Abstract
North Atlantic right whales (NARW), Eubalaena glacialis, were nearly exterminated by historical whaling. Their abundance slowly increased up until 2010, to a maximum of fewer than 500 whales, and since then they have been in decline. We assessed the extent to which the relatively slow increase demonstrated by NARW was intrinsic, and how much could be due to anthropogenic impacts. In order to do so, we first compared calf counts of three populations of Southern right whales (SRW), E. australis, with that of NARW, over the period 1992–2016. By this index, the annual rate of increase of NARW was approximately one-third of that of SRW. Next we constructed a population projection model for female NARW, using the highest annual survival estimates available from recent mark–resight analysis, and assuming a four-year calving interval. The model results indicated an intrinsic rate of increase of 4% per year, approximately twice that observed, and that adult female mortality is the main factor influencing this rate. Necropsy records demonstrate that anthropogenic mortality is the primary cause of known mortality of NARW. Anthropogenic mortality and morbidity has limited the recovery of NARW, and baseline conditions prior to their recent decline were already jeopardizing NARW recovery.
Keywords: population projection model, whale conservation, entanglement mortality, geographical comparison
1. Introduction
The near-extinction of several species of baleen whales by commercial whaling removed these animals as functional components of marine ecosystems [1]. Today many populations are increasing in abundance, but some are recovering more slowly than others [2]. Are these differences in recovery due to ongoing anthropogenic impacts or intrinsic ecological factors [3]? Determining the extent to which anthropogenic impacts impede whales' recovery is important. For biodiversity conservation, some whale species (or populations) are at risk of extinction as they number in the dozens [4,5], or in the low hundreds and are declining [6]. As ecosystem service providers, baleen whales can be important ecosystem engineers, for example by provision of iron in systems where it is lacking [7], or by cycling nutrients through the water column [8]. Ensuring whales' recovery will mean that they may resume their prior ecosystem roles, thereby contributing to marine ecosystem integrity. Here we compare patterns of recovery of four populations of two species of right whales, Eubalaena, and attempt to assess the extent to which differences in recovery of one are due to anthropogenic activity rather than ecologically intrinsic factors.
There are three species of Eubalaena: North Atlantic, E. glacialis (NARW); North Pacific, E. japonica (NPRW); and Southern, E. australis (SRW) right whales. All right whale populations were reduced substantially by historical whaling [2]. There were two populations of NARW, one of which (the eastern) appears to be extinct [9], while the western population off the eastern seaboard of North America has been the subject of substantial research effort throughout their known range since the 1980s [10]. NARW abundance increased between 1990 and 2010 at approximately 2.8% per year, and since then has declined [6]. The species' abundance was estimated at 458 individuals in 2015, using a Bayesian mark–resight analysis of photo-identification data [6]. Of the three species, the habitat of North Atlantic right whales is the most heavily industrialized [10].
Similarly, there are two populations of NPRW; the eastern population of NPRW is believed to number only around 30 individuals [4], and the status of the western population is uncertain, although it appears to be larger than the eastern [11]. There are no records of NPRW births in recent years, and the locality of the calving ground(s) for NPRW remains unknown. Also, there is no time series of NPRW abundance. As there are no data with which NPRW could be compared with other right whales, they are not considered further in this paper.
There appear to be seven populations of SRW, distinguished by the locations of their calving grounds and genetic studies [12]. These are off: the eastern coast of South America (Argentina and Brazil [13]); Tristan da Cunha [14]; the southern coast of Africa (South Africa and Namibia [15,16]); the southwestern and south-central coast of Australia [17]; the southeast and east coast of Australia [18,19]; the Auckland Islands and New Zealand [12]; and the Peruvian and Chilean coasts of western South America [20]. The status of the population that calves around Tristan da Cunha is unknown, as is the extent of immigration between these whales and those observed off either Africa or South America [14,21]. Data collection for the whales off Chile and Peru has been sporadic, and this population is extremely small [20].
Most SRW calve in shallow inshore waters, and calving sites are predictable in time and space. Female right whales with calves tend to remain at the surface and move slowly. This behaviour, coupled with the ease with which right whales can be identified from aerial photographs of their head [6], makes females with calves of the year on the calving grounds relatively easy to survey [22]. This has resulted in long-term survey programmes instituted for those populations where it has been logistically and financially feasible.
Multi-decadal survey data exist for SRW populations off eastern South America [23], southern Africa [24], and southwest Australia [17,25]. An incomplete time series (1995–1998, and the 2006 onwards) exists for SRW calving around the Auckland Islands [26,27]. There are very few data for the SRW populations calving off south-east/east Australia nor for the Peruvian and Chilean coasts of western South America, and no data for the SRW population calving off Tristan da Cunha, so these populations are not considered further.
Here we use calf counts to compare the recovery of NARW with the three populations of SRW for which comparable time-series data are available. To do this, we use the only measure that is directly comparable among populations: a raw minimum count of calves known to be born each year. Further, we question the extent to which the lower rate of increase over time of NARW is intrinsic, or anthropogenically driven. To do this, we construct a matrix population model [28] for female NARW to establish a maximum intrinsic rate of increase for NARW given the conditions in which they currently live, and compare this with the observed rate of increase.
2. Methods
2.1. Data
We analysed four time-series of counts of calves born each year, from 1992 or 1993 to 2013 or 2016 inclusive. These were: North Atlantic right whales (NARW); SRW off eastern South America (SRWSAm); southern Africa (SRWSAf); and southwest Australia (SRWOz). We did not include the Auckland Islands population as the gap in that time series was too large. We selected 1992 as our starting year as it marked the start of intensive aerial surveys of NARW calving habitat over the winter [29], and comparable datasets are available for the four time series analysed. SRW calve in the austral winter (i.e. mid-year), so for them the calving year is the year in which calving occurs. NARW calve in the boreal winter, with calving starting in December. Here, we follow Pace et al. [6] and take December as the starting point for the ‘calving year’, so the 2016 calving year started in December 2016 and ended in March 2017. Details of the survey methods used to collect calf counts over time from each site are provided in electronic supplementary material 1.
