Abstract
Autism spectrum disorder (ASD) is a neurodevelopmental disorder associated with social communication deficits and restricted/repetitive behaviors and is characterized by large-scale atypical subcortical-cortical connectivity, including impaired resting-state functional connectivity between thalamic and sensory regions. Previous studies have typically focused on the abnormal static connectivity in ASD and overlooked potential valuable dynamic patterns in brain connectivity. However, resting-state brain connectivity is indeed highly dynamic, and abnormalities in dynamic brain connectivity have been widely identified in psychiatric disorders. In this study, we investigated the dynamic functional network connectivity (dFNC) between 51 intrinsic connectivity networks in 170 individuals with ASD and 195 age-matched typically developing (TD) controls using independent component analysis and a sliding window approach. A hard clustering state analysis and a fuzzy meta-state analysis were conducted respectively, for the exploration of local and global aberrant dynamic connectivity patterns in ASD. We examined the group difference in dFNC between thalamic and sensory networks in each functional state and group differences in four high-dimensional dynamic measures. The results showed that compared with TD controls, individuals with ASD show an increase in transient connectivity between hypothalamus/subthalamus and some sensory networks (right postcentral gyrus, bi paracentral lobule, and lingual gyrus) in certain functional states, and diminished global meta-state dynamics of the whole-brain functional network. In addition, these atypical dynamic patterns are significantly associated with autistic symptoms indexed by the Autism Diagnostic Observation Schedule. These converging results support and extend previous observations regarding hyperconnectivity between thalamic and sensory regions and stable whole-brain functional configuration in ASD. Dynamic brain connectivity may serve as a potential biomarker of ASD and further investigation of these dynamic patterns might help to advance our understanding of behavioral differences in this complex neurodevelopmental disorder.
1. Introduction
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder characterized by co-occurring cardinal symptoms of social/communication deficits and restricted/repetitive behaviors (Association 2013). Large-scale ASD-related brain abnormalities in both anatomical and functional organization have been identified in previous studies (Casanova et al., 2009; Courchesne et al., 2011; Kennedy et al., 2006; Khan et al., 2013). ASD not only affects brain activities in local regions, but also impairs interactions between multiple brain regions and networks (Kana et al., 2006; Khan et al., 2013; Koshino et al., 2005; Villalobos et al., 2005). Increasing evidence suggests that the exploration of atypical brain connectivity in ASD can provide more insights into the pathophysiology of this disorder.
Although prior neuroimaging studies using functional magnetic resonance imaging (fMRI) have identified numerous ASD-related abnormal connectivity in cortical regions (Just et al., 2004; Kana et al., 2006; Kleinhans et al., 2008; Koshino et al., 2005; Koshino et al., 2007), recent studies also implicated the disruption of the subcortical-cortical brain connections in ASD. In particular, the thalamus and basal ganglia, generally conceptualized as a relay between cortical and subcortical areas, have been associated with cognitive impairments in ASD (Minshew et al., 2010; Tsatsanis et al., 2003; Woodward et al., 2016). Thalamus is a sensory gate that receives and delivers sensory information to cortical regions. A growing literature has identified atypical sensory processing in ASD and suggested the abnormal thalamic-to-cortical connectivity to be a potential cause. The atypical thalamic-to-cortical pathway might result in high sensory input from subcortical regions to the sensory regions that could override higher order cognitive processes (Cerliani et al., 2015). Studies also showed that the thalamic-to-cortical connectivity is involved in cerebello–thalamo–cortical pathways. The impairments of cerebello–thalamo–cortical pathways have been identified in ASD (Bailey et al., 1998; Schmahmann 1996), which provides evidence supporting atypical thalamic-to-cortical connectivity in ASD. Indeed, aberrant patterns of resting-state brain connectivity, especially between thalamic and sensory regions, have been widely reported (Di Martino et al., 2011; Nair et al., 2013; Padmanabhan et al., 2013). However, findings in this field are inconsistent and even contradictory to some extent. For example, Nail et al. identified hypoconnectivity between the thalamus and several somatosensory regions in ASD in the resting-state (Nair et al., 2013). In contrast, Cerliani et al. demonstrated increased functional connectivity between thalamic and sensory networks, which are correlated with the severity of autistic traits (Cerliani et al., 2015) and Tomasi et al. found thalamus has higher functional connectivity with insula, somatosensory, motor, premotor, and auditory areas and the middle cingulum (Tomasi et al., 2017). Although this inconsistency may be partially due to the inherent heterogeneity of ASD (Di Martino et al., 2014) or the diversity in functional connectivity subtypes (Khan et al., 2015), we hypothesized that the dynamic patterns in brain connectivity, which are incompletely captured by an averaged, static connectivity approach, might be a possible source of these disparities. Besides functional connectivity between certain brain regions, the global properties of the whole-brain functional network in individuals with ASD have also been widely explored (Peters et al., 2013; Rudie et al., 2013; Zhou et al., 2014). Previous studies have characterized the brain as a complex network with a hierarchical modular organization and suggested that quantifying the global properties of brain network can improve the understanding of fundamental brain mechanism (Wang et al., 2010; Watts et al., 1998). However, most of these studies assessed static properties of the whole-brain functional network over the entire scan and this static assumption might blur out potentially important changes of whole-brain network, such as how the whole-brain network changes globally over time and whether the prevalence of these changes is related to ASD.
Brain connectivity is not static but varies remarkably among brain states (Calhoun et al., 2014; Rissman et al., 2004; Smith et al., 2009). Dynamic patterns of functional brain connectivity are even more prominent in the resting-state, during which the mental activity is unconstrained (Allen et al., 2014; Hutchison et al., 2013a; Hutchison et al., 2013b; Kang et al., 2011). Mounting evidence supports the concept that ASD can be characterized by atypical brain dynamics (Uhlhaas et al., 2012; Watanabe et al., 2017). For example, a recent study identified increased temporal variability of brain connectivity in individuals with ASD (Falahpour et al., 2016), suggesting that brain connectivity may not be disrupted, but rather be highly unstable in ASD. Widespread altered dynamic functional connectivity between cortical regions has also been identified in our and others’ previous work, mainly within the default mode network (Yao et al., 2016) and control networks (de Lacy et al., 2017). By using the sliding window based approaches, previous studies also identify atypical dynamic brain patterns in other brain disorders (Damaraju et al., 2014; Du et al., 2017; Rashid et al., 2014). For example, Du et al. (Du et al., 2016) applied k-means to extract connectivity states from dynamic connectivity within the default mode network (DMN) of 82 healthy controls (HCs) and 82 schizophrenia (SZ) patients, and found that HCs spent more time in a state that reflected stronger connectivity between anterior and posterior brain regions, while SZ patients spent more time in a disconnected state. Our recent study developed a framework based on the sliding window approach for characterizing time-varying brain activity and exploring its associations with time-varying brain connectivity. We applied this framework to a resting-state fMRI dataset including 151 SZ patients and 163 age- and gender-matched HCs and found that amplitude of low frequency fluctuation (ALFF) and functional connectivity were correlated in time and these relationships are significantly different in SZ (Fu et al., 2017).
To date, no ASD studies have examined dynamic thalamic-sensory connectivity and dynamic global properties of the whole-brain network in the resting-state. We aimed to investigate group differences between typically developing (TD) controls and individuals with ASD in dynamic brain connectivity from a macro and micro perspective. The novelty of current study is twofold. First, our study investigated ASD related abnormalities using time-resolved resting-state functional connectivity between thalamic networks and sensory networks instead of assuming static functional connectivity across the whole scan. Second, our study not only focused on local functional connectivity between specific pairs of regions or networks, but also explored the atypical global dynamics of the whole-brain network in ASD. We calculated time-varying correlation coefficients between windowed time-courses (TCs) of different brain components estimated via group independent component analysis (GICA) as the measure of dynamic functional network connectivity (dFNC) between brain networks. A hard clustering state analysis and a fuzzy metastate analysis were then conducted to investigate the local and global patterns of dynamic brain connectivity. The hard clustering state analysis explores the transient dFNC in discrete states, while the fuzzy meta-state analysis investigates global state-space metrics. These two approaches are capable of capturing reliable dynamic connectivity patterns which are robust against variation in data quality, analysis, grouping, and decomposition methods (Abrol et al., 2017). We hypothesized that individuals with ASD would display atypical dynamic thalamic-sensory connectivity and atypical whole-brain dynamisms vs. TD controls, and these abnormal patterns in local and global dynamic functional connectivity would be associated with ASD symptoms.
