Abstract
A 53-year-old woman with a history of allergic disease was admitted to our hospital because of syncope induced by sustained ventricular tachycardia. The clinical course and the laboratory data did not correspond to those of acute myocarditis. Although eosinophils in the peripheral blood count were not increased, the diagnosis of eosinophilic myocarditis was made following a right ventricular endomyocardial biopsy that showed a remarkable infiltration of eosinophils. While giant cells were another histopathological feature of this case, they were considered to be an expression of the disease severity. This is a rare case of eosinophilic myocarditis, without peripheral eosinophilia.
<Learning objective: Eosinophils in the peripheral blood usually increase in eosinophilic myocarditis. We describe a case of eosinophilic myocarditis without hypereosinophilia. Even in the absence of hypereosinophilia, endomyocardial biopsy should be performed during the investigation of unexplained myocardial disease.>
Keywords: Eosinophilic myocarditis, Eosinophilia, Transvenous endomyocardial biopsy, Giant cell
Introduction
Eosinophilic myocarditis is a rare form of myocarditis, associated with various clinical manifestations. In many cases of eosinophilic myocarditis, the patient develops typical features including acute myocarditis and eosinophilia. Hypereosinophilia in particular gives an important clue to the diagnosis. However, in the absence of peripheral eosinophilia, the diagnosis of eosinophilic myocarditis is occasionally determined by endomyocardial biopsy. Here we present the case of a patient with eosinophilic myocarditis, in whom eosinophilia did not occur and diagnosis was dependent upon biopsy.
Case report
A 53-year-old woman, who was admitted to a local hospital with syncope, was transferred to our hospital because of sustained ventricular tachycardia (VT). Prodrome of cold-like symptoms such as cough and fever did not occur. She had been admitted to our hospital 8 months earlier because of palpitations and dyspnea. At that time, triplets of ventricular extrasystole were noted on the electrocardiogram (ECG), and an echocardiogram showed left ventricular asynergy with akinesis of the mid-portion of the anterior to inferior wall. Coronary angiography showed normal coronary arteries. We recommended that she undergo endomyocardial biopsy, but the patient declined. Therefore, she had been diagnosed with heart failure due to unspecified cardiomyopathy, and treated with a beta-blocker.
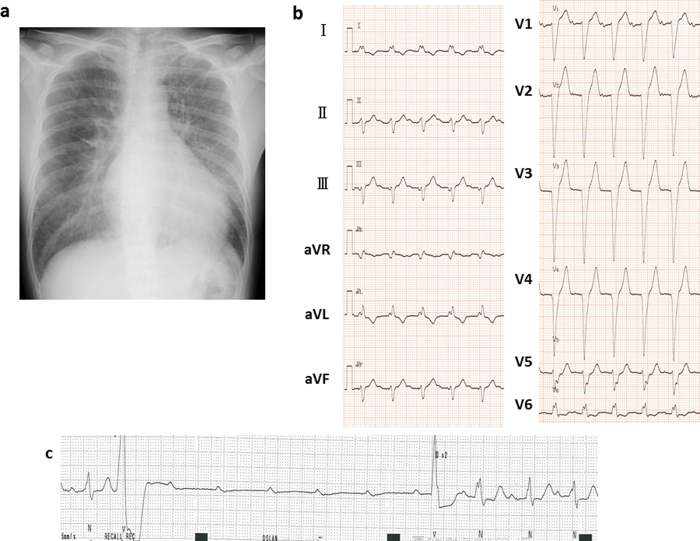
On physical examination, the patient was afebrile with a regular pulse of 104 beats/min and a blood pressure of 104/62 mmHg. The O2 saturation was 98% on 3 L/min of O2 via nasal cannulae. Cardiorespiratory examination revealed bilateral moist rales, third and fourth heart sounds, and marked pretibial edema. The chest radiograph showed cardiomegaly (cardio-thoracic ratio 59%) with pulmonary congestion (Fig. 1a). ECG showed normal sinus rhythm and new findings of first-degree atrioventricular (AV) block (PQ interval: 240 ms) with complete left bundle branch block (QRS width: 140 ms) (Fig. 1b). Echocardiography showed diffuse hypokinesis of the left ventricular wall with the same asynergy as before; the ejection fraction (EF) was reduced to 33%. The white blood cell count was 10,200/μL with no eosinophils; eosinophilia was never detected throughout the clinical course. C-reactive protein was negative, and creatine phosphokinase was not elevated.
Fig. 1.