2.2. Analysis
We tested whether the slopes of the relationships of calf counts over time (i.e. average annual increases and their uncertainty) differed between the four populations of right whales under study. Analyses were run in R version 3.4.3 [30], using the libraries MASS [31], ggplot2 [32]; ggfortify [33,34]; sjPlot [35] and phia [36]. Populations were treated as categorical predictors (Sites) with four categories: NARW, SRWOz, SRWSA and SRWSAm. Calf counts were available for Sites over the period 1992–2016 (figure 1), with the exceptions of SRWSA, for which data were from 1992 to 2013, and SRWOz, for which the data were from 1993 to 2016. We tested whether the trend in calf counts over time differed among populations using Generalized Linear Models (GLMs). We ran a Negative Binomial GLM with log-link, including an interaction effect of population and Year. We adjusted p-values when making multiple comparisons, using Benjamini & Hochberg's False Discovery Rate [37] (in phia::testInteractions). Data and R code are included in electronic supplementary material 2.
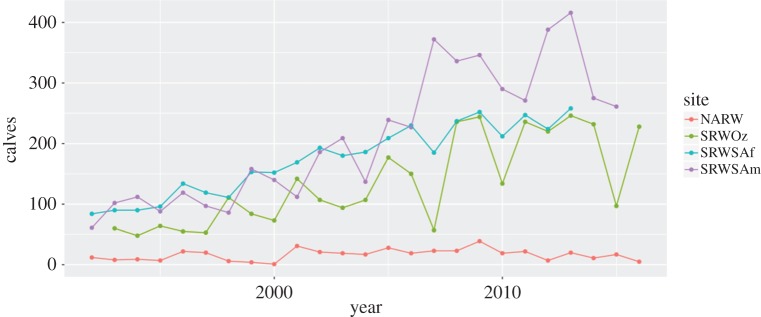
Figure 1.
Right whale, Eubalaena, calf counts over time, 1992–2016, raw data. NARW: North Atlantic right whale, E. glacialis; SRWOz: Southern right whale, E. australis, Southwest Australia; SRWSAf: Southern right whale, South Africa; SRWSAm: Southern right whale, eastern South America. R code for the figure is provided in electronic supplementary material 2.
2.3. Population projection models
We constructed a simple, three-stage population projection model for female right whales (figure 2). We selected the highest annual survival rates estimated for NARW from the Bayesian mark–resight analysis of photo-identifications published recently [6]. These were: calves: 0.96299; juveniles: 0.97507; and adult females: 0.97314, all from 2008. We assumed a juvenile stage duration of nine years [22,25,38,39], and a maximum longevity of 69 years [10]. Survival and transition probabilities for stages were calculated (using Equations 1 and 2 in [40]) (see R code in electronic supplementary material 3). We assumed a calving interval of four years, for the following reason. The mean calving interval for calving females from the NARW Catalog is 4.69 years (P Hamilton 2018, unpublished data). The mean observed calving intervals for SRW include 3.16 years for South Africa [39], 3.42 years for Argentina [41], 3.31 years for the Auckland Islands [27] and 3.3 years for Australia [25]. Rather than assume that NARW can reproduce as rapidly as SRW, we use four years as the approximate mid-point between the values for NARW and SRW.
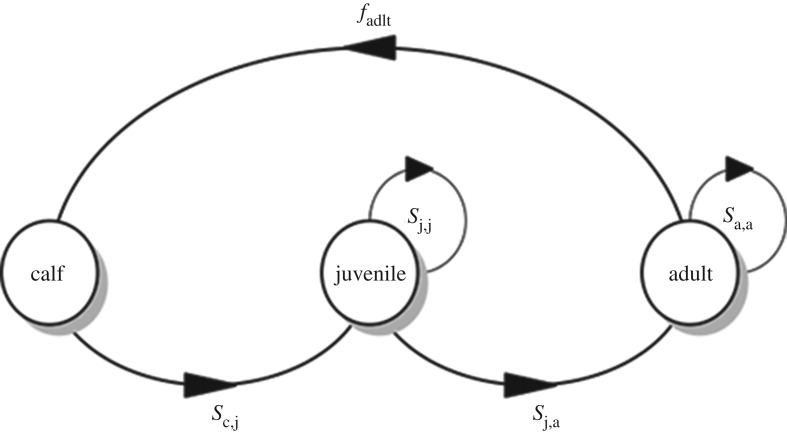
Figure 2.
Simplified, stage-structured model of the demography of female North Atlantic right whales, Eubalaena glacialis. Females are born as calves and remain in the calf state for one year, then transition to the juvenile state, where they remain for eight years, after which, on becoming pregnant, they enter the adult stage. Maximum longevity is assumed to be 69 years. Sc,j is the probability of transitioning from calf to juvenile, Sj,j the probability of remaining in the juvenile state, Sj,a the probability of transitioning from juvenile to adult, and Sa,a the probability of remaining in the adult state; fadlt is the probability of an adult whale giving birth to a calf. Solid black lines with arrows indicate the direction of transitions. R code for the figure is provided in electronic supplementary material 3.
The model was run in R 3.4.3 (R Core Team 2017), using the libraries diagram [42], popbio [43] and popdemo [44]. R code to run the model is included in electronic supplementary material 3.
3. Results
3.1. Calving over time
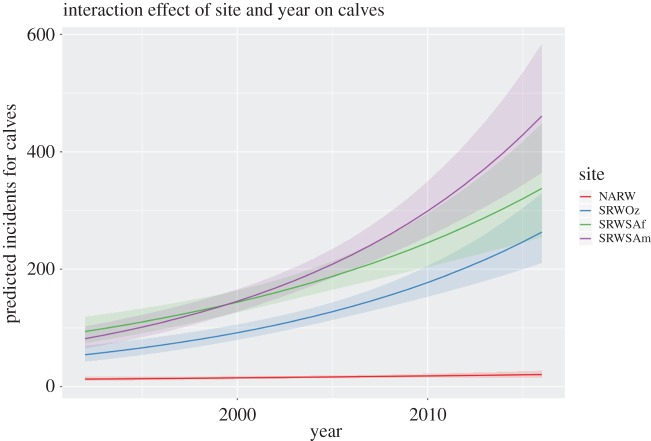
The slope of the GLM of calf counts over time (figure 3) for NARW was significantly different from all SRW Sites, none of which were significantly different from each other (table 1). The calf counts for NARW increased at 1.98% per year (s.e. 1.030), while SRW increased at 5.34% (s.e. 0.964), 6.58% (s.e. 0.861) and 7.21%/year (s.e. 0.845, table 2) for the South Africa, Southwest Australia and eastern South America populations, respectively.
