Abstract
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by atypical functional integration of brain regions. The vast majority of neuroimaging studies of ASD have focused on older children, adolescents, and adults with the disorder. Very little work has explored whole-brain functional connectivity of young children with ASD. Here we collected resting-state functional magnetic resonance imaging data from 58 young children (mean age 4.98 years; 29 with ASD; 29 matched healthy controls (HC)). All children were under sedation during scanning. A functional ‘connectedness’ method was first used to seek for brain regions showing atypical functional connectivity (FC) in children with ASD. Then a recurrent-seek strategy was applied to reveal atypical FC circuits in ASD children. FC matrices between regions-of-interest (ROIs) were compared between ASD and HC. Finally, a support vector regression (SVR) method was employed to assess the relationship between the FC circuits and ASD symptom severity. Two atypical FC circuits comprising 23 ROIs in ASD were revealed: one predominantly comprised brain regions involved with social cognition showing under-connectivity in ASD; the other predominantly comprised sensory-motor and visual brain regions showing over-connectivity in ASD. The SVR analysis showed that the two FC circuits were separately related to social deficits and restricted behavior scores. These findings indicate disrupted FC of neural circuits involved in the social and sensorimotor processes in young children with ASD. The finding of the atypical FC patterns in young children with ASD underscores the utility of studying younger children with the disorder, and highlights nuanced patterns of brain connectivity underlying behavior closer to disorder onset.
Keywords: young children, autism spectrum disorder, resting state fMRI, support vector regression analysis
Lay summary
Autism spectrum disorder (ASD) is an early-onset neurodevelopmental disorder. Understanding brain functional alterations at early ages is important for understanding biological mechanisms of ASD. Here we found two atypical brain functional circuits in young children with ASD that were related to social and sensorimotor function. These results show how atypical patterns of brain functional connectivity in young children with of ASD may underlie core symptoms of the disorder.
Introduction
Autism spectrum disorder (ASD) is a neurodevelopment disorder characterized by severe social communication deficits and restricted and repetitive behaviors and interests (American Psychiatric Association 2013). The estimated prevalence of ASD is 1-2% of the population (Kim YS et al. 2011), and the earliest age that the ASD could be diagnosed is about 2-4 years old (Rutter M 1978). Despite the burgeoning neuroimaging literature, the neural pathology of ASD remains unclear, hindering efforts at early diagnosis and treatment of the disorder in young children.
ASD is generally recognized to be related to atypical brain functional integration (Kana RK et al. 2014). Abundant studies based on resting-state functional magnetic resonance imaging (fMRI) data have reported atypical functional connectivity (FC) between brain regions in individuals with ASD. Yerys et al. reported reduced FC between the medial prefrontal cortex (mPFC) and precuneus (Prc) / posterior cingulate cortex (PCC), temporal pole in 8-13 years old children with ASD (Yerys BE et al. 2015). Von dem Hagen reported reduced FC between the mPFC and temporo-parietal junction (TPJ), amygdala and insula in adults with ASD (von dem Hagen EA et al. 2013). Our previous studies also demonstrated altered FC in individuals with ASD whose age ranged from 7 to 35 years old, especially within the default mode network and salience network (Uddin LQ et al. 2013a; Chen H et al. 2015; Chen H et al. 2017b; Duan X et al. 2017). However, most ASD FC studies have concentrated on older children or adults over 7 years of age. As ASD is an early-onset disorder, these studies of older individuals might reflect causal or compensatory differences in brain FC, but not disorder-related differences (Uddin LQ et al. 2017).
Recent studies reported weaker FC in toddlers with ASD (1-3.5 years old) compared with healthy controls (HC) (Dinstein I et al. 2011). FC studies of children aged 7-13 years old have reported increased FC, which is in contrast to results from adolescents and adults with the disorder (Nomi JS et al. 2015). Uddin et al. proposed that children with ASD showed over-connectivity while adults with ASD showed under-connectivity (Uddin LQ et al. 2013b). Structural neuroimaging studies have shown different atypical structural connectivity patterns between children and adults with ASD. Li et al. reported increased white matter connectivity in preschool children with ASD (Li SJ et al. 2018), while a previous study of adults with ASD showed decreased structural connectivity (Lewis JD et al. 2013). These previous mixed under- and over-connectivity results suggest that age plays an important role in FC deficits characteristic of ASD (Keown CL et al. 2013; Supekar K et al. 2013; Nomi JS et al. 2015).
FC patterns of pre-school age children with ASD have not yet been explored. The mismatch between the age of subjects in previous studies and the age when behavioral changes appear of ASD is an obstacle to clinical translation of results from basic research (Uddin LQ et al. 2017). It is important to study the FC patterns of young children with ASD in order to understand the neurobiological changes emerging during the same period when behavioral changes permitting diagnosis first appear.
Traditional FC studies have applied three kinds of analysis methods: seed-based FC (Chen H et al. 2016), ROI-based FC (Chen H et al. 2017a) and voxel-based FC maps such as FC density (Tomasi D et al. 2010). Seed-based FC can reveal atypical FC at the voxel level, but is region-specific; ROI-based FC is a whole brain large-scale analysis method, however it is at the ROI-level which depends on the definition of ROIs and does not take advantage of the high spatial resolution of fMRI; voxel-based FC maps such as FC density are whole brain voxel-level methods, however these sum the atypical and typical connections, decreasing the sensitivity to detect atypical connections (Gotts SJ et al. 2012). Here we propose a recurrent-seek strategy combining the FC ‘connectedness’ map and seed-based FC map methods. Using this strategy, we reveal whole-brain atypical FC circuits in young children with ASD at the voxel level with relatively high sensitivity.
Based on previous FC studies of children ages 7-13 (Supekar K et al. 2013; Uddin LQ et al. 2013a), we hypothesized that young children with ASD would show aberrant FC patterns compared with HC, which would link to symptom severity in ASD.
Methods
Participants
Thirty-eight male children with ASD were recruited from the Children Development and Behavior Research Center of Harbin Medical University (Heilongjiang, China) and forty-one male HCs were recruited from the local kindergartens. None of the HCs reported history of severe medical problems or neurological/psychiatric conditions. All participants were native Chinese speakers.
