Abstract
Host cell exit is a critical step in the life-cycle of intracellular pathogens, intimately linked to barrier penetration, tissue dissemination, inflammation, and pathogen transmission. Like cell invasion and intracellular survival, host cell exit represents a well-regulated program that has evolved during host-pathogen co-evolution and that relies on the dynamic and intricate interplay between multiple host and microbial factors. Three distinct pathways of host cell exit have been identified that are employed by three different taxa of intracellular pathogens, bacteria, fungi and protozoa, namely (i) the initiation of programmed cell death, (ii) the active breaching of host cellderived membranes, and (iii) the induced membrane-dependent exit without host cell lysis. Strikingly, an increasing number of studies show that the majority of intracellular pathogens utilize more than one of these strategies, dependent on life-cycle stage, environmental factors and/or host cell type. This review summarizes the diverse exit strategies of intracellular-living bacterial, fungal and protozoan pathogens and discusses the convergently evolved commonalities as well as system-specific variations thereof. Key microbial molecules involved in host cell exit are highlighted and discussed as potential targets for future interventional approaches.
Keywords: host cell, egress, exit, pathogen, vacuolar escape, compartment, programmed cell death, membrane lysis.
INTRODUCTION
Infectious diseases caused by viruses, bacteria, fungi, or parasites still represent a major cause of morbidity worldwide and account for almost 50,000 deaths every day [1]. During the course of infection, many bacterial, fungal and protozoan pathogens rely on a life-cycle phase, during which they parasitize host cells, typically but not always contained by membranous vacuoles. The intracellular lifestyle provides several advantages, including accessibility to nutrients and evasion from the human humoral and cellular immune system. Research during the last decades has revealed manifold examples illustrating the intricate and sophisticated strategies of microorganisms to invade their host cells and to manipulate the intracellular environment in order to promote microbial survival and to evade destruction by the host immune system (reviewed in [2]). Although crucial to ensure life-cycle progression, and, in consequence, dissemination and transmission, host cell exit has hitherto remained largely unresolved. Recent results, which were mainly gained from studies on Listeria, Toxoplasma and Plasmodium, provide firm evidence that host cell exit, initially viewed as a largely passive process, represents an active and dynamic interplay between the pathogen and the infected cell. Three major exit pathways, (i) the induction of programmed cell death (PCD), (ii) the active breaching of host cell-derived membranes, and (iii) the induced membrane-dependent exit without host cell lysis, have been identified. The pathways follow a spatially and temporally defined coordinated process and involve the interaction of pathogen- and host-derived effector molecules (reviewed in [3-7]).
Host cell exit by human pathogens is typically linked to tissue inflammation, organ dysfunction, and host-to-host transmission and thus significantly contributes to disease burden and the epidemiology of infectious diseases. Hence, a detailed knowledge of the underlying mechanisms of microbial cell exit is essential to understand the pathogenesis of infectious diseases. This is demonstrated by the critical role of exit-associated microbial effector molecules for the survival and spread of pathogens. An example is the recent finding that chemical inhibition of plasmepsin (PM) proteases can interrupt the egress of malaria parasites from the enveloping erythrocyte [8, 9]. The present review therefore aims at attracting the well-deserved attention to this essential and understudied life-cycle phase of intracellular pathogens. We will explore the diverse exit strategies of intracellular-living bacterial, fungal, and protozoan pathogens, and discuss key microbial and host effector molecules. Further, we will assess the cellular and molecular aspects of microbial host cell exit and evaluate potential targets for future interventional strategies to combat infections by intracellular pathogens.
EXIT PATHWAYS
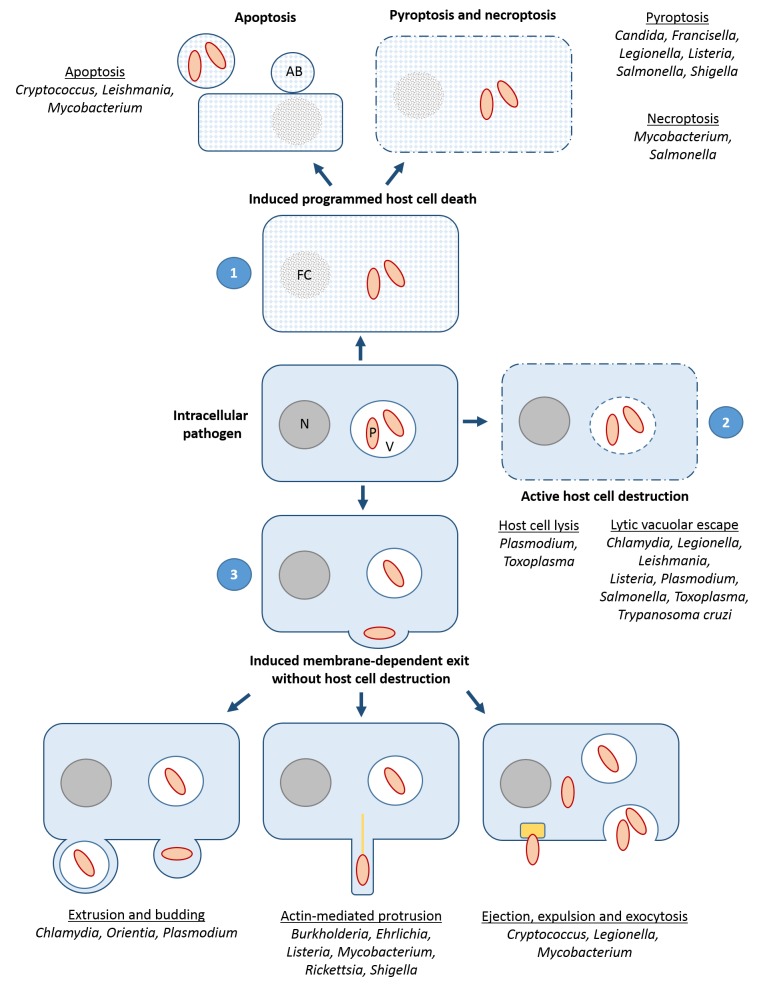
Current data indicate that at least three principal exit pathways are used by intracellular pathogens (outlined in Fig. 1) (reviewed in [3-7]): (i) PCD including the non-lytic apoptosis and the lytic necroptosis and pyroptosis pathways, employed by bacterial, fungal and protozoan pathogens; (ii) the active breaching of host cell-derived membranes such as the endosomal, the vacuolar and/or the host cell plasma membrane, as shown for a variety of bacterial and protozoan parasites; (iii) the induced membrane-dependent exit without host cell lysis, e.g. via actin-based protrusions, extrusions, budding, exocytosis, expulsion or ejection, as has been demonstrated for some bacteria, for the yeast Cryptococcus and for Plasmodium. While the first two pathways ultimately result in killing of the host cell, the third pathway in general leaves the host cell intact. Intriguingly, recent work suggests that the majority of intracellular pathogens is able to utilize more than one of these pathways, dependent on life-cycle stage, environmental factors and/or host cell type.
