Abstract
The development of metal-free organic reactions is one of the hotspots in the synthesis of cyclic compounds. ROTf (alkyl trifluoromethanesulfonates), due to their good electrophilicity, are powerful alkylating reagents at heteroatoms such as nitrogen, oxygen, sulfur and phosphorus to induce an electrophilic centre for carbon–carbon or carbon–heteroatom bond formation. Inspired by this chemistry, a variety of research concentrating on heterocycles synthesis has been carried out. In this review, we mainly summarize the ROTf-induced annulation of heteroatom reagents such as nitriles, carbodiimides, azobenzenes, isothiocyanates, aldehydes, isocyanates and phosphaalkene with themselves or alkynes to afford cyclic compounds.
Keywords: trifluoromethanesulfonates, heteroatom reagents, annulation, cyclic compounds
1. Introduction
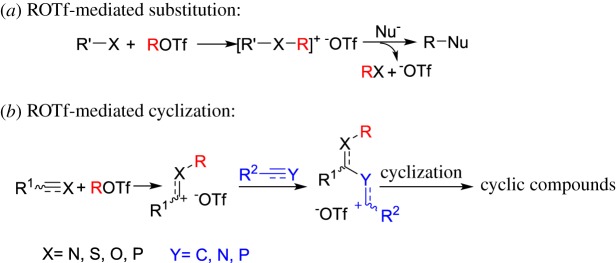
Heterocycles are ubiquitous in natural products, pharmaceuticals, organic materials and numerous functional molecules. Therefore, organic chemists have been making extensive efforts to produce these heterocyclic compounds by developing new and efficient synthetic transformations. Transition-metal-catalysed reactions are some of the most attractive methodologies for synthesizing heterocycles, because a transition-metal-catalysed reaction can directly construct complicated molecules from readily accessible starting materials under mild conditions [1–6]. Nevertheless, transition-metal-catalysed reactions are still limited in applications and confront challenges to some extent, because transition-metals are expensive, toxic, inconvenient for operation and environment damage. In this regard, a transition-metal-free methodology for the construction of important heterocyclic compounds in drug discovery and material science has attracted attention [7–15]. ROTf (alkyl trifluoromethanesulfonates) are powerful alkylating reagents, which are frequently used in alkylation of nucleophiles [16–18]. Benefiting from fluorine in –OTf to stabilize the negative charge, –OTf possesses excellent leaving ability, which makes the ROTf much more reactive as alkylation reagents than alkyl iodide and (MeO)2SO2. As useful and versatile precursors in a variety of organic transformations, many methods have been developed to prepare ROTf, such as the reaction of orthoformates and triflic anhydrides under solvent-free condition [19–26]. The intrinsic electrophilicity of ROTf is often used as alkylation reagents for diverse substrates containing heteroatoms such as P [27], O [28], N [29–31], S [32,33], Te [34], Ge [35], Bi [36], Si [37] and Se [38,39], which straightforwardly affords the corresponding alkylated products with aid of base or stable triflates irreversibly that even could be applied as ionic liquids [40,41]. Moreover, the resulting triflates could be further transformed into other products by substitution [30,42–44] (scheme 1a). On the other hand, unsaturated heteroatom-containing reagents, such as nitriles, aldehydes, isocyanates and isothiocyanates, could be captured by ROTf to generate electrophiles bearing carbon cations [29,31,45,46], which could be subsequently reacted with appropriate unsaturated substrates to produce cyclic compounds by tandem electrophilic reactions/cyclization (scheme 1b); although HOTf or HNTf2 as a catalyst or promoter also demonstrated excellent electrophilic cyclization involving alkynes or alkenes. However, preparing highly functionalized heterocycles is still difficult. ROTf-induced cyclization featuring metal-free, easy to handle and good selectivity provided a feasible approach to diverse heterocyclic compounds. In this review, we mainly focus on the ROTf-induced annulation of unsaturated heteroatom reagents such as nitriles, carbodiimides, azobenzenes, isothiocyanates, aldehydes, isocyanates and phosphaalkene with themselves or alkynes to afford cyclic compounds.
Scheme 1.
ROTf-mediated reactions.
2. ROTf-induced annulation of nitrogen-containing substrates with unsaturated compounds
2.1. ROTf-induced annulation of nitriles
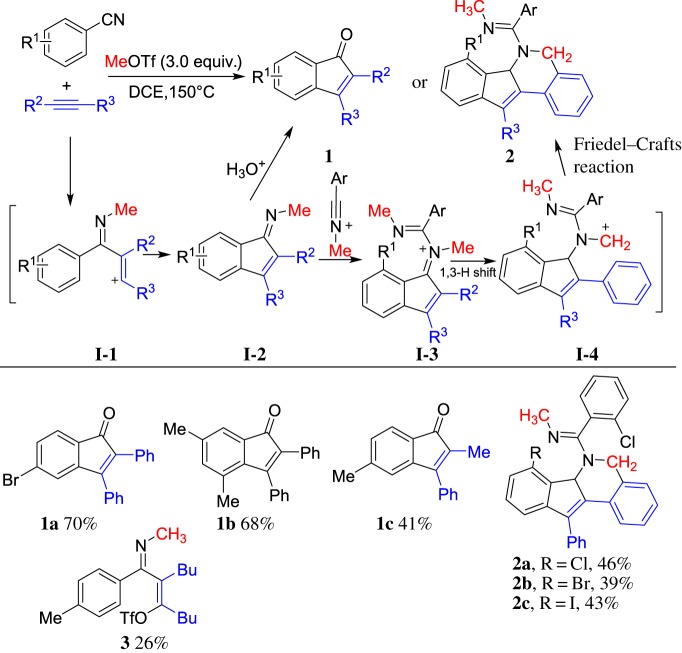
Nitriles as unsaturated heteroatom reagents could react with ROTf to form N-alkylated nitriliums, which were well investigated by Booth et al. [31] in 1980. However, electrophilicity of N-alkylated nitriliums has rarely been used in further reactions until recently. In 2014, our group [47] reported MeOTf-induced carboannulation of arylnitriles and aromatic alkynes to construct indenones 1 (scheme 2). Triflate 3 was isolated when 5-decyne was used indicating I-1 might be an intermediate in this reaction. A range of functionalized indenone derivatives was obtained. When ortho-substituted arylnitriles were used, indenone imine I-2 would further cyclize with another molecule of nitriliums to give indeno[1,2-c]-isoquinolines 2 with the construction of one carbocycle and one heterocycle. Although transition-metal-catalysed annulation of benzimide or arylcarbonyl and arylnitrile with alkynes to the formation of indenones has been reported (for examples, see [48–55]), this reaction reveals a simple reaction process for the synthesis of indenones under metal-free conditions.
Scheme 2.
MeOTf-induced cyclization of arylnitriles and alkynes.