Figure 3.
Right whale, Eubalaena, calf counts over time, 1992–2016. Model predicted slopes for all four sites (solid lines). Shaded areas are 95% confidence intervals of the slopes. Acronyms are as per figure 1. R code for the figure is provided in electronic supplementary material 2.
Table 1.
Results of the Negative Binomial Generalized Linear Model with log-link, including an interaction effect of population and Year. p-values were adjusted for multiple comparisons, using Benjamini & Hochberg's False Discovery Rate (in phia::testInteractions). NARW: North Atlantic right whale; SRWOz: Southern right whale, Southwest Australia; SRWSAf: Southern right whale, South Africa; SRWSAm: Southern right whale, eastern South America. R code for the GLM is provided in electronic supplementary material 2.
| value | d.f. | Chisq | Pr(>Chisq) | |
|---|---|---|---|---|
| NARW-SRWOz | −0.046037 | 1 | 11.7675 | 0.0018082** |
| NARW-SRWSAf | −0.033561 | 1 | 5.6593 | 0.0347260* |
| NARW-SRWSAm | −0.052324 | 1 | 15.4249 | 0.0005151*** |
| SRWOz-SRWSAf | 0.012476 | 1 | 0.9317 | 0.4013015 |
| SRWOz-SRWSAm | −0.006287 | 1 | 0.2717 | 0.6022247 |
| SRWSAf-SRWSAm | −0.018763 | 1 | 2.1409 | 0.2151238 |
| residuals | 87 |
*p < 0.05; **p < 0.01; ***p < 0.001.
Table 2.
Back-transformed interaction means and standard errors for the Negative Binomial Generalized Linear Model (with log-link) of calf counts over time, 1992–2016. The point estimate (mean) is the average annual rate of increase, and the s.e. is the standard error of this estimate. Multiplication by 100 gives the annual percentage increase for each whale population over time. Acronyms are as per table 1. R code for the GLM is provided in electronic supplementary material 2.
| site | mean | s.e. |
|---|---|---|
| NARW | 0.0198 | 0.01030 |
| SRWOz | 0.0658 | 0.00861 |
| SRWSAf | 0.0534 | 0.00964 |
| SRWSAm | 0.0721 | 0.00845 |
3.2. Population projection models
Table 3 shows the matrix model used in the best NARW female survival analysis. The matrix presented in table 3 is the mathematical representation of the simplified, stage-structured model of the demography of female NARW, given the values listed above and calculating survival and transition probabilities for stages (using Equations 1 and 2 in [40]). Table 3's representation, using the same notation as figure 2, is:
The intrinsic rate of increase for NARW is the dominant eigenvalue of this matrix [43] derived from this model which is 1.040 (i.e. 4% annual increase). Elasticity analysis [28] measures the proportional change in the intrinsic rate of increase driven by the proportional change of each of the matrix elements (i.e. survival of different life stages and fecundity). Given the stable stage distribution estimated from the matrix (the right eigenvector) and the reproductive value (the left eigenvector), elasticities can be calculated [28,43] (see electronic supplementary material 3 for R code). As elasticities sum to one [28], their meaning is straightforward to interpret. In this instance, elasticity analysis demonstrated that adult female mortality was proportionally the most important influence on asymptotic population growth rate (table 4), and accounted for about two-thirds of the change in NARW's intrinsic rate of increase.
Table 3.
The matrix model used in the best NARW female survival analysis. R code calculating the matrix is provided in electronic supplementary material 3.
| calf | immature | adult | |
|---|---|---|---|
| calf | 0.00000 | 0.00000 | 0.1250 |
| immature | 0.96299 | 0.86368 | 0.0000 |
| adult | 0.00000 | 0.11139 | 0.9664 |
Table 4.
Results of elasticity analysis of the matrix used in the best NARW female survival analysis. R code for calculating the elasticity analysis is provided in electronic supplementary material 3.
| calf | immature | adult | |
|---|---|---|---|
| calf | 0.00000 | 0.00000 | 0.04740 |
| immature | 0.0474 | 0.23262 | 0.00000 |
| adult | 0.00000 | 0.04740 | 0.62517 |
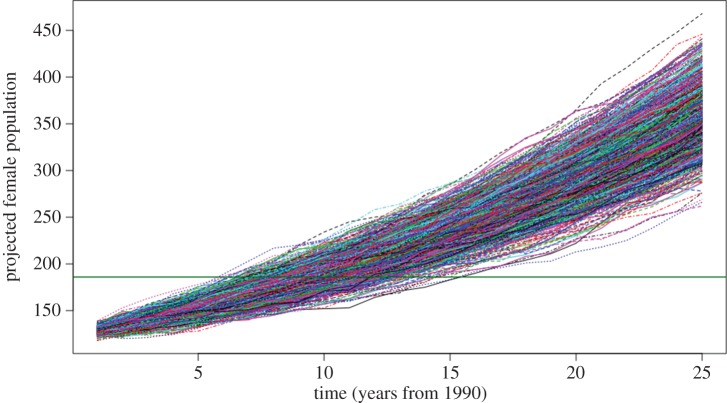
In order to compare this intrinsic rate of increase with that observed, we ran 1000 stochastic projections of the matrix, from 1990 to 2015 (figure 4). First, we derived the proportions of each life-history stage from the stable stage distribution of the matrix [28]. From a starting population of 123 females in 1990 [6], this gave a 1990 population of eight calves, 46 juveniles and 69 adults (see electronic supplementary material 3). The median projected estimate 25 years later was 326 (95% quantiles: 266–393), female right whales of all stages (i.e. calves, juveniles and adults), compared to the observed 186 (95% credible intervals: 174–195, [6]).
Figure 4.
