Abstract
In this study, a newly isolated oleaginous fungus, Mucor circinelloides (M. circinelloides) Q531, was able to convert mulberry branches into lipids. The highest yield and the maximum lipid content produced by the fungal cells were 42.43 ± 4.01 mg per gram dry substrate (gds) and 28.8 ± 2.85%, respectively. The main components of lignocellulosic biomass were gradually reduced during solid-state fermentation (SSF). Cellulose, hemicellulose and lignin were decreased from 45.11, 31.39 and 17.36% to 41.48, 28.71, and 15.1%, respectively. Gas chromatography analysis showed that the major compositions of the fermented products were palmitic acid (C16:0, 18.42%), palmitoleic acid (C16:1, 5.56%), stearic acid (C18:0, 5.87%), oleic acid (C18:1, 33.89%), linoleic acid (C18:2, 14.45%) and γ-linolenic acid (C18:3 n6, 22.53%) after 2 days of SSF. The fatty acid methyl esters contained unsaturated fatty acids with a ratio of 75.95%. The composition and content obtained in this study are more advantageous than those of many other biomass lipids. Meanwhile, the oleaginous fungus had a high cellulase activity of 1.39 ± 0.09 FPU gds−1. The results indicate that the enzyme activity of the isolated fungus was capable of converting the cellulose and hemicelluloses to available sugar monomers which are beneficial for the production of lipids.
Keywords: microbial lipid, solid-state fermentation, mulberry branches, oleaginous fungi, lignocellulose
1. Introduction
Mulberry, an important economic crop, is planted widely in many different countries. China has the largest planted area of mulberry with about 626 000 ha [1,2]. In addition to the fruit, other parts of the mulberry tree are also harvested. Mulberry leaves are used in sericulture, and abundant mulberry branches are by-products of this process. Although mulberry branches have long been used in Chinese medicine [3,4], most are harvested for firewood or result in agro-waste every year, which results in notable environmental problems [5]. Therefore, efficient use of excess mulberry branches remains an important challenge. Many studies have been focused on exploring functional materials from mulberry trees in the past few years. Lignocellulose in mulberry can be used to produce slow-release urea fertilizer [6], biochar [5], cellulose whiskers [7], natural fibre [8] and scrimber [9]. Also, pectin [2], antiviral flavonoids [4] and mulberroside A [10] can be directly extracted from the bark or xylem of mulberry branches.
As lignocellulose biomass, mulberry branches are abundant in hemicellulose, cellulose and lignin. Mulberry branches contain 54.3% cellulose and 28.9% lignin [5]. These soluble and insoluble carbohydrates are widely recognized as a promising feedstock for biofuel and bioenergy [11–13]. Recently, published studies reported that lignocellulose biomass could be bioconverted into lipids by oleaginous microorganisms that can accumulate more than 20% (w/w) of lipids of its total dry biomass weight [14]. The principal oleaginous microbial species are bacteria, yeasts, fungi and microalgae. Microbial lipids, namely single cell oil (SCO), are considered as a promising feedstock for biodiesel production [15]. Usually, biodiesel is produced from vegetable oils from oleaginous plants, but its development and application have been hindered by the high cost of the feedstock. Therefore, it is necessary to explore new raw materials that reduce the price of biodiesel without competing with food production [16]. SCO constitutes a promising alternative for producing biodiesel due to some advantages such as short producing period, little required labour and ease of scale-up [17]. However, the SCO source material is usually starch or glucose, which is still consumed as human food. Therefore, waste lignocellulose biomass has become the ideal SCO source material. Also, the use of mulberry branches to produce SCO is a useful way of disposing of them.
Typically, lignocelluloses are first hydrolysed by acid or alkali, and then, the lignocellulosic hydrolysates, which contain the mono-sugar and xylose, will be fermented into microbial lipids by submerged fermentation (SmF). However, during pretreatment, various inhibitors are generated, mainly weak acids, furan derivatives and phenolic compounds. Most of these inhibitors are toxic to microbes, hindering their cell growth and lipid accumulation. Moreover, the high costs of pretreatment and the SmF system have become the major obstacles for the wide application of biodiesel production. To overcome the shortcomings of SmF, solid-state fermentation (SSF) has been explored regarding cultivation of oleaginous fungus [18–20] because a solid medium better simulates the natural habitat of the fungi [21]. SSF has several advantages over SmF, which include lower wastewater output, reduced energy requirements, simpler fermentation media, easier aeration and reduced bacterial contamination [22]. Furthermore, SSF has also many advantages such as smaller bioreactor volume, no need of organic solvents (which generally confer some level of toxicity for the extract) and higher productivity [23,24]. Try et al. studied the production of γ-decalactones using SSF by Y. lipolytica W29, and the production amount reached the high concentration (5 g l−1) [25]. Lin et al. investigated that Aspergillus oryzae A-4 yielded a lipid of 36.6 mg per gram dry substrate (gds), and a cellulase activity of 1.82 FPU gds−1 with 25.25% of holocellulose use in the substrates was detected on the 6th day in SSF of the wheat straw and bran mixture without pretreatment [19].
Mucor sp. is a natural fungal species, which can be used to produce phytase in SSF processes and has been successfully employed in the production of lipids in SmF processes [17,26]. Several species of this genus have also been proved to be able to accumulate lipids up to 30–60% of biomass [27–31]. However, research studies of Mucor sp. on producing microbial lipid by SSF have not been reported; moreover, lipid productivities of other species by SSF of biomass were still low [32,33]. In this study, we investigated the feasibility of direct bioconversion of mulberry branches into microbial lipids by using a newly isolated filamentous fungus that has been identified as Mucor circinelloides Q531 (M. circinelloides Q531). Furthermore, the characteristics of the microbial lipids from mulberry branches are studied to determine whether the lipids can be used as a feedstock for biodiesel.
2. Material and methods
2.1. Lignocellulosic material
Mulberry branches were obtained from Jiangsu province in China and air-dried for 5 days. The raw materials were ground into a size of 40 mesh, and then oven-dried at 80°C for 24 h before being stored in a dryer at room temperature.