2. Material and Methods
2.1 fMRI Dataset
The Autism Brain Imaging Data Exchange (ABIDE), a publicly available database sharing collected resting-state fMRI data from 539 individuals with ASD and 573 age-matched TD controls was used in this study. Institutional review board approval was provided by each data contributor. Before dataset acquisition, consortium members agreed on a ‘base’ phenotypic protocol by identifying overlaps in measures across sites, which include age at scan, sex, IQ and diagnostic information. From the ABIDE database, we selected data samples according to the following criteria: 1) male subjects with DSM-IV-TR diagnosis; 2) subjects with mean full scale intelligence quotient (FIQ) within 2 standard deviations (s.d). of the overall ABIDE sample mean (if FIQ is missing, FIQ was estimated by averaging available performance IQ (PIQ) and verbal IQ (VIQ) scores); 3) subjects with mean frame-wise displacement (FD) smaller than 0.4465, corresponding to 2 s.d. above the sample mean; 4) subjects with more than 150 time points in fMRI acquisition; 5) subjects with fMRI data scanned using a repeat time (TR) = 2 sec; 6) subjects with functional data providing near full brain successful normalization (by comparing the individual mask with the group mask. Detailed procedures are provided in the supplementary materials). These criteria yielded a total of 365 (170 ASD and 195 TD) age matched (p = 0.7821) subjects. Mounting evidence has demonstrated that ASD is a disorder of sexual differentiation. It is far more prevalent in males than in females and this higher prevalence might be reflected by the sex difference in functional connectivity of individuals with ASD (Alaerts et al., 2016; Halladay et al., 2015; Tomasi et al., 2012). Considering that the confounding effect of gender in ASD is still not well understood and it might affect our dynamic functional connectivity analysis, we used a careful subject selection criterion by choosing only male subjects. Among these selected subjects, 133 subjects had Autism Diagnostic Observation Schedule (ADOS) score passing quality control. ADOS score is an instrument for assessing the autistic symptoms according to the individuals’ performance of a series of semi-structured and general tasks. These tasks are designed and used to evaluate an individuals’ social and communication behaviors related to the ASD diagnosis. Demographic information for all selected subjects is shown in Table I.
Table I.
Participant Demographics
| Phenotypic | ASD (Mean and s.d) | TD (Mean and s.d) |
|---|---|---|
| Age (170 ASD, 195 TD) | 15.57 (7.35) | 16.02 (5.90) |
| FIQ (170 ASD, 195 TD) | 106.47 (14.37) | 110.65 (12.09) |
| Mean FD (170 ASD, 195 TD) | 0.10 (0.07) | 0.08 (0.06) |
| Eyes state (170 ASD, 195 TD) | Eyes close =151, eyes open=19 | Eyes close =163, eyes open=32 |
| ADOS Total (115 ASD, 18 TD) | 11.97 (3.60) | 1.22 (1.13) |
2.2 Preprocessing
Resting-state fMRI data were preprocessed using the Connectome Computation System pipeline (http://lfcd.psych.ac.cn/ccs.html). The preprocessing steps included dropping time points (6 time points were discarded to exclude scans impacted by T1 effects), denoising, slice timing, motion correction, brain extraction and intensity normalization. Data were spatially normalized into the standard Montreal Neurological Institute (MNI) space, resampled to 3 mm × 3 mm × 3 mm voxels using the nonlinear (affine + low-frequency direct cosine transform basis functions) registration implemented in the SPM12 toolbox. The resting-state data were further spatially smoothed with a 5 mm full width at half max Gaussian kernel.
2.3 The Framework of Hard Clustering State Analysis and Fuzzy Meta-state Analysis
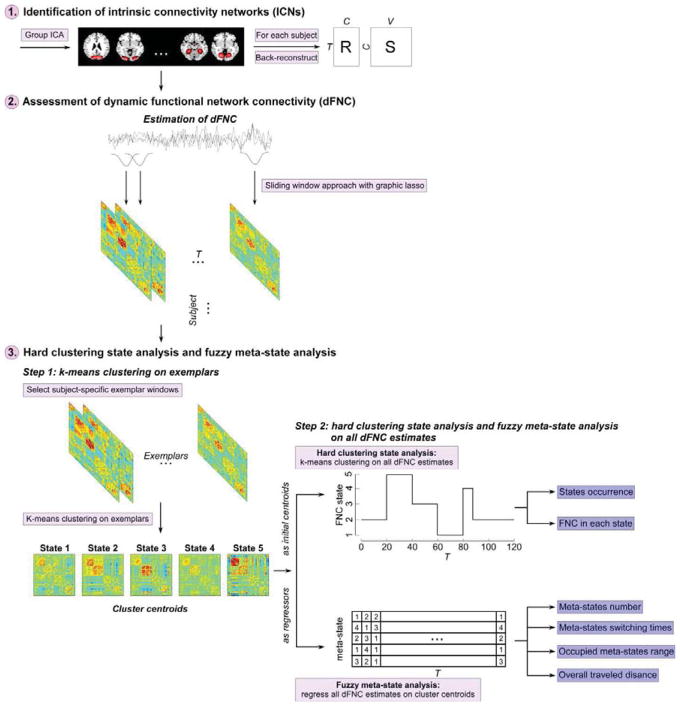
The framework to investigate atypical dynamic brain connectivity in ASD was illustrated using a flowchart in Figure 1. Three major steps were included in this framework. Step 1) group independent component analysis (GICA) was performed and intrinsic connectivity networks (ICNs) were selected according to the spatial maps of components. Time courses (TCs) of selected ICNs were estimated for the following dynamic functional network connectivity (dFNC) analysis (details of GICA and ICNs selection were introduced in section 2.4). Step 2) time-varying correlation coefficients between TCs were calculated using a sliding window approach as the measure of dFNC between ICNs (details of dFNC calculation were introduced in section 2.5). Step 3) the hard clustering state analysis and the fuzzy metastate analysis were conducted on dFNC estimates (time-varying correlation coefficients) to investigate local and global dynamic patterns of functional connectivity (details of the hard clustering state analysis were introduced in section 2.6 and details of the fuzzy meta-state analysis were introduced in section 2.7).
Figure 1.
The flowchart to investigate aberrant dynamic brain connectivity in ASD
2.4 Group Independent Component analysis
Preprocessed resting-state fMRI data were decomposed into linear mixtures of spatial independent components (ICs) and their corresponding single subject TCs and spatial maps were estimated using the GICA back-reconstruction approach implemented in the Group ICA of fMRI Toolbox (GIFT) for further analysis (http://mialab.mrn.org/software/gift) (Calhoun et al., 2012; Calhoun et al., 2001). A relative high model order (number of ICs, C = 100) was used to obtain a functional parcellation of brain components corresponding to known anatomical and functional segmentations (Allen et al., 2014). Before doing GICA to extract ICs, we conducted two levels principal component analysis (PCA) on the functional data for dimension reduction. First, subject-specific data was reduced to 120 principal components with maximal variability using PCA with standard economy-size decomposition (this number should be higher than the group ICA dimensionality as detailed in (Erhardt et al., 2011)). Second, the reduced subject-specific data were concatenated across time and the group data were reduced to 100 principal components using expectation maximization algorithm (Erhardt et al., 2011; Rachakonda et al., 2016). The reduced group data were decomposed into 100 ICs using the infomax algorithm (Langlois et al., 2010; Lee et al., 1999) and group level spatial maps were estimated. We applied ICASSO with 10 ICA runs followed by best-run selection to stabilize the estimation (Wu et al., 2011). Subject-specific spatial maps and TCs were back- reconstructed based on group level spatial maps using the GICA approach (Calhoun et al., 2001).
After obtaining the spatial maps and TCs of all subjects, we calculated the one-sample t-test maps for each spatial map across subjects and the mean power spectra of the corresponding TC. The one-sample t-test was used to identify significantly activated voxels of each spatial map. We selected a set of ICs as ICNs by considering their peak activations and power spectrum. ICNs should exhibit peak activations in grey matter, low spatial overlap with known vascular, ventricular, motion, and susceptibility artifacts, and their TCs are dominated by low-frequency fluctuations (Allen et al., 2014). ICNs were categorized into different domains based on anatomy and prior knowledge of their function (Damaraju et al., 2014). The following post-ICA processing steps were conducted on the TCs of the selected ICNs to further remove remaining noise sources: 1) detrending linear, quadratic, and cubic trends; 2) conducting multiple regressions of the 6 realignment parameters and their temporal derivatives; 3) de-spiking detected outliers; 4) low-pass filtering with a cut-off frequency of 0.15 Hz. It should be noted that a band-pass filter of 0.01 to 0.1 Hz is one of the most commonly used filters in resting-state studies but it is mainly used for seed-based analyses. For ICA analyses, we have previously looked at this in detail in this paper (Allen et al., 2011), which showed that the more stringent 0.1Hz cutoff is removing a substantial amount of the BOLD signal content, and a 0.15Hz cutoff provides a better trade-off between removing noise and including signals of interest.