Projected population growth of female North Atlantic right whales. Projection uses the ‘best NARW female survival matrix' (table 3). Projection is for all female NARW: calves, juveniles and adults. The projection assumes a female population of NARW of 123 individuals in 1990 [6]. The proportions of each life-history stage for this starting population were calculated from the stable stage distribution of the matrix [28]. This estimated a starting population of 8 calves, 46 juveniles and 69 adults, and projects forward a further 25 years post-1990, to 2015. The horizontal green line demarcates 186 females, the estimated abundance of female NARW in 2015 [6]. R code for the figure is provided in electronic supplementary material 3.
4. Discussion
4.1. Why are NARW recovering more slowly than SRW?
The rate at which NARW calf counts have increased over the past 25 years (around 2% per year) is substantially less than that for the three SRW populations for which a comparable time series is available (between 5.3–7.2%/year). NARW calf production has increased slowly, with occasional periods of both relatively high and very low annual calf production (figure 1). Over the same time, the three SRW populations' patterns of recovery have been similar, with the numbers of calves born increasing relatively steadily, from dozens in the 1980s–1990s to hundreds recently.
To what extent is this difference in annual increase due to relatively greater ongoing anthropogenic impacts on NARW, rather than being ecologically intrinsic? NARW and SRW are similar animals, and were thought to be one species until molecular genetic analyses demonstrated otherwise [45]. The body condition of NARW, as estimated using visual health assessments, has been declining over recent years, and periods of reduced calving success coincide with periods during which all individuals of the species showed poorer overall health [46]. That female baleen whales forgo reproduction in response to poor body condition is well established [47]. In the southwest Australian population of SRW, longer and rounder females invested more energy into their calves than did smaller females in poorer condition [48]. Also, calves of larger, more rotund females gained more body volume over the three-month period at the calving ground than did calves of shorter, more thin females [48].
Stochasticity in NARW calving over time has been correlated with availability of Calanus finmarchicus [49]. Likewise, climate [50,51] and krill density [50] have been correlated with calf production of SRW off South America. Further studies comparing all SRW populations would be enlightening, but are beyond the scope of this paper. These findings help explain the stochasticity (figure 1) in calving time series, that is, the pattern of the residuals around the GLM's fit. What drives the slope of the calving trajectory is a different question, and the elasticity analysis of our matrix model demonstrates that the primary driver is adult female survival.
Furthermore, the current abundance of NARW, even at its recent maximum (just under 500 individuals, [6]) is substantially less than projections of their previous historical abundance (e.g. 9000–21 000, [52]). That zooplankton productivity and availability in the western North Atlantic has declined to the extent that food limitation is the sole reason for NARW's poor record of calving has not been demonstrated. In what other ways could adult female NARW be energy-limited?
The energy budget of any animal involves both energy intake and expenditure. Recently, a hitherto-ignored source of significant energy expenditure for NARW has been identified: entanglement in fishing gear [53]. Almost all individual NARW (83%) have been entangled at least once, and with many (59%) entangled two or more times [54]. The energetic demand from the drag associated with entanglement can be comparable to the cost of a one-way migration, and is sufficient to impact the likelihood that a female can successfully reproduce [53]. Entanglements can last from months to years, and recovery from entanglements can take similar time, so the time over which an entanglement episode affects a female NARW is also an issue [53]. For SRW, entanglement in fishing gear, when compared with NARW, is almost non-existent [55,56].
Adult female survival, rather than calving interval, is well established as having greatest influence on NARWs' intrinsic rate of increase [57]. Elasticity analysis of our model (table 4) shows adult female survival to be more than an order of magnitude more important than calving rate. Projections of our matrix model suggest that, had the survival of female NARW remained at the highest rates observed over the time series (0.975 for juveniles, 0.973 for adult females), and calving intervals been around four years, the species' numbers could have increased at 4%/year. Were that the case, our stochastic projections from that model indicate that there would have been almost twice the number of females in the species in 2015 as there actually were.
4.2. Implications for conservation
Thus, while some of the stochasticity in NARW calving may be environmental [48], the general slope of the recovery trajectory is driven by female mortality, particularly of adults. What drives female mortality in NARW? Over a period of 40 years, 1970–2009 inclusive, ∼80% (70 of 87) of NARW mortalities for which the cause of mortality is known (there were 122 mortalities identified overall), were anthropogenic ([58] numbers extracted from table 2 of that paper). This proportion is likely biased low as it does not differentiate calves of the year, which are more prone to natural mortality [55,59], from other age cohorts. With almost no observations from any other sources of mortality, a reasonable inference is that the vast majority of non-calf female NARW mortality is anthropogenic.
Similarly, most mortalities of SRW that have been observed are of calves of the year. The significant mortalities of calves of the year observed at Península Valdés, Argentina, in recent years [60] appear to be influenced by local environmental changes (including the behaviour of kelp gulls, Larus dominicanus, [61]). Most mortalities (for which an age class of the carcass could be determined) observed over 36 years (1963–1998 inclusive) off South Africa [56], and 57 years (1950–2006 inclusive) off southern Australia [55] were of calves of the year: 31 of 53 carcasses, and 16 of 28, respectively. The deaths of very few whales in either country were definitively anthropogenic: definitely eight, and possibly 16 of 55 SRW deaths off South Africa [56], and three of 28 off Australia [55].
Best et al. [56, p. 176], referring to SRW off South Africa, stated that ‘the current degree of anthropogenic mortality does not seem to pose a major conservation concern for this population’. While this statement is somewhat dated now, our analysis (figure 3) suggests it remains valid. Survival of adult female SRW off South Africa (data 1971–1998) was estimated at 0.986 (95% CI: 0.976–0.999), [62], or an annual mortality rate of 0.014, approximately half of the lowest annual mortality estimated (0.02686, in 2008) for adult female NARW. Note that, by using the highest estimates of female survivals from the Pace et al. [6] time series, we may still be overestimating what the mortality rate (i.e. underestimating survival) of female NARW would be if there were no anthropogenic mortality, and thereby underestimating NARW's possible intrinsic rate of increase. However, we chose our approach as we cannot rule out the possibility that there is at least some natural difference in the survival of NARW and SRW.