2.2. Isolation and identification of the oleaginous fungi
The oleaginous fungi were isolated from rotten branches and leaves found on Purple Mountain, located in Nanjing, China. The collection of rotten material was inoculated onto potato dextrose agar medium (PDA medium, pH adjusted to 6.5) containing 0.0001% chloramphenicol, using the spread-plate and streak-plate techniques, and then incubated for 6 days at 28°C. Fungal strains were stained with the Sudan black B technique [34] to screen for fungi with high lipid content. The nine strains with high lipid content were identified as those with large lipid globules, isolated and purified at 28°C on PDA medium and stored at 4°C.
The selected oleaginous fungi were identified based on the 18S rDNA sequences of their internal transcribed spacer (ITS) regions [35]. Briefly, mycelia were harvested by filtration from a liquid culture after 2 days of growth and were transferred to sterile mortar before liquid nitrogen was added. Mycelia were ground into a fine powder. After being ground in the presence of liquid nitrogen, the genomic DNA was extracted using an Ezup column fungi genomic DNA purification kit (Sangon Biotech, Shanghai, China). The ITS region was amplified using the universal primers ITS1 (5′-TCCGTAGGTGAACCT GCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′). Polymerase chain reaction (PCR) amplification was performed by initial denaturation at 94°C for 3 min followed by 30 cycles of 94°C for 1 min, 55°C for 30 s and 72°C for 1 min, with a final extension at 72°C for 10 min. PCR fragments were purified using a SanPrep column DNA gel extraction kit (Sangon Biotech). The obtained sequences were BLAST-searched against the National Center for Biotechnology Information database. Closely related multiple sequences were aligned and corrected. The phylogenetic tree was constructed using the neighbour-joining program in MEGA 5.0 [36].
2.3. Solid-state fermentation
Three grams of dried lignocellulosic materials and 6 ml of mineral salt solution (MS solution contained (NH4)2SO4, 1.7 g; KH2PO4, 2.0 g; MgSO4·7H2O, 0.5 g; CaCl2·2H2O, 0.2 g; FeSO4·7H2O, 0.01 g; ZnSO4·7H2O, 0.01 g; MnSO4·4H2O, 0.001 g; CuSO4·5H2O, 0.0005 g; 0.1% Tween-80 (w/v), pH adjusted to 6.5) were well mixed and added to culture dishes. After sterilization, the medium was cooled down and inoculated with 0.5 ml of a spore suspension containing 107 spores gds−1. The culture was incubated at 28°C, 70–80% humidity for 12 days in an artificial climate incubator. Lipid yield, cellulase activity, fungal biomass, residual sugars, component of biomass and fatty acid composition were detected at different times during SSF.
2.4. Analysis methods
2.4.1. Determination of lipid yield
The samples, which consisted of the whole fermented lignocellulosic material and fungal biomass, were washed twice with distilled water in order to remove the remaining salts, centrifuged again and freeze-dried to a constant weight for 48 h. Extraction of total lipids was performed according to the previous method [37] with the following modifications. The dry sample (0.5 g) was soaked with 6 ml of 4 M HCl in 50 ml centrifuge tubes for 30 min, transferred into boiling water for 20 min, and cooled instantly to −20°C for 10 min. After the first extraction, the sample was vortexed with 12 ml methanol/chloroform (2 : 1, v/v), the remaining cell lipids were further extracted once with 12 ml methanol/chloroform (1 : 1, v/v); and then twice with 12 ml methanol/chloroform (1 : 2, v/v) [38]. Each extraction step consisted of incubation for approximately 1 day at room temperature, after which they were centrifuged at 5000g for 5 min. The bottom organic phase was carefully sucked out through the pipette, then 6 ml 0.1% NaCl solution was added, followed by centrifugation at 5000g for 5 min. Finally, the chloroform phase was transferred to new centrifuge tubes by a pipette. The solvent was removed by the nitrogen purge method, and the total lipid content was measured gravimetrically. The lipid content of the cells in SSF was expressed as percentage of gram lipids per gram dry cell weight (%). The lipid yield in SSF was expressed as milligram lipids per gram dry substrate (mg gds−1). The lipids in the raw material were subtracted from the total lipids extracted before calculation.
2.4.2. Determination of cellulase activity
To prepare the enzyme extract in SSF, the fermented residue was suspended in 100 ml citrate buffer (pH 4.8, 0.1 M), and the mixture was shaken at 37°C for 2 h at 180 r.p.m. before being centrifuged. The clarified supernatant was used for measurement of the cellulase activity by filter paper assay according to the US National Renewable Energy Laboratory (NREL) [39]. The reactions were incubated at 50°C for 1 h, and the released reducing sugars were determined by the 3,5-dinitrosalicylic acid (DNS) method using glucose as the standard for cellulase activities [40]. One unit (U) of enzyme activity was defined as the amount of enzyme required to release 1 µmol of reducing sugar per minute under assay conditions. The enzymatic activity was expressed as filter paper unit per gram of dry substrate (FPU gds−1).
2.4.3. Determination of fungal biomass
Fungal growth estimation in SSF was carried out by estimation of N-acetyl glucosamine released by the acid hydrolysis of chitin present in the cell wall of the fungi [26,41]. The obtained results were depicted as mg glucosamine per gram initial dry substrate (mg gds−1).
2.4.4. Determination of cellulose, hemicellulose and lignin
For cellulose content assayed by means of an HNO3-ethanol method, hemicellulose was measured according to a two-brominating method and lignin was determined by the 72% (w/w) H2SO4 method [42].
2.4.5. Determination of residual sugars
The residual sugars in the fermented residue were extracted by ultrapure water and were analysed using high performance liquid chromatography (HPLC) (Shimadzu LC-20A) equipped with a strong acid cation-exchange resin column (Bio-Rad Aminex HPX-87H) operating at 55°C and a differential refractive index detector (RID-10A) operating at 40°C. The mobile phase was dilute H2SO4 solution of 0.005 mol l−1 at the flow rate of 0.6 ml min−1.