2.5 Dynamic Functional Network Connectivity Estimation
For each subject i = 1 … N, dFNC was estimated via a sliding window approach. The six head motion parameters were regressed out from the TCs to remove the influence of head motion on dFNC estimates. Since subjects’ data from different sites might have different scan lengths, we only selected the first 150 time points of each individual’s component TCs for the dFNC estimation to control the impacts caused by different scan lengths. We used a tapered window, which was obtained by convolving a rectangle (window size = 20 TRs = 40 s) with a Gaussian (σ = 3), to localize the dataset at each time point. We slid the window in steps of 1 TR, resulting in total T = 131 windows. The window size was selected according to previous studies showing that the window size in the range of 30 s to 1 min was a reasonable choice for capturing dynamic patterns in functional connectivity (Allen et al., 2014; Damaraju et al., 2014; Hutchison et al., 2013a). Other window sizes were also tested and the results were consistent among a wide range of window sizes (16 TR to 24 TR: 32 s to 48 s). The results of other window sizes were provided in supplementary materials. We calculated the covariance matrices Σi(t), t = 1 … T, from windowed data to measure the dFNC between ICNs. Since the windowed data might not have enough information to characterize the full covariance matrix, we used graphical LASSO method (using L1 norm to promote sparsity) to estimate the regularized precision matrix first and calculated the covariance matrix from the precision matrix. The regularization parameter λ was optimized for each subject by using a cross-validation framework. The dFNC estimates of each window for each subject, , t = 1 … T, were concatenated to form a C × C × T array (where C denotes the number of ICNs and T denotes the number of windows), which represented the changes in brain connectivity between ICNs as a function of time.
2.6 Hard Clustering Analysis
To assess the reoccurring dFNC patterns and investigate transient thalamic-sensory connectivity, we conduct a hard clustering state analysis on the windowed dFNC estimates. The basic idea of the hard clustering state analysis is to assume that functional brain network will enter in different states with distinct dFNC patterns. The windowed covariance matrices were clustered into a set of separate clusters using the k-means clustering method with the L1 norm as the distance function. Our previous work has demonstrated that different distance functions would provide extremely similar results (Allen et al., 2014). Our analysis used the dFNC between ICNs for clustering (total features = C × (C−1)/2). To reduce the redundancy between windows, the subject-specific array was firstly subsampled along the window dimension. Subject-specific exemplars were chosen as those time windows with local maxima in dFNC variance across all dFNC pairs. Then the k-means clustering was conducted on selected exemplars and was repeated 100 times (with random initialization of centroid position) to obtain the group cluster centroids (functional states). The obtained group centroids were further used as the initial centroids to cluster all subjects’ windowed dFNC (total instances = N × T). The optimal number of clusters was estimated by the elbow criterion, which is defined as the ratio of within clustering distance to between clusters distance. The number of clusters was determined as k = 5, which was consistent with our previous dFNC study on schizophrenia (Damaraju et al., 2014). We have used other methods encoded in the GIFT toolbox to estimate the number of clusters (Akaike information criterion, Bayesian information criterion, Dunns index, and silhouette) and calculated the average of all estimates. The results are the same with the result obtained by elbow criterion. Furthermore, in the supplementary materials, we also provided results using 4 and 6 as the number of clusters, because the number in the range of 4 to 6 were shown to be reasonable choices in previous dynamic functional connectivity studies. The overall results in the supplementary materials demonstrate that the identified atypical patterns in dynamic functional connectivity are consistent over the investigated range of the number of clusters.
To investigate whether individuals with ASD enter in different functional states more or less frequently, we examined the group difference in percentage occurrence of functional states. The percentage occurrence of each functional state was calculated by dividing the number of total windows by the number of time windows which were assigned to each state. To count the occurrence of one state, we only used the subjects’ data with at least one window belonging to that state. A general linear model (GLM) was applied to examine the effect of diagnosis on occurrences of functional states (control covariates: age, FIQ, mean FD, eyes state, and site). The diagnosis variable is binary, in which ASD was set as 1 and TD was set as 0. We conducted the statistical analysis on each site to examine the consistency of these results in the supplementary materials. The overall results showed that although the effect sizes are different across sites, the majority of sites have consistent results with the results in the main text. We also investigated whether percentage occurrences of functional states are associated with autistic traits, which were measured by total ADOS score. The GLM was used to examine the effect of ADOS score on the abnormal occurrences in both ASD group (control covariates: age, FIQ, mean FD, eyes state, and site) and in all samples (control covariates: diagnosis, age, FIQ, mean FD, eyes state, and site). For multiple comparisons, results were corrected using the false discovery rate (FDR) (Benjamini et al., 1995) with correction threshold q = 0.05.
We further explored the presence of abnormal patterns in transient dFNC by examining the group difference in dFNC between thalamic ICNs and ICNs within sensory domains in each functional state. A GLM was applied to examine whether dFNC differences are associated with diagnosis (control covariates: age, FIQ, mean FD, eyes state, and site). We conducted the same statistical analysis on each site and the overall results are consistent (results are provided in the supplementary materials). We then used GLM to examine the effect of ADOS score on those aberrant dFNC in both ASD group (control covariates: age, FIQ, mean FD, eyes state, and site) and in all samples (control covariates: diagnosis, age, FIQ, mean FD, eyes state, and site). This was performed for each dFNC with significant group difference. Results were corrected using the FDR for multiple comparisons. The GLM analysis was not conducted in the TD group because only a few TD subjects have ADOS score measured (only 18 subjects).
2.7 High-dimensional Fuzzy Meta-state Analysis
A fuzzy meta-state analysis (Miller et al., 2016) was further performed to investigate atypical dynamic patterns of the whole brain functional network. This analysis assumes that the whole-brain dFNC in each time window is formed by weighted sums of basic dFNC patterns, and the basic patterns would simultaneously contribute to the magnitude and direction of each dFNC across time. In a complementary analysis to the hard clustering analysis performed above, we used the group cluster centroids obtained previously (Section 2.6) as basis dFNC patterns and performed a soft analysis where the dFNC at each time window is the weighted sum of all functional states. In each time window, by regressing dFNC estimates on the group cluster centroids, we obtained a five-dimensional weight vector representing the contributions of the basic dFNC patterns to the windowed dFNC. Then, to convert the real value weight vectors to discrete meta-states, we replaced the vector weights with a value in ± (1, 2, 3, 4). The weight vector was converted to , where , k 1 … 5 representing the quartile of the weights falls into. The vectors are defined as meta-states.
Four dynamism measures were calculated based on the meta-states vector to evaluate the global dynamic properties of the whole functional brain network: 1) meta-states number; 2) meta-states switching times; 3) occupied meta-states range; 4) overall traveled distance. Meta-states number records the number of distinct meta-states an individual passed through. Meta-states switching times measures how many times individuals switch among different meta-states. Occupied meta-states range evaluates how divergent the meta-states occupied are. Overall traveled distance measures total switching distance among different meta-states. Additional details of the meta-state framework and the four global metrics of connectivity dynamism can be referred to (Abrol et al., 2017; Miller et al., 2016).
We employed a GLM to examine the effect of diagnosis on these four dynamism measures. Age, FIQ, mean FD, eyes state and site were set as covariates and their influences were regressed out. To investigate whether these global dynamic properties are associated with autistic symptoms, the GLM was further used to examine the effect of ADOS score on these four metrics in both ASD group (control covariates: age, FIQ, mean FD, eyes state, and site) and in all samples (control covariates: diagnosis, age, FIQ, mean FD, eyes state, and site). Results were corrected using FDR with q = 0.05.
3. Results
3.1 Spatial ICA and ICNs
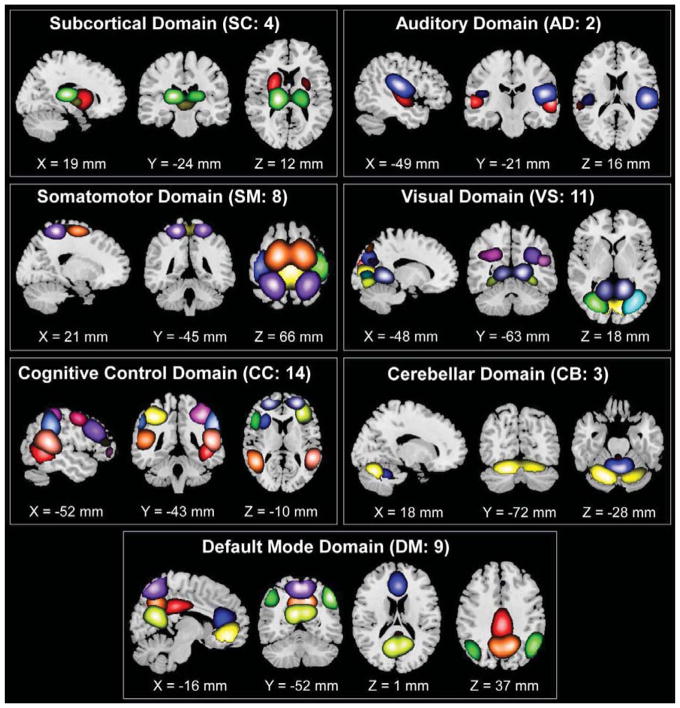
The resulting spatial maps are shown in Figure 2. Overall 51 ICs were identified as ICNs, and were categorized into the following 7 domains: subcortical domain (SC), auditory domain (AD), visual domain (VS), somatomotor domain (SM), cognitive control domain (CC), default-mode domain (DM), and cerebellar domain (CB). The identified ICNs had low spatial over-lap with known vascular, ventricular and brain edge, and were with their activation peaks fell on gray matter. The detailed spatial maps, component labels and peak coordinates of each ICN are provided in the supplementary materials.
