Abstract
Cardiac dysfunction is a common accompaniment to severe sepsis. Clinical management of the same complicating pregnancy presents unique challenges balancing maternal and fetal well-being. Can short-term intravenous (IV) tri-iodothyronine (T3) be used in the management of these patients? T3 has been reported in varied clinical settings to favorably affect cardiac lusitropy, inotropy, and chronotropy without significant side effects.
We report a case of acute severe left ventricular dysfunction in a pregnant woman with severe acute respiratory distress syndrome on veno-venous extracorporeal membrane oxygenation managed with short-term IV T3. Hemodynamic stability was rapidly achieved and the improvement in contractility imaged in real time by transesophageal echocardiography.
<Learning objective: Tri-iodothyronine (T3) rapidly affects cardiac inotropy, lusitropy, chronotropy, and systemic vascular resistance. Widespread application of intravenous T3 for treatment of heart failure is currently limited by a paucity of scientific literature. Selective short-term intravenous T3 use is an underutilized adjunct in the management of acute left ventricular dysfunction.>
Keywords: Tri-iodothyronine, Cardiomyopathy, Transesophageal echocardiography, Pregnancy, ECMO
Introduction
Severe sepsis is characterized by an inappropriate immune response to an infective stimulus complicated by end organ dysfunction. Cardiac dysfunction, etiologically complex, is commonly encountered in the septic patient and remains an independent marker of poor outcomes [1]. When the above complicates pregnancy, clinical challenges balancing maternal and fetal risks arise to optimize outcomes. Pressor uptitration increases myocardial work while inotropic infusion in the setting of a profound vasodilatory state might worsen hypotension. Can short-term intravenous (IV) tri-iodothyronine (T3) be utilized for the management of such a patient?
We report the case of a 23-week pregnant woman on veno-venous extracorporeal membrane oxygenation (V-V ECMO) for severe acute respiratory distress syndrome (ARDS), complicated by acute severe left ventricular dysfunction of likely septic etiology for which short-term IV T3 was used. A rapid improvement in biventricular systolic function was noted within minutes of T3 administration. Hemodynamic stability was immediately achieved and the improvement in contractility imaged in real time by transesophageal echocardiography (TEE).
Case report
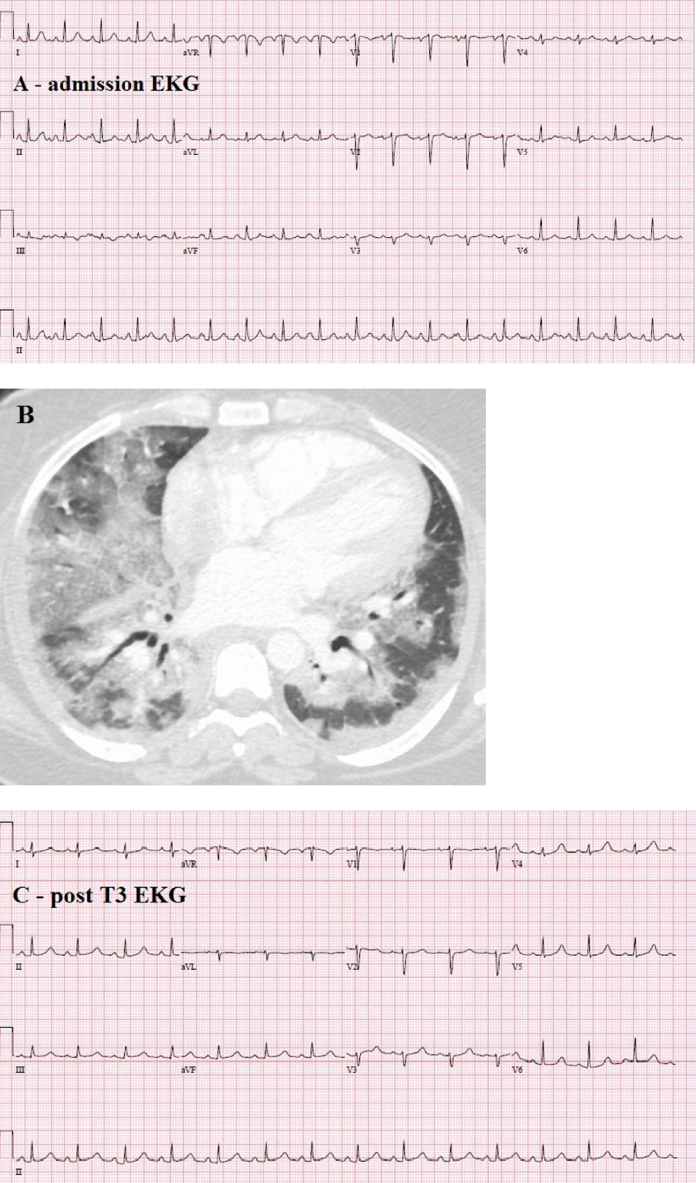
