Abstract
A 49-year-old man was admitted with symptomatic, sustained monomorphic ventricular tachycardia. He had a previous history of AMP-kinase disease associated with hypertrophic cardiomyopathy and complete heart block, and a pre-existing dual chamber pacemaker. He also had a mechanical tricuspid valve replacement and mitral valve replacement, for severe tricuspid regurgitation from right ventricle (RV) lead-induced injury to the tricuspid valve and a fibroblastoma on the mitral valve. His pre-existing RV lead was maintained between the prosthetic valve annulus and the native annulus. Inability to place an implantable cardioverter-defibrillator (ICD) in the RV due to the presence of a mechanical tricuspid valve replacement represented a rare but challenging clinical scenario. Surgical epicardial lead placement or the use of a subcutaneous ICD (S-ICD) were possible alternatives. Traditional ICD lead placement was favored because of the broad QRS from RV pacing meaning that use of the S-ICD was not possible due to failure of the electrocardiogram to lie within the bounds of the screening template, and the perceived high risk of repeat thoracotomy. We describe the technique for ICD lead placement in a mid-lateral cardiac venous branch of the coronary sinus with the ability to deliver anti-tachycardia pacing and cardiac resynchronization. To our knowledge this is the first report of an ICD in the mid-lateral cardiac vein, with cardiac resynchronization.
<Learning objective: This case describes the technique for implantable cardioverter-defibrillator placement in the coronary sinus with biventricular pacing in a patient with a mechanical tricuspid and pre-existing right ventricular endocardial lead. This technique represents a viable alternative to repeat thoracotomy and surgical lead placement, where the risks of complication, prolonged hospital stay and lead failure are high. It also offers the ability to deliver anti-tachycardia pacing and cardiac resynchronization.>
Keywords: Implantable cardioverter-defibrillator, Mechanical tricuspid valve replacement, Hypertrophic cardiomyopathy, AMP-kinase disease
Introduction
Inability to place an implantable cardioverter-defibrillator (ICD) in the right ventricle (RV) due to the presence of a mechanical tricuspid valve replacement represents a rare but challenging clinical scenario. Surgical epicardial lead placement or the use of a subcutaneous ICD (S-ICD) represent possible alternatives. Previous case reports have documented placement of an ICD lead in the middle branch of the coronary sinus [1], the caval vein [2], and the azygous vein [3]. We report a case of ICD lead placement in a mid-lateral cardiac venous branch of the coronary sinus (CS) with the ability to deliver anti-tachycardia pacing and cardiac resynchronization.
Case report
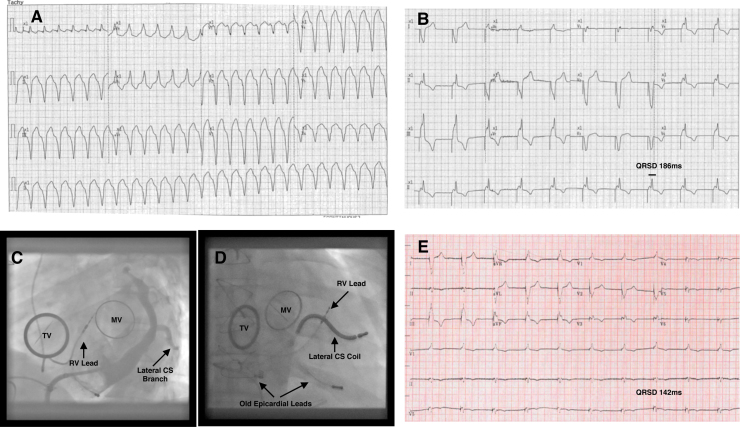
A 49-year-old male was admitted with two episodes of symptomatic sustained monomorphic ventricular tachycardia (VT) (Fig. 1A), requiring direct current (DC) cardioversion. He had a past history of AMP-kinase disease associated with hypertrophic cardiomyopathy and complete heart block. Initially an epicardial pacemaker was implanted and this was subsequently upgraded to a dual chamber pacemaker. Due to the development of permanent atrial fibrillation with transient ischemic attacks, anticoagulation therapy was initiated. In 2010, he underwent mechanical tricuspid valve replacement (TVR) and mitral valve replacement (MVR), for severe tricuspid regurgitation from RV lead-induced injury to the tricuspid valve and a fibroblastoma on the mitral valve. A 35 mm St Jude Medical® (SJM; St. Jude Medical Inc.; Minneapolis, MN) mechanical prosthesis was used in the tricuspid position and the pre-existing RV lead was maintained between the prosthetic valve annulus and the native annulus. At the time of surgery, two previously placed epicardial leads and the endovascular right atrial (RA) lead were removed, as he was now in permanent atrial fibrillation (AF). Echocardiography on admission revealed an ejection fraction of 40%. Due to permanent RV pacing his QRS duration was 186 ms (Fig. 1B).
