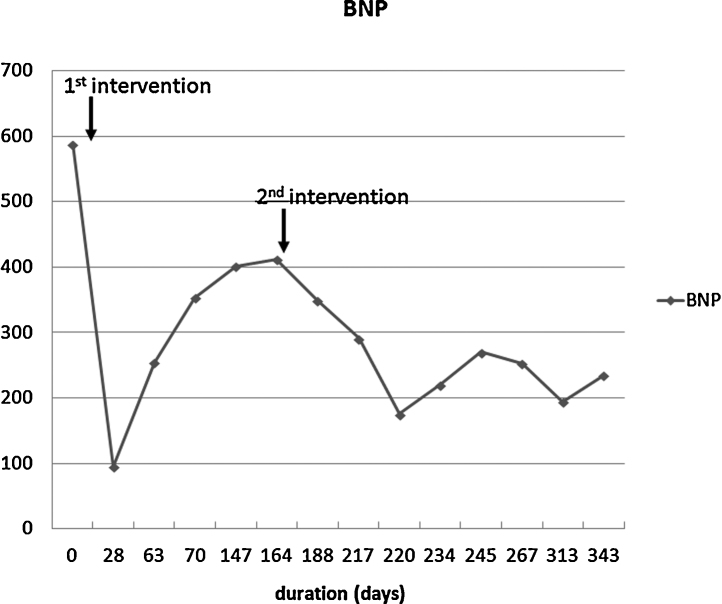
Minor pulmonary embolism occurred despite outflow control during the second procedure. However, the BNP gradually decreased and the patient was discharged one month after the second procedure (Fig. 3). The patient is currently being followed every two or three months in the outpatient clinic.
Fig. 3.
Clinical course after treatment. After first intervention, B-type natriuretic peptide (BNP) level rapidly decreased, but soon increased due to the recanalization of the right internal iliac artery. However, BNP gradually decreased after second treatment.
Discussion
AVF/Ms that cause heart failure are typically congenital, post-traumatic vascular injuries, or iatrogenic disorders after catheterization or surgery [1], [2], [3]. Pelvic AVF/Ms are rare vascular lesions and are notoriously difficult to diagnose and treat [6]. Pelvic AVF/Ms are potentially life threatening due to the potential for spontaneous hemorrhaging and high-output heart failure [7]. Our patient had undergone hysterectomy because of uterine IVL, and this tumor is a rare neoplasm characterized by invasion of the venous channels by a benign smooth muscle tumor originating from a uterine myoma or vessel wall [7]. IVL extends into the gonadal or iliac veins to the vena cava and right atrium and may cause the symptoms of congestive heart failure, eventually leading to a fatal outcome. CT and angiography characteristically demonstrate a hypervascular tumor.
The patients with high-output heart failure caused by AVF/M have been treated with various techniques, including TAE [6] and the placement of stent grafts with sclerotherapy [8]. In the present case, transarterial and percutaneous direct-puncture embolization and sclerotherapy were performed using the NBCA–lip and metallic coils under nidus flow control with transarterial and transvenous occlusion balloons, and this hybrid therapy resulted in the improvement of congestive heart failure. The NBCA–lip is feasibly used for the embolization of the spinal dural AVF [9] and direct-puncture embolization with the NBCA–lip also apply to preoperative devascularization of craniofacial AVM as an effective and safe technique [10]. For safe and effective embolization, the compression of draining venous channels is important because symptomatic pulmonary complications may occur after injection of the NBCA–lip, particularly when delivery systems without flow arrest are used in high-flow vascular brain lesions [10]. Thus, in the present case, we occluded the feeding arteries and draining veins using the occlusion balloon catheters. The complete embolization or sclerotherapy of huge AVM is sometimes difficult, but it is impossible to discontinue the procedure once it is started, because insufficient embolization may cause pulmonary embolism and worsen the symptoms of pulmonary congestion. The repeated embolization and sclerotherapy for the pelvic AVM were completed to improve the symptoms of high-output heart failure in the present case.
The combined therapy using transcatheter and percutaneous direct-puncture embolization and sclerotherapy with the B-RTO technique was needed for reducing the high flow shunt of a huge pelvic AVM and improved the symptoms of cardiac congestion caused by high-output heart failure.
Conflicts of interest
We have nothing to disclose.
Author contribution
M. Okada contributed to the procedure, manuscript preparation and editing. M. Kato contributed to the procedure. K. Uchida, Y. Sufu, and S. Okuda contributed to the patient care and clinical study. M. Yano and N. Matsunaga contributed to the guarantor of integrity of the entire study (manuscript editing and review).
References
- 1.Montejo Baranda M., Perez M., De Andres J., De la Hoz C., Merino J., Aguirre C. High out-put congestive heart failure as first manifestation of Osler-Weber-Rendu disease. Angiology. 1984;35:568–576. doi: 10.1177/000331978403500904. [DOI] [PubMed] [Google Scholar]
- 2.Lindenauer S.M., Thompson N.W., Kraft R.O., Fry W.J. Late complications of traumatic arteriovenous fistulas. Surg Gynecol Obstet. 1969;129:525–532. [PubMed] [Google Scholar]
- 3.Sy A.O., Plantholt S. Congestive heart failure secondary to an arteriovenous fistula from cardiac catheterization and angioplasty. Cathet Cardiovasc Diagn. 1991;23:136–138. doi: 10.1002/ccd.1810230217. [DOI] [PubMed] [Google Scholar]
- 4.Radtke W.E., Smith H.C., Fulton R.E., Adson M.A. Misdiagnosis of atrial septal defect in patients with hereditary telangiectasia (Osler-Weber-Rendu disease) and hepatic arteriovenous fistulas. Am Heart J. 1978;95:235–242. doi: 10.1016/0002-8703(78)90468-4. [DOI] [PubMed] [Google Scholar]
- 5.Zentler-Munro P.L., Howard E.R., Karani J., Williams R. Variceal haemorrhage in hereditary haemorrhagic telangiectasia. Gut. 1989;30:1293–1297. doi: 10.1136/gut.30.9.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Do Y.S., Park K.B., Cho S.K. How do we treat arteriovenous malformations (tips and tricks)? Tech Vasc Interv Radiol. 2007;10:291–298. doi: 10.1053/j.tvir.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Ozaki K., Oda H., Hatada K., Sakai K., Takahashi K., Miida T., Higuma N. Highly vascular pelvic tumor causing high-output heart failure because of massive arteriovenous shunting: a case report. Circ J. 2003;67:554–555. doi: 10.1253/circj.67.554. [DOI] [PubMed] [Google Scholar]
- 8.Choi S.Y., Do Y.S., Lee do Y., Lee K.H., Won J.Y. Treatment of a pelvic arteriovenous malformation by stent graft placement combined with sclerotherapy. J Vasc Surg. 2010;51:1006–1009. doi: 10.1016/j.jvs.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 9.Song J.K., Gobin Y.P., Duckwiler G.R., Murayama Y., Frazee J.G., Martin N.A., Viñuela F. N-butyl 2-cyanoacrylate embolization of spinal dural arteriovenous fistulae. Am J Neuroradiol. 2001;22:40–47. [PMC free article] [PubMed] [Google Scholar]
- 10.Han M.H., Seong S.O., Kim H.D., Chang K.H., Yeon K.M., Han M.C. Craniofacial arteriovenous malformation: preoperative embolization with direct puncture and injection of n-butyl cyanoacrylate. Radiology. 1999;211:661–666. doi: 10.1148/radiology.211.3.r99jn07661. [DOI] [PubMed] [Google Scholar]