A 31-year-old Portuguese, 23 weeks, pregnant female presented to our hospital with severe resting dyspnea. She had recently completed a prescription of azithromycin for an upper respiratory infection. Upon presentation, she was febrile [Tmax 38.8 °C (102 °F)], normotensive (blood pressure 116/75 mmHg), and tachycardic (116–120 beats/min sinus tachycardia). Peripheral oxygen saturation on room air was 47%. An arterial blood gas test reported pH 7.25/PaCO2 51 mmHg/PaO2 26 mmHg with a PaO2/FIO2 ratio 51. Admission electrocardiogram reported sinus tachycardia without significant ST-T changes (Fig. 1A). Emergent endotracheal intubation was undertaken. Computed tomography scan showed diffuse bilateral ground glass consolidations and ruled out pulmonary embolus (Fig. 1B). System review was positive for fever, productive cough, and peripheral cyanosis. Admission drug screen was negative with an uneventful pregnancy reported to date. Past history was positive for mild intermittent asthma and an uncomplicated Cesarean section 9 years previously.
Fig. 1.
(A) Admission electrocardiogram (ECG). (B) Computed tomography scan demonstrating diffuse bilateral ground glass consolidation. (C) Post-T3 ECG.
Treatment per sepsis guidelines was instituted. Hypoxia remained refractory despite bilevel ventilation, recruitment maneuvers, and rescue inhalational nitric oxide (NO) at 20 parts-per-million (ppm) used to alleviate ventilation-perfusion mismatch. Fetal health was ascertained to be normal and noncontributory to her hypoxia. Biomarker assay returned troponin I 1.08 ng/ml (high normal <0.03 ng/ml) and B-type natriuretic peptide (BNP) 130 pg/ml (high normal <400 pg/ml) with normal serum creatinine 0.63 mg/dl (55 μmol/L). An echocardiogram revealed moderate to severe diffuse left ventricular hypokinesis, estimated ejection fraction ∼34%, and no significant valvular pathology. The right ventricle was mildly dilated and mildly hypokinetic. Acute coronary ischemia, myocarditis, dilated and peripartum cardiomyopathy were considered, but remained lower on our differential diagnosis. Cardiac dysfunction was attributed to the severe sepsis and hypoxemia and labeled “septic cardiomyopathy.”
A right heart catheterization prior to ECMO revealed right atrial pressure 10 mmHg, pulmonary artery pressure 25/15/21 mmHg, pulmonary capillary wedge pressure 15 mmHg, and SVO2 of 73%. V-V ECMO was initiated using a 27Fr MAQUET Avalon Elite bicaval dual lumen catheter via the right internal jugular vein. The ventilator was adjusted to rest settings (TV 3–4 ml/kg) and NO at 20 ppm was continued. She became acutely hypotensive post-ECMO initiation for which vasopressin was initiated. Milrinone was subsequently initiated for management of acute biventricular dysfunction. To further optimize oxygen delivery and reduce myocardial stress, she was transfused 2 units of packed red blood cells for baseline hemoglobin of 9.6 g/dl. Empirical broad spectrum antibiotic coverage was started with vancomycin, piperacillin-tazobactam, azithromycin, and oseltamivir at treatment doses.