Figure 1. FIGURE 1: The three strategies of host cell exit.
(1) Induction of programmed cell death, including the non-lytic apoptosis and the lytic necroptosis and pyroptosis pathways; (2) Active host cell destruction, comprising breaching of host cell membranes such as the vacuolar and/or the host cell plasma membrane; (3) Induced membrane-dependent exit without host cell destruction, e.g. by actin tail (yellow line)- mediated protrusions, extrusions, budding, exocytosis, expulsion or ejection via an ejectosome (yellow box). Pathogens for which distinct pathways were demonstrated are indicated. AB, apoptotic body; FC, fragmented chromatin; N, nucleus; P, pathogen; V, vacuole.
Induction of programmed host cell death
PCD represents an intrinsic, regulated process of cell death with typical morphological changes and an important contribution to tissue ontogeny, cellular immunity and organ homeostasis. Different forms of PCD, i.e. apoptosis, necroptosis and pyroptosis, have been described that are initiated by specific signals and signal transduction cascades and that exhibit defined phenotypic characteristics (reviewed in [10]). PCD is also observed during infection, where it allows exfoliation and removal of infected cells. Here, a typical distinction is the one between lytic and non-lytic cell death, which has a profound impact of the immune response and, in consequence, on the course of infection. Lytic death is associated with early cell membrane permeabilization and release of cell debris, whereas non-lytic cell death maintains the surface integrity of the dying cell until its uptake by a phagocyte. Non-lytic cell death is largely confined to apoptosis whereas both pyroptosis and necroptosis cause early loss of plasma membrane integrity. Notably, even late stage apoptosis has a programmed lytic component, referred to as secondary necrosis. Accumulating evidence suggests that microbial targeting of PCD-inducing signal transduction pathways represents a cell exit strategy for a number of pathogens (Fig. 1).
Apoptosis
Apoptosis represents an evolutionarily conserved mechanism that contributes significantly to organ development and tissue homeostasis. Apoptosis also plays a prominent role in preventing pathogen progeny, as previously observed during viral infections [11]. It is characterized by typical morphological changes, including the disintegration of the apoptotic cell into condensed apoptotic bodies, which are subsequently taken up by phagocytes, particularly macrophages. Via uptake of these apoptotic bodies, viable pathogens could be transferred to a phagocytic cell in a non-inflammatory context. Apoptosis would therefore not release the intracellular bacterium to the extracellular space, but would rather lead to its re-uptake and thus cell-to-cell spread. The pathogen will remain within a membrane-enclosed particle and end up in a phagosome, where it has to deal with multiple degradative mechanisms. This is illustrated by the phagocyte-infecting gram-positive bacterium Mycobacterium tuberculosis, which is taken up within apoptotic bodies via efferocytosis [12]. M. tuberculosis is subsequently killed by the macrophage. In contrast, the protozoan parasite Leishmania spp., the causative agent of the vector-borne tropical disease leishmaniosis, is internalized by macrophages packed in apoptotic bodies and subsequently establishes itself in the phagosomal compartment [13, 14]. Furthermore, the fungal pathogen Cryptococcus neoformans, which can cause infections of a number of organs, including the lung and central nervous system, can exit the cell through lytic and non-lytic mechanisms, and the lytic mechanism has been found to be of apoptotic nature, even though lysis does not normally occur during apoptosis [15].
Necroptosis
Necroptosis shows phenotypic characteristics of necrotic cell death (thus also termed programmed necrosis) and is induced by a specific signaling pathway that includes the receptor-interacting protein kinase 3 (RIP3/RIPK3) and the mixed lineage kinase domain like pseudokinase (MLKL) (reviewed in [16, 17]). It has to be kept in mind that most mechanistic studies of necroptosis use agonists of deathreceptor signalling to induce necroptosis. In this situation, necroptosis only occurs if caspase-8, which belongs to a group of PCD-related cysteine-aspartic proteases, is inhibited via chemical or gene-knock out approaches. Although such approaches have improved our understanding, this type of experimental design is not physiological. The role of necroptosis during infection therefore requires further investigations.
Necroptosis has been observed during infection with the facultative intracellular gram-negative bacterium Salmonella enterica serovar Typhimurium (S. Typhimurium), a pathogen causing gastroenteritis in humans following ingestion of contaminated food or water. Following penetration of the intestinal mucosal barrier, S. Typhimurium is taken up by macrophages and induces necroptosis [18]. Unexpectedly, this induction depends on type I interferon and the inhibition of necroptosis reduces the bacterial organ load [19]. However, it remains unresolved whether this is because cell exit is blocked or because macrophages survive and kill the bacterium.
A number of studies have investigated the role of necroptosis during mycobacterial infection. The most intriguing finding is that death of neutrophils that have engulfed virulent M. tuberculosis is instrumental to the transfer of the bacteria into macrophages, where they proliferate and induce persistent infection [20]. Cell death in neutrophils is induced via reactive oxygen species (ROS). Because the study was performed in human cells where the possibilities of genetic modification are limited, it is not clear though, if death of the neutrophil mechanistically represents necroptosis. As shown, chemical inhibition of neutrophilic ROS production prevents cell death. In addition, inhibition of RIPK1 (which is often involved in necroptosis) reduced bacterial transfer [20].
Another possible way of inducing necroptosis is through the cytosolic protein Z-DNA-binding protein ZBP1 (also known as DAI). Upon binding of specific conformations of nucleic acids, ZBP1 can activate the RIPK3/MLKL signalling axis [21]. While this has only been described for viruses so far [22], it would not be surprising if ZBP1 also had a function in bacterium- or parasite-induced PCD, given the overlap of viral and bacterial pattern recognition. In fact, ZBP1 was upregulated in M. tuberculosis-infected macrophages [23], although no specific function has been assigned.
Pyroptosis
Pyroptosis involves pores in the plasma membrane formed by members of the gasdermin family, following their proteolytic cleavage by caspases. Pyroptosis thus requires the proteolytic activity of at least one caspase and a number of caspases have been found to be able to induce gasdermin-cleavage and pyroptosis, e.g. caspase-1 and -11 (caspase-11 is only found in mice, its orthologues in humans are caspase-4 and -5), but also to some degree by the apoptotic caspase-3 [10, 24-26]. These caspases are activated by multiprotein complexes known as inflammasomes that are formed by oligomerization of caspase-adapter proteins (reviewed in [27, 28]).
Intriguingly, pyroptotic caspases can be activated upon recognition of microbial ‘patterns’ in the cytosol. An example is the recognition of cytosolic lipopolysaccharide by caspase-11 (reviewed in [28]). A substantial number of bacteria have been found to activate pyroptosis, among them Legionella, Francisella, Shigella, Salmonella and Listeria (reviewed in [3, 4, 7]). Previous investigations of pyroptosis during microbial infection have focused on its potential role in host defense rather than microbial host cell exit. The deletion of various components of pyroptosis signaling enhances the sensitivity against bacteria; the deletion of caspase-11 for instance sensitizes host cells and mice against enteric Salmonella infection [29] as well as against infection with Legionella pneumophila [30].
