Abstract
Sinus of Valsalva aneurysm (SVA) is a rare cardiac anomaly that is usually congenital, but may be acquired. They are usually asymptomatic unless they compress adjacent structures, develop thrombosis, or rupture. A ruptured SVA (RSVA) can lead to rapid hemodynamic deterioration and often needs to be addressed emergently. Surgical correction has traditionally been the treatment of choice for RSVA; however, lately they have been successfully closed percutaneously using various transcatheter devices. Few cases of RSVA during pregnancy have been reported which were conservatively or surgically managed. There is no documented case of transcatheter closure of RSVA during pregnancy. We report the first case of successful percutaneous device closure of RSVA using an Amplatzer duct occluder in a pregnant woman presenting with heart failure due to RSVA at 26 weeks of gestation.
<Learning objective: Ruptured sinus of Valsalva aneurysm (RSVA) is traditionally repaired by surgery but more recently amenable to percutaneous intervention. Management of RSVA during pregnancy is complex and has been managed by surgery in the past incurring significant risk to fetus due to effects of cardiopulmonary bypass. We report a case of RSVA in pregnancy that was closed by transcatheter closure for the first time, thereby significantly reducing maternal and fetal risks. While risks are present during pregnancy, emergently indicated life-saving invasive cardiac procedures should not be denied solely on the pregnant state.>
Keywords: Pregnancy, Ruptured aneurysm, Sinus of Valsalva aneurysm, Percutaneous, Transcatheter intervention
Introduction
A sinus of Valsalva aneurysm (SVA) is a rare cardiac anomaly, usually congenital in origin, rarely acquired, most often arising from right coronary sinus (80.7%), less often from non-coronary sinus (15.8%), and rarely from left (3.5%) sinus [1]. SVA are asymptomatic unless they compress adjacent structures, form a thrombus, or rupture causing left to right shunting or aortic valve insufficiency and congestive heart failure that often requires urgent surgical resolution. There are few documented cases of ruptured SVA (RSVA) in pregnant patients some of whom were managed by surgical repair [2] and for others intervention was postponed until after delivery [3]. We present for the first time, a case of RSVA that was closed by transcatheter closure during pregnancy.
Case report
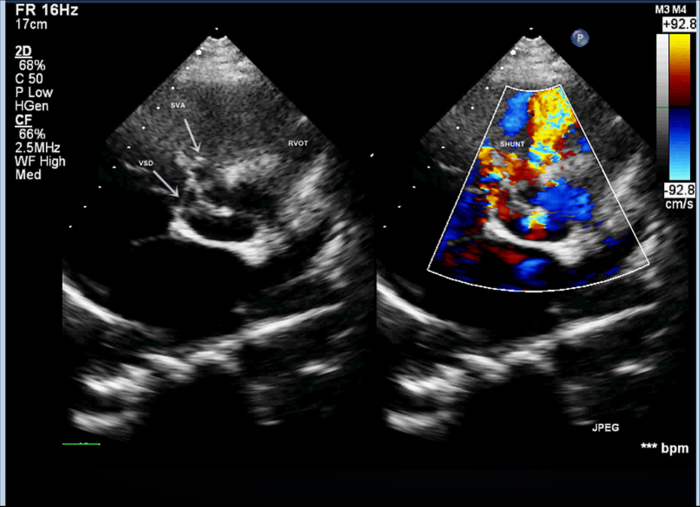
A 25-year-old, 26-week pregnant primigravida weighing 58 kg with a past history of small perimembranous ventricular septal defect (VSD) was transferred to our center from an obstetric hospital for evaluation and management of acute heart failure. On examination, she was mildly dyspneic at rest (respiratory rate: 22/min), tachycardic (heart rate: 120/min), and normotensive (BP: 110/60 mmHg). Her jugular venous pressure was elevated; precordium was hyperdynamic with a prominent systolic and diastolic thrill. S1 and S2 were normal; a diffuse grade 4/6 continuous murmur was heard. She had 3-cm hepatomegaly and bilateral mild pitting pedal edema. She did not have any symptoms or signs of connective tissue disorder. Electrocardiogram showed normal sinus rhythm with infrequent premature ventricular contractions. A transthoracic echocardiogram (TTE) revealed mildly dilated left ventricle with normal function, a 7-mm perimembranous ventricular septal defect (VSD), SVA of right coronary sinus that had ruptured into the right ventricular outflow tract (RVOT) through a 7-mm defect (Fig. 1, and see supplementary data online, Video 1). Color Doppler revealed continuous flow from the aortic root into RVOT, mild aortic regurgitation and mild pulmonary hypertension. There was no pericardial effusion. Simultaneous ultrasound and fetal echocardiogram confirmed fetal well-being and absence of fetal cardiac defects. Her symptoms of acute heart failure progressed and her hemodynamic condition worsened overnight despite medical therapy, necessitating intervention. After some consideration of surgical vs. catheter closure, we decided to proceed with the percutaneous route, because the latter procedure was safer for the mother and also carried less fetal risk.