Four children with ASD were excluded due to low image quality based on manual checks. One HC subject and one ASD subject were excluded due to maximal head motion exceeding 2mm displacement or 2 degrees of rotation. Seven subjects (3 HCs and 4 ASDs) were excluded as fewer than 150 time points (less than 5 minutes of fMRI data) remained after scrubbing. This resulted in a sample included 29 children with ASD and 37 HCs. As the age, handedness, and mean framewise displacement (FD) were not well matched between groups, a data-driven method was applied to create a well-matched dataset as in previous studies (Nomi JS et al. 2015; Chen H, et al. 2017a), resulting in the exclusion of 8 HCs. The final sample included 29 children with ASD and 29 age-, handedness-, and meanFD-matched HCs. Detailed demographic information for included subjects is listed in Table.1. The Peabody Picture Vocabulary Test (PPVT) score was not matched between groups.
Table.1.
demographic information
| ASD | HC | p value | |
|---|---|---|---|
| Count | 29 | 29 | - |
| Age | 4.98±1.29 | 4.99±1.01 | 0.989a |
| Age (range) | 3.47-7.9 | 3.18-6.88 | - |
| Handness (R/L) | 23/6 | 26/3 | 0.277b |
| meanFD | 0.13±0.05 | 0.14±0.04 | 0.533a |
| meanFD (range) | 0.06-0.23 | 0.06-0.21 | - |
| Scrubbed timepoints | 3.28±9.00 | 3.52±7.93 | 0.914a |
| PPVT | 63.6±23.3 | 99.7±26.9 | <0.001a |
| PPVT (range) | 33-156 | 58-154 | |
| ADI-R | |||
| SOCIAL | 22.39±3.39 | - | - |
| COMM | 15.09±4.21 | - | - |
| RRB | 7.48±2.31 | - | - |
two-sample t-test
chi-square test
FD: Framewise Displacement; PPVT: Peabody Picture Vocabulary Test; RRB: Restricted and Repetitive Behaviors
Ethical Considerations
The purpose of the study was fully explained to the participants’ guardians and written informed consents were obtained. The study was approved by the ethics review committee of Harbin Medical University.
Data Acquisition
The resting-state fMRI data were collected at the Department of MR Diagnosis of the Second Hospital affiliated with the Harbin Medical University using a 3.0 Tesla Achieva Magnetic Resonance System (Philips, The Netherlands) with a gradient-echo echo-planar pulse sequence: TR = 2s; TE = 30ms; flip angle = 90 degrees; 39 axial slices; slice thickness = 3mm (with 1mm gap); field of view (FOV) = 240×240 mm; voxel size = 3.75×3.75×4mm. Two hundred and ten volumes (7 minutes) were obtained. Anatomical T1 and DTI images of the whole brain were also acquired. Each child received 50mg/kg chloral hydrate by a nurse trained and certified to administer sedation. The sedation was performed by following guidelines and protocols established by the radiology sedation committee at the hospital. A caregiver for each participant was present throughout the entire scan inside the scanner room.
Clinical Assessment
ASD diagnosis was based on the DSM-5 (American Psychiatric Association 2013) using Autism Diagnostic Interview Revised (ADI-R), Autism Diagnostic Observation Schedule (ADOS), Autism Behavior Checklist (ABC), Childhood Autism Rating Scale (CARS) scales. Children with ASD were diagnosed by two or more pediatric or psychiatry associate chief physicians. Children with known psychiatric, neurological (e.g., epilepsy, Tourette’s syndrome), or genetic (e.g., fragile X, Rett syndrome) disorders were excluded. Children with a history of a loss of consciousness for more than 5 minutes and currently taking psychoactive medication were also excluded. Symptom severity was evaluated using the ADI-R scores for all children with ASD by physicians who were professionally trained (Lord C et al. 1994). The ADI-R contains 93 items and mainly focuses on three functional domains: 1) Language/Communication; 2) Reciprocal social interactions; 3) Restricted, repetitive, and stereotyped behaviors and interests (Lord C et al. 1994).
We utilized the Peabody Picture Vocabulary Test (PPVT) to estimate the verbal intelligence level of children (Dunn LM et al. 2006). Recent studies show a strong correlation between PPVT and verbal IQ in autism, which validates the utility of PPVT as a proxy of verbal IQ in studies of ASD (Krasileva KE et al. 2017). During the PPVT, children were presented a series of pictures and pointed to the picture which he thought was the most relevant to a specific word. The PPVT does not require an oral or written response, and can be rapidly administered and utilized to evaluate children with language problems.
fMRI Data Preprocessing
Resting-state fMRI data were processed using the Data Processing Assistant for Resting-State fMRI toolbox Advanced edition (DPARSFA, http://rfmri.org/DPARSF) (Chao GY et al. 2010). The steps included: 1) Discarding the first 10 images; 2) Slice timing correction; 3) Head motion correction with 6 rigid parameters; 4) Normalization to warp images into standard Montreal Neurological Institute (MNI) space; 5) Smoothing with a Gaussian kernel (FWHM = 6mm); 6) Detrending; 7) Regression of signals from white matter, cerebrospinal fluid and 24 head motion parameters (Friston KJ et al. 1996; Li R et al. 2018; Liao W et al. 2018); 8) Filtering (0.01~0.1Hz) (Pang Y et al. 2018); 9) Scrubbing to remove volumes whose FD > 0.5 with prior 1 and later 2 volumes (Power JD et al. 2012). Here we did not apply the global signal regression (GSR) as growing evidence has shown that the global signal may also contain valuable information (Fox MD et al. 2009; Scholvinck ML et al. 2010), especially in studies of ASD (Gotts SJ et al. 2013).
Functional Connectedness Map
A grey matter mask was created based on the tissue probability maps in the Statistic Parameter Map (SPM) 12 toolbox. The threshold was set at 0.25 and the cerebellum was excluded. We calculated the whole-brain functional ‘connectedness’ map for each subject (Gotts SJ et al. 2012). For each voxel, we computed the Pearson correlation coefficients with every other voxel in the grey matter mask. The correlation coefficients were then transformed using Fisher’s Z formation to yield normally distributed values. The mean value of these transformed correlation coefficients was stored back in the voxel. A two-sample t-test was then utilized to explore the regions showing significant differences in the ‘connectedness’ values between ASD and HC groups. An AlphaSim multi-test correction was applied on the resulting T-map (voxel level p< 0.005, cluster level p< 0.05, cluster size >103 voxels).