Another case in point is the opportunistic yeast pathogen Candida albicans, which induces pyroptosis in macrophages instrumental to facilitate release of the microbe [31]. It therefore seems very likely that pyroptosis, while involved in the antimicrobial host response and the initiation of inflammation, in addition plays a substantial role in cell exit. Pyroptosis is associated with secretion of proinflammatory cytokines that drive inflammation and pyroptotic PCD is expected to further augment inflammation through the release of both microbial components, making them available to the immune system and constituents of the dying cell (damage-associated molecular patterns; reviewed in [32]). Although inflammation is primarily a defense reaction with detrimental consequences to the pathogen, its downstream effects such as changes in the metabolism or influx of immune cells might actually favor growth and tissue spread of the pathogen. Microbedirected skewing of the immune response by specific signals might further diminish the antimicrobial effect and enhance the pathogen’s benefit. Notably, pyroptosis, unlike apoptosis and probably necroptosis, is limited to specific cell types, such as macrophages, since not all cells express or can activate inflammasome components.
Active host cell destruction
Active host cell destruction describes an exit process, during which microbial molecules penetrate or perforate membranes, such as the host cell plasma membrane or the membrane of the vacuolar compartment, in consequence destroying host cell and compartment, respectively (Fig. 1). Exceptionally, active destruction can include the walls of intra- or extracellular pathogen-containing cysts. Particularly, three types of proteins were described that mediate active host cell lysis, i.e. proteases, phospholipases, and pore-forming proteins (Table 1).
Table 1.
Microbial key factors of host cell exit.
| Type | Factor | Pathogen (stage) | Pathway | Function |
|---|---|---|---|---|
| Protease | CPAF | Chlamydia trachomatis | Lytic vacuolar escape | Processes host cell proteins, e.g. vimentin and LAP-1 |
| SUB1 | Plasmodium falciparum (RBC merozoite) | Host cell lysis | Processes effectors, e.g. SERA5, SERA6, MSP1 | |
| SERA5 | Plasmodium falciparum (RBC merozoite) | Host cell lysis | Protease-like w/o activity, unknown regulatory function | |
| SERA6 | Plasmodium falciparum (RBC merozoite) | Host cell lysis | Spectrin cleavage, suggested to mediate destabilization of RBC cytoskeleton | |
| SERA5 | Plasmodium berghei (oocyst) | Cyst destruction | Involved in sporozoite egress from the oocyst, unknown function | |
| PM VIII | Plasmodium berghei (oocyst) | Cyst destruction | Involved in sporozoite egress from the oocyst, unknown function | |
| PMX | Plasmodium falciparum (RBC merozoite) | Host cell lysis | Processing of effectors, e.g. SUB1 | |
| Phospholipase/Cholesterol Acyltransferase | PI-PLC PlcA | Listeria monocytogenes | Lytic vacuolar escape | Suggested to stalling autophagosome formation during vacuolar escape |
| PC-PLC PlcB | Listeria monocytogenes | Lytic vacuolar escape | Suggested to stalling autophagosome formation during vacuolar escape | |
| PlaA | Legionella pneumophila | Lytic vacuolar escape | Involved in vacuolar membrane rupture, counteracted by SdhA | |
| SseJ | Salmonella enterica serovar Typhimurium | Lytic vacuolar escape | Involved in SCV membrane rupture, counteracted by SifA | |
| PbPL | Plasmodium berghei (liver stage merozoite) | Lytic vacuolar escape | Involved in PVM rupture | |
| LCAT | Toxoplasma gondii (tachyzoite) | Host cell lysis | Involved in PVM rupture, unknown function | |
| Poreformer/Cytolysin | LLO | Listeria monocytogenes | Lytic vacuolar escape | Lyses membranes of primary and secondary vacuoles |
| ESAT-6 | Mycobacterium tuberculosis | Lytic vacuolar escape | Lyses vacuolar membrane | |
| CFP-10 | Mycobacterium tuberculosis | Lytic vacuolar escape | Lyses vacuolar membrane | |
| T3SS1 | Salmonella enterica serovar Typhimurium | Lytic vacuolar escape | Involved in vacuolar escape, unknown function | |
| IpaB | Shigella flexneri | Lytic vacuolar escape | Formation of pore complex in vacuolar membrane, cholesterol binding | |
| IpaC | Shigella flexneri | Lytic vacuolar escape | Formation of pore complex in vacuolar membrane, cholesterol binding | |
| IpaD | Shigella flexneri | Lytic vacuolar escape | Suggested to regulator formation of pore complex in vacuolar membrane | |
| Leishporin | Leishmania | Lytic vacuolar escape | Involved in phagolysosomale escape, unknown function | |
| PPLP1 | Plasmodium falciparum (sporozoite) | Lytic vacuolar escape | Perforation of transient vacuolar membrane | |
| PPLP2 | Plasmodium falciparum/berghei (gametocyte) | Host cell lysis | Perforation of the RBCM | |
| Tc-Tox | Trypanosoma cruzi (metacyclic trypomastigote) | Lytic vacuolar escape | Involved in vacuolar escape, unknown function | |
| Non-lytic egress | Sec14, Plb1 | Cryptococcus neoformans | Vomocytosis | Non-lytic escape from macrophages and amoebae |
| LepA | Legionella pneumophila | Endocytosis | Non-lytic escape from amoebae | |
| LepB | Legionella pneumophila | Endocytosis | Non-lytic escape from amoebae | |
| ESAT-6 | Mycobacterium tuberculosis | Ejection | Non-lytic escape from amoebae | |
| ESX-1 | Mycobacterium tuberculosis | Ejection | Non-lytic escape from amoebae | |
| Cytoskeleton modulator | BimA | Burkholderia | Protrusion | Involved in actin tail formation, mimicry of Ena/VASP actin polymerases |
| ActA | Listeria monocytogenes | Protrusion | Involved in actin tail formation, WASP mimicry | |