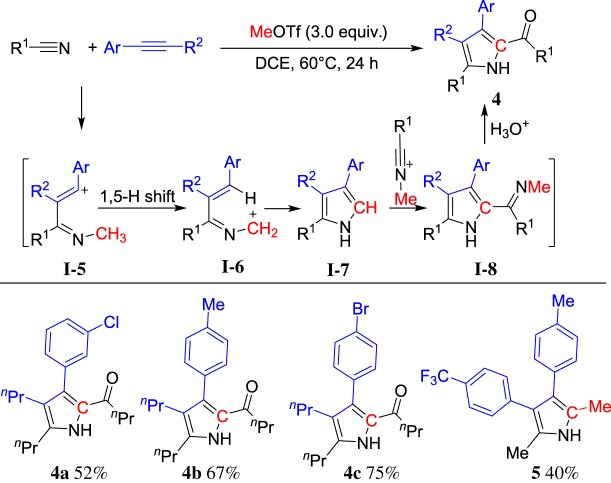
Arylnitriles and alkynes could be induced by MeOTf to afford indenones via intermediate I-5. We envisioned the utilization of alkylnitriles, which lack an aryl group for the ring closure, might lead to a different way for ring formation. To our delight, the reaction of alkylnitriles, alkynes and MeOTf indeed afforded tetrasubstituted NH-pyrroles 4 with high regioselectivity. The structure includes one carbon from ROTf to join the pyrroles [56] (scheme 3). It is noteworthy that the cyclized pyrrole captures another nitrilium leading to substituted 2-acyl-NH-pyrroles after hydrolysis. When EtOTf was used instead of MeOTf, the product 5 was obtained. This reaction provides a practical and convenient method for the synthesis of multiply substituted 2-acylpyrroles from readily available starting materials in a one-pot reaction.
Scheme 3.
ROTf-induced cyclization of alkylnitriles and alkynes.
Furthermore, when 1,2-diphenylethyne and 1,2-di-p-tolylethyne were employed to react with MeOTf and alkylnitriles at 130°C, the isoquinolines 6 were obtained in good yields via intermediate I-9. The representative results are summarized in scheme 4. In the cases, the Friedel–Crafts reaction is favoured to give 6-membered products.
Scheme 4.
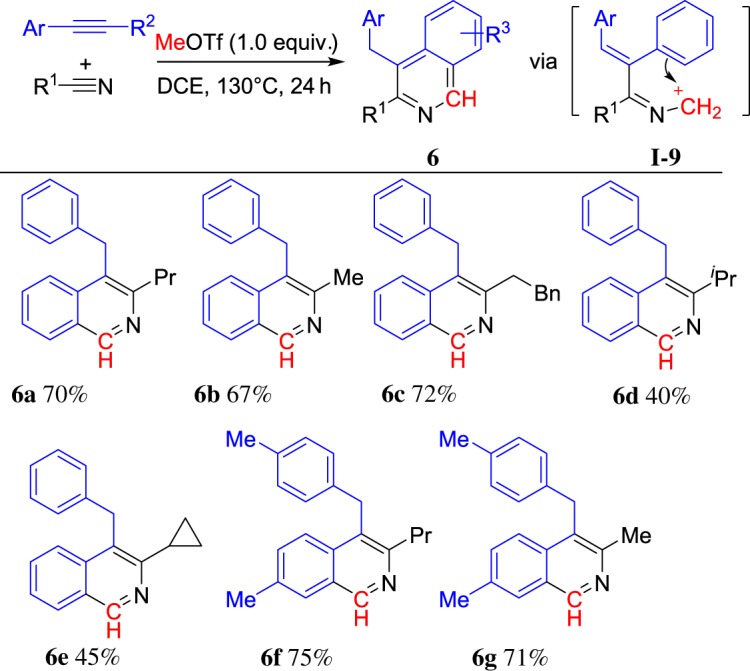
MeOTf-induced cyclization of alkylnitriles and diarylalkynes.
2.2. ROTf-induced annulation of carbodiimides
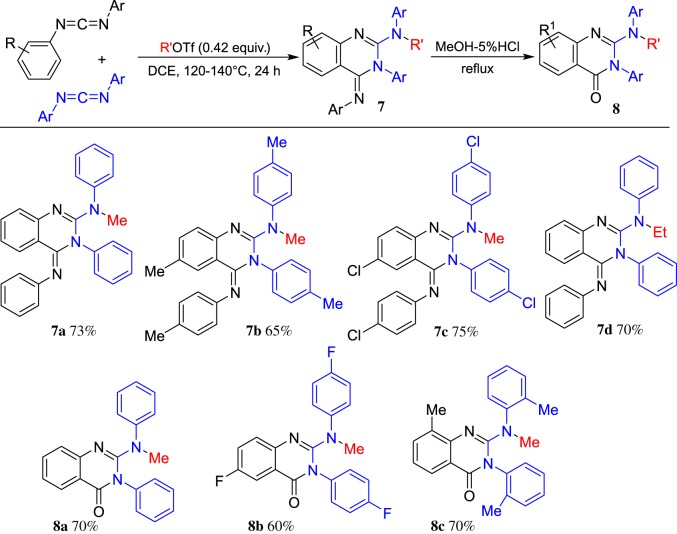
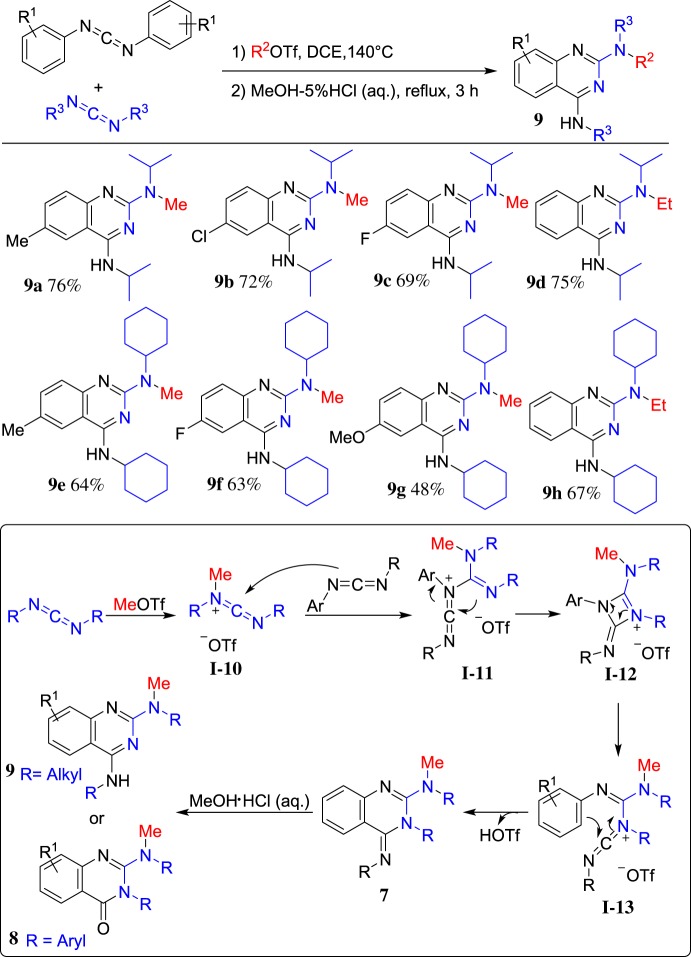
More recently, ROTf-induced electrophilic cyclization was extended to carbodiimides. Our group demonstrated an efficient ROTf-triggered intermolecular cyclization of carbodiimides to afford a range of 2-amino-4-imino-quinazolines 7, 2-aminoquinazolinones 8 and 2,4-diaminoquinazolines 9, respectively, which are important motifs in pharmaceuticals [57]. When N,N'-diarylcarbodiimides were employed, the reaction proceeded smoothly to afford the corresponding 2-amino-4-imino-quinazolines 7, which could be hydrolysed to generate the corresponding 2-aminoquinazolinones 8. The representative results are shown in scheme 5.