2.4.6. Determination of fatty acid composition
Prior to GC analysis, the extracted lipid samples were transformed into their corresponding methyl esters by using the modified protocol [43]. Briefly, 0.4 M KOH-methanol reagent was added to the lipid samples at 65°C for 30 min, followed by treatment with 14% BF3-methanol reagent at 65°C for 10 min and extraction with n-hexane/saturated NaCl solution (2 : 1). The samples were centrifuged (3500g) and the supernatant was obtained. Fatty acid profiles of microbial oils were performed by a GC-2010 gas chromatograph (Shimadzu Inc., Kyoto, Japan) equipped with a cross-linked capillary Rtx-WAX column (30 m length, 0.32 mm internal diameter, 0.5 µm film thickness) and a flame ionization detector. The operating conditions were as follows: inlet temperature at 250°C, detector temperature at 280°C and initial oven temperature at 50°C held for 1 min then raised to 200°C at a rate of 25°C min−1, continuous ramping of temperature to 230°C at a rate of 2°C min−1, then held at 230°C for 23 min. Chromatographic peaks and retention times were identified by the comparison to a fatty acid methyl ester (FAME) standard mixture (Supelco 37-Component FAME Mix, Sigma-Aldrich, St. Louis, MO, USA), and individual peaks were quantified by means of external standards and their corresponding calibration curves.
3. Results and discussion
3.1. Screening and characterization of isolated fungi
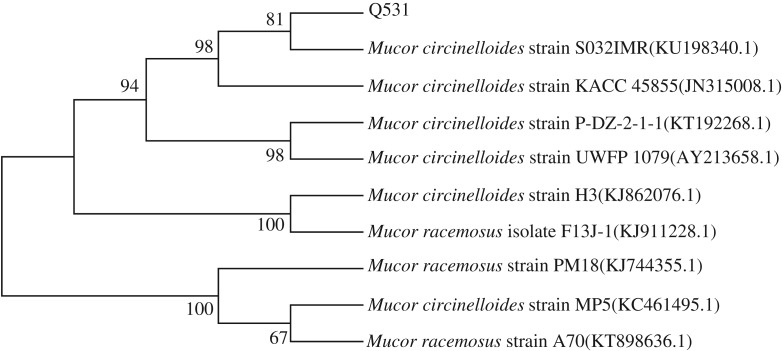
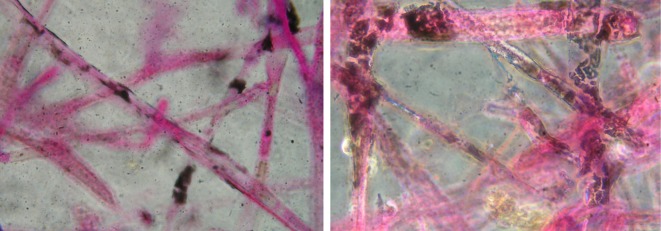
In this study, nine types of different fungi were isolated. They were inoculated in SSF. After 6 days, the lipid content of these strains was detected (see electronic supplementary material, figure S1). One of the isolated fungi, Q531, which contained the highest lipid content, was selected for further study. The 18S rDNA sequence of the newly isolated strain was submitted to the GenBank under the accession number KU523400. The BLAST search results indicated that the sequence of this strain was found to share 99% max ident with those of M. circinelloides. The 18S rDNA sequence and its homologous sequences were analysed using MEGA 5.0 software and a phylogenetic tree was established, as shown in figure 1. The oleaginous fungi were stained with Sudan black B to detect the presence of blue-black lipid globules within the cells. Figure 2 shows lipid globules detected within the cells of M. circinelloides Q531.
Figure 1.
Neighbour-joining phylogenetic tree based on the 18S rDNA sequences shows the relationship between the newly isolated M. circinelloides Q531 and other members of the genus Mucor. The number of replicates is 1000. Numbers following the names of the strains are accession numbers of published sequences in the GenBank database.
Figure 2.
Photographs of lipid globules in the newly isolated M. circinelloides Q531 undergoing SSF taken by a microscope with a ×1000 oil immersion lens.
3.2. Lipid production and biomass by M. circinelloides Q531 in solid-state fermentation
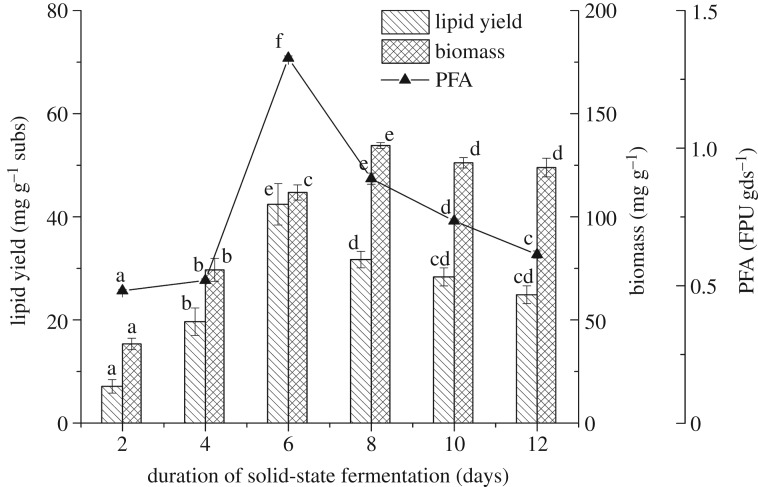
As seen in figure 3, M. circinelloides Q531 has the key characteristics of a typical oleaginous species. Both the fungal biomass and lipid yield increased within 6 days, and the highest lipid yield was 42.43 ± 4.01 mg gds−1. However, lipid yield decreased dramatically after day 6, while the biomass and lignocellulose use kept increasing, which might be attributed to the use of the storage lipids for biomass production. The biomass concentration drastically increased to the maximum of 134.56 ± 1.41 mg g−1 and then started to decline slightly after day 8. The variation tendency of lipid content was similar to lipid yield in SSF. The lipid content of the fungal cells gradually increased to a maximum of 28.8 ± 2.85% after 6 days, as shown in tables 1 and 2. Compared with the previous investigations [32,44], the lipid content of this study is more than that of many other Mucor sp. that are used in active research. The results indicated that M. circinelloides Q531 was capable of converting widely available mulberry branches into microbial lipids.
Figure 3.
Lipid yield, biomass and cellulase activity of M. circinelloides Q531 in solid-state fermentation medium over time.
Table 1.
The lipid content (%) by M. circinelloides Q531 through SSF over time.
| solid-state fermentation (days) | lipid content (%) |
|---|---|
| 2 | 10.95 ± 0.64 |
| 4 | 14.12 ± 1.79 |
| 6 | 28.8 ± 2.85 |
| 8 | 20.51 ± 0.93 |
| 10 | 17.09 ± 1.64 |
| 12 | 15.33 ± 1.28 |
Table 2.