| InlC | Listeria monocytogenes | Protrusion | Involved in actin tail formation, cortex destabilization | |
| RickA | Rickettsia | Protrusion | Involved in actin tail formation, WASP mimicry | |
| Sca2 | Rickettsia | Protrusion | Involved in actin tail formation, actin nucleator | |
| Sca4 | Rickettsia | Protrusion | Involved in protrusion engulfment, interaction with vinculin | |
| IcsA | Shigella flexneri | Protrusion | Involved in actin tail formation, N-WASP activation | |
| VirA | Shigella flexneri | Protrusion | Involved in actin tail formation, microtubule degradation, cysteine protease-like | |
| MTRAP | Plasmodium falciparum/berghei (gametocyte) | Host cell lysis | Involved in PVM rupture, suggested to mediate contact between PVM and parasite cytoskeleton | |
| Further/unknown | GEST | Plasmodium berghei (gametocyte) | Host cell lysis | Involved in PVM rupture, unknown function |
| PAT | Plasmodium falciparum (gametocyte) | Host cell lysis | Involved in osmiophilic body discharge | |
| Transsialidase | Trypanosoma cruzi (metacyclic trypomastigote) | Lytic vacuolar escape | Involved in vacuolar escape, unknown function |
Host cell lysis
Active host cell lysis, which includes the destruction of both host cell plasma membrane and vacuolar membrane, is typical for Apicomplexan parasites and has best been studied for the intraerythrocytic blood and gametocyte stages of malaria parasites, particularly of P. falciparum, the causative agent of malaria tropica (Fig. 2). These stages reside within a parasitophorous vacuole (PV) inside red blood cells (RBCs). Following replication in the RBCs, the generated merozoites ultimately have to escape these to disseminate. RBC exit by merozoites follows an inside-out mode, meaning the PV membrane (PVM) ruptures prior to the RBC membrane (RBCM) (reviewed in [6]). The egress cascade begins with the activation of the plasmodial cGMP-dependent protein kinase G (PKG) by a yet unknown signal [33]. Concurrently, the calcium-dependent protein kinase CDPK5 becomes activated due to an increase of intracellular calcium [34, 35]. The concerted activation of both kinases triggers a protease cascade that mediates merozoite egress. Specialized secretory organelles, called exonemes, discharge their content into the PV lumen, including the subtilisin-like protease SUB1 [36]. SUB1 has several targets, like the multiprotein merozoite surface complex called MSP1/6/7 and members of the soluble papain-like PV-resident SERA (sera-repeat antigen) proteins. The aspartic protease PMX, which is also located in the exonemes, mediates the proteolytic maturation of SUB1 [8, 36-39]. In the PV, SERA5 and SERA6 as well as the merozoite surface-resident 200-kDa protein MSP1 become processed by SUB1 [40-42]. Within minutes following the increase of intracellular calcium, the PVM ruptures by a yet unknown mechanism and the RBCM is perforated [43-46]. The processed SERA6, which has β-spectrin cleaving activity, promotes destabilization of the RBC cytoskeleton, while SERA5 exhibits additional regulatory functions [41, 42, 46]. Eventually, the RBCM ruptures at a single breaking point and curls back, a rapid process that disperses the merozoites [47, 48]. Interestingly, merozoite egress from the RBC does not involve actin-myosin motor-driven motility, contrary to sporozoite egress from the oocyst (see below) [49]. RBC exit by merozoites can be inhibited by cysteine and serine protease inhibitors. These include the recently identified PMX-targeting aminohydantoins as well as the hydroxyl-ethyl-amine-based compound 49c, which targets PMIX in addition to PMX [8, 9].
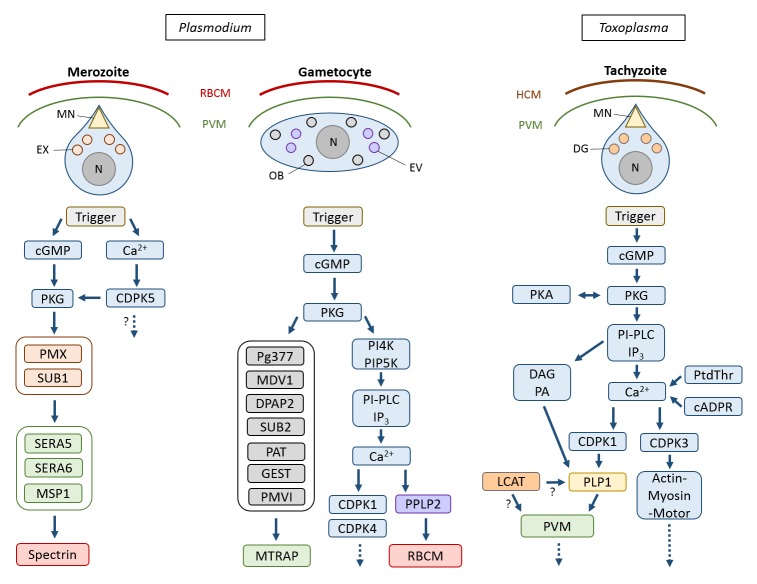
Figure 2. FIGURE 2: Active host cell lysis by Apicomplexan parasites.
The key molecules and the sequence of action during host cell egress by merozoites and gametocytes of Plasmodium and by tachyzoites of Toxoplasma gondii, respectively, are indicated. Question marks indicate steps with ambiguous or unknown interactions. DG, dense granule; EV, egress vesicle; EX, exoneme; HCM, host cell membrane; MN, microneme; N, nucleus; OB, osmiophilic body; PVM, parasitophorous vacuole membrane; RBCM, red blood cell membrane.
Egress of gametocytes from the RBC occurs at the onset of gametogenesis, once the plasmodia have been taken up by a blood-feeding mosquito, and also follows an insideout mode. In the mosquito midgut, the gametocytes are activated by external stimuli, particularly a drop in temperature and the contact with the mosquito-derived molecule xanthurenic acid (XA) (reviewed in [50-52]). While a plasmodial receptor that binds XA has not been identified, it was shown that gametocyte activation leads to cGMP synthesis and PKG activation [53, 54]. PKG controls the synthesis of phosphatidylinositol-(4,5)-bisphosphate (PIP2) via phosphorylation of the phosphoinositol kinases PI4K and PIP5K [55]. A phosphoinositide-specific phospholipase PI-PLC is further activated during the egress cascade, leading to the hydrolysis of PIP2 into diacylglycerol (DAG) and inositol-(1,4,5)-trisphosphate (IP3) [56]. IP3 is suggested to be responsible for the opening of calcium channels in the endoplasmic reticulum, although no plasmodial orthologue of an IP3 channel has been identified so far. The calcium ions then regulate two kinases, CDPK1 and CDPK4, which induces the initiation of DNA synthesis in the replicating male gametes and the release of translational repression in female gametes [57, 58]; these events mark the onset of male and female gametogenesis, respectively.