Scheme 5.
ROTf-induced cyclization of diarylcarbodiimides.
To further extend the substrate scope, a combination of two different carbodiimides has been achieved. A range of diarylcarbodiimides and dialkylcarbodiimides was investigated under the optimized reaction condition. The representative results are shown in scheme 6. A plausible mechanism was proposed. First, the carbodiimide is methylated by MeOTf and subsequently attacked by another molecule of carbodiimide to give intermediate I-11. Then, the intramolecular nucleophilic attack takes place to afford the four-membered intermediate I-12, which generates carbenium I-13 after ring opening via C–N bond cleavage. Finally, intramolecular Friedel–Crafts annulation occurs to form the corresponding quinazolinone imine 7, which could give 2,4-diamino-quinazoline 9 (R = alkyl group) and 2-amino-quinazolinone 8 (R = aryl group) after hydrolysis. This annulation reaction appears a general entry to the synthesis of 2-aminoquinazolinones and 2,4-diaminoquinazolines in a one-pot reaction under metal-free conditions.
Scheme 6.
ROTf-induced cyclization of diarylcarbodiimides and dialkylcarbodiimides.
2.3. ROTf-induced annulation of azobenzenes
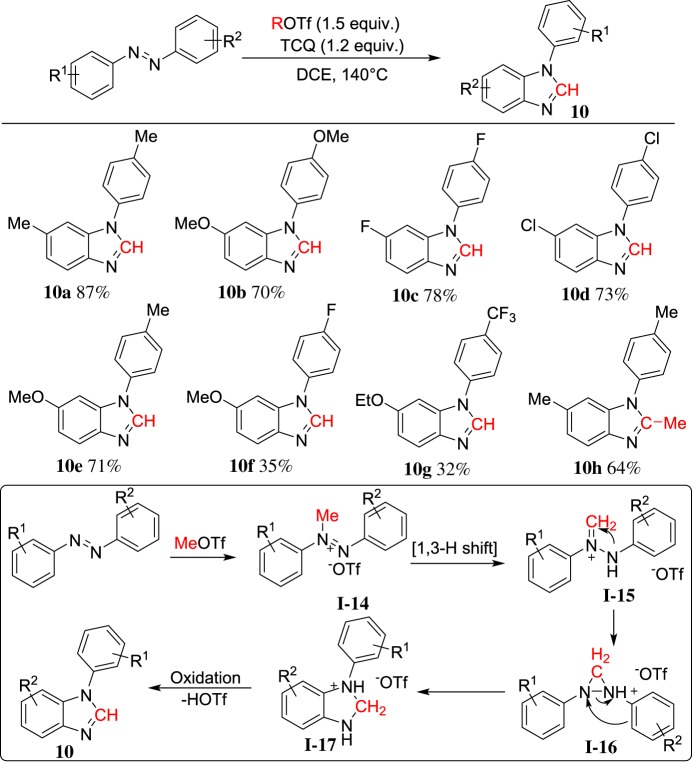
Apart from nitriles and carbodiimides, azobenzenes are also significant nitrogen-containing compounds, which possess Lewis alkalinity. In 2014, we demonstrated MeOTf-induced cyclization of azobenzenes by N=N bond cleavage with aid of TCQ (tetrachloro-1,4-benzoquinone) as oxidant to afford N-arylbenzimidazoles 10 [58] (scheme 7). When unsymmetrical azobenzenes were used, cyclization tends to occur on the electron-rich anisolyl ring (10e–10g). EtOTf could facilitate N=N bond cleavage as well to generate 2-methylbenzimidazole 10h. Although the reaction mechanism is not clear, a plausible mechanism is shown in scheme 7. The carbon atom from MeOTf inserts into the N=N bond and then cyclization to form N-arylbenzimidazole. This is the first example of N=N bond cleavage by a light main group element.
Scheme 7.
ROTf-induced cyclization of azobenzenes.
3. ROTf-induced annulation of sulfur-containing substrates with alkynes
3.1. ROTf-induced annulation of arylisothiocyanates
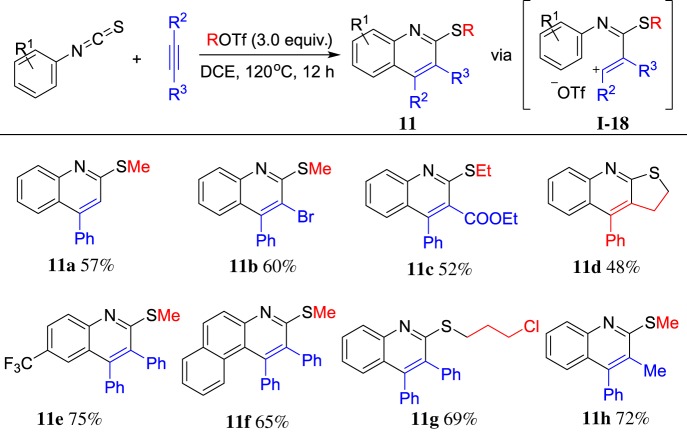
The isothiocyanates possess the chemical group –N=C=S, which represents versatile reactivity in the synthesis of nitrogen- or sulfur-containing heterocycles. Comparatively speaking, the sulfur atom is strongly nucleophilic. Recently, we reported MeOTf as an electrophile [59] to react with aryisothiocyanates and alkynes leading to diverse highly substituted quinolones. The representative results are shown in scheme 8. A tandem electrophilic activation/cyclization via intermediate I-18 is believed to be a possible process. This reaction demonstrated superiority on substrate scope for isothiocyanates. Furthermore, unsymmetrical alkynes such as terminal alkynes, (bromoethynyl) benzenes, even alkynes containing ester group could be applied in this reaction. In addition, alkyltriflates bearing C–C triple bond gave polycyclic quinolines 11d via sequent cyclization process. Great effort has been paid in the transformation of a thioalkoxyl group such as oxidation, reduction and cross-coupling reaction, which make this method more powerful in organic synthesis [59]. This reaction represents a concise, metal-free and one-pot method for synthesis of functionalized quinolines.
Scheme 8.
