Abstract
We herein report a case of Werner's syndrome (WS) with cardiac syndrome X (CSX) and heart failure with preserved ejection fraction (HFpEF), receiving nicorandil treatment.
A 58-year-old woman with chest discomfort on exercise was suspected of having effort-angina pectoris because of broad ST-depression in electrocardiogram of exercise test and reversible defect in the posterior-wall portion of left ventricle in exercise thallium201 myocardial scintigraphy. This patient also exhibited HFpEF, diagnosed by increased ratio of early-transmitral-flow-velocity to tissue-Doppler early-diastolic mitral annular velocity (E/e′) in echocardiography and plasma B-type natriuretic peptide (BNP) levels. However, coronary angiography revealed no organic stenosis in epicardial coronary arteries, and coronary physiological measurements by PressureWire™ (St. Jude Medical, St Paul, MN, USA) demonstrated that coronary flow reserve (CFR) was greatly decreased. Because impaired CFR represents coronary microvascular dysfunction in the absence of obstructive coronary narrowing, we diagnosed CSX, and initiated the administration of nicorandil to improve coronary microcirculation. After three-month-treatment of nicorandil, the patient's symptoms were diminished, and reversible defect in exercise myocardial scintigraphy was improved. Furthermore, both E/e′ and BNP were decreased, indicating the improvement of HFpEF via the restoration of microvascular dysfunction.
Thus, nicorandil administration could bring beneficial effects in WS with CSX and HFpEF, accompanied by coronary microcirculation dysfunction.
<Learning objective: Contrary to previous case reports regarding Werner's syndrome (WS) with obstructive coronary artery disease (CAD), we herein report a case of WS with cardiac syndrome X (CSX) without obstructive CAD, complicated with heart failure with preserved ejection fraction (HFpEF). Because impaired coronary microcirculation is known to be associated with left ventricular hypertrophy and HFpEF, nicorandil could improve not only CSX but HFpEF via the restoration of coronary microvascular dysfunction.>
Keywords: Werner's syndrome, Nicorandil, Cardiac syndrome X, Heart failure with preserved ejection fraction
Introduction
Werner's syndrome (WS) is an autosomal recessive hereditary disease whose major symptom is the onset of early aging. Since this syndrome was first reported in 1904 by Otto Werner describing cases of patients, who had dermatosclerotic change sooner than their actual ages, additional WS case reports have accumulated worldwide, the majority being from Japan [1]. The main causes of death in WS are generally malignant tumors and atherosclerotic diseases including cerebral and myocardial infarction, and the average age of death is 54 years [2].
Case report
A 58-year-old woman suffering from WS, who had a short stature of 146 cm, complained of chest discomfort and pain, and shortness of breath on exertion during the month prior to admission. She had gray hair since junior-high school, a cataract at age 18 years, and a surgical partial thyroidectomy for thyroid tumor when she was 23 years old. She lost her hair at the age of 46, and had femoral head replacement therapy due to necrosis of bone. Furthermore, she had refractory skin ulcer since she was 49 years old, and diagnosed to have WS from her typical symptoms of this skin disease. The diagnosis was confirmed by the test of genomic DNA, by the finding of homozygous G to C mutation at the junction of intron 25 and exon 26 of WRN gene, which is the most common in Japanese WS patients [3].