Weight and component loss of mulberry branches caused by M. circinelloides Q531 under SSF.
| component loss (%) |
||||
|---|---|---|---|---|
| SSF time (days) | weight loss (%) | cellulose | hemicellulose | lignin |
| 6 | 12.45 ± 1.10 | 12.45 ± 1.10 | 16.14 ± 0.85 | 15.43 ± 0.42 |
| 12 | 18.37 ± 0.90 | 18.37 ± 0.90 | 25.79 ± 1.13 | 29.22 ± 1.11 |
3.3. The relationship between cellulase activity and lipid yield in solid-state fermentation
As shown in figure 3, the cellulase activity was rapidly increased (up to 1.39 ± 0.09 FPU gds−1 after 6 days of SSF). Meanwhile, the production of lipids was also increased to 42.43 ± 4.01 mg gds−1. However, the cellulase activity decreased dramatically following the decline of lipid yield after day 6 (p < 0.05), while the biomass kept increasing. As the direct conversion of lignocellulosic, biomass relies on the enzyme activity of the fungi to break down cellulose and hemicelluloses into available sugar monomers, including glucose, xylose and arabinose, for uptake, after which microorganisms use these sugars to produce lipid. Although a high carbon-to-nitrogen ratio is suitable for lipid accumulation, the enzyme activity is a crucial factor for bioconversion of lignocellulosic biomass into lipids. Consequently, low cellulase activity would cause the limitation of available material in SSF and lead to the stored lipids being converted to new biomass. Similarly, Lin et al. [19] found that the lipid degradation did occur in SSF of A. oryzae A-4 in accordance with the decrease in its own cellulase activity. The results of this study indicate that M. circinelloides Q531 was capable of converting widely available lignocellulosic biomass of mulberry branches into microbial lipids. Moreover, high cellulose activity enhanced lipid production. It turned out that the committed step of lipid yield was microbial conversion of raw materials into sugar monomers by microorganisms in SSF. As shown in figure 4, the residual sugar concentration during SSF increased for 6 days, and then reduced, which was similar to the change of lipid product. The results showed that microbial production of lipid was accomplished by converting lignocellulose into sugar.
Figure 4.
Residual sugars produced by M. circinelloides Q531 in solid-state fermentation medium over time.
3.4. The composition of lignocellulose during the solid-state fermentation process
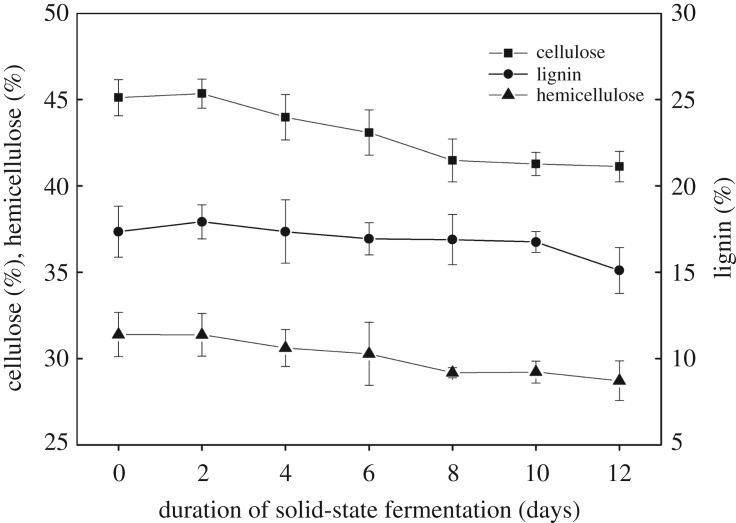
Before the beginning of SSF, the main content of lignocellulosic biomass (mulberry branches) consisted of cellulose, hemicellulose and lignin at 45.11, 31.39 and 17.36%, respectively. Cellulose, the main fraction of plant cell walls, is linear and crystalline and is a homo-polymer of repeating units of glucose linked by β (1–4) glycosidic bonds [45]; thus, the oleaginous fungi firstly use cellulose as a carbon source to grow. As seen in figure 5, the content of cellulose, in comparison to hemicellulose and lignin, was significantly decreased from 45.11% to 41.48% after 8 days. At the same time, the fungal biomass concentration rapidly increased to the maximum, but the lipid yield increased at first and then decreased, which might be attributed to the use of the storage lipids for biomass production (figure 3). Hemicellulose is a highly branched heteropolymer composed of D-xylose, D-arabinose, D-glucose, D-galactose and D-mannose [46], and its content gradually decreased from 31.39% to 28.71% in SSF for 12 days. Lignin, formed by polymerization of phenolic compounds, is hydrophobic in nature and is tightly bound to the cellulose and hemicellulose, protecting them from microbial [47] and chemical [48] degradation. It is hard to remove or modify the lignin (delignification) by microorganisms without different types of pretreatment methods. In figure 5, the results showed that the content of lignin started to decline observably after 10 days, and the content decreased from 17.36% to 15.1% in SSF. The content of cellulose, hemicellulose and lignin decreased by 5.92, 5.11 and 2.25%, respectively. This study indicated that M. circinelloides Q531 can use lignocellulosic biomass and change the chemical structure of mulberry branch in SSF. In addition, high degradation rate of cellulose and hemicellulose might benefit lipid production within a certain range of time.
Figure 5.
The change in lignocellulosic biomass (cellulose, hemicellulose and lignin) composition in solid-state fermentation over time.