Figure 2.
Spatial maps of the 51 identified ICNs, sorted into seven resting-state domains. Each color in the spatial maps corresponds to a different ICN.
3.2 Group Difference in Occurrences of Functional States
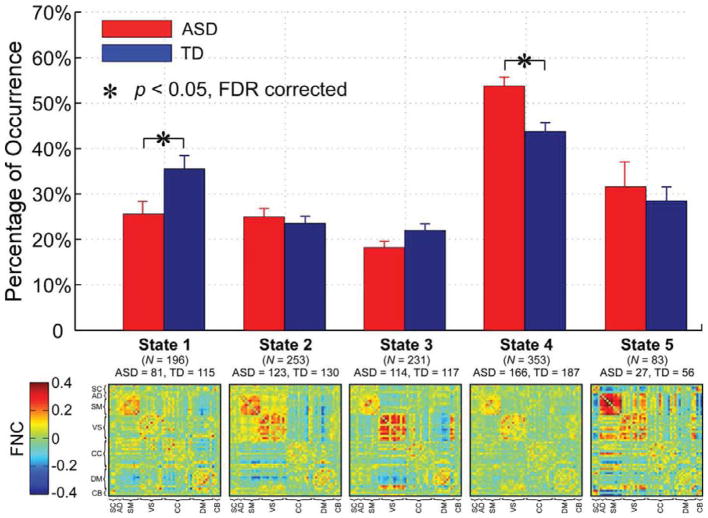
The group difference in occurrences of functional states and the dFNC patterns of each functional state are shown in Figure 3. Note that different functional states had different connectivity patterns. Functional connectivity between DM and VS were negative only in state 2 and state 3. Functional connectivity between CB and SM, and between SB and sensory domains (AD, VS, and SM) were only negative in state 5. Strong positive functional connectivity within sensory domains was observed in states 1, 2, 3 and 5. Functional connectivity was weaker in state 1 and 4, especially in state 4, which was a state with the least negative connectivity between DM and other domains. Among five functional states, two states’ occurrences were associated with the diagnosis effect. Compared with TD controls, individuals with ASD had less occurrence in state 1 (p = 0.0204, FDR corrected, q = 0.05), and more occurrence in state 4 (p = 0.0011, FDR corrected, q = 0.05). There was no significant association identified between occurrences of functional states and ADOS score.
Figure 3.
Upper: group difference in percentage of occurrences of five functional states. Bar represents the mean occurrence of each state, while error bar represents the standard error of mean of occurrence. Two out of five states have significant group difference. Asterisks indicate the significance (FDR corrected, q = 0.05). Lower: the cluster centroids of five functional states, along with the count of subjects that have at least one window clustered into each state.
3.3 Increased Transient dFNC between Thalamic and Sensory Regions in ASD, and Its Association with ADOS Score
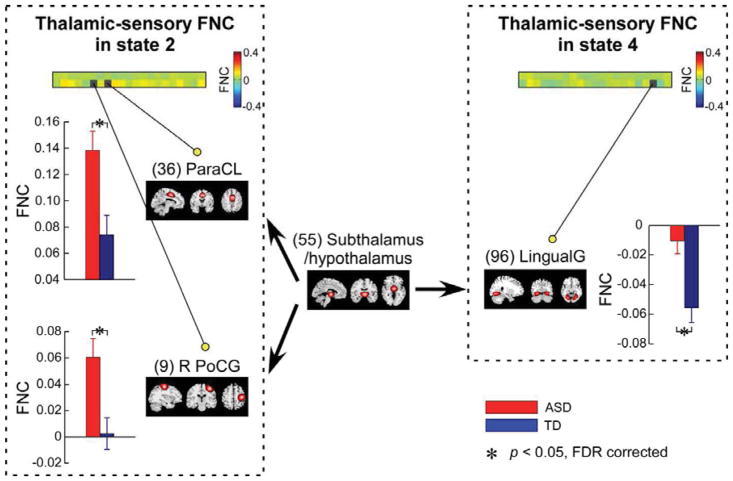
The group differences in dFNC between thalamic ICNs and ICNs within sensory domains are displayed in Figure 4. In this study, two thalamic ICNs and 21 sensory ICNs were identified by GICA. Interestingly, the group difference was only observed in one thalamic ICN (hypothalamus and subthalamus) related dFNC. Relative to TD controls, individuals with ASD always had increased dFNC between hypothalamus/subthalamus and sensory ICNs in certain functional states. In state 2, dFNC between hypothalamus/subthalamus and two ICNs within sensory domains were significantly larger in ASD. These ICNs included right postcentral gyrus (R PoCG) (p = 3.10 × 10−4, FDR corrected, q = 0.05), bi paracentral lobule (Para CL) (p = 0.0021, FDR corrected, q = 0.05). In state 4, dFNC between hypothalamus/subthalamus and lingual gyrus (LingualG) was less negative in the ASD group (p = 3.82 × 10−4, FDR corrected, q = 0.05).
Figure 4.
Group difference in functional connectivity between hypothalamus/subthalamus and ICNs within sensory networks in state 2 and state 4. Bar represents the mean dFNC within this functional state, while error bar represents the standard error of mean of dFNC. Asterisks indicate significance (FDR corrected, q = 0.05).
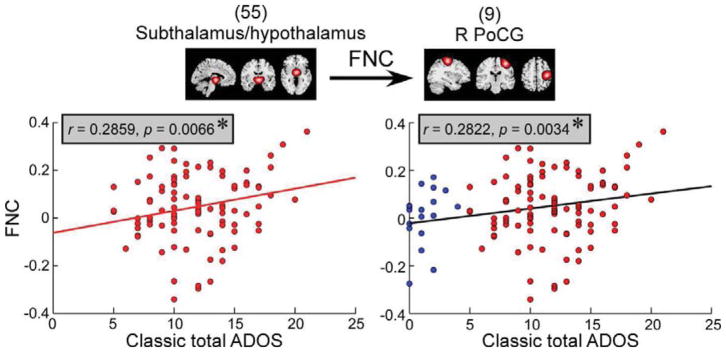
ADOS score clearly distinguished individuals with ASD and TD controls at the group level (p = 6.12 × 10−8). The scatter plots of the associations between the abnormal dFNC and ADOS score are shown in Figure 5. ADOS score was positively correlated with dFNC between hypothalamus/subthalamus and R PoCG in state 2 for ASD group (p = 0.0066, FDR corrected, q = 0.05) and for the whole samples (p = 0.0034, FDR corrected, q = 0.05). It should be noted that only the dynamic brain connectivity between R PoCG and a thalamic ICN was atypical in ASD and associated with the autistic symptoms.
Figure 5.
The scatter plots illustrate the association between classic total ADOS and functional connectivity between hypothalamus/subthalamus and right postcental gyrus (R PoCG) in functional state 2 in ASD group/whole samples. Red circles represent the individuals with ASD with dFNC in functional state 2 and ADOS score measured. Blue circles represent the TD controls with dFNC in functional state 2 and ADOS score measured. Asterisks indicate the significance (FDR corrected, q = 0.05).