Fig. 1.
(A) 12-lead electrocardiogram (ECG) of the patient's monomorphic ventricular tachycardia (VT) on presentation. (B) Subsequent paced ECG showing paced QRS-duration (QRSD) of 186 ms. (C) Selective angiography of the mid-lateral branch of the coronary sinus (CS) in the left anterior oblique (LAO) projection, with mechanical tricuspid valve (TV) and mitral valve (MV) visible, along with pre-existing right ventricular (RV) lead. (D) LAO projection of the final position of the implantable cardioverter-defibrillator coil in the mid-lateral branch of CS, with TV and MV visible, along with right ventricular (RV) lead and old epicardial leads. (E) Bi-ventricular pacing at follow-up with QRSD 142 ms.
A decision was made to implant an ICD lead in the coronary sinus (CS) instead of a surgically placed epicardial lead to avoid the risk of repeat surgery and the concern that epicardial leads are unreliable and have limited longevity [4]. This option had the advantage of being able to deliver anti-tachycardia pacing for VT episodes as well as offering biventricular pacing, unlike the S-ICD. Additionally, due to the permanent pacing with a broad QRS interval, he failed the screening for S-ICD.
Left-arm venography confirmed a patent left subclavian system. Before re-opening the existing pacemaker pocket, the CS was imaged by cannulating the right femoral vein using a fixed curve St Jude SL3 sheath and a steerable electrophysiology (EP) catheter. CS venography demonstrated a large caliber mid-lateral cardiac venous branch (Fig. 1C and Video 1). Following extraction of the existing pacemaker and a single left axillary puncture, the coronary sinus was re-cannulated with a steerable EP catheter and a Boston Scientific Accuity Pro sheath (Natick, MA, USA) (Video 2). Subsequently a Medtronic (Medtronic, Inc., Minneapolis, MN, USA) sub-selection catheter was used to help intubate the mid lateral coronary vein (Video 3). This sheath was then exchanged for an 11F 75 cm Mullins sheath (Medtronic, Inc., Minneapolis, MN, USA) over a 0.035 in guidewire. The hemostatic valve at the proximal end of the Mullins sheath was cut off before introduction to allow it to be split afterwards. Approximately 7 cm of the distal end of the sheath was removed so that it was short enough to permit delivery of 9F Medtronic Sprint-Quattro Secure S single coil 6935 ICD lead, as no commercially available sheath was available for this purpose. The lead was advanced to the distal portion of the mid-lateral cardiac vein (Video 4) and the sheath split and removed (Video 5). Lead parameters showed an impedance of 1292 Ω, with a sensed R wave amplitude of 4.5 mV and a threshold of 2.25 V at 0.4 ms. Fig. 1D shows the final left anterior oblique view of the lead. Defibrillation threshold testing (DFT) was not initially performed, as the patient had sub-therapeutic anticoagulation leading up to the procedure. The device was subsequently programmed to ventricular rate modulated pacing (VVIR) at 60/min, with a left ventricular (LV) offset of −10 ms to promote biventricular pacing and QRS narrowing. At follow-up, 12-Lead electrocardiogram (ECG) showed narrowing of the QRS duration to 142 ms (Fig. 1E). A post-implant device check revealed that the patient had had successful anti-tachycardia pacing for further sustained VT and a DFT performed when international normalized ratio was therapeutic and successful with a single 25 J shock.
Supplementary videos related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jccase.2015.08.004.