Fig. 1.
Transthoracic echocardiographic images in parasternal short-axis view demonstrating small perimembranous ventricular septal defect and ruptured aneurysm of right coronary sinus of Valsalva with left to right shunt into the right ventricular outflow tract.
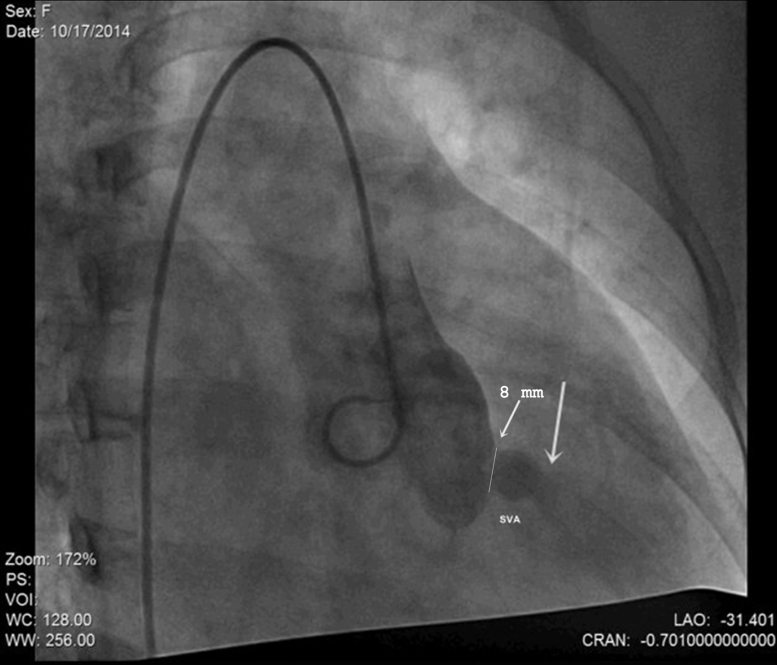
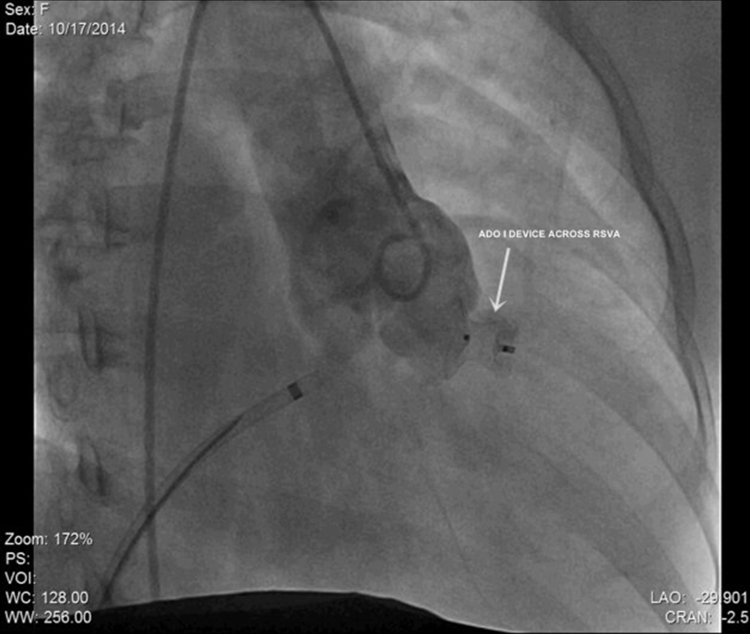
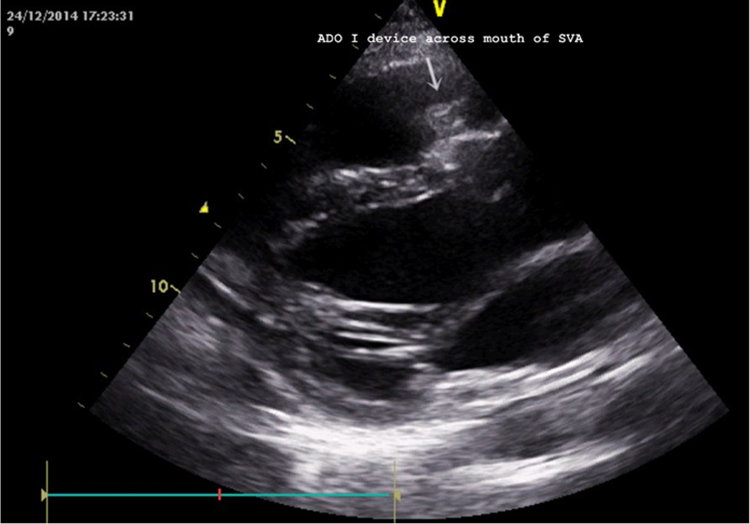
General anesthesia was avoided; the procedure was performed under local anesthesia with TTE guidance after her abdomen was properly shielded with lead. 7F short sheaths were secured in right femoral vein and bilateral femoral arteries and heparin administered. Aortic root angiogram was done which showed SVA from right coronary sinus measuring 8 mm at its origin, with contrast spilling into RVOT from the ruptured site (Fig. 2 and see supplementary data online, Video 2). The coronary arteries appeared normal. Antegrade approach was employed. Arterio-venous loop was created by passing through the defect from the aortic side using an angled tip 0.035″ glide wire (Terumo Inc., Tokyo, Japan) in 6F Judkins right coronary catheter. Glide wire was exchanged with a 260 cm, 0.035″ Amplatz support stiff guide wire (Cook Medical Inc., Bloomington, IN, USA) over which a 7F Amplatzer long sheath (St. Jude Medical, Inc. St. Paul, MN, USA) was tracked from the venous side and parked in ascending aorta. We chose to use an Amplatzer Duct Occluder (ADO1; St. Jude Medical, Inc.) because we thought it suited best for the “wind-sock”-like defect this patient had, with a broader aortic end. We chose to oversize it to 4 mm greater (ADO 12/14) than the defect size (8 mm) because the margins of this wind-sock-like defect appeared flimsy. The device was loaded over the cable, passed through the long sheath and deployed across the defect. Angiogram performed via pigtail catheter introduced through the second femoral arterial sheath confirmed optimum position and absence of residual shunt. After TTE revealed no impingement of device on aortic leaflets and no change in degree of aortic insufficiency, the