3.4 Decreased Functional Network Dynamic, and Its Association with ADOS Score
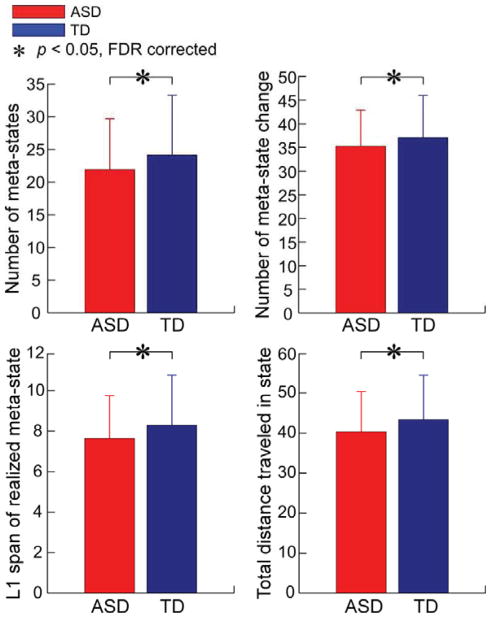
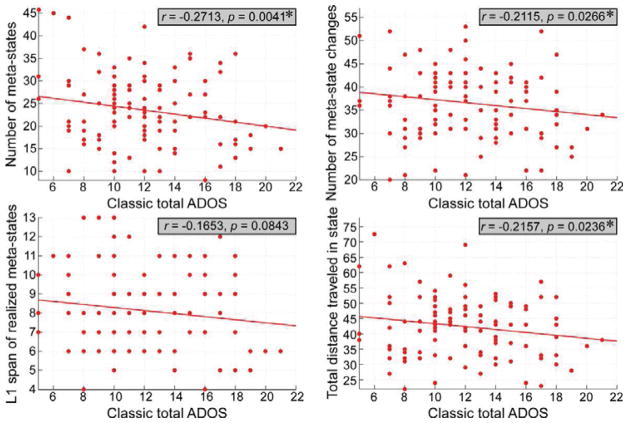
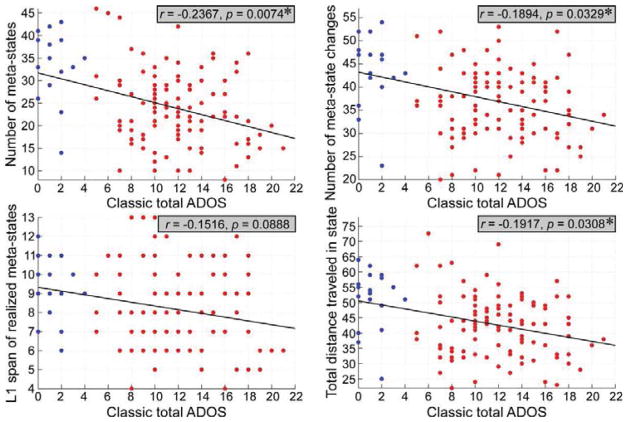
The meta-state results showed that the four dynamism measures consistently decrease in ASD group. Results are displayed in Figure 6. Individuals with ASD passed through fewer distinct meta-states (measured by the number of distinct meta-states subjects occupy, mean TD = 24.14 total states, mean ASD = 21.92 total states; diagnosis effect was FDR corrected, q = 0.05) and traveled less frequently between occupied meta-states (measured by the number of times that subjects switch from one metastate to another, mean TD = 37.12 changes, mean ASD = 35.24 changes; diagnosis effect was FDR corrected, q = 0.05). In addition, individuals with ASD stayed in a smaller radius of the meta-state space (measured by largest L1-distance between meta-state, mean TD = 8.30 diameter, mean ASD = 7.64 diameter; diagnosis effect was FDR corrected, q = 0.05) and traversed less overall distance (measured by summed L1 distance between all meta-states, mean TD = 43.39 total distance, mean ASD = 40.36 total distance; diagnosis effect was FDR corrected, q = 0.05). We further investigated whether the connectivity dynamism measures are associated with ASD symptoms and the results are displayed in Figure 7 and Figure 8. Three dynamism measures were significantly and negatively correlated with ADOS score in both ASD group and whole samples (except for the occupied meta-states range, although there is still a negative correlation between occupied meta-states range and ADOS score). Subjects with more severe autistic symptoms would have fewer dynamic patterns of the whole-brain functional network.
Figure 6.
Group difference in four metrics of connectivity dynamism (number of meta-states, changes of meta-states, L1 span of meta-states and total distance traveled among meta-states). Bar represents the mean of each dynamism measure, while the error bar represents the standard error of mean of each measure. Asterisks indicate the significance (FDR corrected, q = 0.05).
Figure 7.
The scatter plots illustrate the association between total ADOS score and each dynamism measure in ASD group. Asterisks indicate the significance (FDR corrected, q = 0.05).
Figure 8.
The scatter plots illustrate the association between total ADOS score and each dynamism measure in the whole samples. Red circles represent the individuals with ASD, while blue circles represent the TD controls. Asterisks indicate the significance (FDR corrected, q = 0.05).
4. Discussion
In this study, we investigated ASD-related abnormalities in resting-state dynamic brain functional connectivity. Our results showed that both TD controls and individuals with ASD had similar brain functional states, but they spent markedly different lengths of time in different states. Compared with TD controls, individuals with ASD showed hyperconnectivity between hypothalamus/subthalamus and sensory regions only in certain functional states. By analyzing the global dynamic properties of the whole-brain functional network, we also found that the functional brain network of individuals with ASD operated within a limited dynamic range with less dynamic fluidity. Interestingly, the identified abnormalities in dynamic brain functional connectivity were associated with the severity of autistic traits indexed by ADOS score.
4.1 Reoccurring Functional States
Our findings, together with dynamic states research on other psychiatric disorders (Damaraju et al., 2014; Rashid et al., 2014; Yu et al., 2015), suggest the importance of evaluating transient aspects of connectivity. Brain connectivity is highly variable over time, which might represent flexibility in functional coordination between distinct brain systems. In this study, we observed that the functional connectivity varied among functional states. For example, negative dFNC between sensory regions and regions within the sub-cortical network was only observed in functional state 5. Sensory regions were highly synchronous in functional state 2, 3 and 5, but their synchronous patterns were different. State 4 was the most frequently reoccurring state, which resembled the static dFNC patterns, consistent with our previous findings (Allen et al., 2014; Damaraju et al., 2014). TD controls and individuals with ASD showed significantly different occurrences in two functional states. Individuals with ASD spent more time in state 4 with weak dFNC patterns and less negative dFNC between DMN and other networks, while TD controls spent more time in state 1 with both positive and negative dFNC patterns. The weak and diffuse functional state has been associated with increased self-focused thoughts in a previous study (Marusak et al., 2017). Individuals with ASD have been shown to suffer increased levels of self-focus, which might be associated with the depression caused by autism (Meyer et al., 2006; Varga 2011). We speculate that the increased occurrence of functional state 4 in ASD group may be due to more time spent by individuals with ASD on self-focused thinking during the resting-state.
4.2 Hyperconnectivity between Hypothalamus/Subthalamus and Sensory Regions
Relative to TD controls, individuals with ASD had increased transient dFNC between thalamic and several sensory regions. By using a relatively high model order (100 components), we identified two different thalamic networks (dorsal thalamus and hypothalamus/subthalamus) and found that the aberrant connectivity was only related to hypothalamus/subthalamus. These results are in line with previous studies showing increased static functional connectivity between thalamus and sensory cortex in ASD, such as auditory, visual and somatomotor networks (Cerliani et al., 2015; Tomasi et al., 2017) to some extent. More interestingly, our results revealed additional information of the atypical thalamic-sensory connectivity that such abnormalities only exist during specific functional states, not across the whole resting-state. Our results suggested two possible explanations of previous contradictory findings on static thalamic-sensory connectivity. Firstly, ASD does not impair all the interactions between thalamic networks and sensory networks. Only hypothalamus/subthalamus related brain connectivity is affected by the ASD. A recent study showed that the subtypes and locations of brain networks (e.g. DMN) have a significant influence on the functional connectivity and such influence might result in inconsistent findings across different studies (Chen et al., 2017). An ASD study also showed that the spatial scales of functional connectivity affect the observed abnormalities in ASD (Khan et al., 2015). Abnormalities in hypothalamus such as diminished gray matter and atypical activity (Ellegood et al., 2015; Kurth et al., 2011; Mazurek et al., 2013) have been previously reported in ASD with the speculation that these findings might be associated with the sensory over-responsivity. Our results might support this hypothesis by providing evidence for the presence of atypical connections between hypothalamus and sensory regions. Secondly, the observed reoccurring functional states have been suggested to be associated with the mental processes (Allen et al., 2014; Marusak et al., 2017). Our results suggest that ASD does not affect the thalamic-sensory pathways consistently throughout the scan, but rather affects parts of these pathways during certain mental processes. Heterogenous sensory processing differences are widely reported in ASD, such as hyper- or hyporesponsiveness to sensory stimuli (Baranek et al., 2013; Green et al., 2013; Marco et al., 2011; Rogers et al., 2005), suggesting the atypical sensory processing to be potential biomarkers of ASD, which could serve as a diagnostic criterion (Association 2013; Marco et al., 2011). Altered sensory processing in ASD might stem from aberrantly enhanced sensory input from subcortical regions to sensory cortex during some mental processes, as reflected in our findings of hyperconnectivity between hypothalamus/subthalamus and sensory regions during certain functional states.
The observed abnormalities in dFNC were also associated with the severity of autistic symptoms measured by ADOS score. Previous studies have shown that the variability of autistic symptoms indexed by other measures can be captured by static resting-state functional connectivity (Cerliani et al., 2015; Di Martino et al., 2009). In the present study, we found that individuals with ASD had significantly larger ADOS score compared with TD controls and such aberrant high ADOS score was associated with enhanced dFNC between hypothalamus/subthalamus and R PoCG (consistent in both ASD group and whole sample). Our results suggest that dynamic functional connectivity can capture the variability of the ADOS score and might serve as a potential biomarker for diagnosing ASD and predicting autistic symptoms. The observed association between ADOS and dFNC included the component which was localized to the right hemisphere, compatible with the idea that impairments of right hemisphere might be associated with behavioral findings in ASD. Functional impairments of right hemispheric regions have been reported in several studies (Orekhova et al., 2009; Stroganova et al., 2007), and these impairments have been suggested to be related to abnormalities characteristic of ASD.