ROTf-induced cyclization of arylisothiocyanates and arylalkynes.
3.2. ROTf-induced annulation of alkylisothiocyanates
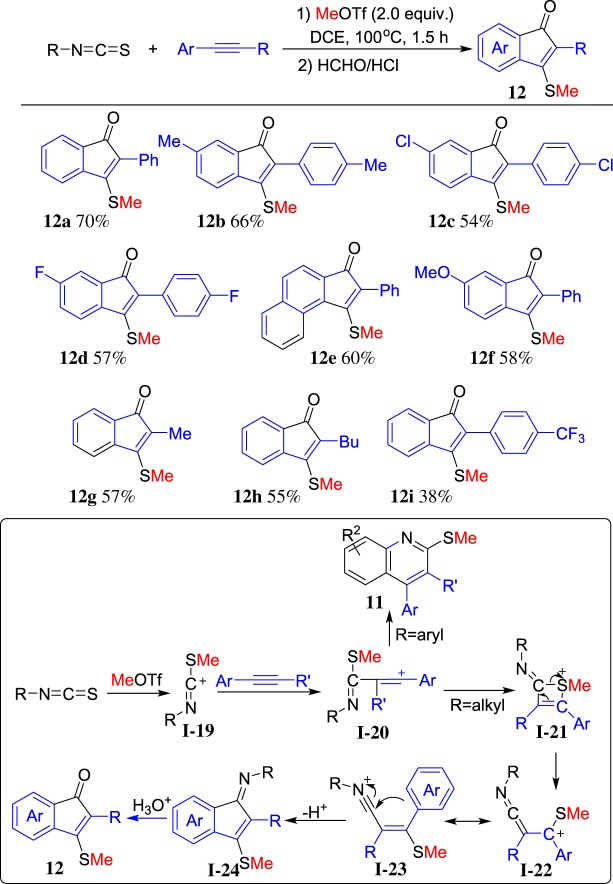
Substitution of arylisothiocyanates with alkylisothiocyanates that lack an aryl group for the ring closure might lead to a new reaction mode. To our delight, the reaction proceeded well to afford indenone 12 after hydrolysis [60]. A range of arylalkynes could be employed in this reaction. The representative results are summarized in scheme 9. A plausible mechanism is also described in scheme 9. MeOTf as an electrophile reacts with isothiocyanate to form methylthio-substituted carbenium ion I-19, which followed the reaction with arylalkyne to form intermediate I-20. Utilization of the arylisothiocyanate affords quinoline 11. Without aryl group in the alkylisothiocyanate, the nucleophilicity-strong sulfur atom attacks carbenium of I-20 to form four-membered thiete I-21, which could be followed by ring opening with the C–S bond cleavage to form carbenium I-23 via intermediate I-22. Finally, intramolecular Friedel–Crafts reaction of I-23 affords indenone imine I-24, which undergoes hydrolysis to form indenone 12. This reaction represents the first example of cleavage C–S bond in the isothiocyanate for construction of the carbocyclic compound under metal-free conditions.
Scheme 9.
ROTf-induced annulation of alkylisothiocyanates and arylalkynes.
More recently, Li and co-workers [61] reported MeOTf-induced intramolecular cyclization of isothiocyanates to afford 1-(methylthio)-3,4-dihydroisoquinolines 13 (scheme 10). The reaction may process by a tandem electrophilic activation and intramolecular Friedel–Crafts reaction.
Scheme 10.
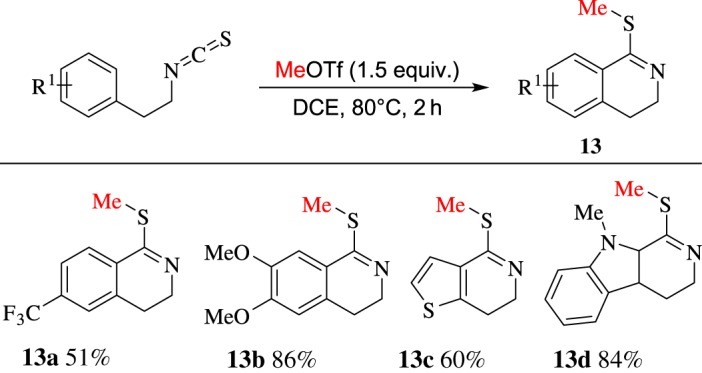
MeOTf-induced intramolecular cyclization of isothiocyanates.
4. ROTf-induced annulation of oxygen-containing substrates
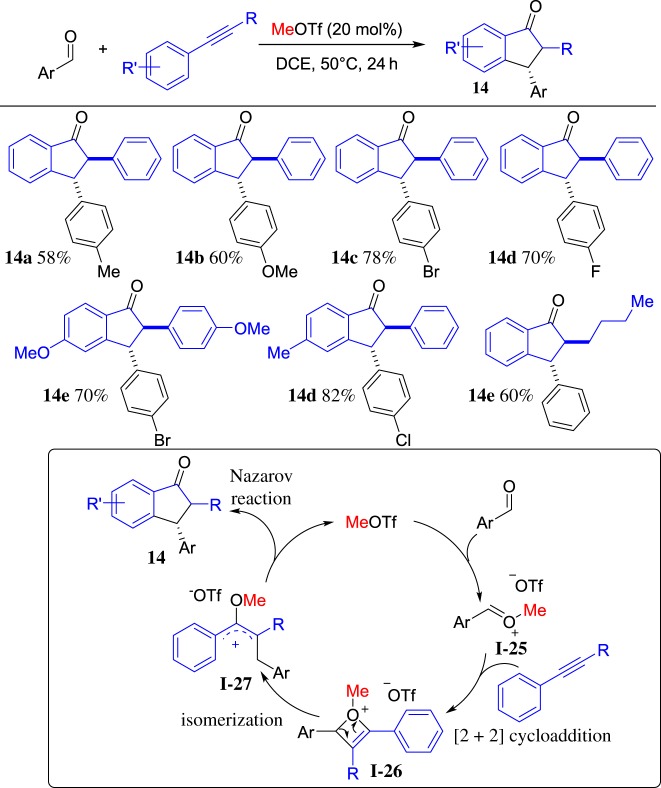
4.1. ROTf-induced annulation of aldehydes
Apart from S- and N-reagents that could react with ROTf straightforwardly, O-reagents also demonstrated a good affinity with ROTf. As a part of ongoing projects on the alkyltriflate-triggered annulation, a reaction of MeOTf, aldehydes and arylalkynes was investigated [62] and a variety of 2,3-disubstituted 1-indanones was obtained. The representative results are shown in scheme 11. It is noteworthy that a catalytic amount of MeOTf was employed and the reaction proceeded in satisfactory yield. Although the reaction mechanism is not clean, a plausible mechanism is shown at the bottom of scheme 11. First, MeOTf as an electrophile reacts with an aldehyde to afford the oxonium I-25, which couples with alkyne to form the highly active oxetenium intermediate I-26 via [2 + 2] cycloaddition. Then, the intermediate I-26 undergoes spontaneous isomerization to form the 4π-Nazarov intermediate I-27, followed by Nazarov cyclization to give 1-indanone 14 and regeneration of MeOTf. This reaction provides a practical and convenient method for the synthesis of 2,3-disubstituted 1-indanones from readily available starting materials via MeOTf-induced catalysis.