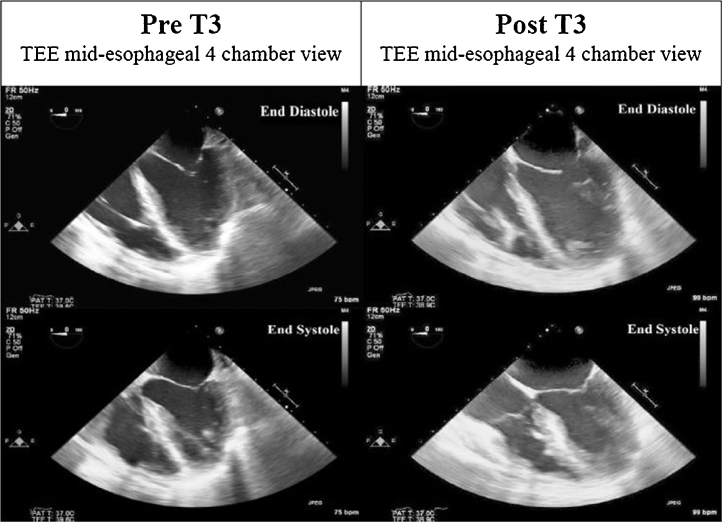
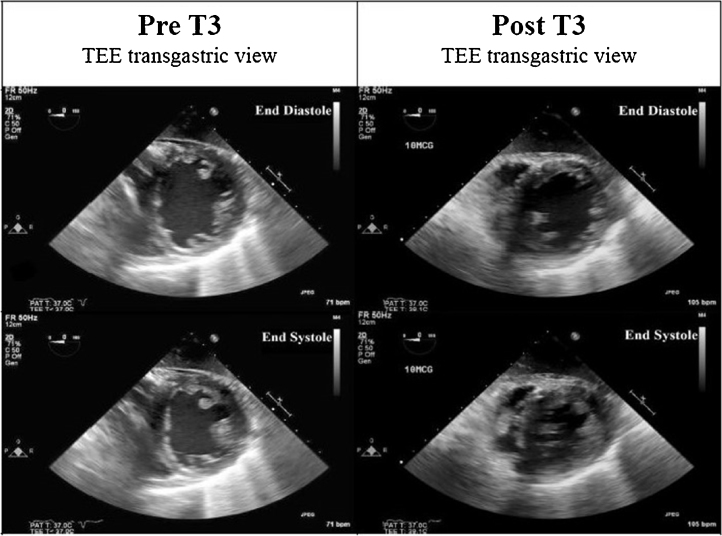
Twenty-four hours after V-V ECMO, she remained persistently hypotensive (blood pressure 70–88/49–56 mmHg) despite intensive medical management (vasopressin 0.1 units/min; milrinone 0.375 mcg/kg/min) and an arterial PaO2 130 mmHg. Vasopressin and milrinone were changed to dobutamine and dopamine to offset splanchnic vasoconstriction and vasodilatory effects, respectively. An emergent transesophageal echocardiogram was indicative of severe global left ventricle hypokinesis (ejection fraction ∼25–30%) with mild to moderate right ventricular hypokinesis (Fig. 2, Fig. 3) (Videos 1 and 2). Based on favorable reports in nongravid adults with heart failure, and, in an effort to prevent pressor escalation, which could have compromised fetal health, a decision was made to use IV T3 (Pregnancy Class A). Repeat thyroid function testing was not undertaken prior to IV T3 with normal prenatal thyroid function results. A 10 mcg bolus was administered with concurrent TEE imaging. An immediate and remarkable improvement in biventricular systolic function was seen (Fig. 2, Fig. 3) (Videos 3 and 4). T3 was continued as a drip at 2.5 mcg/h, and titrated empirically to a heart rate ∼100 beats/min for the next 48 h. Dopamine was discontinued within 24 h of T3 administration. Intravenous furosemide was used for diuresis and heparin was continued per ECMO protocol.
Fig. 2.
Pre- and post-T3 transesophageal echocardiography (TEE) 4-chamber views at end diastole and end systole.
Fig. 3.
Pre- and post-T3 transesophageal echocardiography (TEE) transgastric views at end diastole and end systole.
Supplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jccase.2015.09.005.