4.3 Abnormalities in Global Dynamic Properties of dFNC
In this study, we applied a fuzzy meta-state method to analyze the dynamic properties of the whole-brain functional network in autism. This method is based on the assumption that a given time point of the functional network could be formed by a small set of brain connectivity patterns (Miller et al., 2016; Preti et al., 2016). A meta-state, therefore, captures the whole pattern of activity levels across connectivity patterns. The meta-state analysis is an advanced technique to probe the evolution of whole-brain network configuration and may improve our understanding of the brain function from a global perspective (Lottman et al., 2017; Miller et al., 2014; Miller et al., 2016). We found significant group difference in all of the dynamism measures, suggesting globally-reduced brain functional fluidity and dynamic range. A previous study suggested that moving more frequently across functional states with strong and clear connectivity patterns would lead to larger meta-state space changes (Preti et al., 2016).
The observed abnormal global dynamics are supported by previous findings in both animal models and human subjects, which suggested that the autism may be characterized by “undifferentiated brain states” (Rubenstein et al., 2003; Uddin et al., 2014). The ASD-related diminished changes in brain connectivity have been reported in the literature and such abnormalities might underlie the symptoms associated with autism. Individuals with ASD have been identified to exhibit fewer changes in functional connectivity configuration between task-state and resting-state, and such weak modulation of brain states was suggested to contribute to the repetitive behaviors in ASD (Uddin et al., 2014). Another study has identified atypical modulation of functional connectivity in individuals with ASD when they swift from resting-state to the task state (You et al., 2013). Resting-state is a highly non-stationary condition which includes distinct brain states corresponding to different mental processes. Therefore, it is reasonable to speculate that the dynamics of brain connectivity configuration during the resting-state might also have aberrant patterns associated with ASD. The observed diminished dynamics of the resting-state functional network in our study extend previous “task vs rest” findings to single resting-state condition and indicate that the weak modulation of brain network configuration in ASD not only exist between task and rest, but also within the resting-state.
We found significant associations between the dynamism measures and ADOS symptom scores, consistent with previous observations of associations between atypical brain dynamics and autistic symptoms (Watanabe et al., 2017). Watanabe et al. reported that high-functioning adults with ASD showed fewer transitions among brain states in the resting-state, with these neural dynamics associated with the severity of autism (Watanabe et al., 2017). Although this study characterized the brain states using the brain activity not the functional connectivity, its results still implied critical links between autistic symptoms and decreased brain dynamics, which matched the current findings of associations between ADOS score and diminished dynamics of whole-brain functional configuration. Our current findings are also compatible with the idea of a relationship between functional network discriminability and restricted behaviors in ASD (Uddin et al., 2014), and provide new evidence of a potential link between changes in whole-brain functional network configuration and social & communication deficits that characterize this disorder.
4.4 Limitations and Future Directions
In this study, there are some issues which may deserve investigation in future work. Although we speculated that these dFNC abnormalities might be a potential cause of the presence of atypical sensory processing in ASD, it is still not clear whether and how these abnormalities in dFNC influence the sensory processing during sensory stimulation. Our hypothesis could be further corroborated by conducting functional state analysis on task designed fMRI which specifically probes sensory processes (Baron-Cohen et al., 2009; Bor et al., 2008; Gomot et al., 2008; Minshew et al., 2008).
Our previous work has also reported abnormal dynamic functional connectivity in schizophrenia (Damaraju et al., 2014), including the abnormal occurrence of functional states, hyperconnectivity between thalamic and sensory regions within certain functional states, diminished dynamic fluidity and decreased dynamic range, which was similar to our present observed abnormalities in ASD. Schizophrenia and autism have overlapping molecular-genetic substrates and a number of behavioral phenotypic correspondences (De Lacy et al., 2013) and it is therefore intriguing that we found similar high-dimensional dynamic properties between these conditions. Future work will inform the specificity of these findings.
We examined the consistency of our results across sites in the supplementary materials. The majority of sites showed consistent results with the results in the main text. However, the effect size is somewhat variable across sites. We argue that these differences may be due to several reasons. Firstly, previous studies have shown that ASD is a common and heterogeneous neurodevelopmental disorder and the variation of functional connectivity within ASD has been widely reported (Geschwind et al., 2007; Hull et al., 2017; Lenroot et al., 2013; Lynch et al., 2013; Masi et al., 2017). The heterogeneity of ASD etiology, behaviors and cognition might be a possible cause of the difference in the effect sizes. Secondly, the number of subjects is significantly different across sites. Moreover, k-means clustering can result in fewer subjects for statistical analysis because not all the subjects have at least one window assigned to a specific functional state. This might further unbalance the number of subjects across sites. Some sites have limited number of subjects and thus they could not fully characterize the population. Thirdly, different sites used different scanners and parameters (for example, the number of slice and the number of measurements). Although we have tried to control these difference (for example, we selected subjects with more time measurements and used the same number of measurements from each subject), such difference might still influence the properties of the dataset and impact the effect size. That is why we need to control the confounding effects from sites and conduct analysis on subjects from all sites.
In the current study, we considered gender as a confounding effect which might affect our dynamic functional connectivity analysis and thus used a careful subject selection criterion by choosing only male subjects. To guarantee the accuracy of dynamic functional connectivity estimation and the further analysis, we used very strict criteria for subject selection (as described in section 2.1). If we used the same criteria to select female subjects, only 93 female subjects remain. More importantly, among these 93 females, only 15 of them have ADOS score measured, which is also not enough for a correlation analysis. Therefore, in this study, we did not conduct a separate analysis for females. In future studies with more subjects and greater statistical power, we would like to investigate whether female ASD subjects have similar or distinct atypical dynamic patterns in functional connectivity.
5. Conclusion
Conventionally, atypical functional connectivity in ASD has been investigated from the static perspective. In this study, by combining a sliding window approach and the k-means clustering method, we investigated group differences between TD controls and individuals with ASD in dynamic patterns of local and global brain connectivity. Our results showed that individuals with ASD have higher transient connectivity between hypothalamus/subthalamus and right postcentral gyrus, bi paracentral lobule and lingual gyrus only in several functional states. We also found that global functional network of individuals with ASD exhibits fewer dynamic patterns and is more stable during the resting-state. Moreover, these observed atypical patterns in dynamic functional connectivity were highly correlated with autistic symptoms indexed by the ADOS score. Overall, our results suggest transiently increased thalamic-sensory connectivity and decreased whole-brain dynamism in autism. We propose that such abnormal dynamic functional connectivity in autism can be related to atypical sensory processing and weak modulation of brain network configuration during the resting-state.