Scheme 11.
MeOTf-induced cyclization of aldehyde with arylalkynes.
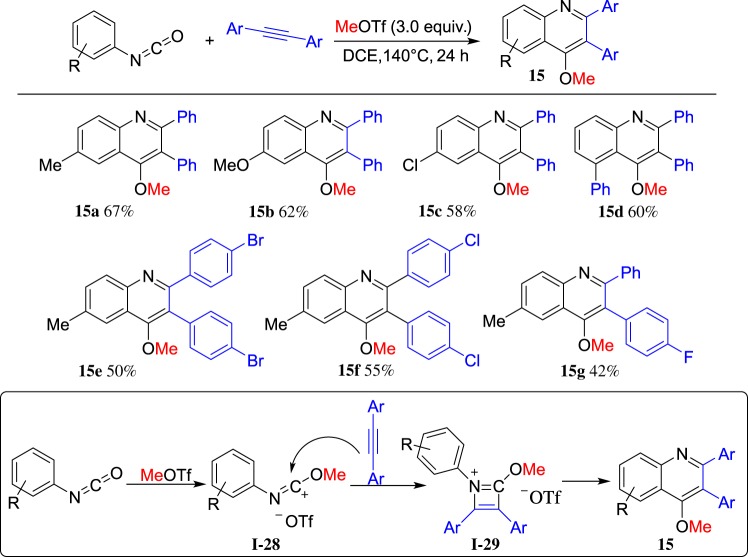
4.2. ROTf-induced annulation of arylisocyanates
Isocyanate is the functional group with the formula R–N=C=O, in which N and O may both be alkylated by ROTf. We investigated a reaction of MeOTf, arylisocyanates and arylalkynes [63]. Notably, a range of 4-methoxyl-2,3-diarylquinolines 15 was obtained in good yields and the representative results are shown in scheme 12. Based on the results, a tandem [2 + 2] cycloaddition and intramolecular Friedel–Crafts reaction may be included in the reaction pathway. It is noteworthy that this reaction has limitations with only diarylalkynes and MeOTf for the construction of 4-methoxyl-2,3-diarylquinolines 15.
Scheme 12.
MeOTf-induced cyclization of arylisocyanates and arylalkynes.
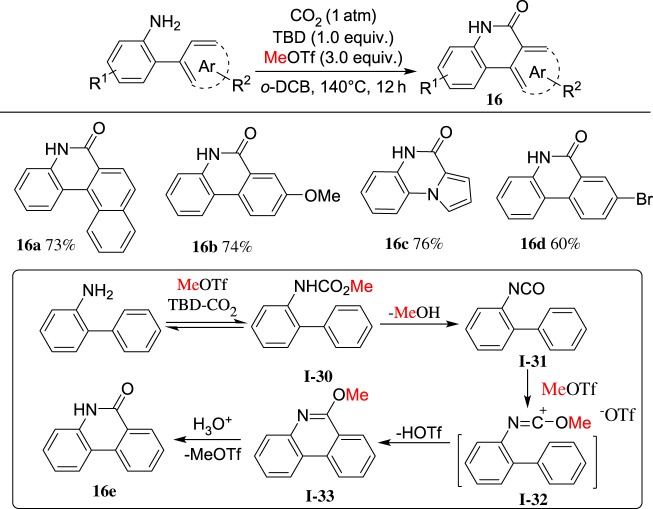
Phenanthridinones are extensively found in natural products and bioactive molecules. We envisioned that MeOTf-induced intramolecular annulation of 2-phenyl aryllisocyanates would provide a pathway for the synthesis of phenanthridinones. During the course of our study on the CO2 chemistry [64–67], we found a one-pot method for the synthesis of phenanthridinones [68] based on the MeOTf- and TBD-mediated carbonylation of ortho-arylanilines with CO2. The representative results and reaction pathway are shown in scheme 13. This reaction shows MeOTf-induced carbonylation reaction of o-arylanilines applying CO2 as the ideal carbonyl source to synthesize phenanthridinones containing a free (NH)-lactam motif under metal-free conditions.
Scheme 13.
MeOTf-induced cyclization with o-arylanilines and CO2.
5. ROTf-induced annulation of phosphorus-containing substrates
In 2006, Bates & Gates [69] used the strong electrophilicity of MeOTf in the synthesis of highly strained four-membered phosphorus heterocycles with phosphaalkenes as a precursor (scheme 14). The structure of the unprecedented diphosphetanium salt 17 was identified by single crystal X-ray diffraction. Although the reaction mechanism is not clean, this reaction demonstrated a convenient method for synthesis of highly strained phosphorus heterocycles, which may be used as a propagating species in the cationic polymerization of phosphaalkenes.
Scheme 14.
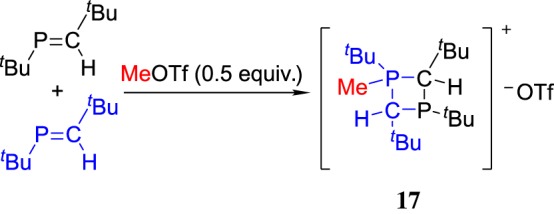
MeOTf-induced cyclization with phosphaalkenes.
6. Conclusion and future outlook
ROTf has been a powerful reagent in organic synthesis featuring efficient, metal-free and easy of handing. A range of heteroatom-containing unsaturated reagents such as nitriles, carbodiimides, azobenzenes, isothiocyanates, aldehydes, isocyanates and phosphaalkene could be alkylated by the ROTf to generate reactive intermediates, which are capable of capturing other electrophilic substrates to afford cyclic compounds with a rational design. The strong electrophilicity of ROTf has bestowed the heteroatom-containing reagent quite unique versatility as a catalyst, promoter or reactant in a wide range of organic transformations, including carbon–carbon and carbon–heteroatom bond formation processes. The synthetic methodology to various carbocyclic and heterocyclic compounds has been widely developed. Although ROTf has exhibited great value for the synthesis of cyclic compounds, further exploration is required for the utilization of functionalized ROTf reagents and other heteroatom reagents such as organoselenium, organophosphorus substrates for synthesis of functionalized compounds. Furthermore, ROTf-induced unsaturated heteroatom-containing reagents to generate electrophiles bearing carbon cations could be involved in new approaches in many synthetic organic reactions under mild and metal-free conditions. Moreover, ROTf-induced [2 + 2] cycloaddition to afford four-member ring intermediates proposed to elucidate surprising rearrangements still needs firm evidence. We anticipate that ROTf can be extended to more organic reactions in organic synthesis.
Supplementary Material
Acknowledgements
We thank all the cited authors for providing the research results for our reference.
Data accessibility
This article does not contain any additional data.