Recurrent-seek strategy
Comparing the whole-brain ‘connectedness’ values can reveal brain regions with atypical FC in ASD. However, as this measure averages normal and abnormal FCs in ASD, some regions with atypical FC in ASD would be blurred. To reveal specific brain regions showing atypical FC in ASD, we utilized a recurrent-seek strategy (Fig.1). The idea behind the method is that if one region shows deficits, then regions that are connected to this region have a high likelihood of being affected. Using the clusters showing significant differences in functional ‘connectedness’ between ASD and HC, we defined ROIs by expanding the peak MNI coordinates into spheres with radius of 6mm. For each ROI, we calculated the seed-based FC map for each subject and then compared the difference using two-sample t-tests with AlphaSim corrections (voxel level p < 0.005, cluster level p < 0.05). ROIs were included if the peak MNI coordinates of the clusters were at least 25mm apart (here 25mm was set to avoid overlapping ROIs). For these new ROIs, we repeated the seed-based FC analysis to find more ROIs. This procedure was repeated until no other ROI was added (Fig.1). Using this strategy, we could reveal ASD-related FC circuits at the voxel level. Then we computed a permutation test to determine whether the circuits we revealed were larger than random cases. The permutation test is similar to the network-based statistic approach (Zalesky A et al. 2010). Each time we shuffled the ASD and HC subjects and randomly divided them into new “ASD” and “HC” groups. Then we compared the functional connectedness maps between these two groups and picked the peak voxel showing the largest statistically significant difference. These peak voxels were treated as the starter ROIs in the recurrent-seek strategy, and the count of ROIs revealed were then calculated. This procedure was repeated 100 times to construct the random distribution of the count of ROIs. The final p value was calculated by comparing the original ROI count with the random cases.
Figure.1.
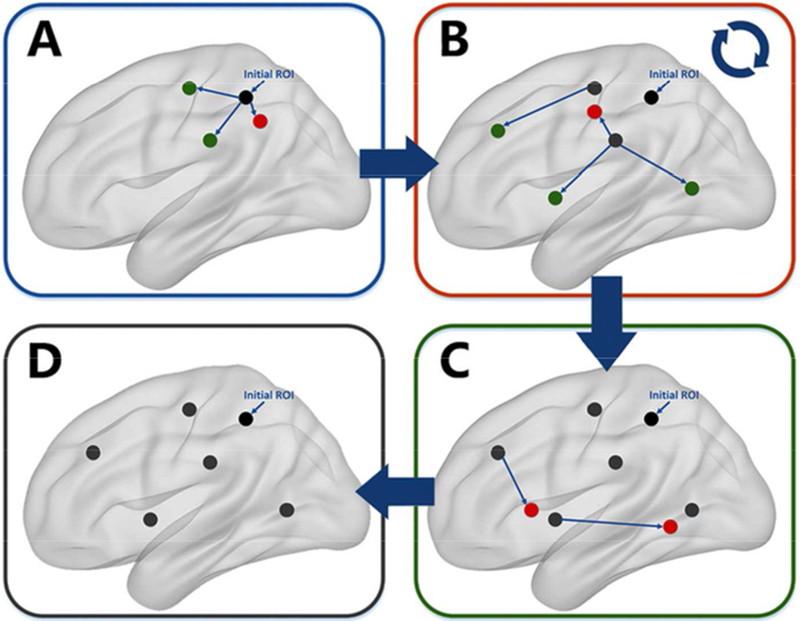
Flowchart of recurrent-seek strategy
A: For the ROI revealed by the functional ‘connectedness’ map (black), seed-FC analysis was applied based on the initial ROI to reveal new ROIs showing atypical FC by picking the peak points (expanded to 6-mm radius sphere) of the atypical clusters in ASD. B: The newly revealed ROIs located too close to the existing ROIs were excluded (red). For the remaining newly revealed ROIs (green), seed-FC analysis were applied to reveal new ROIs. C: Step B was repeated until no new ROIs could be found. D: Finally, the ROIs with atypical FC in ASDs were revealed and utilized for subsequent analysis.
Previous studies suggest the existence of atypical brain circuits in ASD (Gustafsson L 1997; Lee DA et al. 2003; Gotts SJ et al. 2012). Based on these theories, we conducted a cluster analysis to determine whether the ROI revealed could form circuits (Gotts SJ et al. 2012). ROI-by-ROI functional connectivity matrices were constructed for each participant by averaging the time course within each ROI and then calculating the all-to-all correlation matrices. A fisher’s Z transformation was utilized to convert the correlation coefficients into normally distributed Z values. These matrices were averaged across participants within each group. The interrelationship between these ROIs was visualized through multidimensional scaling and K-means cluster analysis based on the group-averaged matrix of HCs to avoid the bias of the disorder condition (Gotts SJ et al. 2012). Based on the silhouette criteria (Rousseeuw PJ 1987), a two-cluster solution was utilized.
A one-sample t-test was applied on the ROI-by-ROI functional connectivity matrices across all participants to determine whether the connectivity was positive or negative. A mask was created by including only significant FCs (p< 0.05, uncorrected). A two-sample t-test with Bonferroni correction was utilized to find connections showing the largest differences between ASD and HC within the mask created by the one-sample t-test. For the two-sample t-test, it should be noted that for negative connections, connections with higher FC values were treated as decreased connectivity in ASD.
Relationship with ASD symptoms
As two atypical FC circuits related to social and sensorimotor processing were revealed, we next tested the relationship between these two circuits and ASD symptoms. For the cluster that was more related to social processing, the relationship between FC values and ADI-R social scores was tested; for the cluster that was more related to sensorimotor processing, the relationship between FC values and ADI-R RRB scores was tested. To compare with other ASD phenotypes, we also tested the relationship between FC values and other ADI-R sub-scores. A linear support vector regression (SVR) was applied by utilizing the liblinear toolbox with default parameters (Fan RE et al. 2008; Uddin LQ et al. 2013a). A leave-one-out cross validation (LOOCV) strategy was used to assess the performance of the prediction (Chen H et al. 2015). For each trial, one subject was picked as the test dataset while the remaining subjects were used as the training dataset to construct the prediction model. This procedure was repeated n times, with n equal to the number of subjects. For each subject, we obtained a predicted ADI-R score. The correlation coefficient was calculated between the actual ADI-R score and the predicted scores. A permutation test with 1000 loops was applied to assess the significance level of the correlation coefficients (Chen H et al. 2015).