Supplementary Material
Acknowledgments
This work is supported by the National Institutes of Health (NIH) grants (R01EB006841, R01REB020407, and P20GM103472 PI: Calhoun), National Science Foundation (NSF) grant 1539067 and National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR000421 to NdL. We would like to thank Drs. Marlene Behrmann and Leonardo Cerliani for their efforts in the collection, organization, and sharing of their datasets and the NITRC team (www.nitrc.org) for providing the data sharing platform for the ABIDE initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrol A, Damaraju E, Miller RL, Stephen JM, Claus ED, Mayer AR, Calhoun VD. Replicability of time-varying connectivity patterns in large resting state fMRI samples. Neuroimage. 2017;163:160–176. doi: 10.1016/j.neuroimage.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaerts K, Swinnen SP, Wenderoth N. Sex differences in autism: a resting-state fMRI investigation of functional brain connectivity in males and females. Social cognitive and affective neuroscience. 2016;11:1002–1016. doi: 10.1093/scan/nsw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, Havlicek M, Rachakonda S, Fries J, Kalyanam R. A baseline for the multivariate comparison of resting-state networks. Frontiers in systems neuroscience. 2011;5:2. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD. Tracking whole-brain connectivity dynamics in the resting state. Cerebral cortex. 2014;24:663–676. doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association, A. P. Diagnostic and statistical manual of mental disorders (DSM-5®) American Psychiatric Pub; 2013. [DOI] [PubMed] [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P. A clinicopathological study of autism. Brain: a journal of neurology. 1998;121:889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Watson LR, Boyd BA, Poe MD, David FJ, McGuire L. Hyporesponsiveness to social and nonsocial sensory stimuli in children with autism, children with developmental delays, and typically developing children. Development and Psychopathology. 2013;25:307–320. doi: 10.1017/S0954579412001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Ashwin E, Ashwin C, Tavassoli T, Chakrabarti B. Talent in autism: hyper-systemizing, hyper-attention to detail and sensory hypersensitivity. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2009;364:1377–1383. doi: 10.1098/rstb.2008.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the royal statistical society. Series B (Methodological) 1995;57:289–300. doi: 10.2307/2346101. [DOI] [Google Scholar]
- Bor D, Billington J, Baron-Cohen S. Savant memory for digits in a case of synaesthesia and Asperger syndrome is related to hyperactivity in the lateral prefrontal cortex. Neurocase. 2008;13:311–319. doi: 10.1080/13554790701844945. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar J. A method for making group inferences from functional MRI data using independent component analysis. Human brain mapping. 2001;14:140–151. doi: 10.1002/hbm.1048. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T. Multisubject independent component analysis of fMRI: a decade of intrinsic networks, default mode, and neurodiagnostic discovery. IEEE Rev Biomed Eng. 2012;5:60–73. doi: 10.1109/RBME.2012.2211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Miller R, Pearlson G, Adali T. The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron. 2014;84:262–274. doi: 10.1016/j.neuron.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova M, Trippe J. Radial cytoarchitecture and patterns of cortical connectivity in autism. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2009;364:1433–1436. doi: 10.1098/rstb.2008.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerliani L, Mennes M, Thomas RM, Di Martino A, Thioux M, Keysers C. Increased functional connectivity between subcortical and cortical resting-state networks in autism spectrum disorder. JAMA psychiatry. 2015;72:767–777. doi: 10.1001/jamapsychiatry.2015.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JE, Glover GH, Greicius MD, Chang C. Dissociated patterns of anti-correlations with dorsal and ventral default-mode networks at rest. Hum Brain Mapp. 2017;38:2454–2465. doi: 10.1002/hbm.23532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, Barnes CC, Pierce K. Neuron number and size in prefrontal cortex of children with autism. Jama. 2011;306:2001–2010. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- Damaraju E, Allen E, Belger A, Ford J, McEwen S, Mathalon D, Mueller B, Pearlson G, Potkin S, Preda A. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. NeuroImage: Clinical. 2014;5:298–308. doi: 10.1016/j.nicl.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lacy N, King BH. Revisiting the relationship between autism and schizophrenia: toward an integrated neurobiology. Annual review of clinical psychology. 2013;9:555–587. doi: 10.1146/annurev-clinpsy-050212-185627. [DOI] [PubMed] [Google Scholar]
- de Lacy N, Doherty D, King B, Rachakonda S, Calhoun V. Disruption to control network function correlates with altered dynamic connectivity in the wider autism spectrum. Neuroimage Clin. 2017;15:513–524. doi: 10.1016/j.nicl.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Shehzad Z, Kelly C, Roy AK, Gee DG, Uddin LQ, Gotimer K, Klein DF, Castellanos FX, Milham MP. Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults. Am J Psychiatry. 2009;166:891–899. doi: 10.1176/appi.ajp.2009.08121894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Kelly C, Grzadzinski R, Zuo X-N, Mennes M, Mairena MA, Lord C, Castellanos FX, Milham MP. Aberrant striatal functional connectivity in children with autism. Biological psychiatry. 2011;69:847–856. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Yan C-G, Li Q, Denio E, Castellanos FX, Alaerts K, Anderson JS, Assaf M, Bookheimer SY, Dapretto M. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Molecular psychiatry. 2014;19:659–667. doi: 10.1038/mp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Pearlson GD, Yu Q, He H, Lin D, Sui J, Wu L, Calhoun VD. Interaction among subsystems within default mode network diminished in schizophrenia patients: a dynamic connectivity approach. Schizophrenia research. 2016;170:55–65. doi: 10.1016/j.schres.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Fryer SL, Fu Z, Lin D, Sui J, Chen J, Damaraju E, Mennigen E, Stuart B, Mathalon DH. Dynamic functional connectivity impairments in early schizophrenia and clinical high-risk for psychosis. NeuroImage. 2017 doi: 10.1016/j.neuroimage.2017.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegood J, Anagnostou E, Babineau B, Crawley J, Lin L, Genestine M, DiCicco-Bloom E, Lai J, Foster J, Penagarikano O. Clustering autism-using neuroanatomical differences in 26 mouse models to gain insight into the heterogeneity. Molecular psychiatry. 2015;20:118. doi: 10.1038/mp.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD. Comparison of multi-subject ICA methods for analysis of fMRI data. Human brain mapping. 2011;32:2075–2095. doi: 10.1002/hbm.21170. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falahpour M, Thompson WK, Abbott AE, Jahedi A, Mulvey ME, Datko M, Liu TT, Müller R-A. Underconnected, But Not Broken? Dynamic Functional Connectivity MRI Shows Underconnectivity in Autism Is Linked to Increased Intra-Individual Variability Across Time. Brain connectivity. 2016;6:403–414. doi: 10.1089/brain.2015.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Tu Y, Di X, Du Y, Pearlson G, Turner J, Biswal BB, Zhang Z, Calhoun V. Characterizing dynamic amplitude of low-frequency fluctuation and its relationship with dynamic functional connectivity: an application to schizophrenia. Neuroimage. 2017 doi: 10.1016/j.neuroimage.2017.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Current opinion in neurobiology. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Gomot M, Belmonte MK, Bullmore ET, Bernard FA, Baron-Cohen S. Brain hyper-reactivity to auditory novel targets in children with high-functioning autism. Brain. 2008;131:2479–2488. doi: 10.1093/brain/awn172. [DOI] [PubMed] [Google Scholar]
- Green SA, Rudie JD, Colich NL, Wood JJ, Shirinyan D, Hernandez L, Tottenham N, Dapretto M, Bookheimer SY. Overreactive brain responses to sensory stimuli in youth with autism spectrum disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52:1158–1172. doi: 10.1016/j.jaac.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halladay AK, Bishop S, Constantino JN, Daniels AM, Koenig K, Palmer K, Messinger D, Pelphrey K, Sanders SJ, Singer AT. Sex and gender differences in autism spectrum disorder: summarizing evidence gaps and identifying emerging areas of priority. Molecular autism. 2015;6:36. doi: 10.1186/s13229-015-0019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull JV, Jacokes ZJ, Torgerson CM, Irimia A, Van Horn JD. Resting-state functional connectivity in autism spectrum disorders: A review. Frontiers in psychiatry. 2017;7:205. doi: 10.3389/fpsyt.2016.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, Della Penna S, Duyn JH, Glover GH, Gonzalez-Castillo J. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage. 2013a;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Gati JS, Everling S, Menon RS. Resting-state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Human brain mapping. 2013b;34:2154–2177. doi: 10.1002/hbm.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Wang L, Yan C, Wang J, Liang X, He Y. Characterizing dynamic functional connectivity in the resting brain using variable parameter regression and Kalman filtering approaches. Neuroimage. 2011;56:1222–1234. doi: 10.1016/j.neuroimage.2011.03.033. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proceedings of the National Academy of Sciences. 2006;103:8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Gramfort A, Shetty NR, Kitzbichler MG, Ganesan S, Moran JM, Lee SM, Gabrieli JD, Tager-Flusberg HB, Joseph RM. Local and long-range functional connectivity is reduced in concert in autism spectrum disorders. Proceedings of the National Academy of Sciences. 2013;110:3107–3112. doi: 10.1073/pnas.1214533110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Michmizos K, Tommerdahl M, Ganesan S, Kitzbichler MG, Zetino M, Garel K-LA, Herbert MR, Hämäläinen MS, Kenet T. Somatosensory cortex functional connectivity abnormalities in autism show opposite trends, depending on direction and spatial scale. Brain. 2015;138:1394–1409. doi: 10.1093/brain/awv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, Greenson J, Dawson G, Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131:1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. fMRI investigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cereb Cortex. 2007;18:289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Narr KL, Woods RP, O’Neill J, Alger JR, Caplan R, McCracken JT, Toga AW, Levitt JG. Diminished gray matter within the hypothalamus in autism disorder: a potential link to hormonal effects? Biol Psychiatry. 2011;70:278–282. doi: 10.1016/j.biopsych.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois D, Chartier S, Gosselin D. An introduction to independent component analysis: InfoMax and FastICA algorithms. Tutorials in Quantitative Methods for Psychology. 2010;6:31–38. doi: 10.20982/tqmp.06.1.p031. [DOI] [Google Scholar]