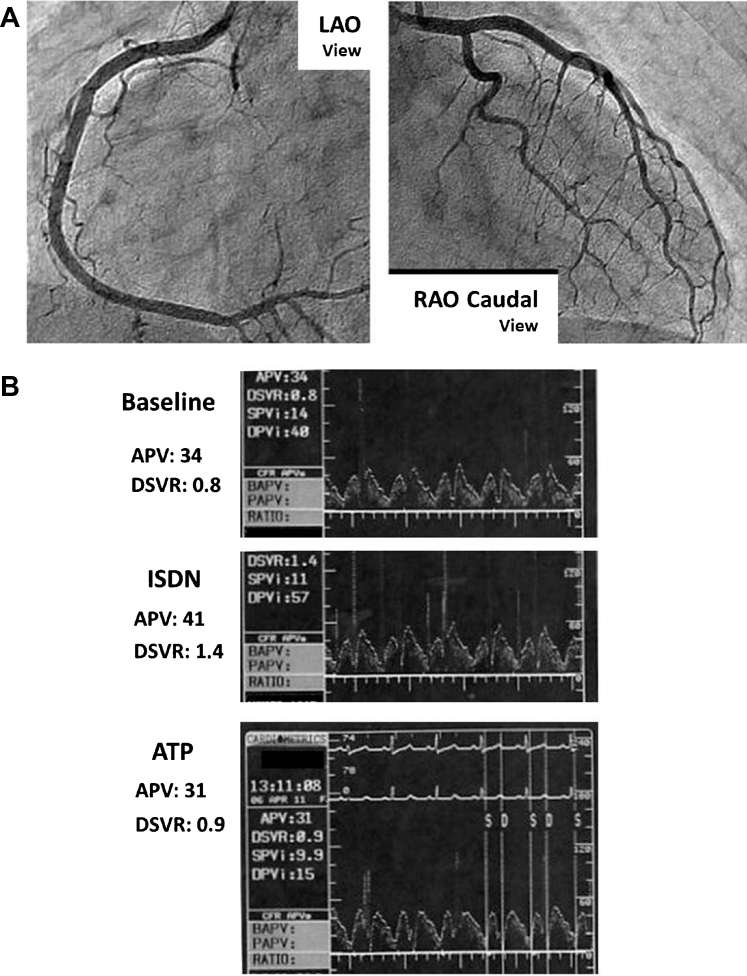
In order to evaluate the atherosclerotic change of major arteries systemically, we conducted cervical echography and magnetic resonance angiography of cerebral, carotid, and lower-limb arteries and aorta, but these studies showed no apparent macrovascular atherosclerotic changes. By contrast, both brachial-ankle pulse wave velocity (baPWV; right side: 2585 cm/s, left side: 2701 cm/s) measured by ankle brachial index measurement (BP-203RPE II; Omron Colin, Tokyo, Japan) and peripheral vascular endothelial function estimated by Endo-PAT2000 (Itamar Medical, Caesarea, Israel) as fingertip reactive hyperemia peripheral arterial tonometry index (RHI; 1.59) were impaired. Moreover, significant ST depressions were found in II, III, aVf, and V4–6 leads in electrocardiogram of the treadmill exercise test with chest pain symptoms, and exercise thallium201 myocardial scintigraphy showed a reversible defect in the posterior wall portion of left ventricle (LV), indicating exercise-induced myocardial ischemia (Fig. 1). Thereafter, we initiated the administration of aspirin 100 mg/day, a β-blocker, bisoprolol 5 mg/day, and a hydroxymethylglutaryl coenzyme A reductase inhibitor, pitavastatin 2 mg/day for her as the patient with effort angina pectoris (AP), but this WS patient's symptoms were not diminished. Ultrasound cardiography revealed that the ratio of early transmitral flow velocity to tissue Doppler early diastolic mitral annular velocity (E/e′) was 22.2, LV wall motion had normal contraction [LV ejection fraction (EF) estimated by modified Simpson method was 69.7%], and no asynergy, indicating cardiac diastolic dysfunction without reduced LVEF. Hence, we defined this WS patient associated with heart failure (HF) with preserved LVEF (HFpEF) according to the criteria of the European Working Group [4]: (1) symptoms of HF; (2) normal or mildly reduced LV systolic function (LVEF >50% and LV end-diastolic volume index <97 mL/m2); and (3) evidence of abnormal LV diastolic distensibility. To confirm a diagnosis of obstructive coronary artery disease (CAD) in this WS patient, we then performed heart catheterization including coronary angiography (CAG). However, CAG revealed that there was no organic stenosis in epicardial coronary arteries including the left circumflex coronary artery (LCX), which was considered to have significant stenosis from the results of above-mentioned thallium scintigraphy (Fig. 2A). When there is no organic stenosis in epicardial coronary artery, we generally perform coronary flow reserve (CFR) measurement only in left anterior descending coronary artery (LAD) vessel, which has the greatest perfusion territory among epicardial coronary arteries, under adenosine triphosphate (ATP) provocation to estimate coronary microvascular function, because regions of cardiac microvascular dysfunction are independent of blood supply from epicardial coronary arteries. Hence, we further inserted PressureWire™ Certus (St. Jude Medical, St Paul, MN, USA) to LAD, positioned in the distal more than two-thirds of the LAD, and performed coronary physiological measurements. The basal diastole-to-systole velocity ratio (DSVR) and averaged peak velocity (APV) were 0.8 and 34 cm/s, respectively before intracoronary injection of any cardiovascular agents, and elevated to 1.4 and 41 cm/s, respectively after the infusion of isosorbide dinitrate into left coronary artery. ATP for 2 min was then administered into central venous to induce steady state maximal hyperemia, CFR was calculated as the ratio of hyperemic to baseline APV. After 2 min infusion of 180 μg/kg/min ATP, DSVR and APV were changed inversely to 0.9 and 31 cm/s, revealing that CFR estimated in distal LAD was only 0.76. In the absence of obstructive coronary narrowing, impaired CFR represents coronary microvascular dysfunction [5]. These data demonstrated that coronary microcirculation was quite impaired without epicardial coronary organic stenosis in this WS patient (Fig. 2B). Furthermore, LAD flow pattern in this case was atypical, and such flow pattern is often seen in right coronary artery. Similar flow pattern of LAD with RCA in this case could be induced by cardiac diastolic dysfunction, because LAD flow pattern in HFpEF without right-sided cardiac disturbance has the possibility to be changed by LV structural or mechanical remodeling. In addition, CFR was completely abolished in LAD, hence reversible defect in the region other than LCX could appear if myocardial scintigraphy was performed under ATP provocation or greater exercise load.