Results
Comparing the functional ‘connectedness’ values between ASD and HC groups, we found that the left supramarginal gyrus (peak MNI coordinates: x = −51, y = −36, z = 54, T = 4.28) showed altered functional ‘connectedness’ values in the ASD group compared with HCs (voxel level p< 0.005, cluster p<0.05). This ROI was considered as a starting point to search for other ROIs showing atypical FC in ASD using the recurrent-seek strategy. Finally, 23 ROIs were found to show atypical FC in the ASD group (Table.2). The count was significantly larger than random cases (p <0.05).
Table.2.
ROIs with aberrant FC in ASD compared with controls
| ROI number | ROI name | x | y | z |
|---|---|---|---|---|
| Cluster1 | ||||
| 1 | HippoCampus | 27 | −18 | −15 |
| 2 | Precuneous Cortex | 0 | −57 | 51 |
| 3 | Inferior Temporal Gyrus, anterior division | 45 | −9 | −39 |
| 4 | Medial Prefrontal cortex | 3 | 60 | −6 |
| 5 | Lateral Occipital Cortex, superior division | −39 | −69 | 24 |
| 6 | MiddleTemporal Gyrus | 57 | −36 | 6 |
| 7 | Lateral Occipital Cortex, superior division | 48 | −75 | 18 |
| 8 | Frontal Pole | −27 | 42 | 42 |
| 9 | Middle Temporal Gyrus | −60 | −27 | −12 |
| 10 | Middle Temporal Gyrus | −60 | −60 | 9 |
| 11 | Middle Frontal Gyrus | 33 | 27 | 51 |
| Cluster2 | ||||
| 12 | Insular Cortex | −30 | 18 | 9 |
| 13 | Occipitotemporal | 42 | −60 | −3 |
| 14 | Lateral Occipital Cortex, superior division | 24 | −78 | 33 |
| 15 | Postcentral Gyrus | 30 | −39 | 60 |
| 16 | Frontal Pole | 42 | 36 | 24 |
| 17 | Paracingulate Gyrus | 0 | 30 | 36 |
| 18 | *Supramarginal Gyrus, anterior division | −51 | −36 | 54 |
| 19 | Lateral Occipital Cortex, inferior division | −24 | −87 | 3 |
| 20 | Superior Frontal Gyrus | −21 | 9 | 51 |
| 21 | Precentral Gyrus | −51 | −3 | 39 |
| 22 | Supramarginal Gyrus, posterior division | 63 | −36 | 39 |
| 23 | Superior Frontal Gyrus | 30 | 0 | 63 |
Regions with index from 1-11 belong to Cluster1 while regions with index from 12-23 belong to Cluster2. Regions with star represent the seed region obtained from the functional ‘connectedness’ analysis.
As shown in Table.2, ROIs were clustered into two clusters by utilizing the k-means method: Cluster1 primarily comprised social and cognitive association areas. These included the ‘default mode’ regions, such as medial prefrontal cortex and precuneus cortex, associated with social function (Mars RB et al. 2012), the temporal regions related to language processing (Turken AU et al. 2011), and the hippocampus related to working memory function (Baddeley A et al. 2011). Meanwhile, Cluster2 primarily comprised sensorimotor and visual regions, including pre- and postcentral gyrus, and supramarginal gyrus (Picard N et al. 1996).
Comparing the ROI-by-ROI functional connectivity matrices, we found lower functional connectivity between ROIs within Cluster1 and higher functional connectivity between ROIs within Cluster2 in the ASD group (Fig.2). As shown in Tab.3, for connections within Cluster1, HCs showed positive connectivity while ASDs showed weaker connections. For connections within Cluster2, ASDs showed strong positive connectivity while HCs showed weaker or negative connectivity.
Figure.2.
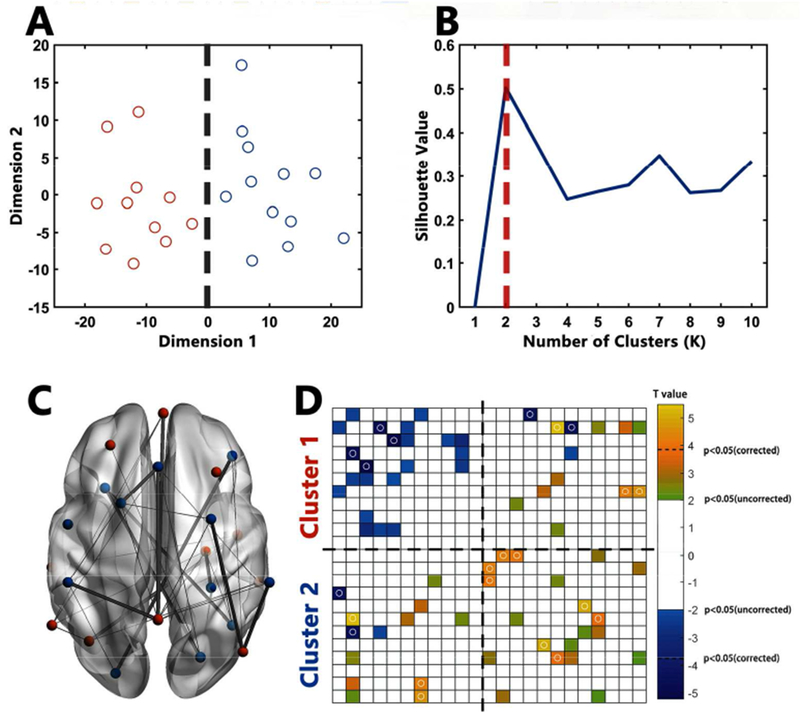
Atypical FCs in ASD
A: Points in 2D scatter plot represent 23 ROIs after multi-dimension scaling. Near points in the plot indicating similarity in the pattern of FC. Red points represent ROIs belonging to Cluster1 while blue points represent ROIs belonging to Cluster2; B: Silhouette plot of the k-means clustering. Multi-dimension scaling and Silhouette plots showed high consistence in the existence of two clusters of these 23 ROIs. C: The location of ROIs and atypical ROI-by-ROI connections in ASD. Red ROIs represent regions belonging to Cluster1 while blue ROIs represent regions belonging to Cluster2. For connections, thin lines represent connections showing significant differences between HC and ASD at the significance level at p < 0.05 (uncorrected); thick lines represent connections showing significant differences between HC and ASD at the significance level at p < 0.05 (Bonferroni corrected). D: Atypical FCs in ROI-by-ROI matrices in ASD. Yellow blocks represent connections showing higher FC in ASD (p < 0.05, uncorrected) while blue blocks represent connections showing lower FC in ASD (p < 0.05, uncorrected). Blocks with circles represent connections surviving Bonferroni correction. The order of ROIs is in accordance with Table 2.