- Lee T-W, Girolami M, Sejnowski TJ. Independent component analysis using an extended infomax algorithm for mixed subgaussian and supergaussian sources. Neural computation. 1999;11:417–441. doi: 10.1162/089976699300016719. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Yeung PK. Heterogeneity within autism spectrum disorders: what have we learned from neuroimaging studies? Frontiers in human neuroscience. 2013;7:733. doi: 10.3389/fnhum.2013.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lottman KK, Kraguljac NV, White DM, Morgan CJ, Calhoun VD, Butt A, Lahti AC. risperidone effects on Brain Dynamic connectivity—a Prospective resting-state fMri study in schizophrenia. Frontiers in Psychiatry. 2017;8:14. doi: 10.3389/fpsyt.2017.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CJ, Uddin LQ, Supekar K, Khouzam A, Phillips J, Menon V. Default mode network in childhood autism: posteromedial cortex heterogeneity and relationship with social deficits. Biological psychiatry. 2013;74:212–219. doi: 10.1016/j.biopsych.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco EJ, Hinkley LB, Hill SS, Nagarajan SS. Sensory processing in autism: a review of neurophysiologic findings. Nature Publishing Group; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Calhoun VD, Brown S, Crespo LM, Sala-Hamrick K, Gotlib IH, Thomason ME. Dynamic functional connectivity of neurocognitive networks in children. Human Brain Mapping. 2017;38:97–108. doi: 10.1002/hbm.23346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi A, DeMayo MM, Glozier N, Guastella AJ. An overview of autism spectrum disorder, heterogeneity and treatment options. Neuroscience bulletin. 2017;33:183–193. doi: 10.1007/s12264-017-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek MO, Vasa RA, Kalb LG, Kanne SM, Rosenberg D, Keefer A, Murray DS, Freedman B, Lowery LA. Anxiety, sensory over-responsivity, and gastrointestinal problems in children with autism spectrum disorders. Journal of abnormal child psychology. 2013;41:165–176. doi: 10.1007/s10802-012-9668-x. [DOI] [PubMed] [Google Scholar]
- Meyer JA, Mundy PC, Van Hecke AV, Durocher JS. Social attribution processes and comorbid psychiatric symptoms in children with Asperger syndrome. Autism. 2006;10:383–402. doi: 10.1177/1362361306064435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RL, Yaesoubi M, Calhoun VD. Higher dimensional analysis shows reduced dynamism of time-varying network connectivity in schizophrenia patients. Engineering in Medicine and Biology Society (EMBC). 2014 36th Annual International Conference of the IEEE; IEEE; 2014. [DOI] [PubMed] [Google Scholar]
- Miller RL, Yaesoubi M, Turner JA, Mathalon D, Preda A, Pearlson G, Adali T, Calhoun VD. Higher dimensional meta-state analysis reveals reduced resting fMRI connectivity dynamism in schizophrenia patients. PloS one. 2016;11:e0149849. doi: 10.1371/journal.pone.0149849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew NJ, Hobson JA. Sensory sensitivities and performance on sensory perceptual tasks in high-functioning individuals with autism. Journal of autism and developmental disorders. 2008;38:1485–1498. doi: 10.1007/s10803-007-0528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew NJ, Keller TA. The nature of brain dysfunction in autism: functional brain imaging studies. Current opinion in neurology. 2010;23:124. doi: 10.1097/WCO.0b013e32833782d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Treiber JM, Shukla DK, Shih P, Müller R-A. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain. 2013;136:1942–1955. doi: 10.1093/brain/awt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova E, Stroganova T, Prokofiev A, Nygren G, Gillberg C, Elam M. The right hemisphere fails to respond to temporal novelty in autism: Evidence from an ERP study. Clin Neurophysiol. 2009;120:520–529. doi: 10.1016/j.clinph.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Padmanabhan A, Lynn A, Foran W, Luna B, O’Hearn K. Age related changes in striatal resting state functional connectivity in autism. Front Hum Neurosci. 2013;7:814. doi: 10.3389/fnhum.2013.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Taquet M, Vega C, Jeste SS, Fernández IS, Tan J, Nelson CA, Sahin M, Warfield SK. Brain functional networks in syndromic and non-syndromic autism: a graph theoretical study of EEG connectivity. BMC medicine. 2013;11:54. doi: 10.1186/1741-7015-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preti MG, Bolton TA, Van De Ville D. The dynamic functional connectome: State-of-the-art and perspectives. NeuroImage. 2016;160:41–54. doi: 10.1016/j.neuroimage.2016.12.061. [DOI] [PubMed] [Google Scholar]
- Rachakonda S, Silva RF, Liu J, Calhoun VD. Memory efficient PCA methods for large group ICA. Frontiers in neuroscience. 2016;10:17. doi: 10.3389/fnins.2016.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid B, Damaraju E, Pearlson GD, Calhoun VD. Dynamic connectivity states estimated from resting fMRI Identify differences among Schizophrenia, bipolar disorder, and healthy control subjects. Frontiers in human neuroscience. 2014;8:897. doi: 10.3389/Fnhum.2014.00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’esposito M. Measuring functional connectivity during distinct stages of a cognitive task. NeuroImage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Ozonoff S. Annotation: What do we know about sensory dysfunction in autism? A critical review of the empirical evidence. Journal of Child Psychology and Psychiatry. 2005;46:1255–1268. doi: 10.1111/j.1469-7610.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- Rubenstein J, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes, Brain and Behavior. 2003;2:255–267. doi: 10.1034/j.1601-183X.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudie JD, Brown J, Beck-Pancer D, Hernandez L, Dennis E, Thompson P, Bookheimer S, Dapretto M. Altered functional and structural brain network organization in autism. Neuroimage Clin. 2013;2:79–94. doi: 10.1016/j.nicl.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Human brain mapping. 1996;4:174–198. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroganova TA, Nygren G, Tsetlin MM, Posikera IN, Gillberg C, Elam M, Orekhova EV. Abnormal EEG lateralization in boys with autism. Clinical Neurophysiology. 2007;118:1842–1854. doi: 10.1016/j.clinph.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Gender differences in brain functional connectivity density. Human brain mapping. 2012;33:849–860. doi: 10.1002/hbm.21252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Reduced Local and Increased Long-Range Functional Connectivity of the Thalamus in Autism Spectrum Disorder. Cereb Cortex. 2017 doi: 10.1093/cercor/bhx340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsatsanis KD, Rourke BP, Klin A, Volkmar FR, Cicchetti D, Schultz RT. Reduced thalamic volume in high-functioning individuals with autism. Biol Psychiatry. 2003;53:121–129. doi: 10.1016/S0006-3223(02)01530-5. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Lynch CJ, Cheng KM, Odriozola P, Barth ME, Phillips J, Feinstein C, Abrams DA, Menon V. Brain state differentiation and behavioral inflexibility in autism. Cereb Cortex. 2014;25:bhu161. doi: 10.1093/cercor/bhu161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron. 2012;75:963–980. doi: 10.1016/j.neuron.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Varga S. Pretence, social cognition and self-knowledge in autism. Psychopathology. 2011;44:46–52. doi: 10.1159/000317777. [DOI] [PubMed] [Google Scholar]
- Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Müller R-A. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage. 2005;25:916–925. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zuo X, He Y. Graph-based network analysis of resting-state functional MRI. Front Syst Neurosci. 2010;4:16. doi: 10.3389/fnsys.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Rees G. Brain network dynamics in high-functioning individuals with autism. Nature communications. 2017;8:16048. doi: 10.1038/ncomms16048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’networks. Nature. 1998;393:440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Giraldo-Chica M, Rogers B, Cascio CJ. Thalamocortical Dysconnectivity in Autism Spectrum Disorder: An Analysis of the Autism Brain Imaging Data ExchangeThalamocortical dysconnectivity in ASD. 2016 doi: 10.1016/j.bpsc.2016.09.002. [DOI] [PMC free article] [PubMed]
- Wu X, Li R, Fleisher AS, Reiman EM, Guan X, Zhang Y, Chen K, Yao L. Altered default mode network connectivity in Alzheimer’s disease—a resting functional MRI and Bayesian network study. Human brain mapping. 2011;32:1868–1881. doi: 10.1002/hbm.21153. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Hu B, Xie Y, Zheng F, Liu G, Chen X, Zheng W. Resting-State Time-Varying Analysis Reveals Aberrant Variations of Functional Connectivity in Autism. Front Hum Neurosci. 2016;10:463. doi: 10.3389/fnhum.2016.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You X, Norr M, Murphy E, Kuschner ES, Bal E, Gaillard WD, Kenworthy L, Vaidya CJ. Atypical modulation of distant functional connectivity by cognitive state in children with Autism Spectrum Disorders. Front Hum Neurosci. 2013;7:482. doi: 10.3389/fnhum.2013.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Erhardt EB, Sui J, Du Y, He H, Hjelm D, Cetin MS, Rachakonda S, Miller RL, Pearlson G. Assessing dynamic brain graphs of time-varying connectivity in fMRI data: application to healthy controls and patients with schizophrenia. Neuroimage. 2015;107:345–355. doi: 10.1016/j.neuroimage.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Yu F, Duong T. Multiparametric MRI characterization and prediction in autism spectrum disorder using graph theory and machine learning. PLoS One. 2014;9:e90405. doi: 10.1371/journal.pone.0090405. ARTN e90405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.