device was released (Fig. 3 and see supplementary data online, Video 3). All pregnancy precautions, such as keeping flouroscopy time short (22 min), reducing number of cineangiograms (6 at 15 fps), radiation dose (dose area product-23,796 mGy cm2), and contrast volume were taken. Soon after the procedure, the patient reported improvement in her symptoms; clinically diastolic component of murmur disappeared (holosystolic murmur of VSD persisted). TTE after the procedure revealed the device to be in a good position without any residual shunt (Fig. 4). Fetal echocardiography confirmed an active fetus with normal fetal heart rate and rhythm. We decided to follow the small VSD medically as the shunt was not hemodynamically significant enough to close. Aspirin at 150 mg once daily was commenced, which was continued throughout pregnancy. Mother and fetus were regularly followed and elective cesarean section was performed at near term to deliver a healthy 3-kg baby.
Fig. 2.
Angiographic image in 30° right anterior oblique projection demonstrating right coronary sinus of Valsalva aneurysm (SVA) and contrast spilling into right ventricular outflow tract through the ruptured sinus (thick arrow), mouth of aneurysm measuring 8 mm (thin arrow).
Fig. 3.
Post-procedure angiographic image in 30° right anterior oblique projection demonstrating a well seated Amplatzer Duct Occluder (ADO) I device across the ruptured aneurysm of right coronary sinus of Valsalva (RSVA) without any residual shunt.
Fig. 4.
Post-procedure transthoracic echocardiogram in parasternal long-axis view showing device in good position across the mouth of sinus of Valsalva aneurysm.
Discussion
Incidence of SVA ranges from 0.23% to 1.5%, being higher in Asians [1]. Pathogenesis likely involves incomplete fusion of distal bulbar septum and truncal ridges, leading to weakness between aortic media and annulus fibrosus resulting in aneurysmal enlargement of sinus. SVA ruptures in 35% of cases, leading to acute symptoms of dyspnea, palpitation, chest pain, and fatigue in one fourth of the patients.