Table.3.
FC values of each atypical connections
| ID | Connection | HC group (mean ± std) | ASD group (mean ± std) | p values |
|---|---|---|---|---|
| Within Cluster1 | ||||
| 1 | ROI1-ROI2 | 4.22±5.00 | 1.52±3.72 | 0.023 |
| 2 | ROI1-ROI7 | 4.55±5.71 | 1.31±5.83 | 0.037 |
| 3 | ROI2-ROI4 | 8.20±6.69 | 1.11±5.02 | 2.756e-5 |
| 4 | ROI2-ROI6 | 6.77±6.06 | 3.58±4.74 | 0.030 |
| 5 | ROI3-ROI5 | 6.22±4.36 | 0.03±4.63 | 2.593e-6 |
| 6 | ROI3-ROI6 | 5.78±5.77 | 2.61±4.03 | 0.019 |
| 7 | ROI3-ROI9 | 5.73±4.57 | 2.56±6.16 | 0.031 |
| 8 | ROI3-ROI10 | 6.81±5.49 | 2.65±5.34 | 0.005 |
| 9 | ROI4-ROI10 | 5.79±6.30 | 2.10±6.24 | 0.029 |
| 10 | ROI5-ROI6 | 4.23±6.69 | 0.92±5.68 | 0.047 |
| 11 | ROI5-ROI10 | 9.90±6.50 | 5.42±7.55 | 0.019 |
| Within Cluster2 | ||||
| 12 | ROI12-ROI13 | −1.22±5.05 | 4.19±4.64 | 8.214e-5 |
| 13 | ROI12-ROI14 | −0.89±5.44 | 4.43±4.48 | 1.526e-4 |
| 14 | ROI12-ROI20 | 0.33±7.66 | 5.47±7.00 | 0.010 |
| 15 | ROI13-ROI23 | 3.96±6.30 | 8.53±5.38 | 0.004 |
| 16 | ROI14-ROI17 | 0.23±5.97 | 3.67±4.58 | 0.017 |
| 17 | ROI16-ROI19 | −0.57±3.61 | 4.53±4.16 | 6.237e-6 |
| 18 | ROI17-ROI20 | 0.91±7.93 | 8.62±6.98 | 2.371e-4 |
| 19 | ROI18-ROI19 | 0.45±3.95 | 3.53±6.37 | 0.031 |
| 20 | ROI18-ROI20 | 2.14±7.02 | 7.11±5.43 | 0.004 |
| 21 | ROI20-ROI23 | 2.41±8.00 | 6.30±6.11 | 0.042 |
| Cluster1 × Cluster2 | ||||
| 22 | ROI1-ROI15 | −3.81±4.65 | 1.05±3.89 | 6.647e-5 |
| 23 | ROI2-ROI17 | 0.01±4.83 | 6.88±4,69 | 1.037e-6 |
| 24 | ROI2-ROI18 | −5.77±4.66 | 1.07±6.75 | 3.580e-5 |
| 25 | ROI2-ROI20 | 2.83±5.12 | 6.97±7.32 | 0.016 |
| 26 | ROI2-ROI22 | −0.79±6.66 | 4.94±6.42 | 0.002 |
| 27 | ROI2-ROI23 | 2.10±5.72 | 5.22±5.10 | 0.033 |
| 28 | ROI4-ROI18 | −5.54±6.00 | −2.10±5.88 | 0.032 |
| 29 | ROI6-ROI17 | 0.92±4.27 | 3.89±5.10 | 0.003 |
| 30 | ROI7-ROI16 | −0.78±6.13 | 4.42±6.44 | 0.003 |
| 31 | ROI7-ROI22 | 0.93±7.42 | 8.81±6.64 | 7.785e-5 |
| 32 | ROI7-ROI23 | 1.75±6.05 | 8.57±5.29 | 2.730e-5 |
| 33 | ROI8-ROI14 | 1.57±5.12 | 4.55±4.69 | 0.025 |
| 34 | ROI10-ROI17 | −0.21±5.67 | 2.96±4.12 | 0.018 |
The index of ROIs is according to Table.2. FC values are Fisher Z transformed values. P values were calculated using two-sample t-test.
Using the linear SVR method, we confirmed that FCs within Cluster1 were related to ADI-R social scores (r = 0.65, permutation p < 0.05, 1000 loops) and FCs within Cluster2 were related to ADI-R RRB scores (r = 0.70, permutation p < 0.05, 1000 loops). The performance of the linear SVR method is shown in Fig.3. We found FCs within Cluster1 were not significantly related to ADI-R RRB scores (r = 0.43, permutation p > 0.05, 1000 loops) and FCs within Cluster2 were not significantly related to ADI-R social scores (r = −0.17, permutation p >0.05, 1000 loops);
Figure.3.
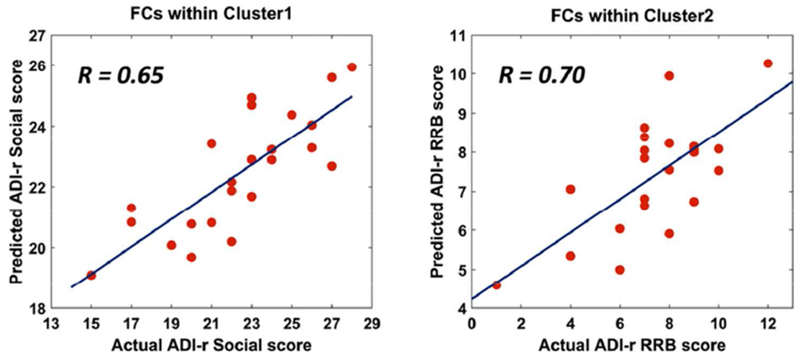
Relationship between FCs within two clusters and ASD symptoms
Correlations between actual ADI-R scores and predicted ADI-R scores using linear SVR.
