Abstract
Few data on HIV incidence among men who have sex with men and inject drugs (MSM-PWID) are available. Drawing on a prospective cohort in Vancouver, Canada, we examined the relationship between MSM status and HIV incidence among PWID using Kaplan-Meier analyses and extended Cox regression. Data were collected from 1996–2014 and analyzed in 2017. Of 1131 HIV-negative male PWID, 8.6% (n=97) reported sex with men over the study period. MSM status was crudely associated with HIV incidence (Hazard Ratio [HR] = 1.81; 95% CI = 1.08 – 3.03), but not after adjustment for daily cocaine injection and syringe borrowing (Adjusted HR = 1.33; 95% CI = 0.78 – 2.28). Findings highlight the need for harm reduction interventions and socio-behavioral research focused on MSM-PWID.
Keywords: injection drug use, men who have sex with men, HIV, incidence
INTRODUCTION
Men who have sex with men (MSM) comprise the majority of new HIV infections in high-income countries, while infections among people who inject drugs (PWID) also are contributing substantially to the burden of HIV [1]. In Canada, an estimated 67.3% of new HIV infections in 2014 were attributed to the MSM, PWID, and MSM-PWID exposure categories combined [2]. Although population size estimates of MSM-PWID across Canada are unavailable, it is estimated that 1.2% of residents of the province of British Columbia are PWID [3], and that 2.9% of adult males in Vancouver, British Columbia are MSM [4]. Thus, the relatively high proportion of new infections occurring among MSM-PWID—2.5% of all new infections in Canada—is indicative of the particularly high HIV acquisition risk among those who belong to both of these key populations.
Despite these elevated risks, longitudinal studies of HIV among MSM-PWID are rare. Reports to date have often relied on repeated cross-sectional surveys, finding that MSM-PWID continue to demonstrate higher HIV prevalence and risk behaviors than non-MSM PWID, despite declines in some settings [5,6]. Available incidence data largely date from the first two decades of the HIV pandemic [7].
HIV vulnerability among MSM-PWID is related to dual sexual and injection risks, with high per-act transmission risks associated with HIV exposure through both receptive anal intercourse and syringe-sharing [8]. Social and behavioral factors may further contribute to differential vulnerability for MSM-PWID, including greater engagement in drug-related risk behaviors (e.g., syringe borrowing [9]), drug use in sexual situations [10], and higher HIV prevalence within their sexual and injecting networks. The extent to which sexual versus injection-related behavioral risks contribute to increased HIV acquisition among MSM-PWID is unclear.
Therefore, we sought to describe HIV incidence among MSM-PWID in a Canadian setting, and to identify whether reporting sex with men is associated with HIV incidence after considering potential drug-related and sociodemographic confounders.
METHODS
Data were drawn from the Vancouver Injection Drug Users Study (VIDUS), an open prospective cohort of HIV-negative PWID recruited through snowball sampling and street outreach in Vancouver, Canada, beginning in May 1996. Methods have been described previously [11]. Briefly, individuals were eligible if they had injected illicit drugs at least once in the previous month, were at least 18 years old, and resided in the Vancouver region. At baseline and semiannually, subjects gave blood samples for HIV serology and completed an interviewer-administered questionnaire. Participants received a $20–30CDN stipend. The study has been approved by the University of British Columbia/Providence Healthcare Research Ethics Board.
The questionnaire collected information on demographics, drug use and HIV risk behaviors, and social-structural exposures. Sexual orientation identity was queried at baseline. Sexual activity items were included at baseline and at each follow-up visit through 2014; however, the structure and wording of these items changed over time.
Participants were included in this analysis if they were male, HIV-negative at baseline, recruited between 1 May 1996 and 1 June 2014, and attended at least two follow-up visits. For these analyses, participants were categorized as MSM if (1) they indicated ever having had sex with a man at baseline and reported sex (oral or anal) with another man at least once over the study period, or (2) if they identified their sexual orientation as “gay or homosexual” at baseline and reported recent sexual activity (without specifying partner gender) over the study period. We treated MSM in this manner, rather than as a time-updated variable, because of changes in the relevant questionnaire items over time.
We first examined baseline characteristics stratified by MSM status, using Pearson’s chi-squared test (for binary variables), Fisher’s exact test (for binary variables with expected cell counts ≤5), and the Mann-Whitney test (for continuous variables).