RSVA has been reported in pregnancy [2], [3], [4], [5], [6], [7], [8], [9], [10]; it is unknown if pregnancy precipitates rupture. Increase in cardiac output and decrease in systemic vascular resistance, during pregnancy, may precipitate cardiac failure in patients with intracardiac shunts. Pregnancy also causes hormonally induced change in mechanical properties of connective tissue, which could theoretically lead to rupture of SVA more often during pregnancy.
Management of RSVA during pregnancy can be challenging. Most reported cases were followed conservatively until delivery without intervention [3]. However, in patients such as ours who present with progressive heart failure, definitive management during pregnancy becomes imperative. Treatment of choice for RSVA has traditionally been surgical correction [11]; however, cardiac surgery with extra corporeal support in a pregnant patient constitutes a complex task, as the sum effects of anesthesia, surgery, and cardiopulmonary bypass (CPB) on two individuals under biologically distinct situations have to be considered. Hemodilution, changes in coagulation, complement activation, release of vasoactive substances, particulate and air embolism, and hypotension during CPB add to the deleterious effect of pre-existing hyperdynamic circulation of pregnancy [12]. Emergent CPB invariably causes fetal bradycardia, likely due to placental hypoperfusion and hypoxia [12]. Non-pulsatile flow of CPB rapidly triggers severe placental dysfunction due to vasoconstriction and catecholamine-mediated increase in systemic vascular resistance both of which may cause fetal death [12]. However, with improving anesthetic and surgical techniques, risk to the mother has been reduced to levels similar to that of non-pregnant counterparts; nonetheless, the fetal mortality remains high (19–33%) [12], [13]. We found only two reports of surgical repair during pregnancy using CPB and deep hypothermic circulatory arrest who subsequently delivered normal infants at term [2], [14].
This is the first report of successful transcatheter closure of RSVA during pregnancy. Cardiac catheterization in pregnancy also poses a unique challenge due to potential risks from radiation and medication (acute and chronic) use. Exposure of pregnant women to radiation often causes high levels of anxiety, but it is reassuring that the harmful effects of radiation such as spontaneous abortion, carcinogenesis, genetic effects, and teratogenicity are typically not observed with the doses used for the majority of diagnostic and therapeutic procedures. Risk for the fetus is limited when the exposure dose to the mother is <50 mGy and for most cardiac procedures this usually does not exceed 20 mGy [15]. At these doses there is no observed increased risk of fetal malformation; a small increase in risk of childhood cancer cannot be excluded. Effective doses to fetus of over 50–100 mSv are known to increase the incidence of fetal malformation, while the median exposure of fetus in most therapeutic cardiac catheterization procedures is 6.0 mSV [16]. Despite the low risk of radiation, all precautions such as restricting the X-ray beam size, directing primary beam away from the fetus, selecting appropriate exposure factors, minimizing fluoroscopy time, abdominal shielding, and minimizing iodinated contrast agents must be taken. The best period to perform invasive procedures is the beginning of the second trimester, when organogenesis is complete but the uterus is still small. While risks are present during pregnancy, emergently indicated invasive cardiac procedures should not be denied or delayed solely on the pregnant state, as many of these procedures have highly time-responsive benefits that can be lost by irrational delays.
Another issue that needs special address during pregnancy is the safety to the fetus of long-term oral medications such as aspirin to prevent device thrombosis. Oligohydramnios and minor bleeding complications after delivery may occur with aspirin; however, aspirin at 60–150 mg /day is widely used for prevention of pregnancy-induced hypertension with no observed risk of maternal or neonatal adverse effects [17]. There have been no reports of premature closure of the fetal ductus arteriosus with the maternal use of aspirin.
To conclude, transcatheter closure of RSVA is safe and effective even during pregnancy. It can be life-saving for a pregnant woman with this condition without incurring the high fetal morbidity and mortality associated with maternal cardiac surgery using CPB. It is also applicable for patients with poor general condition, the elderly, and those with co-morbidities who are considered at high risk for cardiac surgery. Extended follow-up is required to assess the long-term outcome of these patients.