Discussion
In this study, we explored atypical whole-brain FC in young children with ASD. Utilizing a recurrent-seek strategy and k-means clustering, 23 ROIs showing atypical FC in ASD belonging to two neural circuits were revealed: Cluster1 related to social functioning while Cluster2 related to sensorimotor and visual functioning. Linear SVR analysis confirmed that FC within Cluster1 was related to social deficits in ASD and FC within Cluster2 was related to restricted and repetitive behaviors in ASD. By comparing the ROI-by-ROI matrices, we found that children with ASD showed higher intra-Cluster2 FC and lower intra-Cluster1 FC. Thus, our results show aberrant connections within two neural circuits in young children with ASD.
Social interaction deficits are key characteristics of ASD. Previous studies based on adults with ASD have revealed reduced connections between brain regions involved in social functions (Gotts SJ et al. 2012). A recent study examining preschool-age children with ASD also reported decreased FC of the amygdala, a key region of the “social brain” (Shen MD et al. 2016). Our results based on young children are in line with these previous reports of weak FC within social brain circuits in ASD. We found that the weakest connection in ASD was between mPFC and precuneus, two core nodes of the default mode network (DMN). The DMN is suggested to be related to self-consciousness and studies reveal a large degree of overlap between the DMN and the “social brain”, especially the mPFC and precuneus (Mars RB et al. 2012). Moreover, we found that the strength of FC within this circuit was correlated to ADI-R social scores in ASD. These results indicate that the abnormalities within social brain circuits occur at early ages in children with ASD, and this weak pattern may last from early age to adulthood (Kennedy DP et al. 2008).
Interestingly, in addition to the weak connections within social brain circuits, we found higher connectivity of sensorimotor and visual circuits in ASD. The most connected regions were the insular cortex and occipital cortex. The insular cortex plays an important role in multimodal sensory processing (Bushara KO et al. 2001), sensory binding (Bushara KO et al. 2003) and motor controlling (Anderson TJ et al. 1994) while the occipital cortex is the key region receiving visual input. The atypical patterns of these sensorimotor and visual circuits might relate to the sensory hyper/hypo-sensitivity in individuals with ASD (Shen MD et al. 2016). The deficits observed in sensorimotor regions are in accordance with a previous structural neuroimaging study that reported increased gray matter volume in the left superior temporal gyrus and left postcentral gyrus in children with ASD (Wang J et al. 2017). Previous studies have demonstrated significant correlations between atypical sensory and restricted behaviors in ASD. Using factor analysis, Brian et al. reported significant correlations between hyper-responsive sensory symptoms and restricted behaviors in children with ASD (Boyd BA et al. 2010). Penelope et al. also reported significant correlations between sensory profiles and ADI-R RRB scores (Hannant P et al. 2016). We tested the relationship between FCs within the sensorimotor/visual circuit and ADI-R RRB scores and confirmed this significant relationship. Studies have reported abnormal bimodal patterns of sensory profiles (hyper/hypo-sensitivity) in ASD (Tomchek SD et al. 2007). The FC profile in ASD shows heterogeneity between subjects (Hahamy A et al. 2015). Previous studies suggested that ASD is a disorder with atypical neural circuits (Gustafsson L 1997; Lee DA et al. 2003), and suggested that different subjects with ASD show various atypical spatial regional distribution within the sensorimotor circuit. These varying patterns might contribute to different sensory profiles within ASD. However, the lack of direct measures of sensorimotor deficits in children with ASD is one limitation of the current study. This relationship should be confirmed in future studies.
With the exception of the social brain regions (e.g., mPFC, precuneus, temporal regions) in Cluster1 and sensorimotor regions (e.g. precentral, postcentral) in Cluster2, there were some common regions between these two clusters such as frontal and occipital regions. Previous large-scale longitudinal studies have shown atypical developmental courses of these regions (Zielinski BA et al. 2014), which might contribute to atypical FC of these regions. It is interesting that the occurrence of both under- and over-connectivity was observed for these regions. We hypothesize that the aberrant developmental trajectories may cause atypical brain functional segregation, leading to different FC patterns within these regions. Previous studies exploring local FC patterns reported atypical local FC within frontal and occipital region in ASD (Keown CL et al. 2013; Dajani DR et al. 2016). Decreased local connectivity might contribute to abnormal functional segregation of these regions, resulting in affecting the balance of functional connectivity and contributing to the coexistence of under/over – connectivity of these regions.
ASD is a neurodevelopment disorder with average onset at 2-4 years of age (American Psychiatric Association 2013). One prominent theory of developmental changes in FC in ASD suggests over-connectivity in young individuals with ASD and under-connectivity in adults with ASD (Keown CL et al. 2013; Kana RK et al. 2014). However, this theory is mostly based on studies which included subjects aged 7 to 12 years old, and have not yet been extended to young children (Uddin LQ et al. 2013b). A recent study exploring amygdala-FC in preschool age children with ASD showed under-connectivity of the amygdala (Shen MD et al. 2016). A previous study on even younger toddlers with ASD also showed decreased interhemispheric FC (Dinstein I et al. 2011). Keown et al. first reported local over-connectivity patterns in children with ASD, and, decreased connectivity of social brain regions (Keown CL et al. 2013). Our finding of decreased FC of social circuits and increased FC of sensorimotor circuits indicate that young children with ASD show different atypical FC patterns of distinct neural circuits. This suggests that the under/over-connectivity theory is unable to fully describe the atypical FC patterns in ASD, especially in ASD children.
It is worth noting that in our study the subjects were in a sedated state, while most previous ASD FC studies analyzed data collected during waking states. Previous studies have indicated that chloral hydrate induced sedation would decrease the brain’s functional interactions in school-aged children (7-15 years old) (Wei Z et al. 2013). Doria et al. compared sedated and deep-sleeping infants and found no significant differences of FCs (Doria V et al. 2010). Although the HCs and ASDs were both under sedation in our study, which could partly decrease the effect of sedation on the differences between HCs and ASDs, our previous study suggested that brain states would affect atypical FC patterns in ASD (Uddin LQ et al. 2014; Chen H et al. 2017a). Thus, we speculate that the atypical patterns we reveal could be specific to deep sleeping children with ASD. Further FC work on awake children with ASD is warranted. Children recruited in our study were all native Chinese speakers. Previous studies suggest that cultural factors could affect the diagnostic process, symptom description, and treatment of ASD (Bernier R et al. 2010). Thus, cultural differences might influence atypical FC patterns in ASD. This suggests the need for FC studies based on cross-cultural ASD datasets.