3.5. GC analysis of FAMEs
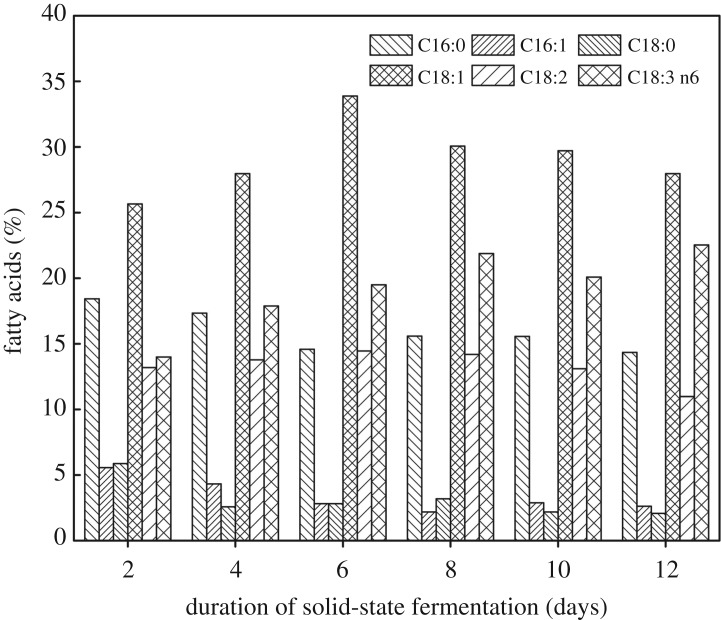
The GC analysis results (figure 6) showed the major fatty acid compositions of total lipids from the SSF products at different fermentation time. After 2 days of SSF, the compositions were palmitic acid (C16:0, 18.42%), palmitoleic acid (C16:1, 5.56%), stearic acid (C18:0, 5.87%), oleic acid (C18:1, 33.89%), linoleic acid (C18:2, 14.45%) and γ-linolenic acid (C18:3 n6, 22.53%). During 12 days of SSF process, the content of five major fatty acids was continuously changed. The contents of palmitic acid (C16:0), palmitoleic acid (C16:1) and stearic acid (C18:0) were continuously reduced. However, the contents of oleic acid (C18:1) and linoleic acid (C18:2) reached a maximum of 33.89% and 14.45%, respectively, after 6 days. On the last day, the content of γ-linolenic acid (C18:3 n6) was 22.52%.
Figure 6.
The composition of major fatty acids of lipids produced by M. circinelloides Q531 in solid-state fermentation over time.
As revealed from the data (table 3), the total unsaturated fatty acid (USFA) content was 75.95% higher than saturated fatty acid (SFA, 24.06%) content. In addition, The USFA contained mono-unsaturated fatty acid (MUFA, 38.97%) and poly-unsaturated fatty acid (PUFA, 36.98%). It is well known that high concentration of oleic acid (33.89%) in the obtained lipids was a desirable property for the production of biodiesel [49]. Moreover, γ-linolenic acid (22.53%), as a critical PUFA, was experimentally proved to have beneficial effects for the prevention and treatment of inflammatory disorders, diabetes, cardiovascular disorders, cancers and some other diseases [50]. Indeed, M. circinelloides was the first microorganism to be used commercially to produce an oil for human consumption—an oil rich in gamma-linoleic acid (GLA, 18:3; cis-6,9,12-octadecatrienoic acid) [51]. Similar fatty acid composition of lipids obtained from the oleaginous fungi was also found when M. circinelloides WJ11 was cultivated on K & R medium [52]. The fatty acid profile obtained in this study is similar to that of M. circinelloides CBS 203.28 cultivated on an acetic acid medium [33].
Table 3.
Fatty acid profile (%) of total lipids produced by M. circinelloides Q531 in solid-state fermentation of mulberry branches. Fatty acids including myristic (C14:0), palmitic (C16:0), palmitoleic (C16:1), stearic (C18:0), oleic (C18:1), linoleic (C18:2), γ-linolenic (C18:3 n6). SFA, saturated fatty acids; MUFA, mono-unsaturated fatty acids; PUFA, poly-unsaturated fatty acids.
| solid-state fermentation (days) |
||||||
|---|---|---|---|---|---|---|
| fatty acid type | 2 | 4 | 6 | 8 | 10 | 12 |
| C14:0 | 1.63 | 1.51 | 1.37 | 1.3 | 1.05 | 1.39 |
| C16:0 | 18.42 | 17.33 | 14.59 | 15.59 | 15.58 | 14.36 |
| C16:1 | 5.56 | 4.32 | 2.81 | 2.18 | 2.89 | 2.62 |
| C18:0 | 5.87 | 2.58 | 2.81 | 3.19 | 2.19 | 2.08 |
| C18:1 | 25.65 | 27.97 | 33.89 | 30.07 | 29.71 | 27.97 |
| C18:2 | 13.19 | 13.77 | 14.45 | 14.19 | 13.1 | 10.98 |
| C18:3 n6 | 14 | 17.88 | 19.51 | 21.87 | 20.09 | 22.53 |
| SFA | 33.76 | 28.74 | 24.06 | 25.89 | 26.52 | 26.87 |
| MUFA | 34.57 | 35.43 | 38.97 | 34.74 | 35.90 | 34.46 |
| PUFA | 31.67 | 35.83 | 36.98 | 39.38 | 37.59 | 38.67 |
Lipid and cellulase production was compared in table 4 through SSF of lignocellulosic biomass by oleaginous fungi. As far as we know, the first attempt at direct lipid production from lignocellulosic biomass was by Peng et al. [32]. Their strains were able to directly produce lipids from a wheat straw and bran mixture with the lipid yield of 19–42 mg gds−1. This was probably due to the cellulase activity of their strains (0.31–0.69 FPU gds−1). Lin et al. [19] found that A. oryzae A-4 could produce the amount of lipid yield (36.6 mg gds−1) by its own cellulose (1.69 FPU gds−1). Dey et al. [53] discovered that Alternaria sp. was able to produce high cellulase activity of 1.21 FPU gds−1 and lipid yield of 60.3 mg gds−1. M. elongate PFY was another strain that has been reported for its lipid yield of 70.7 mg gds−1 from rice straw but gave no available information of its enzyme activity [54]. A. tubingensis TSIP9 converted palm pressed fibre (PPF) and palm empty fruit bunches (EFB) into lipids with a maximum yield of 31.1 mg gds−1 and 37.5 mg gds−1, respectively; however, the strain produced very low cellulase activity (less than 1.3 U gds−1) from both PPF and EFB [55]. Cheirsilp et al. [18] also found that A. tubingensis TSIP9 could use the mixture of palm empty fruit bunch and palm kernel cake to produce a lipid yield of 39.5 mg gds−1, but the cellulose activity is very low (2.35 U gds−1). In this study, it was clear that the cellulase activity and lipid yield of M. circinelloides Q531 were higher than the majority of those previously reported. It should be also noted that M. circinelloides Q531 is capable of converting lignocellulosic biomass into lipids by its own secretory cellulase. This study is the first to report that the newly isolated M. circinelloides directly converted lignocellulosic material into lipids with the highest yield, without exogenous cellulose and without pretreatment.
Table 4.