Discussion
The presence of a tilting disk valve prosthesis is an absolute contraindication to endocardial lead placement in view of the risk of acute valve failure, damage to the lead, and death. Repeat thoracotomy is associated with significant risks, long hospital stays, and a high lead failure rate [4]. The advantage of the approach used in the present case was that it is the only solution that offers anti-tachycardia pacing and bi-ventricular pacing, in addition to defibrillation. Although S-ICD remains an alternative option to endovascular lead placement, the broad QRS complexes in the present case meant that the patient failed ECG screening for S-ICD.
Several lead configurations have been described previously including the use of a floating double-coil in the inferior vena cava (IVC) [2], azygous vein ICD lead implant [3], the use of a CS defibrillation coil coupled with a left sided array [5], and placement of the ICD lead in the low right atrium [6]. Azygous vein implantation can be challenging technically and adequate defibrillation vectors are hard to achieve without an RV coil. Similar issues can occur with ICD lead implantation at the low right atrium and IVC. One option to negate the issue of inadequate field vector coverage is to tunnel the lead into the abdomen as previously described by Cohen et al. [7] and place the ‘active can’ in the abdomen, but this requires a more complex surgical procedure under general anesthesia and again does not offer cardiac resynchronization needed in our patient. The placement of a left-sided array would have still required a lead in the CS, resulting in longer procedure times and the risk of infection in a patient with two metallic prosthetic valves. Additionally problems have been reported with subcutaneous arrays such as lead dislocation and lead insulation defects, which means that their use requires close surveillance with regular chest radiographs and annual DFTs [8]. Thus, in order to offer the combination of defibrillation, anti-tachycardia pacing, and cardiac resynchronization, we elected to place a standard ICD lead in a lateral branch of the CS.
A technique for implantation of the ICD lead into the middle cardiac vein has been previously described in a series of six patients [1]. At a 5 year follow-up, there were no acute or late complications and no lead displacements [1]. We utilized this approach due to the evidence suggesting its effectiveness and feasibility [1]. Additionally positioning the ICD coil in the inferior epicardium of the LV wall increases the amount of myocardium covered by the current field and allows for greater defibrillation mass across the ventricle. In animal studies, CS ICD electrodes have been shown to have a lower defibrillation threshold than conventional ICD placement [9]. Finally this approach enabled us to deliver biventricular pacing to our patient and anti-tachycardia pacing (ATP), without the need for two separate leads in the CS as previously described [1]. This has potential advantages with regard to complications relating to thrombosis and fibrosis within the CS and mortality associated with possible future lead removal.
This case describes a novel technique for delivery of an ICD lead into the mid-lateral branch of the CS which permits the use of ATP and defibrillation, as well as cardiac resynchronization through biventricular pacing using an LV lead site on the mid-lateral LV wall. In such cases, standard tools for CS cannulation are not suitable as the defibrillating lead is bigger than standard CS leads. We therefore used a Mullins sheath that was available in our institution. It should be noted that other options exist, such as the use of the Worley™ system (9Fr) (Merit Medical Systems, Inc., South Jordan, UT, USA) or a smaller defibrillating lead (Durata™ (SJM; St. Jude Medical Inc.; Minneapolis, MN), Riata™ (SJM; St. Jude Medical Inc.; Minneapolis, MN), Sprint-Fidelis™(Medtronic, Inc., Minneapolis, MN, USA), Reliance 4-Front™ (Boston Scientific, Inc., Natick, MA, USA)), depending on their availability in different centers. In our center, we no longer carry 7F ICD leads routinely and therefore elected to insert a standard 9F lead instead. Although more difficult to position in smaller coronary veins, 9F leads are theoretically more stable once positioned due to their size and weight. Additionally, given the well-documented concerns regarding premature failure and longevity of 7F leads such as the St Jude Riata and Medtronic Sprint-Fidelis leads [10], [11], we elected to use the 9F Medtronic Sprint-Quattro lead, which has excellent longevity data [11]. An important decision-making choice is whether or not to deploy the helix within the CS. We did not deploy the helix as sensing was already provided by the RV lead, lead parameters with an un-deployed helix were acceptable and importantly to minimize the risk of CS trauma and pericardial tamponade. Although lead function is known to be improved with helical deployment, we chose not to take this additional risk for the above reasons. The long-term safety of a defibrillating coil in a coronary vein is unknown as well as the risk for later lead extraction; however, initial case series results are promising [1]. There is a need for new leads to be designed for this application and to minimize ingrowth on the coil when placed in a venous branch of the CS.