To describe the association between MSM status and HIV seroconversion, we used Kaplan – Meier analyses to plot cumulative HIV incidence stratified by MSM status. The log-rank test was used to compare survival curves. We also calculated HIV incidence density stratified by MSM status, using the Poisson distribution to estimate 95% confidence intervals. The date of seroconversion was estimated using the midpoint between the last negative and the first positive antibody test result. The date of study enrolment was set as time zero, and participants who remained seronegative were right censored at the date of their most recent HIV serology result before 31 May 2015.
Next, to account for potential confounders, unadjusted and adjusted relative hazards of HIV infection were calculated using extended Cox regression models. Variables considered as potential confounders included age (per 10-year increase), white race/ethnicity (yes vs. no), residence in the Downtown Eastside (Vancouver’s epicenter of drug use and related HIV infections), homelessness, educational attainment (high school diploma vs. less than high school), recent incarceration (yes vs. no), HCV seropositivity (yes vs. no), daily crack smoking (yes vs. no), daily cocaine injecting (yes vs. no), daily methamphetamine injecting (yes vs. no), daily heroin injecting (yes vs. no), syringe borrowing (yes vs. no), sex work, defined as engaging in sexual activities in exchange for money or goods (yes vs. no), relationship status (partnered vs. other), and multiple sexual partners (≥2 vs. <2). Unless otherwise specified, behavioral variables referred to the previous six-month period and were treated as time-updated covariates.
In line with previous VIDUS studies [12] and considering the association between daily cocaine injection and HIV seroconversion among PWID in Vancouver [11], as well as higher levels of both stimulant use [10] and syringe borrowing [9] among MSM in various settings, the following modelling approach was employed. First, unadjusted relative hazards were estimated. Next, to determine whether differences in HIV incidence by MSM status may be attributable to higher levels of cocaine injection and syringe borrowing among MSM, the relative hazard of HIV seroconversion among MSM versus non-MSM PWID was adjusted for both variables in a stepwise fashion. Finally, to allow for the possibility of suppression effects, a model was built including all variables associated with time to HIV infection in unadjusted Cox analyses at p < 0.05. All analyses were conducted in R 3.2.4 (R Foundation for Statistical Computing, Vienna, Austria). All P-values were two-sided.
RESULTS
Over the study period, 1131 male PWID who were HIV-negative at baseline were enrolled and attended at least two follow-up visits. The median length of follow-up was 84 months (IQR = 31.6–110.1) and 8.6% (n=97) were MSM.
At baseline, MSM were younger (median age [Interquartile range; IQR]=31.1 [24.0 – 38.3] vs. 38.6 [31.7 – 44.5] years; P <0.001) than non-MSM and were less likely to live in the Downtown Eastside (52.6% vs. 63.9%; χ2 = 4.90, P = 0.027), to smoke crack daily (12.4% vs. 21.9%; χ2 = 4.80, P = 0.028), or to be Hepatitis C seropositive (71.1% vs. 80.6%, χ2 = 4.88, P = 0.027). MSM were more likely to report daily cocaine injection (41.2% vs. 29.1%; χ2 = 5.97, P = 0.015), syringe borrowing (53.6% vs. 25.0%; χ2 = 36.01, P <0.001), sex work (41.2% vs. 4.2%; χ2 = 178.69, P <0.001), and multiple sex partners (59.8% vs. 30.6%; χ2 = 34.24, P <0.001) at baseline.