Our projections from the best NARW female survival model suggest there could have been around 326 female NARW in 2015 (and so at least 650 individuals in the species), if whales' survival had been consistently as good as the best observed in the 1990–2015 time series [6]. Had that been the case, the discovery of at least 17 dead right whales in 2017 would have been cause for alarm, but relative risk to the species would have been manageable. Instead, the recent detection of a decrease in NARW abundance since 2010 [6], coupled with the discoveries of mortalities in 2017, means that ‘the North Atlantic right whale is in deep trouble again’ [63].
For comparison, since 2003, there has been substantial mortality of SRW calves of the year on the calving grounds at Península Valdés, Argentina, with over 600 found dead to the end of 2013 [60,61]. Because the population of SRW in the western South Atlantic showed decades of substantial increase (figure 3) prior to these mortalities, the immediate risk to this population is far less than the current risk to NARW.
4.3. Caveats
We recognize that using counts of calves born each year could be biased, compared with estimates of absolute abundance for each right whale population. The three SRW populations are not surveyed on the foraging grounds, and only data from calving surveys are available. It is possible that some births are missed because of this. NARW are surveyed intensively throughout the year [10], so it is much less likely that births are missed. If the proportion of SRW births being missed increased over the time series, the SRW rates of increase over time would be biased low. If the proportion of SRW births missed remained constant over the time series, this would give an underestimate of the number of births, but not their rate of increase. If this bias exists, what it does not do is change the main finding of this comparison, that NARW are increasing more slowly than the SRW populations for which we have good calving time series.
Calf counts for right whales are collected in a similar manner at all four sites, so making direct comparisons with one analysis, as presented here, is superior to comparing rates of increase calculated using different methods [3]. Is it possible to compare a time series of calf counts with an independent time series of abundance estimates for right whales? Estimates of SRW abundance are extrapolated from the abundance of calves [23]. The only population for which there is a comparable, independent, published time series of mark–resight abundance estimates to compare with calf counts is NARW. A 26-year increase (1990–2015, [6]) from 270 [6] at the point estimate of increase from the calving index (i.e. 1.98%/year) gives an estimate of 450 whales [(1.019826) × 270]. This estimate lies inside the 95% credible intervals of the abundance estimate for 2015 (444–471, [6]), demonstrating that the NARW calving time series is a reliable estimator of this species' trajectory over time.
A second caveat is that the values used in our population projection model for NARW population matrix were from observed data from the existing NARW time series. When using those values for the best NARW female survival we assume that the year with best survival values was one when anthropogenic impacts were minimized. In 2008, a year when the probability of photographically identifying each NARW was 94–95% (see [6], electronic supplementary material), there were no detected anthropogenic mortalities of NARW, and no mortalities for which the cause of death could not be determined [64]. The three mortalities detected in 2008 were all neonates that died of natural causes (A Henry 2018, personal communication). Although it is possible that our estimate of a maximum rate of increase for NARW is biased low, it is from a year when anthropogenic impacts of NARW survival seem at their lowest in the time series, and so the closest to what might be ‘natural’.
A third caveat is that some populations of SRW—those off southeastern Australian and Chile/Peru—are not recovering as rapidly as those off eastern South America, southern Africa, southwest Australia and the Auckland Islands/New Zealand. The reasons for these populations' relatively poor recovery are unclear but require assessment.
5. Conclusion
NARW have increased in abundance since 1990 at approximately 2% per year (including the decline in abundance observed since 2010), or approximately a third of the rate of increase demonstrated by at least three populations of their sister species, SRW. Projection models based on the best annual estimates of survival recorded for NARW suggest that they could increase at least 4% per year, over twice that observed. Elasticity analysis shows that adult female mortality is the key driver of the species' rate of change, and necropsy data over decades demonstrate that deaths of non-calf NARW are almost entirely due to anthropogenic causes.
Most studies of baleen whale conservation concentrate specifically on the one population of interest, and NARW are no exception to this norm. There are now multiple populations of some species of baleen whale for which times series of abundance are available [2]. A meta-analysis of recovery of humpback populations suggests differences in rates of recovery for those whales in the Northern and Southern Hemispheres [3]. Such comparisons can offer a broader context to our understanding of the recovery of particular species or populations. In this instance, by comparing NARW's recovery with multiple populations of SRW, we can make relatively strong inference that NARW recovery is unusually slow. Further, by coupling that analysis with our model projecting what NARW could do, given the best survival and calving rates that this species has demonstrated, we can address the question posed at the start of this paper: are these differences in recovery due to ongoing anthropogenic impacts or intrinsic ecological factors? Our results indicate that it is likely that NARW's maximum intrinsic rate of increase is less than that of SRW. However, we can conclude that anthropogenic mortality has limited the recovery of NARW, and that baseline conditions prior to their decline (post-2010) were already jeopardizing NARW recovery. Had NARW increased at the annual rate at which they are capable, the species' numbers would be almost double what they are now, and their current emergency would not be so dire.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
P.H., P.C. and R.M.P. thank the dedicated Catalog team at the Anderson Cabot Center at the New England Aquarium and the North Atlantic Right Whale Consortium for managing and providing access to the North Atlantic right whale data. J.B. thanks Jenny Schmidt, Andrew Halsall (long-term pilot/observers), Prof Phil Hammond (trend analysis) and Dr Mike Double (data analysis and other assistance). K.F. thanks Dr Keiko Sekiguchi, Dr Desray Reeb, Mr Michael Meÿer, Mr Mark Samson, Dr Simon Elwen, Ms Ines Ferreira, Dr L Karczmarski, Ms Ingrid Peters and Ms Meredith Thornton for their participation as observers in the South African aerial surveys. K.R.G. thanks the research team of Projeto Baleia Franca and invited photographers who helped data collection. V.R. thanks John Atkinson, Roger Payne and other researchers and photographers for their invaluable work during aerial surveys of the Península Valdés right whales and the hundreds of others who have assisted in data analysis since 1971. We thank Denise Greig, Sean Hayes, Scott Kraus, Julie van der Hoop, and an anonymous reviewer for providing comments that improved this paper. Vale John Bannister, who left us while this manuscript was in review. The findings and conclusions in the paper are those of the authors and do not necessarily represent the views of the National Marine Fisheries Service, NOAA.