No additional pressor or inotrope uptitration was needed. ECMO weaning was initiated on day 4 and decannulation was achieved on day 6. Dobutamine was discontinued 2 days later. She was extubated on day 10 and discharged from the intensive care unit (ICU) on day 12. Her post-ICU hospitalization was uneventful. A viral etiology for ARDS was considered likely. An exhaustive infectious work up returned negative, although exposure to antibiotics prehospitalization could confound the same. Electrocardiogram post-T3 reported sinus rhythm without significant ST-T wave changes (Fig. 1C). She was discharged to home on day 20 on metoprolol, hydralazine, and nitrates for heart failure management. A predischarge echocardiogram showed normal biventricular systolic function. This rapid normalization supports septic cardiomyopathy as likely etiology of her cardiac dysfunction. Obstetric ultrasound done on day 18 reported mild fetal ventriculomegaly which was not reported on subsequent prenatal testing post-discharge. An elective Caesarian section was undertaken at 39 weeks for decreased fetal movements delivering a baby girl weighing 3779 g/8 lb 5 oz. The baby had Apgar scores of 9/9. Mother and baby were discharged home 3 days post-partum with excellent outcomes.
Discussion
T4 and T3 are the predominant circulating thyroid hormones in humans. T4 deiodination to biologically active T3 is catalyzed by tissue specific Type 1 (D1) and Type 2 (D2) deiodinases. Type 3 (D3) deiodinase converts active T3 to inactive diiodothyronine (T2) and T4 to reverse T3 (rT3). Systemic illnesses, both acute and chronic, are associated with downregulation of D1 and D2 activities and upregulation of D3 deiodinase activity [2], particularly illnesses that include substantial ischemia or infarction [3]. Culminating in low serum T3, high rT3, a low or normal T4, and normal thyroid-stimulating hormone levels, this is commonly referred to as Non-Thyroidal Illness Syndrome, Low T3 Syndrome (LT3S), or the Sick Euthyroid State.
T3 has been shown to affect cardiac inotropy, lusitropy, chronotropy, and systemic vascular resistance through a variety of genomic and nongenomic pathways affecting ionic currents and cardiac structural, contractile, and regulatory proteins. A decrease in systemic vascular resistance, increased left ventricular diastolic relaxation, and systolic ventricular force generation (dp/dt) associated with positive ventriculo-arterial coupling have been shown with T3 administration [2], [4], [5]. This may explain the decrease in aldosterone, norepinephrine, and NT-proBNP levels; a deactivation of the neuroendocrine profile was noted with T3 administration in chronic heart failure [6].
Chronic heart failure patients show biochemical evidence of LT3S [7], [8]. LT3S prevalence in these patients is positively correlated with New York Heart Association symptom class and disease severity [7], [8]. Construed as evidence of “fetal gene phenotype” reactivation minimizing energy expenditure and protein catabolism short term, animal experiments demonstrate deleterious effects over the long term. Moreover, LT3S prevalence and severity has been shown to be an independent predictor of decreased survival in heart failure patients [7], [9]. Iatrogenic supplementation to restore T3 levels to the physiologic range in heart failure has been fraught with challenges, given lack of sufficient scientific evidence regarding dosage, timing, mode, and duration of administration. Nonetheless, small studies have demonstrated an improvement in mean arterial pressure, stroke volume, and cardiac index after the administration of T3 without a significant increase in heart rate, occurrence of arrhythmias, or increased myocardial oxygen demand [2], [6], [10]. T3 supplementation has been studied in adult [10] and pediatric open heart surgical patients [11]. Use has extended to treatment of acute myocardial dysfunction of donor heart allografts post-brain death [12] and management of acute transplant allograft dysfunction.
While it is apparent that no laboratory evidence of a low T3 state was documented in our patient, a decision to use T3 was made based on our prior heart transplant experience backed by the available scientific evidence. Her pregnant state with a viable fetus necessitated a novel therapeutic approach. Pressor uptitration could have potentially compromised utero-placental circulation and jeopardized fetal well-being. A low T3 loading and maintenance dose was chosen based upon our experience in the post-heart transplant population. Titration to physiological replacement levels as has been done elsewhere [6] was not undertaken due to nonavailability of free T3 laboratory testing in house.
Real time TEE imaging during T3 administration helped document an immediate improvement in systolic function. Most likely mediated by nongenomic actions on ion channel conduction properties, a potentiation of the vasopressor and inotropic actions of the other infusions was likely partially responsible. Short-term IV T3 infusion helped achieve hemodynamic stability without adverse effects and facilitated pressor/inotrope de-escalation, successful ECMO decannulation, shortened ICU stay, and early discharge home. Most importantly, she was able to maintain the pregnancy to term with an excellent fetal and maternal outcome.