We believe this is the first case to report placement of a CS ICD lead in a lateral branch of the cardiac vein with the addition of biventricular pacing. In cases where biventricular pacing is preferred and an existing RV lead is in situ, placement of an ICD lead into the lateral branches of the CS should be considered.
Conflict of interest
Dr Segal receives honoraria and consulting fees from Boston Scientific Inc., and Medtronic Inc. and research funds from Medtronic Inc. Dr Srinivasan is funded by the British Heart Foundation as a research Fellow (Grant no: FS/14/9/30407).
References
- 1.Lopez J.A. Implantable cardioverter defibrillator lead placement in the middle cardiac vein after tricuspid valve surgery. Europace. 2012;14:853–858. doi: 10.1093/europace/eus013. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber C., Mehmanesch H., Kolb C., Schmitt C., Lange R. Modified implantation of a transvenous defibrillator in a patient after tricuspid valve replacement. Pacing Clin Electrophysiol. 2000;23:1698–1699. doi: 10.1046/j.1460-9592.2000.01698.x. [DOI] [PubMed] [Google Scholar]
- 3.Kommuri N.V., Kollepara S.L.S., Saulitis E., Siddiqui M. Azygos vein lead implantation for high defibrillation thresholds in implantable cardioverter defibrillator placement. Indian Pacing Electrophysiol J. 2010;10:49–54. [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen M.I., Bush D.M., Vetter V.L., Tanel R.E., Wieand T.S., Gaynor J.W., Rhodes L.A. Permanent epicardial pacing in pediatric patients: seventeen years of experience and 1200 outpatient visits. Circulation. 2001;103:2585–2590. doi: 10.1161/01.cir.103.21.2585. [DOI] [PubMed] [Google Scholar]
- 5.Leng C.T., Crosson J.E., Calkins H., Berger R.D. Lead configuration for defibrillator implantation in a patient with congenital heart disease and a mechanical prosthetic tricuspid valve. Pacing Clin Electrophysiol. 2001;24:1291–1292. doi: 10.1046/j.1460-9592.2001.01291.x. [DOI] [PubMed] [Google Scholar]
- 6.Biffi M., Bertini M., Ziacchi M., Boriani G. Transvenous cardioverter-defibrillator implantation in a patient with tricuspid mechanical prosthesis. J Cardiovasc Electrophysiol. 2007;18:329–331. doi: 10.1111/j.1540-8167.2006.00686.x. [DOI] [PubMed] [Google Scholar]
- 7.Cohen T., Kokotos W., Kersten R. Implantable defibrillator in a patient with a tricuspid valve bioprosthesis. J Invasive Cardiol. 2008;20:E341–E342. [PubMed] [Google Scholar]
- 8.Kettering K., Mewis C., Dörnberger V., Vonthein R., Bosch R.F., Seipel L., Kühlkamp V. Long-term experience with subcutaneous ICD leads: a comparison among three different types of subcutaneous leads. Pacing Clin Electrophysiol. 2004;27:1355–1361. doi: 10.1111/j.1540-8159.2004.00639.x. [DOI] [PubMed] [Google Scholar]
- 9.Arruda M., Otomo K., Reynolds D., Bonner M., Pitha J. Ventricular defibrillation from the middle cardiac vein: a new transvenous epicardial lead configuration to lower the defibrillation threshold. Pacing Clin Electrophysiol. 1998;21(Part II):853. [Google Scholar]
- 10.Borleffs C.J.W., van Erven L., van Bommel R.J., van der Velde E.T., van der Wall E.E., Bax J.J., Rosendaal F.R., Schalij M.J. Risk of failure of transvenous implantable cardioverter-defibrillator leads. Circ Arrhythm Electrophysiol. 2009;2:411–416. doi: 10.1161/CIRCEP.108.834093. [DOI] [PubMed] [Google Scholar]
- 11.Hauser R.G., Maisel W.H., Friedman P.A., Kallinen L.M., Mugglin A.S., Kumar K., Hodge D.O., Morrison T.B., Hayes D.L. Longevity of Sprint Fidelis implantable cardioverter-defibrillator leads and risk factors for failure: implications for patient management. Circulation. 2011;123:358–363. doi: 10.1161/CIRCULATIONAHA.110.975219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.