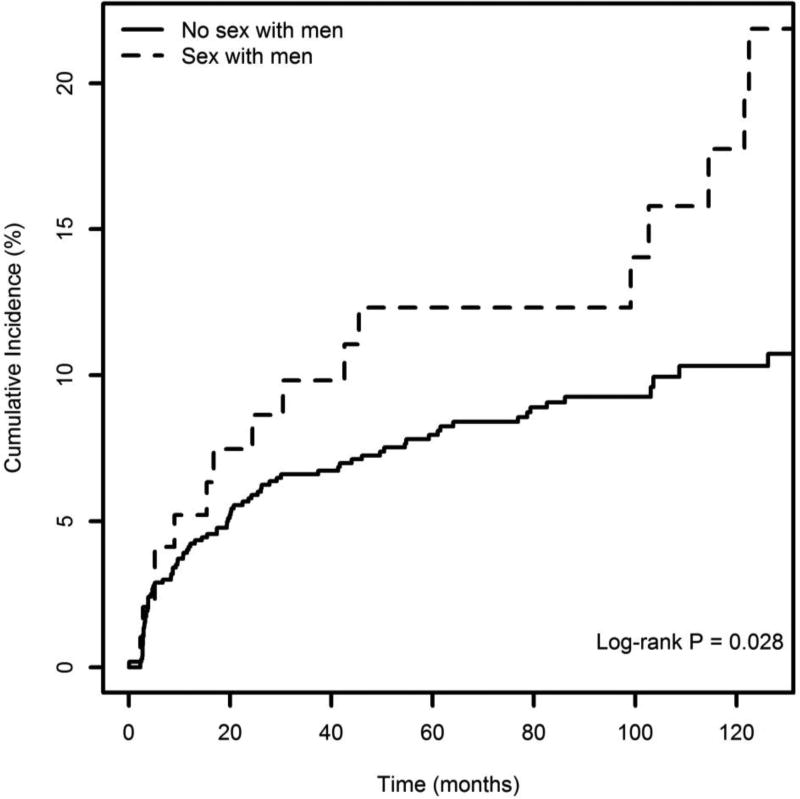
Among all male PWID, there were 102 HIV seroconversions over 8075 person-years of follow-up, for an HIV incidence density of 1.26 per 100 person-years (95% CI: 1.03 – 1.53). The incidence rate among MSM was 1.87 per 100-person-years (95% CI = 1.07 – 3.04), as compared to 1.19 (95% CI = 0.95 – 1.47) among non-MSM. Figure 1 shows cumulative incidence stratified by MSM status through 126 months after study recruitment. Cumulative HIV incidence calculated using the Kaplan-Meier estimator was 21.86% (95% confidence interval [CI]= 11.18 – 31.26) among MSM-PWID and 10.74% (95% CI = 8.30 – 13.11) among other male PWID (log-rank P = 0.028).
Figure 1.
Kaplan–Meier curves of time to HIV infection, stratified by MSM status (n=1131)
As shown in Table 1, MSM status was positively associated with incident HIV in bivariable analyses (Hazard Ratio [HR]= 1.81; 95% CI = 1.08 – 3.03), as were younger age, Downtown Eastside residence, recent incarceration, HCV seropositivity, daily cocaine injection, daily methamphetamine injection, and syringe borrowing (all P < 0.05). In the stepwise analysis of potential confounders, the relative hazard of HIV seroconversion for MSM-PWID was 1.53 (95% CI = 0.91 – 2.58; P = 0.107) after controlling for daily cocaine injection, and 1.33 (95% CI = 0.78 – 2.28; P = 0.301) after additional control for syringe borrowing. Table 1 also shows results of the full extended cox regression model, including all variables associated with HIV seroconversion at P < 0.05 in unadjusted analyses. The association between MSM status and HIV seroconversion was further attenuated in this model (HR = 1.21, 95% CI = 0.70 – 2.09; P = 0.493).
Table 1.
Bivariable and multivariable Cox regression analysis of factors associated with HIV incidence among men who inject drugs in Vancouver, Canada
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
|
|
|
|||
| Characteristic | Hazard Ratio (95% CI) |
p - value | Hazard Ratio (95% CI) |
p - value |
| Sex with men* (yes vs. no) | 1.81 (1.08 – 3.03) | 0.025 | 1.21 (0.70 – 2.09) | 0.493 |
| Age (per 10-year increase) | 0.97 (0.95 – 0.99) | 0.002 | 0.96 (0.94 – 0.99) | 0.002 |
| White race/ethnicity (yes vs. no) | 0.70 (0.47 – 1.03) | 0.071 | -- | -- |
| Downtown eastside residence† (yes vs. no) | 2.00 (1.30 – 3.07) | 0.002 | 2.33 (1.48 – 3.67) | <0.001 |
| Recent homelessness† (yes vs. no) | 0.79 (0.47 – 1.30) | 0.349 | -- | -- |
| Education (high school vs. less) | 1.07 (0.69 – 1.68) | 0.758 | -- | -- |
| Recent incarceration† (yes vs. no) | 1.73 (1.15 – 2.60) | 0.009 | 1.19 (0.77 – 1.85) | 0.432 |
| Hepatitis C positive (yes vs. no) | 4.40 (1.62 – 11.93) | 0.004 | 4.04 (1.45 – 11.26) | 0.008 |
| Daily crack smoking† (yes vs. no) | 0.78 (0.47 – 1.29) | 0.328 | -- | -- |
| Daily cocaine injection† (yes vs. no) | 3.88 (2.62– 5.76) | <0.001 | 2.96 (1.88 – 4.67) | <0.001 |
| Daily crystal methamphetamine injection† (yes vs. no) | 2.19 (1.24 – 3.85) | 0.007 | 0.86 (0.45 – 1.63) | 0.639 |
| Daily heroin injection† (yes vs. no) | 1.28 (0.86 – 1.92) | 0.228 | -- | -- |
| Syringe borrowing† (yes vs. no) | 2.90 (1.85 – 4.54) | <0.001 | 1.98 (1.20 – 3.28) | 0.008 |
| Sex work† (yes vs. no) | 1.26 (0.46 – 3.42) | 0.652 | -- | -- |
| Relationship status (partnered vs. other) | 0.83 (0.51 – 1.35) | 0.461 | -- | -- |
| Multiple sex partners† (2 or more versus 0–1) | 0.97 (0.61 – 1.54) | 0.895 | -- | -- |
Reporting sex with men at baseline or any follow-up visit.