Authors' contributions
S.Z. and S.W. participated in the design of the study and drafted the manuscript. C.X. conceived the study, coordinated the study and helped draft the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the National Natural Science Foundation of China (nos. 21472106 and 91645120).
References
- 1.Lie JJ, Gribble GW. 2000. Palladium in heterocyclic chemistry. New York, NY: Pergamon Press. [Google Scholar]
- 2.Zeni G, Larock RC. 2004. Synthesis of heterocycles via palladium π-olefin and π-alkyne chemistry. Chem. Rev. 104, 2285–2309. ( 10.1021/cr020085h) [DOI] [PubMed] [Google Scholar]
- 3.Nakamura I, Yamamoto Y. 2004. Transition-metal-catalyzed reactions in heterocyclic synthesis. Chem. Rev. 104, 2127–2198. ( 10.1021/cr020095i) [DOI] [PubMed] [Google Scholar]
- 4.Zeni G, Larock RC. 2006. Synthesis of heterocycles via palladium-catalyzed oxidative addition. Chem. Rev. 106, 4644–4680. ( 10.1021/cr0683966) [DOI] [PubMed] [Google Scholar]
- 5.Álvarez-Corral M, Muñoz-Dorado M, Rodríguez-García I. 2008. Silver-mediated synthesis of heterocycles. Chem. Rev. 108, 3174–3198. ( 10.1021/cr078361l) [DOI] [PubMed] [Google Scholar]
- 6.Patil NT, Yamamoto Y. 2008. Coinage metal-assisted synthesis of heterocycles. Chem. Rev. 108, 3395–3442. ( 10.1021/cr050041j). [DOI] [PubMed] [Google Scholar]
- 7.Li CJ, Trost BM. 2008. Green chemistry for chemical synthesis. Proc. Natl Acad. Sci. USA 105, 13 197–13 202. ( 10.1073/pnas.0804348105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonchick AP, Samanta R, Kulikov K, Lategahn J. 2011. Organocatalytic, oxidative, intramolecular C-H bond amination and metal-free cross-amination of unactivated arenes at ambient temperature. Angew. Chem. Int. Ed. 50, 8605–8608. ( 10.1002/anie.201102984) [DOI] [PubMed] [Google Scholar]
- 9.Kim HJ, Kim J, Cho SH, Chang S. 2011. Intermolecular oxidative C-N bond formation under metal-free conditions: control of chemoselectivity between aryl sp2 and benzylic sp3 C-H bond imidation. J. Am. Chem. Soc. 133, 16 382–16 385. ( 10.1021/ja207296y) [DOI] [PubMed] [Google Scholar]
- 10.Kantak AA, Potavathri S, Barham RA, Romano KM, DeBoef B. 2011. Metal-free intermolecular oxidative C-N bond formation via tandem C-H and N-H bond functionalization. J. Am. Chem. Soc. 133, 19 960–19 965. ( 10.1021/ja2087085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Froehr T, Sindlinger CP, Kloeckner U, Finkbeiner P, Nachtsheim BJ. 2011. A metal-free amination of benzoxazoles: the first example of an iodide-catalyzed oxidative amination of heteroarenes. Org. Lett. 13, 3754–3757. ( 10.1021/ol201439t) [DOI] [PubMed] [Google Scholar]
- 12.Xiao Q, Tian L, Tan R, Xia Y, Qiu D, Zhang Y, Wang J. 2012. Transition-metal-free electrophilic amination of arylboroxines. Org. Lett. 14, 4230–4233. ( 10.1021/ol301912a) [DOI] [PubMed] [Google Scholar]
- 13.Sun CL, Shi ZJ. 2014. Transition-metal-free coupling reactions. Chem. Rev. 114, 9219–9280. ( 10.1021/cr400274j) [DOI] [PubMed] [Google Scholar]
- 14.Kim YR, Cho S, Lee PH. 2014. Metal-free azaphosphaannulation of phosphonamides through intramolecular oxidative C-N bond formation. Org. Lett. 16, 3098–3101. ( 10.1021/ol501207w) [DOI] [PubMed] [Google Scholar]
- 15.Youn SW, Lee EM. 2016. Metal-free one-pot synthesis of N,N'-diarylamidines and N-arylbenzimidazoles from arenediazonium salts, nitriles, and free anilines. Org. Lett. 18, 5728–5731. ( 10.1021/acs.orglett.6b02994) [DOI] [PubMed] [Google Scholar]
- 16.Alder RW, Phillips JGE, Huang L, Huang X. 2005. Methyltrifluoromethanesulfonate. In Encyclopedia of reagents for organic synthesis. New York, NY: John Wiley & Sons. [Google Scholar]
- 17.Stang PJ, Hanack M, Subramanian LR. 1982. Perfluoroalkanesulfonic esters: methods of preparation and applications in organic chemistry. Synthesis 2, 0085–0127. ( 10.1055/s-1982-29711) [DOI] [Google Scholar]
- 18.Howells RD, Mc Cown JD. 1977. Trifluoromethanesulfonic acid and derivatives. Chem. Rev. 77, 69–92. ( 10.1021/cr60305a005) [DOI] [Google Scholar]
- 19.Zheng D, Ma H, Ding K. 2017. A practical synthesis of trifluoromethanesulfonate esters. Chin. J. Org. Chem. 37, 1582–1584. ( 10.6023/cjoc201701035) [DOI] [Google Scholar]
- 20.Chapman RD, Andreshak JL, Herrlinger SP, Shackelford SA, Hildreth RA, Smith JP. 1986. Trifluoromethanesulfonate esters from dibromoalkane metatheses with silver triflate: mechanistic and synthetic aspects. J. Org. Chem. 51, 3792–3798. ( 10.1021/jo00370a009) [DOI] [Google Scholar]
- 21.Baraznenok IL, Nenajdenko VG, Balenkova ES. 2000. Chemical transformations induced by triflic anhydride. Tetrahedron 56, 3077–3119. ( 10.1016/S0040-4020(00)00093-4) [DOI] [Google Scholar]
- 22.Aubert C, Begue JP. 1985. A simple preparation of alkyl trifluoromethanesulfonates (triflates) from alkyl trimethylsilyl ethers. Synthesis 8, 759–760. ( 10.1055/s-1985-31336) [DOI] [Google Scholar]
- 23.Burdon J, McLoughlin VCR. 1965. Trifluoromethanesulphonate esters and their alkylating properties. Tetrahedron 21, 1–4. ( 10.1016/S0040-4020(01)82194-3) [DOI] [Google Scholar]
- 24.Dobbs AP, Jones K, Veal KT. 1997. The generation and cyclisation of pyridinium radicals as a potential route to indolizidine alkaloids. Tetrahedron Lett. 38, 5383–5386. ( 10.1016/S0040-4039(97)01178-7) [DOI] [Google Scholar]