Comparison of lipid and cellulase production by different oleaginous fungi grown on variety of inexpensive and non-edible agricultural and forestry residues in SSF.
| lignocellulosic biomass | strain | lipid yield (mg gds−1) | cellulase | reference |
|---|---|---|---|---|
| wheat straw and bran | Microsphaeropsis sp. | 19–42 | 0.31–0.69 (FPU gds−1) | [32] |
| wheat straw and bran | A. oryzae A-4 | 36.6 | 1.69 (FPU gds−1) | [19] |
| rice straw and wheat bran | Alternaria sp. | 60.3 | 1.21 (FPU gds−1) | [53] |
| rice straw | M. elongate PFY | 70.7 | —a | [54] |
| palm pressed fibre | A. tubingensis TSIP9 | 31.1 | <1.3 (U gds−1) | [55] |
| palm empty fruit bunches | A. tubingensis TSIP9 | 37.5 | <1.3 (U gds−1) | [55] |
| palm empty fruit bunch and palm kernel cake | A. tubingensis TSIP9 | 39.5 | 2.35 (U gds−1) | [18] |
| mulberry branches | M. circinelloides Q531 | 42.4 | 1.39 (FPU gds−1) | this study |
aNot available.
4. Conclusion
The newly isolated oleaginous fungus M. circinelloides Q531 exhibited satisfactory lipid production through SSF of mulberry branches. The fungi were able to use lignocellulosic biomass as the sole carbon and nutritional source. The main component of lignocellulosic biomass (including cellulose, hemicellulose and lignin) was continuously reduced during SSF. The maximum values of lipid yield, biomass, lipid content and cellulose activity were 42.43 ± 4.01 mg gds−1, 134.56 ± 1.41 mg gds−1, 28.8 ± 2.85% and 1.39 ± 0.09 FPU gds−1, respectively. In addition, the major fatty acids were palmitic acid (C16:0), oleic acid (C18:1), linoleic acid (C18:2) and γ-linolenic acid (C18:3 n6). M. circinelloides has the ability to greatly contribute toward the economics of biofuel production from cheap and abundant lignocellulosic biomass. Furthermore, the lipid, which was produced by M. circinelloides Q531 from mulberry branches through SSF, not only can be used as a biodiesel fuel but also can be processed into high-value-added products, such as GLA.
Supplementary Material
Ethics
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Data accessibility
DNA sequences: Genbank accessions KU523400.
Authors' contributions
T.T. carried out isolation and identification of the oleaginous fungi, participated in data analysis, carried out solid-state fermentation and determination of fungal biomass, participated in the design of the study and drafted the manuscript; J.M. carried out the acquisition of data, or analysis and interpretation of data; Q.Y. carried out determination of cellulose, hemicellulose and lignin, cellulose activity, lipid yield and fatty acid composition; W.Q. conceived of the study, designed the study, coordinated the study, helped draft the manuscript and revised it critically for important intellectual content, and gave final approval of the version to be published. All authors gave final approval for publication. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests
We have no competing interests.
Funding
The study was supported by a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
References
- 1.Sanchez MD. 2002. World distribution and utilization of mulberry, potential for animal feeding. FAO Anim. Prod. Health Paper 147,1–11. [Google Scholar]
- 2.Liu L, Cao J, Huang J, Cai Y, Yao J. 2010. Extraction of pectins with different degrees of esterification from mulberry branch bark. Bioresour. Technol. 101, 3268–3273. ( 10.1016/j.biortech.2009.12.062) [DOI] [PubMed] [Google Scholar]
- 3.Asano N, et al. 2001. Polyhydroxylated alkaloids isolated from mulberry trees (Morus alba L.) and silkworms (Bombyx mori L.). J. Agric. Food Chem. 49, 4208–4213. ( 10.1021/jf010567e) [DOI] [PubMed] [Google Scholar]
- 4.Du J, He ZD, Jiang RW, Ye WC, Xu HX, But PPH. 2003. Antiviral flavonoids from the root bark of Morus alba L. Phytochemistry 62, 1235–1238. ( 10.1016/s0031-9422(02)00753-7) [DOI] [PubMed] [Google Scholar]
- 5.Wu DL, Wang W, Zhang JH, Fu H, Lv XS, Xu XH. 2012. Preparation of mulberry branch biomass char and its usage in wastewater treatment. Water Environ. Res. 84, 2060–2069. ( 10.2175/106143012X13415215906898) [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Liang X, Yang X, Liu H, Yao J. 2014. An eco-friendly slow-release urea fertilizer based on waste mulberry branches for potential agriculture and horticulture applications. ACS Sustain. Chem. Eng. 2, 1871–1878. ( 10.1021/sc500204z) [DOI] [Google Scholar]
- 7.Li R, Fei J, Cai Y, Li Y, Feng J, Yao J. 2009. Cellulose whiskers extracted from mulberry: a novel biomass production. Carbohydr. Polym. 76, 94–99. ( 10.1016/j.carbpol.2008.09.034) [DOI] [Google Scholar]
- 8.Garcia R, Pizarro C, Lavin AG, Bueno JL. 2012. Characterization of Spanish biomass wastes for energy use. Bioresour. Technol. 103, 249–258. ( 10.1016/j.biortech.2011.10.004) [DOI] [PubMed] [Google Scholar]
- 9.Yu HX, Fang CR, Xu MP, Guo FY, Yu WJ. 2014. Effects of density and resin content on the physical and mechanical properties of scrimber manufactured from mulberry branches. J. Wood Sci. 61, 159–164. ( 10.1007/s10086-014-1455-6) [DOI] [Google Scholar]
- 10.Wang S, Liu XM, Zhang J, Zhang YQ. 2014. An efficient preparation of mulberroside A from the branch bark of mulberry and its effect on the inhibition of tyrosinase activity. PLoS ONE 9, e109396 ( 10.1371/journal.pone.0109396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chundawat SP, Beckham GT, Himmel ME, Dale BE. 2011. Deconstruction of lignocellulosic biomass to fuels and chemicals. Ann. Rev. Chem. Biomol. Eng. 2, 121–145. ( 10.1146/annurev-chembioeng-061010-114205) [DOI] [PubMed] [Google Scholar]