Over the previous six months.
DISCUSSION
In this sample of men who inject drugs in Vancouver, reporting sex with men was associated with shorter time to HIV seroconversion. This is consistent with earlier research showing elevated seroprevalence and incidence among MSM-PWID in other locations [5–7].
MSM status, however, was not associated with seroconversion after adjustment for daily cocaine injection and syringe borrowing, suggesting that drug-related rather than sexual risks accounted for elevated HIV incidence among these MSM-PWID. Previous research documented increased syringe sharing among MSM-PWID in Vancouver [9]. We additionally found that MSM-PWID were more likely to report daily cocaine injection, the primary risk factor for HIV seroconversion among PWID in this setting [11]. It is important to note that drug-related and sexual risks may co-occur among MSM-PWID, particularly in the context of sexualized drug use and among HIV-positive MSM [10]. The extent to which sexualized drug use contributes to HIV risks among PWID populations in this setting is an important topic for future research. These findings indicate a need for interventions to reduce drug-related risks as well as concurrent sexual risks among MSM-PWID, including expanded access to sterile syringes, stimulant use treatment, and pre- and post-exposure prophylaxis.
Visual inspection of the cumulative incidence plots reveals different patterns of HIV incidence. Among MSM, incidence steadily increased early in the study period, plateaued, and then sharply increased again. By contrast, HIV incidence among non-MSM was high in the first two years after study recruitment and then relatively stable. Cocaine injection contributed to the high HIV incidence observed initially in this cohort [11] and affected both MSM and non-MSM. Although patterns should be cautiously interpreted in light of the small number of MSM, the later incidence spike is limited to MSM. This pattern is potentially related to the increase in methamphetamine use in the study setting in the 2000s [13], which disproportionately impacted MSM and is associated with both sexual- and injection-related HIV risks [14].
Some limitations of this study should be noted. First, the observational nature of the data limit causal inference. Participants were not randomly recruited, and thus results cannot be generalized to all local PWID. In particular, MSM-PWID accessible through outreach to PWID may be unrepresentative of all MSM-PWID. In addition, most data were self-reported; however, PWID self-reports can be valid and reliable and our outcome was laboratory-confirmed seroincidence.
More significantly, data on sexual orientation and behaviors were not consistently collected across baseline or follow-up surveys, and thus MSM was operationalized as an “ever” variable. The lack of data on sexual behavior and identity over time precludes examination of temporal patterns in HIV incidence and limits our understanding of the sexuality of MSM-PWID in this sample. Some participants may have had sex with men regularly, and others infrequently. Participants were recruited on the basis of PWID status, and may or may not identify as gay, bisexual, or MSM. It is likely that among MSM-PWID who are gay-identified and/or who regularly have sex with men, sexual risks play a larger role in HIV acquisition risk. Future longitudinal research, including consistent and detailed measurement of sexual orientation identity and behavior, will be required to more precisely characterize HIV-related risks among MSM-PWID.
In summary, our findings underscore that MSM-PWID are a population at particularly high risk of HIV infection. Further, these results indicate that tailored interventions addressing elevated drug-related risks among MSM-PWID are warranted, alongside ongoing behavioral and biomedical efforts to address sexual transmission of HIV among MSM. Additional research on sexual identity, behavior, and HIV risks among MSM-PWID over time would support the development of such interventions.
Acknowledgments
The authors thank the study participants for their contribution to the research, as well as current and past researchers and staff. The study was supported by the US National Institutes of Health (U01DA038886). This research was undertaken, in part, thanks to funding from the Canada Research Chairs program through a Tier 1 Canada Research Chair in Inner City Medicine which supports Dr. Evan Wood. Dr. Ayden Scheim is supported by a Canadian Institutes of Health Research Fellowship and the Pierre Elliott Trudeau Foundation. Dr. Kanna Hayashi is supported by a CIHR New Investigator Award (MSH-141971), a Michael Smith Foundation for Health Research (MSFHR) Scholar Award and the St. Paul’s Hospital Foundation. Dr. Rod Knight is supported by a MSFHR Scholar Award.