Ethics
Aerial surveys of calving areas in US waters were conducted under US National Marine Fisheries Service permits 716 and 1014. Permits for surveys in Argentina were issued annually by the Dirección de Fauna y Flora Silvestre and the Subsecretaría de Turismo y Áreas Protegidas of Chubut Province, Argentina. Aerial surveys conducted in South African waters have been carried out under permits in terms of South African legislation and granted by the relevant authorities, including reserve area management authorities. Surveys off south-western Australia have been conducted under permits from the Parks and Wildlife Service of Western Australia.
Data accessibility
The dataset and code supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
P.C. conceived the paper; C.C. provided data and ideas for proof of concept; P.H., J.B., P.B., K.R.G., K.F., V.R. and E.V. collected, archived, and provided calving data; P.C. analysed the data with input from R.M.P.; P.C. wrote the manuscript with contributions from all authors. All living authors contributed critically to the drafts and gave final approval for publication.
Competing interests
We have no competing interests.
Funding
The North Atlantic Right Whale Catalog is maintained with support from ongoing contracts from NOAA Fisheries. J.B. has been funded since at least 1993 by various Australian Government Environment Agencies, since 2015 the National Environment Marine Sciences Program, Marine Diversity Hub. K.F. thanks the Island Foundation for support during the collection of the South African aerial survey data between 2012 and 2015. Various institutions funded the South African aerial surveys over the data collection period, including Moby Dick Rum, Exclusive Trust, the Island Foundation, the National Research Foundation, members of the Offshore Petroleum Association of South Africa and the International Whaling Commission. The Brazilian Right Whale Catalog have been supported by several companies through funding to Projeto Baleia Franca, in particular PETROBRAS Brazilian Oil Company and Santos Brasil Company. V.R. thanks the many individuals and non-profit organizations who funded the 47 years of aerial surveys of the Argentine right whales, in particular Sarah Haney for her support in many of our lean years. V.R.'s research permits were issued annually by the Dirección de Fauna y Flora Silvestre and the Subsecretaría de Turismo y Áreas Protegidas of Chubut Province, Argentina.
References
- 1.Roman J, et al. 2014. Whales as marine ecosystem engineers. Front. Ecol. Environ. 12, 377–385. ( 10.1890/130220) [DOI] [Google Scholar]
- 2.Thomas PO, Reeves RR, Brownell RL Jr. 2015. Status of the world's baleen whales. Mar. Mamm. Sci. 32, 682–734. ( 10.1111/mms.12281) [DOI] [Google Scholar]
- 3.Wedekin L, Engel M, Andriolo A, Prado P, Zerbini A, Marcondes M, Kinas P, Simões-Lopes P. 2017. Running fast in the slow lane: rapid population growth of humpback whales after exploitation. Mar. Ecol. Prog. Ser. 575, 195–206. ( 10.3354/meps12211) [DOI] [Google Scholar]
- 4.Wade PR, et al. 2010. The world's smallest whale population? Biol. Lett. 7, 83–85. ( 10.1098/rsbl.2010.0477) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosel P, Wilcox L. 2014. Genetic evidence reveals a unique lineage of Bryde's whales in the northern Gulf of Mexico. Endanger. Species Res. 25, 19–34. ( 10.3354/esr00606) [DOI] [Google Scholar]
- 6.Pace RM III, Corkeron PJ, Kraus SD. 2017. State-space mark-recapture estimates reveal a recent decline in abundance of North Atlantic right whales. Ecol. Evol. 7, 8730–8741. ( 10.1002/ece3.3406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavery TJ, Roudnew B, Seymour J, Mitchell JG, Smetacek V, Nicol S. 2014. Whales sustain fisheries: blue whales stimulate primary production in the Southern Ocean. Mar. Mamm. Sci. 30, 888–904. ( 10.1111/mms.12108) [DOI] [Google Scholar]
- 8.Roman J, Nevins J, Altabet M, Koopman H, McCarthy J. 2016. Endangered Right whales enhance primary productivity in the Bay of Fundy. PLoS ONE 11, e0156553 ( 10.1371/journal.pone.0156553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Notarbartolo di Sciara G, Politi E, Bayed A, Beaubrun P-C, Knowlton A. 1998. A winter cetacean survey off southern Morocco, with a special emphasis on Right whales. Rep. Inter. Whal. Commn. 48, 547–551. [Google Scholar]
- 10.Kraus SD, Rolland RR. 2007. The urban whale. Cambridge, MA: Harvard University Press. [Google Scholar]
- 11.Ovsyanikova E, et al. 2015. Opportunistic sightings of the endangered North Pacific right whales (Eubalaena japonica) in Russian waters in 2003–2014. Mar. Mamm. Sci. 31, 1559–1567. ( 10.1111/mms.12243) [DOI] [Google Scholar]
- 12.Carroll EL, Baker CS, Watson M, Alderman R, Bannister J, Gaggiotti OE, Gröcke DR, Patenaude N, Harcourt R. 2015. Cultural traditions across a migratory network shape the genetic structure of southern right whales around Australia and New Zealand. Sci. Rep. 5, 16182 ( 10.1038/srep16182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camara IG, Palazzo JT.. 1985. New information on the presence of Eubalaena australis in the south of Brazil. In Primera Reunion de Trabajo de Expertos Mamferos acuaticos America del Sur, Buenos Aires, June 1984. Buenos Aires, Argentina: Comite Organizador de la Reunion.
- 14.Best PB, Payne R, Rowntree V, Palazzo JT, Both MDC. 1993. Long-range movements of South Atlantic right whales, Eubalaena australis. Mar. Mamm. Sci. 9, 227–234. ( 10.1111/j.1748-7692.1993.tb00451.x) [DOI] [Google Scholar]
- 15.Best PB. 1990. Trends in the inshore right whale population off South Africa, 1969–1987. Mar. Mamm. Sci. 6, 93–108. ( 10.1111/j.1748-7692.1990.tb00232.x) [DOI] [Google Scholar]
- 16.Roux JP, Braby RJ, Best PB. 2015. Does disappearance mean extirpation? The case of right whales off Namibia. Mar. Mamm. Sci. 31, 1132–1152. ( 10.1111/mms.12213) [DOI] [Google Scholar]
- 17.Bannister JL, Hammond PS, Double MC.2016. Population trend in right whales off southern Australia 1993–2015. Paper SC/66b/BRG/09 presented to the IWC Scientific Committee, May 2016. See http://www.iwc.int .