At least two types of vesicles are discharged during gametocyte egress from the RBC. During the first minutes following gametocyte activation, the osmiophilic bodies release their content into the PV lumen. These contain a variety of egress-related proteins, like Pg377, MDV-1/Peg3, the subtilisin protease SUB2, the dipeptidyl aminopeptidase DPAP2, PMVI, the putative pantothenate transporter PAT and the gamete egress and sporozoite traversal protein GEST [59-67]. Consequently, the PVM ruptures at multiple sites. Although the precise mechanism of PVM rupture is undefined, it appears to involve the merozoite thrombospondin-related adhesive protein MTRAP [64, 65, 68]. It was suggested that the membrane-spanning MTRAP links the gametocyte plasma membrane and the PVM and needs to be dismantled prior to PVM rupture, and indeed a rhomboid-protease cleavage site was identified in MTRAP [69]. In a subsequent calcium-dependent step, a second set of vesicles is released into the RBC cytoplasm. These ‘egress vesicles’, which might represent a sub-type of osmiophilic bodies, contain the plasmodial perforin-like protein PPLP2 [63, 64]. Upon discharge, PPLP2 perforates the RBCM, resulting in the release of the RBC cytoplasm [70, 71]. Noteworthy, the male and female gametes that have meanwhile formed are still contained in the remnants of the RBCM for several more minutes, before the RBCM opens via a single pore to release the fertile gametes [71-73]. The delayed cell exit might represent a way to circumvent the host's immune system. Because RBCM breakdown is sensitive to inhibitors of cysteine and serine proteases, it was suggested that the enzymatic cleavage of RBC cytoskeletal proteins precedes membrane rupture [72, 74].
Host cell exit has further been investigated for the Apicomplexan parasite Toxoplasma gondii, a feline pathogen that, if taken up by humans, can infect various tissues and causes severe toxoplasmosis, particularly in immuno-compromised individuals (Fig. 2). The lecithin-cholesterol acyltransferase LCAT of T. gondii was reported to be released from dense granules, where it associates with the parasite plasma membrane as well as with the PVM prior to egress, suggesting a role in either mediating microneme release or PVM break-down [75, 76]. In addition, the microneme-resident perforin-like protein PLP1 is secreted into the PV, which mediates PVM rupture by forming hexameric pore complexes [77-79]. The micronemes play a central role in host cell exit by T. gondii and hence molecules involved in microneme maturation and functionality, e.g. the phosphoglucomutase-related proteins, the micronemal protein MIC2, or the secretory protein ASP3 [80-82], affect parasite egress. Micronemal discharge is stimulated by a variety of factors, which include acidification, serum albumin and the reduction of potassium levels in the host cell cytoplasm [83-85]. Downstream, a PKG becomes activated, in turn regulating PI-PLC activity, which results in increased cytosolic calcium levels [86]. PKG activity is regulated amongst others via cross talk with the protein kinase PKA. Calcium levels appear to be further regulated by the second messenger cyclic ADP ribose (cADPR) via a yet unknown endoplasmic reticulum receptor [87, 88]. It was suggested that cADPR synthesis itself is controlled by the endogenous stress regulator abscisic acid (ABA) [89]; however, neither an ABA receptor nor the signaling pathway induced by ABA has hitherto been identified. In addition, calcium homeostasis is controlled by phosphatidylthreonine (PtdThr) levels, and abrogation of PtdThr synthesis results in the motility-dependent egress of T. gondii [90-92]. The increased calcium levels activate the kinases CDPK1 and CDPK3. While CDPK1 is required for microneme secretion, CDPK3 facilitates rapid initiation of motility during parasite egress by phosphorylation of myosin and the suppressor of calcium-dependent egress 1 protein [84, 93-96]. Also involved in micronemal secretion is DAG and its downstream product phosphatidic acid, which is recognized by the microneme-associated acetylated pleckstrinhomology domain-containing protein APH prior to microneme secretion [97].
Lytic vacuolar escape
Exit via active destruction has also been described for the escape from the vacuolar compartment by a variety of pathogens, even if the final exit mode follows another route. For example, the plasmodial liver stages use a secreted phospholipase (PbPL) to disrupt the PVM (although the release from infected hepatocytes eventually occurs by budding; see section Extrusion and budding) [98]. Noteworthy in this context, prior to settling down in a host hepatocyte contained in a PV, the sporozoites traverse through a variety of tissue cells. Cell traversal initially occurs within a transient (non-parasitophorous) vacuole, which the sporozoite subsequently escapes, a process requiring the the plasmodial perforin PPLP1 [99, 100].
Furthermore, the intracellular Kinetoplastida parasites Leishmania spp. and Trypanosoma cruzi, which mainly parasitize human phagocytes, are initially contained in a vacuolar compartment of phagosomal and lysosomal origin, respectively, before they escape into the host cell cytoplasm. Their vacuolar escape mechanisms involve parasitederived molecules. For example, L. amazonensis expresses leishporin, a protein with membrane-lytic activity [101, 102]. However, a direct proof of active vacuolar lysis by leishporin has not been provided [103]. In addition, the bloodstream trypomastigotes of T. cruzi express a transsialidase on their surface, which might mediate vacuolar escape my removing sialic acid from the vacuolar membrane [104, 105]. Further, trypomastigotes secrete a poreforming hemolytic toxin Tc-TOX into the vacuole, which is activated by the acidic pH of the lysosomal compartment. It has been proposed that the concerted action of the two proteins mediates rupture of the lysosomal membrane [106, 107]. The detailed mode of action of Tc-TOX, though, is still unknown.
The vacuolar escape of some intracellular bacteria occurs immediately following uptake to evade endosomelysosome fusion and allow replication within the host cell cytosol. This has been shown e.g. for Shigella, Listeria, Francisella, and Rickettsia. In contrast, bacteria like Legionella, Mycobacterium, and Chlamydia that remain within the endosomal vacuole manipulate this compartment to inhibit phagosomal-lysosomal fusion. These bacteria have adapted to this modified endosomal compartment and escape the vacuole only shortly before host cell exit (reviewed in [108]). For some of these bacterial pathogens, cytolytic proteins and phospholipases involved in active vacuolar membrane lysis have been reported.
Lytic vacuolar escape has been investigated in detail for the facultative intracellular gram-positive bacterium Listeria monocytogenes, which is able to infect a variety of cells including phagocytes. While L. monocytogenes enters the host cell within a primary vacuole, the bacterium rapidly escapes the vacuole in order to replicate in the cytosol. Vacuolar escape is essential for intracellular growth, because L. monocytogenes replicates inefficiently when contained in vacuoles [109-111]. Cytosolic L. monocytogenes uses actin-based motility to spread to the neighboring cells (see section Actin-mediated protrusion). Following cell-to-cell transit, L. monocytogenes resides in a vacuole enclosed by two membranes, one layer derived from the donor and one from the recipient cell. L. monocytogenes also rapidly escapes this secondary vacuole and continuous replicating in the cytosol [112]. To allow endosomal escape, L. mono-cytogenes expresses a pore-forming cholesterol-dependent cytolysin, termed listeriolysin O (LLO), which mediates the escape from both, the primary and the secondary vacuoles [113]. Additionally, two phospholipases, PlcA and PlcB, contribute to endosomal membrane disruption [[114, 115]; reviewed in [116]). The two enzymes additionally function in subverting host autophagic defenses by stalling autophagosome formation [117, 118]. The LLO pore-forming activity is enhanced by phagosomal acidification [119-121].