- 12.Service R. 2007. Cellulosic ethanol: biofuel researchers prepare to reap a new harvest. Science 315, 1488–1491. ( 10.1126/science.315.5818.1488) [DOI] [PubMed] [Google Scholar]
- 13.Cardona ÓJSC. 2008. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 99, 5270–5295. ( 10.1016/j.biortech.2007.11.013) [DOI] [PubMed] [Google Scholar]
- 14.Hu C, Zhao X, Zhao J, Wu S, Zhao ZK. 2009. Effects of biomass hydrolysis by-products on oleaginous yeast Rhodosporidium toruloides. Bioresour. Technol. 100, 4843–4847. ( 10.1016/j.biortech.2009.04.041) [DOI] [PubMed] [Google Scholar]
- 15.Li Q, Du W, Liu D. 2008. Perspectives of microbial oils for biodiesel production. Appl. Microbiol. Biotechnol. 80, 749–756. ( 10.1007/s00253-008-1625-9) [DOI] [PubMed] [Google Scholar]
- 16.Yousuf A. 2012. Biodiesel from lignocellulosic biomass – prospects and challenges. Waste Manage. (Oxford) 32, 2061–2067. ( 10.1016/j.wasman.2012.03.008) [DOI] [PubMed] [Google Scholar]
- 17.Vicente G, Bautista LF, Rodríguez R, Gutiérrez FJ, Sádaba I, Ruiz-Vázquez RM, Torres-Martínez S, Garre V. 2009. Biodiesel production from biomass of an oleaginous fungus. Biochem. Eng. J. 48, 22–27. ( 10.1016/j.bej.2009.07.014) [DOI] [Google Scholar]
- 18.Cheirsilp B, Kitcha S. 2015. Solid state fermentation by cellulolytic oleaginous fungi for direct conversion of lignocellulosic biomass into lipids: Fed-batch and repeated-batch fermentations. Ind. Crops Prod. 66, 73–80. ( 10.1016/j.indcrop.2014.12.035) [DOI] [Google Scholar]
- 19.Lin H, Cheng W, Ding HT, Chen XJ, Zhou QF, Zhao YH. 2010. Direct microbial conversion of wheat straw into lipid by a cellulolytic fungus of Aspergillus oryzae A-4 in solid-state fermentation. Bioresour. Technol. 101, 7556–7562. ( 10.1016/j.biortech.2010.04.027) [DOI] [PubMed] [Google Scholar]
- 20.Peng X, Chen H. 2008. Single cell oil production in solid-state fermentation by Microsphaeropsis sp. from steam-exploded wheat straw mixed with wheat bran. Bioresour. Technol. 99, 3885–3889. ( 10.1016/j.biortech.2007.08.015) [DOI] [PubMed] [Google Scholar]
- 21.Robinson T, Singh D, Nigam P. 2001. Solid-state fermentation: a promising microbial technology for secondary metabolite production. Appl. Microbiol. Biotechnol. 55, 284–289. ( 10.1007/s002530000565) [DOI] [PubMed] [Google Scholar]
- 22.Pandey A, Soccol CR, Mitchell D. 2000. New developments in solid state fermentation: I-bioprocesses and products. Process Biochem. 35, 1153–1169. ( 10.1016/S0032-9592(00)00152-7) [DOI] [Google Scholar]
- 23.Mitchell DA, Berovič M, Krieger N. 2006. Solid-state fermentation bioreactor fundamentals: introduction and overview. In Solid-state fermentation bioreactors: fundamentals of design and operation (eds Mitchell DA, Berovič M, Krieger N), pp. 1–12. Berlin, Germany: Springer Berlin Heidelberg. [Google Scholar]
- 24.Martins S, Mussatto SI, Martínez-Avila G, Montañez-Saenz J, Aguilar CN, Teixeira JA. 2011. Bioactive phenolic compounds: production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 29, 365–373. ( 10.1016/j.biotechadv.2011.01.008) [DOI] [PubMed] [Google Scholar]
- 25.Try S, De-Coninck J, Voilley A, Chunhieng T, Waché Y. 2018. Solid state fermentation for the production of γ-decalactones by Yarrowia lipolytica. Process Biochem. 64, 9–15. ( 10.1016/j.procbio.2017.10.004) [DOI] [Google Scholar]
- 26.Roopesh K, Ramachandran S, Nampoothiri KM, Szakacs G, Pandey A. 2006. Comparison of phytase production on wheat bran and oilcakes in solid-state fermentation by Mucor racemosus. Bioresour. Technol. 97, 506–511. ( 10.1016/j.biortech.2005.02.046) [DOI] [PubMed] [Google Scholar]
- 27.Carvalho AK, Rivaldi JD, Barbosa JC, de Castro HF. 2015. Biosynthesis, characterization and enzymatic transesterification of single cell oil of Mucor circinelloides – a sustainable pathway for biofuel production. Bioresour. Technol. 181, 47–53. ( 10.1016/j.biortech.2014.12.110) [DOI] [PubMed] [Google Scholar]
- 28.Xia C, Wei W, Hu B. 2014. Statistical analysis and modeling of pelletized cultivation of Mucor circinelloides for microbial lipid accumulation. Appl. Biochem. Biotechnol. 172, 3502–3512. ( 10.1007/s12010-014-0759-8) [DOI] [PubMed] [Google Scholar]
- 29.Wei H, et al. 2013. Genomic, proteomic, and biochemical analyses of oleaginous Mucor circinelloides: evaluating its capability in utilizing cellulolytic substrates for lipid production. PLoS ONE 8, 1–12. ( 10.1371/journal.pone.0071068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitra D, Rasmussen ML, Chand P, Chintareddy VR, Yao L, Grewell D, Verkade JG, Wang T, van Leeuwen JH. 2012. Value-added oil and animal feed production from corn-ethanol stillage using the oleaginous fungus Mucor circinelloides. Bioresour. Technol. 107, 368–375. ( 10.1016/j.biortech.2011.12.031) [DOI] [PubMed] [Google Scholar]
- 31.Xia C, Zhang J, Zhang W, Hu B. 2011. A new cultivation method for microbial oil production: cell pelletization and lipid accumulation by Mucor circinelloides. Biotechnol. Biofuels 4, 15–24. ( 10.1186/1754-6834-4-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng XW, Chen HZ. 2007. Microbial oil accumulation and cellulase secretion of the endophytic fungi from oleaginous plants. Ann. Microbiol. 57, 239–242. ( 10.1007/BF03175213) [DOI] [Google Scholar]