Funding: This study was funded by the National Institutes of Health (U01DA038886).
Footnotes
Conflict of Interest: All authors declare that they have no conflict of interest.
Compliance with Ethical Standards
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Sullivan T, Jones JS, Baral SD. The global north: HIV epidemiology in high-income countries. Curr Opin HIV AIDS. 2014;9:199–205. doi: 10.1097/COH.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 2.Public Health Agency of Canada. Summary: Estimates of HIV incidence, prevalence and proportion undiagnosed in Canada, 2014 [Internet] Ottawa: 2015. [Retrieved 30 August 2017]. from https://www.canada.ca/content/dam/canada/health-canada/migration/healthy-canadians/publications/diseases-conditions-maladies-affections/hiv-aids-estimates-2014-vih-sida-estimations/alt/hiv-aids-estimates-2014-vih-sida-estimations-eng.pdf. [Google Scholar]
- 3.Janjua NZ, Islam N, Kuo M, Yu A, Wong S, Butt ZA, Gilbert M, Buxton J, Chapinal N, Samji S, Chong M, Alverez M, Wong J, Tyndall MW, Krajden M. Identifying injection drug use and estimating population size of people who inject drugs using healthcare administrative datasets. Int J Drug Policy. 2018;55:31–39. doi: 10.1016/j.drugpo.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Rich AJ, Lachowsky NJ, Sereda P, Cui Z, Wong J, Wong S, Jollimore J, Raymond HF, Hottes TS, Roth EA, Hogg RS, Moore DM. Estimating the size of the MSM population in Metro Vancouver, Canada, using multiple methods and diverse data sources. J Urban Health. 2017 doi: 10.1007/s11524-017-0176-8. online ahead of print June 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maslow CB, Friedman SR, Perlis TE, Rockwell R, Jarlais Des DC. Changes in HIV seroprevalence and related behaviors among male injection drug users who do and do not have sex with men: New York City, 1990–1999. Am J Public Health. 2002;92:382–384. doi: 10.2105/ajph.92.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bluthenthal RN, Kral AH, Gee L, Lorvick J, Moore L, Seal K, et al. Trends in HIV seroprevalence and risk among gay and bisexual men who inject drugs in San Francisco, 1988 to 2000. J Acquir Immune Defic Syndr. 2001;28:264–269. doi: 10.1097/00042560-200111010-00010. [DOI] [PubMed] [Google Scholar]
- 7.Kral AH, Lorvick J, Gee L, Bacchetti P, Rawal B, Busch M, et al. Trends in Human Immunodeficiency Virus seroincidence among street-recruited injection drug users in San Francisco, 1987–1998. Am J Epidemiol. 2003;157:915–922. doi: 10.1093/aje/kwg070. [DOI] [PubMed] [Google Scholar]
- 8.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk. AIDS. 2014;28:1509–1519. doi: 10.1097/QAD.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall BDL, Wood E, Li K, Kerr T. Elevated syringe borrowing among men who have sex with men: a prospective study. J Acquir Immune Defic Syndr. 2007;46:248–252. doi: 10.1097/QAI.0b013e31814a5533. [DOI] [PubMed] [Google Scholar]
- 10.Melendez-Torres GJ, Bourne A. Illicit drug use and its association with sexual risk behaviour among MSM. Curr Opin Infect Dis. 2016;29:58–63. doi: 10.1097/QCO.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 11.Tyndall MW, Currie S, Spittal P, Li K, Wood E, O'Shaughnessy MV, et al. Intensive injection cocaine use as the primary risk factor in the Vancouver HIV-1 epidemic. AIDS. 2003;17:887–93. doi: 10.1097/00002030-200304110-00014. [DOI] [PubMed] [Google Scholar]
- 12.Kerr T, Shannon K, Ti L, Strathdee S, Hayashi K, Nguyen P, et al. Sex work and HIV incidence among people who inject drugs. AIDS. 2016;30:627–34. doi: 10.1097/QAD.0000000000000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairbairn N, Kerr T, Buxton JA, Li K, Montaner JS, Wood E. Increasing use and associated harms of crystal methamphetamine injection in a Canadian setting. Drug Alcohol Depend. 2007;88:313–316. doi: 10.1016/j.drugalcdep.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall BDL, Wood E, Shoveller JA, Patterson TL, Montaner JSG, Kerr T. Pathways to HIV risk and vulnerability among lesbian, gay, bisexual, and transgendered methamphetamine users: a multi-cohort gender-based analysis. BMC Public Health. 2011;11:20. doi: 10.1186/1471-2458-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]