Potential membrane-lytic functions have further been assigned to the type III-secreted translocator proteins IpaB and IpaC of the gram-negative rod Shigella flexneri, which causes shigellosis in humans [122-124]. The two proteins form a complex in the vacuolar membrane that binds cholesterol, resulting in membrane degradation. A third protein, IpaD, seems to have regulatory function during this process [125]. Vacuolar escape has also been reported for mycobacteria [126, 127]. Two secreted membrane-lytic proteins, ESAT-6 (a 6-kDa early secretory antigenic protein) and CFP-10 (a 10-kDa culture filtrate protein), were assigned to this process [128-132]. Both proteins are exported into the host cell cytosol through the type VII secretion system ESX-1 (reviewed in [133, 134]); their exact mode of action, however, is still unclear. Vacuolar escape of myco-bacteria rapidly leads to the initiation of PCD (see section Necroptosis) [135, 136].
Chlamydia trachomatis is an obligate intracellular gram-negative bacterium that infects epithelial cells of the urogenital tract and the conjunctivae. In host cells, the bacteria are contained in vacuolar compartments termed inclusions. C. trachomatis can exit the host cell by either extrusion (see section Extrusion and budding) or by lysis. Here, C. trachomatis first lyses the inclusions by a yet unknown mechanism to escape into the host cell cytoplasm. In preparation for escape, C. trachomatis translocates virulence factors into the host cell cytoplasm, among others via a type III secretion system (T3SS). The chlamydial protease-like activity factor CPAF, a serine protease, is initially secreted into the inclusion lumen and eventually crosses the inclusion membrane [137]. Following its release into the cytosol, CPAF processes a wealth of host proteins to promote chlamydial intracellular growth, particularly vimentin and the lamin-associated protein LAP-1 [138]. Chlamydiae lacking CPAF are unable to egress from the host cell [132]. Host cell exit by C. trachomatis further requires a variety of plasmid-encoded virulence factors like the transcription regulator Pgp4 [139]. Since cell lysis can be blocked by the cysteine protease inhibitor E64, it has been suggested that cytoskeleton degradation precedes rupture of the host cell membrane [140].
In epithelial cells of the intestine, S. Typhimurium resides in a membrane-bound endosome, termed Salmonella-containing vacuole (SCV), where it is able to proliferate [141-143]. During the first hours post-invasion, bacteria of a minor proportion of infected cells escape into the cytosol, where they rapidly replicate. The SCV biogenesis involves numerous bacterial effector molecules translocated by the type III secretion systems 1 and 2 (T3SS1 and T3SS2, respectively) (reviewed in [144, 145]). While cytosolic S. Typhimurium exhibit T3SS1 expression, T3SS2-positive bacteria remain in the SCV [146]. Noteworthy, poreforming activities were demonstrated for T3SS1 mediated effector molecules and it is thought that the T3SS1 plays a role in damaging the nascent (early) SCV [147-150]. In addition, several T3SS2-translocated effectors like the protein Salmonella-induced filaments SifA and SseJ homologous to L. pneumophila PlaA (see below) are involved in SCV stabilization and destabilization, respectively, at later time points. SifA counteracts the phagosome rupture by SseJ, which shows PLA and cholesterol acyltransferase activities [151-153]. SifA binds to SKIP (SifA and kinesin-interacting protein), which in turn interacts with kinesin-1 and the secreted effector protein PipB2. This interaction might regulate SCV stability [154-156].
Legionella pneumophila, a facultative intracellular bacterium, infects amoeba under environmental conditions. Once entering the human lung, it can invade macrophages, thereby causing severe lung infections. In both, macrophages and amoebae, L. pneumophila replicates in a specialized phagosome avoiding fusion with the host endocytic pathway. A type IVB secretion system (Dot/Icm) responsible for the translocation of a multitude of effector proteins into the host cell as well as the type II secretion system Lsp are required for intracellular replication (reviewed in [157]). L. pneumophila exits from the phagosome either by nonlytic exocytosis (see section Exocytosis, expulsion and ejection) or via a pore-forming activity [158-161]. Pore formation might be triggered by phospholipases found in L. pneumophila (reviewed in [162]). Specifically, the type II secreted lysophospholipase PlaA, showing homology to S. Typhimurium SseJ (see above), destabilizes the phagosomal membrane in the absence of the type IVB-secreted protective factor SdhA [163, 164]. The detailed mode of phagosome lysis remains to be investigated.
A special form of lysis-mediated microbial exit is the one from cysts. This has been investigated for the egress of P. berghei from oocysts, parasite-derived cyst-like structures, which form after sexual reproduction and are found in the mosquito midgut attached to the epithelium. Oocyst exit required two proteases, P. berghei SERA5 (an orthologue of P. falciparum SERA8) and PMVIII [165, 166]. Other plasmodial proteins that mediate sporozoite maturation, e.g. the thrombospondin-related protein TRP1 or the LCCL-domain containing proteins, were further assigned to oocyst egress by malaria parasites [167-170]. Noteworthy, the motility of Plasmodium sporozoites, driven by an actinmyosin motor, is necessary for oocyst egress [170].
Induced membrane-dependent exit
Some microbes are able to leave the intact host cell. Such exit strategies include actin-driven membrane protrusions enabling the spread of single bacteria between cells, extrusions and budding of microbes packed in a membranous compartment as well as ejection, expulsion and exocytosis of free microbes (Fig. 1). The detailed mechanisms of induced membrane-dependent exit, however, are to date not well defined and only few key molecules of this exit pathway have been identified (Table 1).
Actin-mediated protrusion
Polar recruitment and polymerization of actin results in a directed locomotion of cytosolic bacteria. Actin polymerization is facilitated by the expression of a microbial surface protein that binds or mimics the host cell actin-related protein (Arp)2/3 complex [171]. Reaching the cell border, the advancing bacterium is able to protrude the host cell plasma membrane by physical force. Since bacteria are unable to penetrate this membrane, they project from the infected cell within the tip of a filopodium-like membrane extension. This cell membrane protrusion is subsequently engulfed by the adjacent cell [172]. Notably, this cell-to-cell spread occurs without contact to the extracellular environment and thus protects the pathogen from exposure to extracellular immune surveillance mechanisms and antimicrobial effector molecules. The most intensively studied microorganisms performing actin-mediated protrusion are Listeria monocytogenes and Shigella flexneri, but a similar ability has been demonstrated for the spotted fever group of rickettsiae, Burkholderia spp., Ehrlichia spp. and Myco-bacterium marinum.