Radiograph showing cardiomegaly and pulmonary congestion (a). Electrocardiogram showing first-degree atrioventricular (AV) block with complete left bundle branch block (b). Electrocardiographic monitoring revealing transient complete AV block (c).
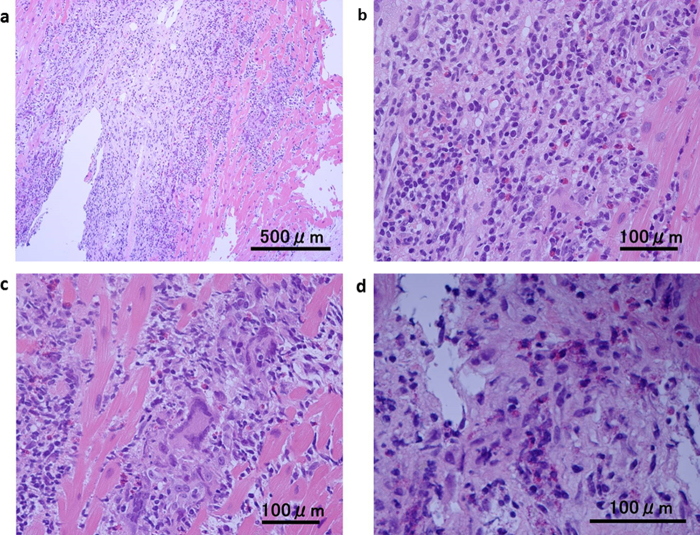
After admission, the patient was treated with diuretics and a continuous infusion of amiodarone and lidocaine. Temporary cardiac pacing was started because ECG monitoring had also revealed a transient complete AV block during episodes of syncope (Fig. 1c). As the complete AV block was persistent over more than 1 week, we decided to implant a permanent pacemaker prior to the following examination. On the device, cardiac resynchronization therapy-defibrillator was chosen because of her reduced EF, wide QRS, and history of sustained VT. Abnormal findings that were revealed after device implantation are as follows. Gallium-67 scintigraphy showed abnormal uptake only in the myocardium (Fig. 2). Right ventricular endomyocardial biopsy revealed a remarkable infiltration of inflammatory cells, including numerous degranulated eosinophils in the myocardium (Fig. 3). In addition, a scattering of giant cells was found, although these were few in number. Based on these findings, despite the absence of eosinophilia, diagnosis of eosinophilic myocarditis was made. Given that the patient had a history of allergic rhinitis and atopic dermatitis, and no history of drug hypersensitivity, parasitic disease, or other potential cause, we concluded that the cause of eosinophilic myocarditis was an allergic disorder. Prednisolone was started at a dose of 30 mg and was gradually tapered to at maintenance dose of 10 mg over the course of 1 year. Although the reduced left ventricular function and the advanced AV block did not improve, the episode of VT disappeared and her congestive heart failure was compensated, in combination with treatment with a beta-blocker, amiodarone, and diuretics.
Fig. 2.

Gallium-67 scintigraphy showing abnormal uptake only in the myocardium (arrows). (a) coronal slice, (b) sagittal slice, (c) transverse slice, and (d) whole body.
Fig. 3.
Histological findings of the endomyocardial biopsy specimens, showing remarkable infiltration of inflammatory cells (a) and many eosinophils (b). A scatter of infiltrated giant cells (c) and degranulation of eosinophils is seen (d) (Hematoxylin and eosin staining: (a) 100×; (b and c) 400×; (d) 600×).
Discussion
In most cases of eosinophilic myocarditis, eosinophils in the peripheral blood increase to various degrees. Although eosinophilia is sometimes delayed from the onset [1], [2], [3], it is extremely rare that eosinophils never increase during the clinical course [4], as in this case. It remains unclear as to why eosinophils infiltrate the myocardium without hypereosinophilia. However it has been suggested that peripheral eosinophils migrate into the tissues in the patient exposed to acute changes, while the bone marrow cannot respond immediately with increased production [5]. In this way, the paradoxical eosinopenia occasionally observed in such patients is explained.
In our case, histopathology demonstrated not only the infiltration of eosinophils, but also the presence of multinucleated giant cells. Giant cell myocarditis is generally fatal and the prognosis is extremely poor. Kodama et al. reported that in an experimental rat model of autoimmune myocarditis, the appearance of the multinucleated giant cells was restricted to a period corresponding to the fulminant phase of inflammation [6]. Accordingly, giant cells can appear in eosinophilic myocarditis if the inflammation is severe. Hyogo et al., for example, reported a case of acute necrotizing eosinophilic myocarditis with giant cell infiltration [7]. We diagnosed this case as eosinophilic myocarditis, in spite of the presence of giant cells, for several reasons including the infiltration of the myocardium with numerous eosinophils, the patient's history of allergic disease, and the condition of the patient, which was not as critical as that associated with giant cell myocarditis.