Fig. 1.
Bull's eye images of exercise thallium201 myocardial scintigraphy, indicating exercise-induced myocardial ischemia in left ventricle posterior wall (red arrow). Upper panel, exercise-phase; lower panel, rest-phase.
Fig. 2.
(A) CAG revealed that there was no organic stenosis in epicardial coronary arteries. (B) DSVR and APV in LAD. Baseline (upper panel), post intracoronary injection of ISDN (mid panel), and post intravenous infusion of ATP (lower panel). CAG, coronary angiography; DSVR, diastole-to-systole velocity ratio; APV, averaged peak velocity; LAD, left anterior descending coronary artery; ISDN, isosorbide dinitrate; ATP, adenosine triphosphate.
Because we could not perform acetylcholine provocation test to exclude coronary spastic angina (CSA) due to chronic kidney disease, we performed a hyperventilation provocation (HV) test, and found that this patient exhibited no symptom and no ST-T change in electrocardiogram by HV test. Taken together with typical chest pain on exertion for effort AP, this WS patient is thought to have cardiac syndrome X (CSX), defined as typical chest pain, transient myocardial ischemia, and angiographically normal coronary arteries with the result of heart catheterization. Therefore, we administered the addition of 15 mg/day nicorandil to improve coronary microcirculation. After three months of treatment with nicorandil, exercise-induced symptoms were diminished, and a reversible defect in exercise myocardial scintigraphy was improved as shown in Fig. 3. Nicorandil treatment for this WS patient improved coronary microvascular dysfunction and effort-induced myocardial ischemia without epicardial coronary stenosis.
Fig. 3.
The changes in myocardial scintigraphy before (left panel) and after (right panel) the treatment with nicorandil in this Werner's syndrome patient.
Discussion
Nicorandil, which acts both as a nitrate and an ATP-sensitive potassium channel opener, dilates coronary resistance vessels [6], and improves microcirculation. Also, nicorandil has been reported to improve the prognosis not only of CAD, but also of chronic HF patients [7]. In this case, nicorandil treatment decreased both E/e′ and B-type natriuretic peptide (BNP) values (before and three months after treatment with nicorandil in E/e′, 22.2–14.8, and before and three months after treatment with nicorandil in BNP, 353.7–49.4 pg/mL) in this WS patient. Although there is no direct evidence of changes in microvascular function by nicorandil treatment in this patient, these data suggested the possibility of cardiac diastolic dysfunction improvement by the restoration of microvascular-induced myocardial ischemia. However, further clinical studies are required to elucidate whether nicorandil treatment can improve LV diastolic dysfunction through its beneficial effects on microcirculation. After three months of treatment with nicorandil, both baPWV and RHI values were slightly improved, indicating the improvement in vascular function by nicorandil in this WS patient. Camici et al. recently reported that the coronary circulation and blood flow were decreased in an experimental model of LV hypertrophy, with complicated cardiac diastolic function [8]. Hence, the improvement in coronary microcirculation could be a useful therapeutic strategy for HF with preserved LVEF, which has great clinical importance in current clinical practice.