Our study explored atypical FC patterns in young children with ASD. By utilizing a recurrent-seek strategy, we explored two atypical FC circuits in ASD: one predominantly containing social brain regions showing under-connectivity, and the other predominantly containing sensorimotor and visual regions showing over-connectivity. These findings indicated neural circuit specific atypical FC patterns in young children with ASD, which could help to understand the neural basis of behavioral symptom emergence in ASD.
Acknowledgements
This work was supported by 863 project (2015AA020505), the Natural Science Foundation of China (Nos. 61533006, 81871432, 81874270 and 61673089), the Fundamental Research Funds for the Central Universities (Nos. ZYGX2016J187 and 2672018ZYGX2018J079), the Natural Science Foundation Heilongjiang Province China (QC2018106), and the National Institute of Mental Health (R01MH107549) to LQU.
Footnotes
All authors declared no conflict of interest.
Reference
- American Psychiatric Association. (2013). The Diagnostic and Statistical Manual of Mental Disorders: DSM 5. Arlington, VA: American Psychiatric Publishing; 991 p. [Google Scholar]
- Anderson TJ, Jenkins IH, Brooks DJ, Hawken MB, Frackowiak RS, Kennard C (1994). Cortical control of saccades and fixation in man. A PET study. Brain : a journal of neurology, 117 ( Pt 5), 1073–1084. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Jarrold C, Vargha-Khadem F (2011). Working memory and the hippocampus. Journal of cognitive neuroscience, 23, 3855–3861. [DOI] [PubMed] [Google Scholar]
- Bernier R, Mao A, Yen J (2010). Psychopathology, families, and culture: autism. Child and adolescent psychiatric clinics of North America, 19, 855–867. [DOI] [PubMed] [Google Scholar]
- Boyd BA, Baranek GT, Sideris J, Poe MD, Watson LR, Patten E et al. (2010). Sensory features and repetitive behaviors in children with autism and developmental delays. Autism research : official journal of the International Society for Autism Research, 3, 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushara KO, Grafman J, Hallett M (2001). Neural correlates of auditory-visual stimulus onset asynchrony detection. The Journal of neuroscience : the official journal of the Society for Neuroscience, 21, 300–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushara KO, Hanakawa T, Immisch I, Toma K, Kansaku K, Hallett M (2003). Neural correlates of cross-modal binding. Nature neuroscience, 6, 190–195. [DOI] [PubMed] [Google Scholar]
- Chao GY, Yu FZ (2010). DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Frontiers in systems neuroscience, 4, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Duan X, Liu F, Lu F, Ma X, Zhang Y et al. (2015). Multivariate classification of autism spectrum disorder using frequency-specific resting-state functional connectivity-A multi-center study. Progress in neuro-psychopharmacology & biological psychiatry, 64, 1–9. [DOI] [PubMed] [Google Scholar]
- Chen H, Nomi JS, Uddin LQ, Duan X, Chen H (2017a). Intrinsic functional connectivity variance and state-specific under-connectivity in autism. Human brain mapping, 38, 5740–5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Uddin LQ, Duan X, Zheng J, Long Z, Zhang Y et al. (2017b). Shared atypical default mode and salience network functional connectivity between autism and schizophrenia. Autism research : official journal of the International Society for Autism Research, 10, 1776–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Uddin LQ, Zhang Y, Duan X, Chen H (2016). Atypical effective connectivity of thalamo-cortical circuits in autism spectrum disorder. Autism research : official journal of the International Society for Autism Research, 9, 1183–1190. [DOI] [PubMed] [Google Scholar]
- Dajani DR, Uddin LQ (2016). Local brain connectivity across development in autism spectrum disorder: A cross-sectional investigation. Autism research : official journal of the International Society for Autism Research, 9, 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Pierce K, Eyler L, Solso S, Malach R, Behrmann M et al. (2011). Disrupted neural synchronization in toddlers with autism. Neuron, 70, 1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria V, Beckmann CF, Arichi T, Merchant N, Groppo M, Turkheimer FE et al. (2010). Emergence of resting state networks in the preterm human brain. Proceedings of the National Academy of Sciences of the United States of America, 107, 20015–20020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Chen H, He C, Long Z, Guo X, Zhou Y et al. (2017). Resting-state functional under-connectivity within and between large-scale cortical networks across three low-frequency bands in adolescents with autism. Progress in neuro-psychopharmacology & biological psychiatry, Part B, 79B, 434–441. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM (1997). Peabody Picture Vocabulary Test-Third Edition (PPVT-III). MN: American Guidance Services. [Google Scholar]
- Fan RE, Chang KW, Hsieh CJ, Wang XR, Lin CJ (2008). LIBLINEAR: A library for large linear classification. The Journal of Machine Learning Research, 9, 1871–1874. [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME (2009). The global signal and observed anticorrelated resting state brain networks. Journal of neurophysiology, 101, 3270–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R (1996). Movement-related effects in fMRI time-series. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine, 35, 346–355. [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Saad ZS, Jo HJ, Wallace GL, Cox RW, Martin A (2013). The perils of global signal regression for group comparisons: a case study of Autism Spectrum Disorders. Frontiers in human neuroscience, 7, 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts SJ, Simmons WK, Milbury LA, Wallace GL, Cox RW, Martin A (2012). Fractionation of social brain circuits in autism spectrum disorders. Brain : a journal of neurology, 135, 2711–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson L (1997). Inadequate cortical feature maps: a neural circuit theory of autism. Biological psychiatry, 42, 1138–1147. [DOI] [PubMed] [Google Scholar]
- Hahamy A, Behrmann M, Malach R (2015). The idiosyncratic brain: distortion of spontaneous connectivity patterns in autism spectrum disorder. Nature neuroscience, 18, 302–309. [DOI] [PubMed] [Google Scholar]
- Hannant P, Cassidy S, Tavassoli T, Mann F (2016). Sensorimotor Difficulties Are Associated with the Severity of Autism Spectrum Conditions. Frontiers in integrative neuroscience, 10, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Uddin LQ, Kenet T, Chugani D, Muller RA (2014). Brain connectivity in autism. Frontiers in human neuroscience, 8, 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E (2008). The intrinsic functional organization of the brain is altered in autism. NeuroImage, 39, 1877–1885. [DOI] [PubMed] [Google Scholar]