Conflict of interest
None.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jccase.2015.08.001.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Chu S.H., Hung C.R., How S.S., Chang H., Wang S.S., Tsai C.H., Liau C.S., Tseng C.D., Tseng Y.Z., Lee Y.T. Ruptured aneurysms of the sinus of Valsalva in Oriental patients. J Thorac Cardiovasc Surg. 1990;99:288–298. [PubMed] [Google Scholar]
- 2.Pamulapati M., Teague S., Stelzer P., Thadani U. Successful surgical repair of a ruptured aneurysm of the sinus of Valsalva in early pregnancy. Ann Intern Med. 1991;115:880–882. doi: 10.7326/0003-4819-115-11-880. [DOI] [PubMed] [Google Scholar]
- 3.Cripps T., Pumphrey C.W., Parker D.J. Rupture of the sinus of Valsalva during pregnancy. Br Heart J. 1987;57:490–491. doi: 10.1136/hrt.57.5.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pelaez Romero R., Grigorov I., Gimenez I., Aquilar J.L., Atanassof P. Ruptured sinus of Valsalva aneurysm and pregnancy: what anesthetic technique should be preferred for a scheduled cesarean section? Rev Esp Anestesiol Reanim. 2010;57:177–180. doi: 10.1016/s0034-9356(10)70193-6. [DOI] [PubMed] [Google Scholar]
- 5.Rambaldi R., Pozatti A., Colletta M., Pallotti G., Perugini E., Pedone C., Greco C., Nardi R., Di Pasquale G. Acute rupture of sinus of Valsalva in the right atrium during attempted pregnancy. J Cardiovasc Med. 2008;9:323–324. doi: 10.2459/JCM.0b013e3280c56d32. [DOI] [PubMed] [Google Scholar]
- 6.Vincelj J., Starcević B., Sokol I., Sutlić Z. Rupture of a right sinus of Valsalva aneurysm into the right ventricle during vaginal delivery: a case report. Echocardiography. 2005;22:844–846. doi: 10.1111/j.1540-8175.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- 7.Divakar S.R., Singh C., Verma C.M., Kulkarni C.D. Cesarean section under epidural anesthesia in a documented case of ruptured aneurysm of the sinus of Valsalva. J Anaesthesiol Clin Pharmacol. 2015;31:119–122. doi: 10.4103/0970-9185.150565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talaulikar V., Verenkar S., Kamat A. Successful outcome of a rare case of ruptured sinus of Valsalva aneurysm in pregnancy. J Obstet Gynaecol India. 2012;62:4–5. doi: 10.1007/s13224-013-0352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goel L., Gautam P., Suchith C. Ruptured aneurysm of right sinus of Valsalva in pregnancy-a case report. Indian J Anaesth. 2009;5:88–93. [PMC free article] [PubMed] [Google Scholar]
- 10.Latzman J., Makaryus A.N., Rosman D. Ruptured sinus of Valsalva aneurysm in a pregnant woman. Tex Heart Inst J. 2006;33:66–69. [PMC free article] [PubMed] [Google Scholar]
- 11.Moustafa S., Mookadam F., Cooper L., Adam G., Zehr K., Stulak J., Holmes D. Sinus of Valsalva aneurysms-47 years of a single center experience and systematic overview of published reports. Am J Cardiol. 2007;99:1159–1164. doi: 10.1016/j.amjcard.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 12.Parry A.J., Westaby S. Cardiopulmonary bypass during pregnancy. Ann Thorac Surg. 1996;61:1865–1869. doi: 10.1016/0003-4975(96)00150-6. [DOI] [PubMed] [Google Scholar]
- 13.Patel A., Asopa S., Tang A.T.M., Ohri S.K. Cardiac surgery during pregnancy. Tex Heart Inst J. 2008;35:307–312. [PMC free article] [PubMed] [Google Scholar]
- 14.Metgud M., Dixit M., Dhulked V., Kamath P. Successful term delivery following ruptured sinus of Valsalva aneurysm repair at 29 weeks of pregnancy. J Turkish-German Gynecol Assoc. 2008;9:103–104. [Google Scholar]
- 15.Pieper P.G., Hoendermis E.S., Drijver Y.N. Cardiac surgery and percutaneous intervention in pregnant women with heart disease. Neth Heart J. 2012;20:125–128. doi: 10.1007/s12471-012-0244-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bacher K., Bogaert E., Lapere R., De Wolf D., Thierens H. Patient-specific dose and radiation risk estimation in pediatric cardiac catheterization. Circulation. 2005;111:83–89. doi: 10.1161/01.CIR.0000151098.52656.3A. [DOI] [PubMed] [Google Scholar]
- 17.Imperiale T.F., Petrulis A.S. A meta-analysis of low-dose aspirin for the prevention of pregnancy-induced hypertensive disease. JAMA. 1991;266:260–264. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.