Although it is considered to resemble the normal aging, there are some differences between normal aging and the symptoms of WS. In normal aging, atherosclerosis is mainly in the major arteries including aortas. On the contrary, atherosclerosis is observed in small and peripheral arterioles in patients with WS. In this case, relatively large arteries including cerebral, cervical, and coronary arteries had no severe atherosclerotic plaques. Hence, the impairments of not only coronary microcirculation but also cardiac diastolic dysfunction in this case were possible to be caused by arteriosclerosis, which is often common in WS patients. Without acetylcholine provocation test during heart catheterization, however, we could not distinguish whether microcirculation dysfunction in this case was induced by coronary microvascular organic change or by microvascular spasm. CSX is an important clinical entity, and coronary microvascular abnormalities have already been suggested to be the underlying pathophysiological mechanism for CSX [9]. This case suggested nicorandil could improve CSX via restoration of coronary microvascular dysfunction in WS, because nicorandil was reported to improve not only microvascular spasm but also atherosclerosis in end-stage renal disease on hemodialysis, which is commonly complicated with coronary microvascular dysfunction. Furthermore, it was possible that other medicines such as calcium channel blockades or nitrates were also possibly effective, if coronary microvascular dysfunction was mainly induced by microvascular spasm.
Although there were some case reports of obstructive CAD treated with any coronary interventions in WS patients [10], this is the first report of WS patients with CSX and cardiac diastolic dysfunction, accompanied by impaired coronary microcirculation. Also, we suggested that nicorandil administration could bring beneficial effects in the case of WS with coronary microcirculation dysfunction.
Conflict of interest
None.
Acknowledgments
None.
References
- 1.Goto M., Matsuura M. Secular trends towards delayed onsets of pathologies and prolonged longevities in Japanese patients with Werner syndrome. BioSci Trends. 2008;2:81–87. [PubMed] [Google Scholar]
- 2.Goto M. Hierarchical deterioration of body systems in Werner's syndrome: implications for normal ageing. Mech Ageing Dev. 1997;98:239–254. doi: 10.1016/s0047-6374(97)00111-5. [DOI] [PubMed] [Google Scholar]
- 3.Huang S., Lee L., Hanson N.B., Lenaerts C., Hoehn H., Poot M., Rubin C.D., Chen D.F., Yang C.C., Juch H., Dorn T., Spiegel R., Oral E.A., Abid M., Battisti C. The spectrum of WRN mutations in Werner syndrome patients. Hum Mutat. 2006;27:558–567. doi: 10.1002/humu.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paulus W.J., Tschöpe C., Sanderson J.E., Rusconi C., Flachskampf F.A., Rademakers F.E., Marino P., Smiseth O.A., De Keulenaer G., Leite-Moreira A.F., Borbély A., Edes I., Handoko M.L., Heymans S., Pezzali N. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 5.Nakanishi K., Fukuda S., Shimada K., Miyazaki C., Otsuka K., Maeda K., Miyahana R., Kawarabayashi T., Watanabe H., Yoshikawa J., Yoshiyama M. Impaired coronary flow reserve as a marker of microvascular dysfunction to predict long-term cardiovascular outcomes, acute coronary syndrome and the development of heart failure. Circ J. 2012;76:1958–1964. doi: 10.1253/circj.cj-12-0245. [DOI] [PubMed] [Google Scholar]
- 6.Taira N. Nicorandil as a hybrid between nitrates and potassium channel activators. Am J Cardiol. 1989;63:18J–24J. doi: 10.1016/0002-9149(89)90200-2. [DOI] [PubMed] [Google Scholar]
- 7.Kasama S., Toyama T., Iwasaki T., Sumino H., Kumakura H., Minami K., Ichikawa S., Matsumoto N., Sato Y., Kurabayashi M. Effects of oral nicorandil therapy on sympathetic nerve activity and cardiac events in patients with chronic heart failure: subanalysis of our previous report using propensity score matching. Eur J Nucl Med Mol Imaging. 2014;41:144–154. doi: 10.1007/s00259-013-2538-0. [DOI] [PubMed] [Google Scholar]
- 8.Camici P.G., Olivotto I., Rimoldi O.E. The coronary circulation and blood flow in left ventricular hypertrophy. J Mol Cell Cardiol. 2012;52:857–864. doi: 10.1016/j.yjmcc.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 9.Luo C., Long M., Hu X., Huang Z., Hu C., Gao X., Du Z. Thermodilution-derived coronary microvascular resistance and flow reserve in patients with cardiac syndrome X. Circ Cardiovasc Interv. 2014;7:43–48. doi: 10.1161/CIRCINTERVENTIONS.113.000953. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka S., Miyairi T., Shimada S., Miura S., Kigawa I., Fukuda S. Off-pump coronary artery bypass grafting in a patient with Werner's syndrome. Gen Thorac Cardiovasc Surg. 2008;56:592–594. doi: 10.1007/s11748-008-0308-x. [DOI] [PubMed] [Google Scholar]