- 18.Jackson JA, Carroll EL, Smith TD, Zerbini AN, Patenaude NJ, Baker CS. 2016. An integrated approach to historical population assessment of the great whales: case of the New Zealand southern right whale. R. Soc. open sci. 3, 150669 ( 10.1098/rsos.150669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watson M, Westhorpe I, Bannister J, Hedley S, Harcourt R. 2013. Final report on the assessment of numbers and distribution of southern right whales in Southeast Australia. Report to the Australian Marine Mammal Centre.
- 20.Vernazzani BG, Cabrera E, Brownell RL Jr. 2013. Eastern South Pacific southern right whale photo-identification catalog reveals behavior and habitat use patterns. Mar. Mamm. Sci. 30, 389–398. ( 10.1111/mms.12030) [DOI] [Google Scholar]
- 21.Barendse J, Best PB. 2014. Shore-based observations of seasonality, movements, and group behavior of southern right whales in a nonnursery area on the South African west coast. Mar. Mamm. Sci. 30, 1358–1382. ( 10.1111/mms.12116) [DOI] [Google Scholar]
- 22.Cooke JG, Rowntree VJ, Payne R. 2001. Estimates of demographic parameters for southern right whales (Eubalaena australis) observed off Península Valdés, Argentina. J. Cetacean Res. Manage. 2, 125–132. [Google Scholar]
- 23.Cooke J, Rowntree V, Sironi M. 2015. Southwest Atlantic right whales: interim updated population assessment from photo-id collected at Península Valdéz, Argentina. Paper SC/66a/BRG/23 presented to the IWC Scientific Committee, May 2015. See http://www.iwc.int .
- 24.Findlay KP, Thornton MT, Peters IT, Best PB.2015. Report of the 2014 Mammal Research Institute Whale Unit Southern right whale survey, Nature's Valley to Muizenberg, South Africa. Paper SC/66a/BRG/4 presented to the IWC Scientific Committee, May 2015. See http://www.iwc.int .
- 25.Charlton CM. 2017. Population demographics of southern right whales (Eubalaena australis) in Southern Australia. PhD Thesis, Centre for Marine Science and Technology, Curtin University. [Google Scholar]
- 26.Carroll EL, Childerhouse SJ, Fewster RM, Patenaude NJ, Steel D, Dunshea GJ, Boren L, Baker CS. 2013. Accounting for female reproductive cycles in a superpopulation capture-recapture framework, Ecol. Appl. 23, 1677–1690. ( 10.1890/12-1657.1) [DOI] [PubMed] [Google Scholar]
- 27.Davidson AR, Rayment W, Dawson SM, Webster T, Slooten E. 2017. Estimated calving interval for the New Zealand southern right whale (Eubalaena australis). N. Z. J. Mar. Freshwater Res. 52, 372–382. ( 10.1080/00288330.2017.1397034) [DOI] [Google Scholar]
- 28.Caswell H. 2001. Matrix population models, 2nd edn Sunderland, MA: Sinauer Associates Inc. [Google Scholar]
- 29.Garrison LP. 2007. The big picture: modeling right whales in space and time. In The urban whale (eds Kraus SD, Rolland RR), pp. 460–487. Cambridge, MA: Harvard University Press. [Google Scholar]
- 30.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/ [Google Scholar]
- 31.Venables WN, Ripley BD. 2002. Modern applied statistics with S, 4th edn New York, NY: Springer. [Google Scholar]
- 32.Wickham H. 2009. ggplot2: Elegant graphics for data analysis. J. Stat. Softw. 35, 65–88. [Google Scholar]
- 33.Horikoshi M, Tang Y. 2016. ggfortify: Data Visualization Tools for Statistical Analysis Results. See https://CRAN.R-project.org/package=ggfortify .
- 34.Tang Y, Horikoshi M, Li W. 2016. ggfortify: Unified interface to visualize statistical result of popular R packages. R J. 8.2, 478–489. [Google Scholar]
- 35.Lüdecke D. 2018. sjPlot: Data Visualization for Statistics in Social Science. R package version 2.4.1. See https://CRAN.R-project.org/package=sjPlot .
- 36.De Rosario-Martinez H. 2015. phia: Post-hoc interaction analysis. R package version 0.2-1. See https://CRAN.R-project.org/package=phia .
- 37.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300. ( 10.2307/2346101) [DOI] [Google Scholar]
- 38.Kraus SD, Pace RM III, Frasier TR. 2007. High investment, low return: the strange case of reproduction in Eubalaena glacialis. In The urban whale (eds Kraus SD, Rolland RR), pp. 172–199. Cambridge, MA: Harvard University Press. [Google Scholar]
- 39.Brandão A, Best PB, Butterworth DS. 2011. Monitoring the recovery of the southern right whale in South African waters. Paper SC/S11/RW18 presented to the IWC Scientific Committee, May 2011. See http://www.iwc.int .
- 40.Crouse DT, Crowder LB, Caswell H. 1987. A stage-based population model for Loggerhead Sea Turtles and implications for conservation. Ecology 68, 1412–1423. ( 10.2307/1939225) [DOI] [Google Scholar]
- 41.Cooke JG, Rowntree VJ. 2003. Analysis of inter-annual variation in reproductive success of South Atlantic right whales (Eubalaena australis) from photo-identifications of calving females observed off Península Valdés, Argentina, during 1971-2000. Paper SC/55/O23 presented to the IWC Scientific Committee, May 2003. See http://www.iwc.int .
- 42.Soetaert K. 2017. diagram: Functions for Visualising Simple Graphs (Networks), Plotting Flow Diagrams. R package version 1.6.4. See https://CRAN.R-project.org/package=diagram .