- 25.Beard CD, Baum K, Grakauskas V. 1973. Synthesis of some novel trifluoromethanesulfonates and their reactions with alcohols. J. Org. Chem. 38, 3673–3677. ( 10.1021/jo00961a003) [DOI] [Google Scholar]
- 26.Ignatyev NV, Barthen P, Kucheryna A, Willner H, Sartori P. 2012. A method for producing perfluoroalkanesulfonic acid esters. Molecules 17, 5319–5337. ( 10.3390/molecules17055319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burford N, Dyker CA, Decken A. 2005. Facile synthetic methods for the diversification of catena-polyphosphorus cations. Angew. Chem. Int. Ed. 44, 2364–2367. ( 10.1002/anie.200462997) [DOI] [PubMed] [Google Scholar]
- 28.Imamoto T, Kikuchi SI, Miura T, Wada Y. 2001. Stereospecific reduction of phosphine oxides to phosphines by the use of a methylation reagent and lithium aluminum hydride. Org. Lett. 3, 87–90. ( 10.1021/ol0068041) [DOI] [PubMed] [Google Scholar]
- 29.Rong MK, Duin K, Dijk T, Pater JJM, Deelman B.-J, Nieger M, Ehlers AW, Slootweg JC, Lammertsma K. 2017. Iminophosphanes: synthesis, rhodium complexes, and ruthenium(II)-catalyzed hydration of nitriles. Organometallics 36, 1079–1090. ( 10.1021/acs.organomet.7b00057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye H, et al. 2013. A safe and facile route to imidazole-1-sulfonyl azide as a diazotransfer reagent. Org. Lett. 15, 18–21. ( 10.1021/ol3028708) [DOI] [PubMed] [Google Scholar]
- 31.Booth BL, Jibodu KO, Proenca MF. 1980. The synthesis and some reactions of N-methylnitrilium trifluoromethanesulphonate salts. J. Chem. Soc. Chem. Commun. 1980, 1151–1153. ( 10.1039/C39800001151) [DOI] [Google Scholar]
- 32.Thomas Z, Gregor L. 1998. Synthesis of β-mannosides via prearranged glycosides. Angew. Chem. Int. Ed. 37, 3129–3132. ( 10.1002/(SICI)1521-3773(19981204)37:22%3C3129::AID-ANIE3129%3E3.0.CO;2-8) [DOI] [PubMed] [Google Scholar]
- 33.Ranade SC, Kaeothip S, Demchenko AV. 2010. Glycosyl alkoxythioimidates as complementary building blocks for chemical glycosylation. Org. Lett. 12, 5628–5631. ( 10.1021/ol1023079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laali K, Chen HY, Gerzina RJ. 1988. Methylation of aromatics with Me3S+, Me3Se+, and Me3Te+ in superacid media. J. Org. Chem. 348, 199–204. ( 10.1016/0022-328X(88)80396-6) [DOI] [Google Scholar]
- 35.Su B, Ganguly R, Li Y, Kinjo R. 2016. Synthesis, characterization, and electronic structures of a methyl germyliumylidene ion and germylone-group VI metal complexes. Chem. Commun. 52, 613–616. ( 10.1039/C5CC08665E) [DOI] [PubMed] [Google Scholar]
- 36.Wallenhauer S, Seppelt K. 1994. Methylbismuth(V) compounds. Angew. Chem. Int. Ed. 33, 976–978. ( 10.1002/anie.199409761) [DOI] [Google Scholar]
- 37.Yamaguchi T, Asay M, Sekiguchi A. 2012. [[(Me3Si)2CH]2iPrSi(NHC)Si=Si(Me)SiiPr[CH(SiMe3)2]2]+: a molecule with disilenyl cation character. J. Am. Chem. Soc. 134, 886–889. ( 10.1021/ja210669n) [DOI] [PubMed] [Google Scholar]
- 38.Spera ML, Harman WD. 1999. Osmium-mediated electrophilic addition reactions with selenophene and activation of the Se-C bond. Organometallics 18, 1559–1561. ( 10.1021/om9806949) [DOI] [Google Scholar]
- 39.Mutoh Y, Murai T. 2003. Acyclic selenoiminium salts: isolation, first structural characterization, and reactions. Org. Lett. 5, 1361–1364. ( 10.1021/ol034334f) [DOI] [PubMed] [Google Scholar]
- 40.Chen ZJ, Xi HW, Lim KH, Lee JM. 2013. Distillable ionic liquids: reversible amide O alkylation. Angew. Chem. Int. Ed. 52, 13 392–13 396. ( 10.1002/anie.201306476) [DOI] [PubMed] [Google Scholar]
- 41.Li G, Xue Z, Cao B, Yan C, Mu T. 2016. Preparation and properties of C=X (X: O, N, S) based distillable ionic liquids and their application for rare earth separation. ACS Sustain. Chem. Eng. 4, 6258–6262. ( 10.1021/acssuschemeng.6b01984) [DOI] [Google Scholar]
- 42.Ulibarri G, Choret N, Bigg DCH. 1996. Activation of imidazolides using methyl trifluoromethanesulfonate: a convenient method for the preparation of hindered esters and amides. Synthesis 11, 1286–1288. ( 10.1055/s-1996-4399) [DOI] [Google Scholar]
- 43.Zhu F, Tao J, Wang Z. 2015. Palladium-catalyzed C-H arylation of (benzo)oxazoles or (benzo)thiazoles with aryltrimethylammonium triflates. Org. Lett. 17, 4926–4929. ( 10.1021/acs.orglett.5b02458) [DOI] [PubMed] [Google Scholar]
- 44.Yu W, Yang S, Xiong F, Fan T, Feng Y, Huang Y, Fu J, Wang T. 2018. Palladium-catalyzed carbonylation of benzylic ammonium salts to amides and esters via C–N bond activation. Org. Biomol. Chem. 16, 3099–3103. ( 10.1039/C8OB00488A) [DOI] [PubMed] [Google Scholar]
- 45.Dondoni A, Catozzi N, Marra A. 2005. Concise and practical synthesis of c-glycosyl ketones from sugar benzothiazoles and their transformation into chiral tertiary alcohols. J. Org. Chem. 70, 9257–9268. ( 10.1021/jo051377w) [DOI] [PubMed] [Google Scholar]
- 46.Murai T, Mutoh Y, Ohta Y, Murakam M. 2004. Synthesis of tertiary propargylamines by sequential reactions of in situ generated thioiminium salts with organolithium and -magnesium reagents. J. Am. Chem. Soc. 126, 5968–5969. ( 10.1021/ja048627v) [DOI] [PubMed] [Google Scholar]
- 47.Yan X, Zou S, Zhao P, Xi C. 2014. MeOTf-induced carboannulation of arylnitriles and aromatic alkynes: a new metal-free strategy to construct indenones. Chem. Commun. 50, 2775–2777. ( 10.1039/C4CC00088A) [DOI] [PubMed] [Google Scholar]