Many cases of eosinophilic myocarditis are associated with peripheral eosinophilia and the prodrome of cold-like symptoms. Corticosteroid therapy is generally effective in acute phase of eosinophilic myocarditis and left ventricular function usually improves soon [8], [9]. However, it was difficult to diagnose without typical findings of eosinophilic myocarditis in our case, so we were not able to start the steroid therapy early. The reduced left ventricular function and AV block did not recover despite the long-term steroid administration. Although there is no evidence of efficacy using high-dose corticosteroids in eosinophilic myocarditis, steroid pulse therapy is sometimes given in severe cases [4], [8]. If we had administered high-dose corticosteroids earlier, left ventricular function might have recovered and device therapy might have been avoided.
In conclusion, we present a case of eosinophilic myocarditis without hypereosinophilia. Even in the absence of hypereosinophilia, eosinophils may infiltrate the myocardium. Therefore, endomyocardial biopsy should be performed during the investigation of unexplained myocardial disease.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Morimoto S., Kubo N., Hiramitsu S., Uemura A., Ohtsuki M., Kato S., Kato Y., Sugiura A., Miyagishima K., Mori N., Yoshida Y., Hishida H. Changes in the peripheral eosinophil count in patients with acute eosinophilic myocarditis. Heart Vessels. 2003;18:193–196. doi: 10.1007/s00380-003-0721-0. [DOI] [PubMed] [Google Scholar]
- 2.Kazama R., Okura Y., Hoyano M., Toba K., Ochiai Y., Ishihara N., Kuroha T., Yoshida T., Namura O., Sogawa M., Nakamura Y., Yoshimura N., Nishikura K., Kato K., Hanawa H. Therapeutic role of pericardiocentesis for acute necrotizing eosinophilic myocarditis with cardiac tamponade. Mayo Clin Proc. 2003;78:901–907. doi: 10.4065/78.7.901. [DOI] [PubMed] [Google Scholar]
- 3.Sohn I.S., Park J.C., Chung J.H., Kim K.H., Ahn Y., Jeong M.H., Cho J.G. A case of acute eosinophilic myopericarditis presenting with cardiogenic shock and normal peripheral eosinophil count. Korean J Int Med. 2006;21:136–140. doi: 10.3904/kjim.2006.21.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe N., Nakagawa S., Fukunaga T., Fukuoka S., Hatakeyama K., Hayashi T. Acute necrotizing eosinophilic myocarditis successfully treated by high dose methylprednisolone. Jpn Circ J. 2001;65:923–926. doi: 10.1253/jcj.65.923. [DOI] [PubMed] [Google Scholar]
- 5.Rothenberg M.E. Eosinophilia. N Engl J Med. 1998;338:1592–1600. doi: 10.1056/NEJM199805283382206. [DOI] [PubMed] [Google Scholar]
- 6.Kodama M., Matsumoto Y., Fujiwara M., Zhang S.S., Hanawa H., Itoh E., Tsuda T., Izumi T., Shibata A. Characteristics of giant cells and factors related to the formation of giant cells in myocarditis. Circ Res. 1991;69:1042–1050. doi: 10.1161/01.res.69.4.1042. [DOI] [PubMed] [Google Scholar]
- 7.Hyogo M., Kamitani T., Oguni A., Kawasaki S., Miyanaga H., Takahashi T., Kunishige H., Andachi H. Acute necrotizing eosinophilic myocarditis with giant cell infiltration after remission of idiopathic thrombocytopenic purpura. Int Med. 1997;36:894–897. doi: 10.2169/internalmedicine.36.894. [DOI] [PubMed] [Google Scholar]
- 8.Kawano S., Kato J., Kawano N., Yoshimura Y., Masuyama H., Fukunaga T., Sato Y., Maruyama H., Mihara K., Ueda A., Toyoda K., Imamura T., Kitamura K. Clinical features and outcomes of eosinophilic myocarditis patients treated with prednisolone at a single institution over a 27-year period. Int Med. 2011;50:975–981. doi: 10.2169/internalmedicine.50.4079. [DOI] [PubMed] [Google Scholar]
- 9.Al Ali A.M., Straatman L.P., Allard M.F., Ignaszewski A.P. Eosinophilic myocarditis: case series and review of literature. Can J Cardiol. 2006;22:1233–1237. doi: 10.1016/s0828-282x(06)70965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]