- 33.Immelman M, du Preez JC, Kilian SG. 1997. Effect of C:N ratio on gamma-linolenic acid production by Mucor circinelloides grown on acetic acid. Syst. Appl. Microbiol. 20, 158–164. ( 10.1016/S0723-2020(97)80061-6) [DOI] [Google Scholar]
- 34.Patnayak S, Sree A. 2005. Screening of bacterial associates of marine sponges for single cell oil and PUFA. Lett. Appl. Microbiol. 40, 358–363. ( 10.1111/j.1472-765X.2005.01671.x) [DOI] [PubMed] [Google Scholar]
- 35.White TJ, Bruns T, Lee S, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. San Diego, CA: Academic Press, Inc. [Google Scholar]
- 36.Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24, 1596–1599. ( 10.1093/molbev/msm092) [DOI] [PubMed] [Google Scholar]
- 37.Stephan J, Mailaender C, Etienne G, Daffe M, Niederweis M. 2004. Multidrug resistance of a porin deletion mutant of Mycobacterium smegmatis. Antimicrob. Agents Chemother. 48, 4163–4170. ( 10.1128/AAC.48.11.4163-4170.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cot M, Loret MO, Francois J, Benbadis L. 2007. Physiological behaviour of Saccharomyces cerevisiae in aerated fed-batch fermentation for high level production of bioethanol. FEMS Yeast Res. 7, 22–32. ( 10.1111/j.1567-1364.2006.00152.x) [DOI] [PubMed] [Google Scholar]
- 39.Adney B, Baker J. 1996. Measurement of cellulase activities. LAP-006 NREL analytical procedure. Golden, CO: National Renewable Energy Laboratory. [Google Scholar]
- 40.Ncube T, Howard RL, Abotsi EK, van Rensburg ELJ, Ncube I. 2012. Jatropha curcas seed cake as substrate for production of xylanase and cellulase by Aspergillus niger FGSCA733 in solid-state fermentation. Ind. Crops Prod. 37, 118–123. ( 10.1016/j.indcrop.2011.11.024) [DOI] [Google Scholar]
- 41.Sakurai Y, Lee TH, Shiota H. 1977. On the convenient method for the glucosamine estimation in koji. Agric. Biol. Chem. 41, 619–624. ( 10.1080/00021369.1977.10862552) [DOI] [Google Scholar]
- 42.Yang H, Yan R, Chen H, Zheng C, Lee DH, Liang DT. 2006. In-depth investigation of biomass pyrolysis based on three major components: hemicellulose, cellulose and lignin. Energy Fuels. 20, 388–393. ( 10.1021/ef0580117) [DOI] [Google Scholar]
- 43.Morrison WR, Smith LM. 1964. Preparation of fatty acid methyl esters and dimethylacetals from lipid with boron fluoride–methanol. J. Lipid Res. 5, 600–608. [PubMed] [Google Scholar]
- 44.Ratledge C, Hopkins S. 2006. Lipids from microbial sources. In Modifying lipids for use in food (ed. Gunstone FD.), pp. 80–113. Abington, UK: Woodhead Publishing. [Google Scholar]
- 45.Coradini ALV, Anschau A, Vidotti ADS, Reis ÉM, da Cunha Abreu Xavier M, Coelho RS, Franco TT. 2015. Microorganism for bioconversion of sugar hydrolysates into lipids. Microorganisms Biorefineries Microbiol. Monogr. 26, 51–78. ( 10.1007/978-3-662-45209-7_3) [DOI] [Google Scholar]
- 46.Chandel AK, Antunes FAF, de Arruda PV, Milessi TSS, da Silva SS, de Almeida Felipe MDG. 2012. Dilute Acid Hydrolysis of Agro-Residues for the Depolymerization of Hemicellulose: State-of-the-Art. In D-Xylitol: fermentative production, application and commercialization (eds da Silva SS, Chandel AK), pp. 39–61. Berlin, Germany: Springer. [Google Scholar]
- 47.Sarkar N, Ghosh SK, Bannerjee S, Aikat K. 2012. Bioethanol production from agricultural wastes: an overview. Renewable Energy 37, 19–27. ( 10.1016/j.renene.2011.06.045) [DOI] [Google Scholar]
- 48.Meng X, Ragauskas AJ. 2014. Recent advances in understanding the role of cellulose accessibility in enzymatic hydrolysis of lignocellulosic substrates. Curr. Opin. Biotechnol. 27, 150–158. ( 10.1016/j.copbio.2014.01.014) [DOI] [PubMed] [Google Scholar]
- 49.Sitepu IR, Sestric R, Ignatia L, Levin D, German JB, Gillies LA, Almada LA, Boundy-Mills KL. 2013. Manipulation of culture conditions alters lipid content and fatty acid profiles of a wide variety of known and new oleaginous yeast species. Bioresour. Technol. 144, 360–369. ( 10.1016/j.biortech.2013.06.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ratledge C, Wynn J. 2002. The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv. Appl. Microbiol. 51, 1–51. ( 10.1016/S0065-2164(02)51000-5) [DOI] [PubMed] [Google Scholar]
- 51.Ratledge C. 2005. Microbial production of gamma-linolenic acid. Boca Raton, FL: CRC press. [Google Scholar]
- 52.Tang X, Chen H, Chen YQ, Chen W, Garre V, Song Y, Ratledge C. 2015. Comparison of biochemical activities between high and low lipid-producing strains of Mucor circinelloides: an explanation for the high oleaginicity of strain WJ11. PLoS ONE 10, 1–12. ( 10.1371/journal.pone.0128396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dey P, Banerjee J, Maiti MK. 2011. Comparative lipid profiling of two endophytic fungal isolates – Colletotrichum sp. and Alternaria sp. having potential utilities as biodiesel feedstock. Bioresour. Technol. 102, 5815–5823. ( 10.1016/j.biortech.2011.02.064) [DOI] [PubMed] [Google Scholar]
- 54.Yao RS, Zhang P, Wang H, Deng SS, Zhu HX. 2012. One-step fermentation of pretreated rice straw producing microbial oil by a novel strain of Mortierella elongata PFY. Bioresour. Technol. 124, 512–515. ( 10.1016/j.biortech.2012.08.142) [DOI] [PubMed] [Google Scholar]
- 55.Kitcha S, Cheirsilp B. 2014. Bioconversion of lignocellulosic palm byproducts into enzymes and lipid by newly isolated oleaginous fungi. Biochem. Eng. J. 88, 95–100. ( 10.1016/j.bej.2014.04.006) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
DNA sequences: Genbank accessions KU523400.