- 43.Stubben CJ, Milligan BG. 2007. Estimating and analyzing demographic models using the popbio package in R. J. Stat. Softw. 22, 11 ( 10.18637/jss.v022.i11) [DOI] [Google Scholar]
- 44.Stott I, Hodgson D, Townley S. 2016. popdemo: Demographic Modelling Using Projection Matrices. R package version 0.2-3. See https://CRAN.R-project.org/package=popdemo .
- 45.Rosenbaum HC, et al. 2000. World-wide genetic differentiation of Eubalaena: questioning the number of right whale species. Mol. Ecol. 9, 1793–1802. ( 10.1046/j.1365-294x.2000.01066.x) [DOI] [PubMed] [Google Scholar]
- 46.Rolland RM, Schick RS, Pettis HM, Knowlton AR, Hamilton PK, Clark JS, Kraus SD. 2016. Health of North Atlantic right whales Eubalaena glacialis over three decades: from individual health to demographic and population health trends. Mar. Ecol. Prog. Ser. 542, 265–282. ( 10.3354/meps11547) [DOI] [Google Scholar]
- 47.Lockyer C. 1986. Body fat condition in Northeast Atlantic Fin Whales, Balaenoptera physalus, and its relationship with reproduction and food resource. Can. J. Fish. Aquat. Sci. 43, 142–147. ( 10.1139/f86-015) [DOI] [Google Scholar]
- 48.Christiansen F, Vivier F, Charlton C, Ward R, Amerson A, Burnell S, Bejder L. 2018. Maternal body size and condition determine calf growth rates in southern right whales. Mar. Ecol. Prog. Ser. 592, 267–281. ( 10.3354/meps12522) [DOI] [Google Scholar]
- 49.Meyer-Gutbrod EL, Greene CH. 2018. Uncertain recovery of the North Atlantic right whale in a changing ocean. Glob. Change. Biol. 24, 455–464. ( 10.1111/gcb.13929) [DOI] [PubMed] [Google Scholar]
- 50.Seyboth E, Groch KR, Dalla Rose L, Reid K, Secchi E. 2016. Southern right whale (Eubalaena australis) reproductive success is influenced by krill (Euphausia superba) density and climate. Sci. Rep. 6, 28205 ( 10.1038/srep28205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leaper R, Cooke J, Trathan P, Reid K, Rowntree V, Payne R. 2006. Global climate drives southern right whale (Eubalaena australis) population dynamics. Biol. Lett. 2, 289–292. ( 10.1098/rsbl.2005.0431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monsarrat S, Pennino MG, Smith TD, Reeves RR, Meynard CN, Kaplan DM, Rodrigues ASL. 2016. A spatially explicit estimate of the prewhaling abundance of the endangered North Atlantic right whale. Conserv. Biol. 30, 783–791. ( 10.1111/cobi.12664) [DOI] [PubMed] [Google Scholar]
- 53.van der Hoop J, Corkeron P, Moore M. 2016. Entanglement is a costly life-history stage in large whales. Ecol. Evol. 7, 92–106. ( 10.1002/ece3.2615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knowlton A, Hamilton P, Marx M, Pettis H, Kraus S. 2012. Monitoring North Atlantic right whale Eubalaena glacialis entanglement rates: a 30 yr retrospective. Mar. Ecol. Prog. Ser. 466, 293–302. ( 10.3354/meps09923) [DOI] [Google Scholar]
- 55.Kemper K, Coughran D, Warneke R, Pirzl R, Watson M, Gales R, Gibbs S. 2008. Southern right whale (Eubalaena australis) mortalities and human interactions in Australia, 1950–2006. J. Cetacean Res. Manage. 10, 1–8. [Google Scholar]
- 56.Best PB, Peddemors VM, Cockcroft VG, Rice N. 2001. Mortalities of right whales and related anthropogenic factors in South African waters, 1963–1998. J Cetacean Res. Manage. (Spec. Iss). 2, 171–176. [Google Scholar]
- 57.Fujiwara M, Caswell H. 2001. Demography of the endangered North Atlantic right whale. Nature 414, 537–541. ( 10.1038/35107054) [DOI] [PubMed] [Google Scholar]
- 58.van der Hoop JM, et al. 2012. Assessment of management to mitigate anthropogenic effects on large whales. Conserv. Biol. 27, 121–133. ( 10.1111/j.1523-1739.2012.01934.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moore MJ, Knowlton AR, Kraus SD, McLellan WA, Bonde RK. 2004. Morphometry, gross morphology and available histopathology in North Atlantic right whale (Eubalaena glacialis) mortalities (1970–2002). J. Cetacean Res. Manage. 6, 199–214. [Google Scholar]
- 60.Rowntree VJ, et al. 2013. Unexplained recurring high mortality of southern right whale Eubalaena australis calves at Península Valdés, Argentina. Mar. Ecol. Prog. Ser. 493, 275–289. ( 10.3354/meps10506) [DOI] [Google Scholar]
- 61.Marón CF, Beltramino L, Di Martino M, Chirife A, Seger J, Uhart M, Sironi M, Rowntree VJ. 2015. Increased wounding of southern right whale (Eubalaena australis) calves by kelp gulls (Larus dominicanus) at Península Valdés, Argentina. PLoS ONE 10, e0139291 ( 10.1371/journal.pone.0139291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Best PB, Brandão A, Butterworth DS. 2001. Demographic parameters of southern right whales off South Africa. J Cetacean Res. Manage. (Spec. Iss) 2, 161–169. [Google Scholar]
- 63.Pennisi E. 2017. North Atlantic right whale faces extinction. Science 358, 703–704. ( 10.1126/science.358.6364.703) [DOI] [PubMed] [Google Scholar]
- 64.Henry AG, Cole TVN, Hall L, Ledwell W, Morin D, Reid A. 2014. Mortality determinations for baleen whale stocks along the Gulf of Mexico, United States east coast, and Atlantic Canadian provinces, 2008–2012. Northeast Fish. Sci. Cent. Ref. Doc. 14-10. ( 10.7289/V5JW8BVD). [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset and code supporting this article have been uploaded as part of the electronic supplementary material.