- 48.Pletnev AA, Tian Q, Larock RC. 2002. Carbopalladation of nitriles: synthesis of 2,3-diarylindenones and polycyclic aromatic ketones by the Pd-catalyzed annulation of alkynes and bicyclic alkenes by 2-iodoarenenitriles. J. Org. Chem. 67, 9276–9287. ( 10.1021/jo026178g) [DOI] [PubMed] [Google Scholar]
- 49.Miura T, Murakami M. 2005. Rhodium-catalyzed annulation reactions of 2-cyanophenylboronic acid with alkynes and strained alkenes. Org. Lett. 7, 3339–3341. ( 10.1021/ol051249u) [DOI] [PubMed] [Google Scholar]
- 50.Tsukamoto H, Kondo Y. 2007. Palladium(II)-catalyzed annulation of alkynes with ortho-ester-containing phenylboronic acids. Org. Lett. 9, 4227–4230. ( 10.1021/ol701776m) [DOI] [PubMed] [Google Scholar]
- 51.Harada Y, Nakanishi J, Fujihara H, Tobisu M, Fukumoto Y, Chatani N. 2007. Rh(I)-catalyzed carbonylative cyclization reactions of alkynes with 2-bromophenylboronic acids leading to indenones. J. Am. Chem. Soc. 129, 5766–5771. ( 10.1021/ja070107n) [DOI] [PubMed] [Google Scholar]
- 52.Morimoto T, et al. 2009. Rh(I)-catalyzed CO gas-free carbonylative cyclization reactions of alkynes with 2-bromophenylboronic acids using formaldehyde. Org. Lett. 11, 1777–1780. ( 10.1021/ol900327x) [DOI] [PubMed] [Google Scholar]
- 53.Li BJ, Wang HY, Zhu QL, Shi ZJ. 2012. Rhodium/copper-catalyzed annulation of benzimides with internal alkynes: indenone synthesis through sequential C-H and C-N cleavage. Angew. Chem. Int. Ed. 51, 3948–3952. ( 10.1002/anie.201200271) [DOI] [PubMed] [Google Scholar]
- 54.Chen S, Yu J, Jiang Y, Chen F, Cheng J. 2013. Rhodium-catalyzed direct annulation of aldehydes with alkynes leading to indenones: proceeding through in situ directing group formation and removal. Org. Lett. 15, 4754–4757. ( 10.1021/ol4021145) [DOI] [PubMed] [Google Scholar]
- 55.Qi Z, Wang M, Li X. 2013. Access to indenones by rhodium(III)-catalyzed C–H annulation of arylnitrones with internal alkynes. Org. Lett. 15, 5440–5443. ( 10.1021/ol4025309). [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Yi X, Luo X, Xi C. 2017. MeOTf-mediated annulation of alkylnitriles and arylalkynes leading to polysubstituted NH-pyrroles. J. Org. Chem. 82, 11 391–11 398. ( 10.1021/acs.joc.7b01845) [DOI] [PubMed] [Google Scholar]
- 57.Zhang X, Wang S, Liu Y, Xi C. 2018. Triflates-triggered intermolecular cyclization of carbodiimides leading to 2-aminoquinazolinone and 2,4-diaminoquinazoline derivatives. Org. Lett. 20, 2148–2151. ( 10.1021/acs.orglett.8b00314) [DOI] [PubMed] [Google Scholar]
- 58.Yan X, Yi X, Xi C. 2014. Direct cleavage of the N=N bond of azobenzenes by MeOTf leading to N-arylbenzimidazoles. Org. Chem. Front. 1, 657–660. ( 10.1039/C4QO00056K) [DOI] [Google Scholar]
- 59.Zhao P, Yan X, Yin H, Xi C. 2014. Alkyltriflate-triggered annulation of arylisothiocyanates and alkynes leading to multiply substituted quinolines through domino electrophilic activation. Org. Lett. 16, 1120–1123. ( 10.1021/ol500221u) [DOI] [PubMed] [Google Scholar]
- 60.Zhao P, Liu Y, Xi C. 2015. MeOTf-induced carboannulation of isothiocyanates and aryl alkynes with C=S bond cleavage: access to indenones. Org. Lett. 17, 4388–4391. ( 10.1021/acs.orglett.5b02201) [DOI] [PubMed] [Google Scholar]
- 61.Wen L, Dou Q, Wang Y, Zhang J, Guo W, Li M. 2017. Synthesis of 1-thio-substituted isoquinoline derivatives by tandem cyclization of isothiocyanates. J. Org. Chem. 82, 1428–1436. ( 10.1021/acs.joc.6b02605) [DOI] [PubMed] [Google Scholar]
- 62.Liu Y, Zhao P, Zhang B, Xi C. 2016. MeOTf-catalyzed annulation of aldehydes and arylalkynes leading to 2,3-disubstituted indanones. Org. Chem. Front. 3, 1116–1119. ( 10.1039/C6QO00253F) [DOI] [Google Scholar]
- 63.Liu Y, Zhang X, Xi C. 2018. MeOTf-induced annulation of arylisocyanates and arylalkynes leading to 4-methoxyl-2,3-diarylquinolines. Tetrahedron Lett. 25, 2440–2442. ( 10.1016/j.tetlet.2018.05.030) [DOI] [Google Scholar]
- 64.Shao P, Wang S, Chen C, Xi C. 2015. Zirconocene-catalyzed sequential ethylcarboxylation of alkenes using ethylmagnesium chloride and carbon dioxide. Chem. Commun. 51, 6640–6642. ( 10.1039/C5CC01153A) [DOI] [PubMed] [Google Scholar]
- 65.Wang S, Shao P, Chen C, Xi C. 2015. Copper-catalyzed carboxylation of alkenylzirconocenes with carbon dioxide leading to α,β-unsaturated carboxylic acids. Org. Lett. 17, 5112–5115. ( 10.1021/acs.orglett.5b02619) [DOI] [PubMed] [Google Scholar]
- 66.Wang S, Du G, Xi C. 2016. Copper-catalyzed carboxylation reactions using carbon dioxide. Org. Biomol. Chem. 14, 3666–3676. ( 10.1039/C6OB00199H) [DOI] [PubMed] [Google Scholar]
- 67.Shao P, Wang S, Chen C, Xi C. 2016. Cp2TiCl2-catalyzed regioselective hydrocarboxylation of alkenes with CO2. Org. Lett. 9, 2050–2053. ( 10.1021/acs.orglett.6b00665). [DOI] [PubMed] [Google Scholar]
- 68.Wang S, Shao P, Du G, Xi C. 2016. MeOTf- and TBD-mediated carbonylation of ortho-arylanilines with CO2 leading to phenanthridinones. J. Org. Chem. 81, 6672–6676. ( 10.1021/acs.joc.6b01318) [DOI] [PubMed] [Google Scholar]
- 69.Bates JI, Gates PD. 2006. Diphosphiranium (P2C) or diphosphetanium (P2C2) cyclic cations: different fates for the electrophile-initiated cyclodimerization of a phosphaalkene. J. Am. Chem. Soc. 128, 15 998–15 999. ( 10.1021/ja0667662) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article does not contain any additional data.