- Keown CL, Shih P, Nair A, Peterson N, Mulvey ME, Muller RA (2013). Local functional overconnectivity in posterior brain regions is associated with symptom severity in autism spectrum disorders. Cell reports, 5, 567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Leventhal BL, Koh YJ, Fombonne E, Laska E, Lim EC et al. (2011). Prevalence of autism spectrum disorders in a total population sample. The American journal of psychiatry, 168, 904–912. [DOI] [PubMed] [Google Scholar]
- Krasileva KE, Sanders SJ, Bal VH (2017). Peabody Picture Vocabulary Test: Proxy for Verbal IQ in Genetic Studies of Autism Spectrum Disorder. Journal of autism and developmental disorders, 47, 1073–1085. [DOI] [PubMed] [Google Scholar]
- Lee DA, Lopez-Alberola R, Bhattacharjee M (2003). Childhood autism: a circuit syndrome? The neurologist, 9, 99–109. [DOI] [PubMed] [Google Scholar]
- Lewis JD, Theilmann RJ, Fonov V, Bellec P, Lincoln A, Evans AC et al. (2013). Callosal fiber length and interhemispheric connectivity in adults with autism: Brain overgrowth and underconnectivity. Human brain mapping, 34, 1685–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Liao W, Yu Y, Chen H, Guo X, Tang YL et al. (2018). Differential patterns of dynamic functional connectivity variability of striato-cortical circuitry in children with benign epilepsy with centrotemporal spikes. Human brain mapping, 39, 1207–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SJ, Wang Y, Qian L, Liu G, Liu SF, Zou LP et al. (2018). Alterations of White Matter Connectivity in Preschool Children with Autism Spectrum Disorder. Radiology, 170059. [DOI] [PubMed] [Google Scholar]
- Liao W, Li J, Duan X, Cui Q, Chen H, Chen H (2018). Static and dynamic connectomics differentiate between depressed patients with and without suicidal ideation. Human brain mapping, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A (1994). Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of autism and developmental disorders, 24, 659–685. [DOI] [PubMed] [Google Scholar]
- Mars RB, Neubert FX, Noonan MP, Sallet J, Toni I, Rushworth MFS (2012). On the relationship between the “default mode network” and the “social brain”. Frontiers in human neuroscience, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi JS, Uddin LQ (2015). Developmental changes in large-scale network connectivity in autism. NeuroImage Clinical, 7, 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Chen H, Wang Y, Long Z, He Z, Zhang H et al. (2018). Transdiagnostic and diagnosis-specific dynamic functional connectivity anchored in the right anterior insula in major depressive disorder and bipolar depression. Progress in neuro-psychopharmacology & biological psychiatry, 85, 7–15. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL (1996). Motor areas of the medial wall: a review of their location and functional activation. Cerebral cortex, 6, 342–353. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59, 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseeuw PJ (1987). Silhouettes: A Graphical Aid to the Interpretation and Validation of Cluster Analysis. Journal of Computational & Applied Mathematics, 20, 53–65. [Google Scholar]
- Rutter M (1978). Diagnosis and definition of childhood autism. Journal of autism and childhood schizophrenia, 8, 139–161. [DOI] [PubMed] [Google Scholar]
- Scholvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA (2010). Neural basis of global resting-state fMRI activity. Proceedings of the National Academy of Sciences of the United States of America, 107, 10238–10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MD, Li DD, Keown CL, Lee A, Johnson RT, Angkustsiri K et al. (2016). Functional Connectivity of the Amygdala Is Disrupted in Preschool-Aged Children With Autism Spectrum Disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 55, 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Khouzam A, Phillips J, Gaillard WD, Kenworthy LE et al. (2013). Brain hyperconnectivity in children with autism and its links to social deficits. Cell reports, 5, 738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND (2010). Functional connectivity density mapping. Proceedings of the National Academy of Sciences of the United States of America, 107, 9885–9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchek SD, Dunn W (2007). Sensory processing in children with and without autism: a comparative study using the short sensory profile. The American journal of occupational therapy : official publication of the American Occupational Therapy Association, 61, 190–200. [DOI] [PubMed] [Google Scholar]
- Turken AU, Dronkers NF (2011). The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Frontiers in systems neuroscience, 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Dajani DR, Voorhies W, Bednarz H, Kana RK (2017). Progress and roadblocks in the search for brain-based biomarkers of autism and attention-deficit/hyperactivity disorder. Translational psychiatry, 7, e1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Lynch CJ, Cheng KM, Odriozola P, Barth ME et al. (2014). Brain state differentiation and behavioral inflexibility in autism. Cerebral cortex, 25, 4740–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Lynch CJ, Khouzam A, Phillips J, Feinstein C et al. (2013a). Salience network-based classification and prediction of symptom severity in children with autism. JAMA psychiatry, 70, 869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V (2013b). Reconceptualizing functional brain connectivity in autism from a developmental perspective. Frontiers in human neuroscience, 7, 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von dem Hagen EA, Stoyanova RS, Baron-Cohen S, Calder AJ (2013). Reduced functional connectivity within and between ‘social’ resting state networks in autism spectrum conditions. Social cognitive and affective neuroscience, 8, 694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fu K, Chen L, Duan X, Guo X, Chen H et al. (2017). Increased Gray Matter Volume and Resting-State Functional Connectivity in Somatosensory Cortex and their Relationship with Autistic Symptoms in Young Boys with Autism Spectrum Disorder. Frontiers in physiology, 8, 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Alcauter S, Jin K, Peng ZW, Gao W (2013). Graph theoretical analysis of sedation’s effect on whole brain functional system in school-aged children. Brain connectivity, 3, 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerys BE, Gordon EM, Abrams DN, Satterthwaite TD, Weinblatt R, Jankowski KF et al. (2015). Default mode network segregation and social deficits in autism spectrum disorder: Evidence from non-medicated children. Neuroimage Clinical, 9, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET (2010). Network-based statistic: identifying differences in brain networks. NeuroImage, 53, 1197–1207. [DOI] [PubMed] [Google Scholar]
- Zielinski BA, Prigge MB, Nielsen JA, Froehlich AL, Abildskov TJ, Anderson JS et al. (2014). Longitudinal changes in cortical thickness in autism and typical development. Brain : a journal of neurology, 137, 1799–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]