Upon release from the endosomal membrane, L. monocytogenes starts polar expression of the surface molecule actin assembly-inducing protein (ActA) [173]. The N-terminus of ActA represents a mimic of the naturally occurring actin nucleation-promoting factor Wiscott-Aldrich syndrome protein (WASP) and binds preformed Arp2/3 complexes [174]. The central domain of ActA additionally binds profilin and vasodilator-stimulated phosphoprotein (VASP), promoting the rapid formation of branched actin polymers. Furthermore, parallel actin polymerization and actin tail assembly is facilitated by the activation of Rho GTPase and the actin polymerization factor formin [112, 175]. The actin comet tail thereby propels the bacterium through the host cell cytosol. Once approaching the vicinity of the plasma membrane the secreted Listeria protein internalin C (InlC) binds to the host adaptor protein Tuba to inhibit the actin polymerization promoting neural WASP (N-WASP) and to the COPII complex component SEC31 [176]. This weakens the cortical tension at the plasma membrane allowing Listeria to protrude the plasma membrane in a filopodia-like fashion [175]. The membrane cytoskeleton linker ezrin accumulates at the protrusion site and stabilizes the growing membrane extension [177]. While it is still unclear, how exactly the bacterium-containing membrane extension is engulfed by the adjacent cell, the process requires active participation of both, the donor and recipient cell. For example, the host casein kinase CSNK1A1 was shown to promote the conversion of protrusions to endosomes in the donor cell [178, 179]. After transfer, Listeria resides in an endosomal compartment composed of a membrane layer of both the donor and recipient cell. This double-layered endosomal membrane is lysed by PlcB, PlcA, and LLO releasing the bacterium in the recipient cell cytosol (see section Lytic vacuolar escape) [115, 180-183]. Importantly, the ability to perform cell-to-cell spread represents a critical component of L. monocytogenes virulence and does not lead to host cell death [181, 184, 185].
Actin-based motility also critically contributes to Shigella flexneri virulence [186]. Here, the Shigella IcsA protein activates N-WASP to recruit Arp2/3 and initiate actin tail formation [187-189]. Simultaneously, Shigella stimulates RhoA GTPases and the mammalian diaphanous-related formins mDia1 and mDia2 to promote actin polymerization in parallel arrays at the protrusion site [190]. The virulence factor VirA of S. flexneri degrades the cytoplasmic microtubule network via its cysteine protease-like activity and might thereby promote cytosolic locomotion [191, 192]. Similar to Listeria, engulfment of the Shigella-containing protrusions by the adjacent cell does not depend on additional bacterial factors. Instead, it requires active participation of the host cell proteins. The requirement for tricellulin, epsin-1, clathrin and dynamin-2 suggest the involvement of a noncanonical clathrin-dependent endocytic pathway in the recipient cell [193, 194].
Other bacteria that employ actin-based motility to allow cell-to-cell transfer include the spotted fever group rickettsiae such as Rickettsia rickettsii, R. conorii or R. parkeri [195]. The molecular basis, however, is less well examined. Early motility has been attributed to polar expression of RickA, which recruits the Arp2/3 complex and induces actin polymerization in an N-WASP-like manner. Later, motility occurs in an Arp2/3 independent fashion via secretion of the autotransporter surface cell antigen (Sca) 2, a formin-like actin nucleator that generates long unbranched actin tails [196, 197]. Subsequent secretion of Sca4 promotes protrusion engulfment through interaction with the cell-adhesion protein vinculin. This relieves intercellular tension and the engulfment of bacteria-containing protrusions [198].
Ehrlichia chaffeensis recruits N-WASP to generate actin polymerization and form bacteria-driven filopodia and allow cell-to-cell spread [199, 200]. The pathogenesis of Burkholderia spp. relies on actin-based motility [201]. Whereas the secreted trimeric autotransporter BimA (Burkholderia intracellular motility A) of the animal pathogen B. thailandensis activates the host Arp2/3 complex, the orthologue BimA of the human-pathogenic B. pseudomallei and B. mallei mimic host Ena/VASP actin polymerases [202]. In contrast to the above-discussed pathogens, cell-to-cell spread is facilitated by fusion of infected and adjacent cells induced by the type VI secretion system (T6SS)-1 [203]. Mycobacterium marinum, a natural fish pathogen and occasional human pathogen, requires either WASP or N-WASP to perform actin-based motility and both the Arp2/3 complex and the vasodilator-stimulated phosphoprotein were identified as constituents of the actin tail [126, 204]. The molecular mechanism of subsequent cell-to-cell spread may differ from the other pathogens.
Extrusion and budding
Host cell exit via extrusion or budding involves the release of membrane-encircled microorganisms (Fig. 1). Here, the membrane coat protects the microbe against humoral factors of the host immune system. To date, only few molecular players have been identified that modulate this type of host cell exit (Table 1).
Extrusions have been reported for Chlamydia trachomatis. Chlamydial extrusions depend on actin polymerization mediated by N-WASP and Rho GTPases, while myosin and septins are involved in regulation and stabilization of the actin filaments [140, 205]. A bacterial protein appears to recruit the myosin-activating machinery to the inclusion to favor extrusion of pathogens over a cell-lytic pathway [206].
Viral-like budding was shown for the scrub typhus-causing bacterium Orientia tsutsugamushi that replicates in the cytosol of a variety of host cells, including phagocytes. Inside its host cell, the bacterium is ‘encapsulated’ by the plasma membrane of the cell. Host cell exit appears to depend on lipid rafts and a bacterial protein that was found to co-localize with caveolins at the site of cell exit, suggesting a role in egress ([207], reviewed in [208]).
Host cell exit by budding was further demonstrated for the intrahepatic merozoites of Plasmodium. Following phospholipase-mediated PV breakdown (see section Lytic vacuolar escape), these lie in the hepatocyte cytoplasm and are subsequently released into the blood stream. The merozoites leave the hepatocyte in host cell plasma membrane-derived vesicles termed merosomes that can contain a few up to several hundreds of merozoites [209]. The parasites induce the separation of the actin cortex from the hepatocyte plasma membrane prior to merozoite formation [210]. The molecular mechanisms that allow the vesicle to bud from the hepatocyte and penetrate the endothelial cell layer to reach the blood stream are, however, unknown. Interestingly, although PCD is induced by the parasite in the infected hepatocyte, the budding merosomes do not expose phosphatidylserine on their surface as would be expected from apoptotic cells [211]. This probably helps the merosomes to remain undetected by macrophages as they leave the liver and travel to the lung tissue [212]. Contrary to the induced membrane-dependent exit by other intracellular pathogens, in this case, the host hepatocyte dies.
Exocytosis, expulsion and ejection
Exocytosis involves the transport of molecules from intracellular endosomal vesicles to the plasma membrane. The membrane of transport vesicles fuses with the plasma membrane to allow cargo release and thereby aids the passage of mostly large and polar substances into or through the plasma membrane (reviewed in [213, 214]). A variety of pathogenic microbes exit host cells by exocytosis and exocytosis-like expulsion (also called vomocytosis) as well as by mechanistically distinct ejection. All these procedures have in common that they release free pathogens without harming the host cell. The processes therefore avoid the release of proinflammatory cellular constituents (reviewed in [4, 5, 215]).