Our report highlights the safe use of IV T3 for acute ventricular dysfunction complicating pregnancy. Concurrent TEE imaging helped document the immediate improvement in cardiac function that has heretofore not been reported. Selective short-term IV T3 may therefore be an important underutilized adjunct in the management of acute left ventricular dysfunction.
Conflict of interest
None.
References
- 1.Merx M.W., Weber C.D. Sepsis and the heart. Circulation. 2007;116:793–802. doi: 10.1161/CIRCULATIONAHA.106.678359. [DOI] [PubMed] [Google Scholar]
- 2.Gerdes A.M., Iervasi G. Thyroid replacement therapy and heart failure. Circulation. 2010;122:385–393. doi: 10.1161/CIRCULATIONAHA.109.917922. [DOI] [PubMed] [Google Scholar]
- 3.Fliers E., Bianco A.C., Langouche L., Boelen A. Thyroid function in critically ill patients. Lancet Diabetes Endocrinol. 2015;3:816–822. doi: 10.1016/S2213-8587(15)00225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galli E., Pingitore A., Iervasi G. The role of thyroid hormone in the pathophysiology of heart failure: clinical evidence. Heart Fail Rev. 2010;15:155–169. doi: 10.1007/s10741-008-9126-6. [DOI] [PubMed] [Google Scholar]
- 5.Katzeff H.L., Powell S.R., Ojamaa K. Alterations in cardiac contractility and gene expression during low-T3 syndrome: prevention with T3. Am J Physiol. 1997;273:E951–E956. doi: 10.1152/ajpendo.1997.273.5.E951. [DOI] [PubMed] [Google Scholar]
- 6.Pingitore A., Galli E., Barison A., Iervasi A., Scarlattini M., Nucci D., L’abbate A., Mariotti R., Iervasi G. Acute effects of triiodothyronine (T3) replacement therapy in patients with chronic heart failure and low-T3 syndrome: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2008;93:1351–1358. doi: 10.1210/jc.2007-2210. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton M.A., Stevenson L.W., Luu M., Walden J.A., Angeles L. Altered thyroid hormone metabolism in advanced heart failure. J Am Coll Cardiol. 1990;16:91–95. doi: 10.1016/0735-1097(90)90462-x. [DOI] [PubMed] [Google Scholar]
- 8.Ascheim D.D., Hryniewicz K. Thyroid hormone metabolism in patients with congestive heart failure: the low triiodothyronine state. Thyroid. 2002;12:511–515. doi: 10.1089/105072502760143908. [DOI] [PubMed] [Google Scholar]
- 9.Iervasi G. Low-T3 syndrome: a strong prognostic predictor of death in patients with heart disease. Circulation. 2003;107:708–713. doi: 10.1161/01.cir.0000048124.64204.3f. [DOI] [PubMed] [Google Scholar]
- 10.Ranasinghe A.M., Quinn D.W., Pagano D., Edwards N., Faroqui M., Graham T.R., Keogh B.E., Mascaro J., Riddington D.W., Rooney S.J., Townend J.N., Wilson I.C., Bonser R.S. Glucose-insulin potassium and tri-iodothyronine individually improve hemodynamic performance and are associated with reduced troponin I release after on-pump coronary artery bypass grafting. Circulation. 2006;114:I245–I250. doi: 10.1161/CIRCULATIONAHA.105.000786. [DOI] [PubMed] [Google Scholar]
- 11.Portman M.A., Slee A., Olson A.K., Cohen G., Karl T., Tong E., Hastings L., Patel H., Reinhartz O., Mott A.R., Mainwaring R., Linam J., Danzi S., TRICC Investigators Triiodothyronine supplementation in infants and children undergoing cardiopulmonary bypass (TRICC): a multicenter placebo-controlled randomized trial: age analysis. Circulation. 2010;122:S224–S233. doi: 10.1161/CIRCULATIONAHA.109.926394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casartelli M., Bombardini T., Simion D., Gaspari M.G., Procaccio F. Wait, treat and see: echocardiographic monitoring of brain-dead potential donors with stunned heart. Cardiovasc Ultrasound. 2012;10:25. doi: 10.1186/1476-7120-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.