Exocytosis-like egress from amoebae has been described for Legionella pneumophila. Within the host cell, L. pneumophila is contained in a specialized phagosome and via a type IVB secretion system secretes effector proteins into the host cell cytoplasm to allow replication (see section Lytic vacuolar escape) (reviewed in [157]). Such effectors frequently harbor signatures of eukaryotic proteins and some of these exert homology to the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins known to mediate the fusion of vesicles to biological membranes [216-219]. These analogous bacterial effectors may drive or even abrogate vesicle fusion events. Two type IVB-secreted L. pneumophila proteins, LepA and LepB, with regions weakly similar to mammalian early endosome antigen 1 (EEA1), required for endosome docking of SNARE proteins, were identified. They play a critical role in the bacterial release from the amoebae Acanthamoeba castellanii or Dictyostelium discoideum [216], indicating that L. pneumophila is released from amoebae by an exocytotic pathway. However, earlier reports described vesicle-associated Legionella expelled from A. castellanii [220]. This raises the question whether the bacteria are indeed released by the above-described process in their free form or rather in a vesicle-enclosed form.
Non-lytic egress of the fungus Cryptococcus neoformans is debated as an important factor determining systemic dissemination of the pathogen [221, 222]. The yeast exerts an exocytosis-like expulsion, called vomocytosis, in macrophages. Non-lytic expulsion of Cryptococcus occurs through fusion of the phagosome and plasma membrane. Mutants defective for the secreted phospholipase PLB1 or the PLB1-exporting secretion system Sec14 exhibit reduced quantities of vomocytosis. The actin cytoskeleton of the host cell is not essential for this process [223-226]. However, macrophage phagosomes containing intracellular cryptococci undergo repeated cycles of actin polymerization, called actin ‘flashes’ dependent on the classical WASP-Arp2/3 complex. Prior to expulsion, the majority of phagosomes is permeabilized, which is immediately followed by an actin flash likely devoted to temporarily inhibit expulsion [225]. Vomocytosis has also been observed upon in vivo infections of mice and zebrafish [227, 228]. The latter study reported that inhibition of the mitogen-activated protein kinase ERK5 increased vomocytosis and decreased pathogen dissemination, indicating that vomocytosis enhancement might represent a therapeutic target.
A particular mode of cell exit was discovered in Mycobacterium tuberculosis and M. marinum. Both are non-lytically ejected from D. discoideum through an F-actin-based structure, the ejectosome [229]. The process involves the so-called region of difference RD1 locus, where components of the mycobacterial type VII secretion system ESX-1 are encoded [127, 130, 135, 229, 230]. Both the ESX-1 secretion system and its secreted effector ESAT-6/EsxA are required for mycobacterial ejection from Dictyostelium and bacterial translocation into the cytosol of mammalian cells. A recent report describes an unexpected role of the autophagic machinery in non-lytic release of Mycobacteria and cell-to-cell transmission in Dictyostelium [231]. Further, the extraordinary importance of maintaining membrane integrity during the process of ejection is highlighted. Specifically, the study indicates that bacteria shortly prior to ejection are escorted by an autophagocytic vacuole, which is recruited in an ESX-1 independent manner. When autophagy is impaired, cell-to-cell transmission is inhibited. In this case, the host plasma membrane becomes compromised and the host cell subsequently dies [231]. These findings illustrate that non-lytic egress by ejection requires host cell-derived membrane protection pathways.
HOST CELL EXIT BY INTRACELLULAR PATHOGENS: THE ACHILLES’ HEEL?
The combined data highlighted in this review suggest that host cell exit by intracellular pathogens represents a fundamental and active step in infection, which, shaped by evolutionary pressure, is crucial for microbial spread and might represent the Achilles' heel of microbial pathogenesis. A limited set of host cell exit pathways appears to be shared by a high variety of phylogenetically different microbes with the involvement of similar types of pathogen-derived proteins, like proteases, pore-forming proteins and phospholipases or actin-binders. This strongly suggests convergent evolution of the exit machineries. The fact that exit strategies employ manipulated secretion or delivery routes as well as the complex cytoskeletal restructuring further point to the intimate interaction between pathogens and their host cells.
While much experimental work lies ahead of us to decipher the molecular mechanisms of host cell exit by intracellular pathogens, evidence emerges that key molecules of host cell exit are promising targets for novel types of interventional strategies. The fact that only few classes of pathogen-derived proteins appear to be involved in the exit processes and that in general their accessibility for inhibitors is known makes host cell exit as a point of attack even more attractive. Indeed, as described earlier, first protease inhibitors have been identified that are able to block the egress of malaria parasites from RBCs [8, 9]. While targeting microbial exit might not protect from primary infection, the cell-entrapment of microbes ensures immediate control of microbial tissue spread and disease progression. Importantly, it should still allow stimulation of a protective adaptive immune response thus providing therapeutic and prophylactic advantages. Such approaches have been exploited in the recent past for liver stage-targeting antimalarial vaccines, using attenuated parasites (reviewed in e.g. [232-234]) and might represent a pioneering strategy to combat life-threatening human infectious diseases. Concluding, present pieces of evidence point to exit strategies of intracellular pathogens as an emerging field of infection biology essential to fully understand and successfully counteract microbial pathogenesis.
Glossary
Abbreviations:
- ABA
– abscisic acid,
- ActA
– actin assembly-inducing protein,
- Arp2/3
– actin-related protein 2/3,
- CPAF
– chlamydial protease-like activity factor,
- N-WASP
– neuronal WASP,
- PCD
– programmed cell death.
- PI
– phosphatidylinositol,
- PK
– protein kinase,
- PV
– parasitophorous vacuole,
- PVM
– PV membrane,
- RBC
– red blood cell,
- RBCM
– RBC membrane,
- WASP
– Wiscott-Aldrich syndrome protein.
Funding Statement
The authors thank M. Blackman (The Francis Crick Institute London) and Ö. Günay-Esiyok (Humboldt-University Berlin) for helpful discussions. The authors acknowledge funding by the priority programme SPP1580 (AF, GH, MWH, GP) and SFB 1129 (FF) of the Deutsche Forschungsgemeinschaft (DFG). GP is recipient of a DFG Heisenberg professorship.
REFERENCES
- 1.World Health Organization. World health statistics 2018. 2018 Available at: http://apps.who.int/iris/bitstream/handle/10665/272596/9789241565585-eng.pdf. [Accessed 26.06.2018] [Google Scholar]
- 2.Schaible UE, Haas A. Wiley-VCH; Hoboken: 2009. Intracellular niches of microbes - A pathogens' guide through the host cell. ISBN 978-3-527-32207-7. [Google Scholar]
- 3.Hybiske K, Stephens RS. Exit strategies of intracellular pathogens. Nat Rev Microbiol. 2008;6(2):99–110. doi: 10.1038/nrmicro1821. [DOI] [PubMed] [Google Scholar]
- 4.Hybiske K, Stephens R. Cellular exit strategies of intracellular bacteria. Microbiol Spectr. 2015;3(6) doi: 10.1128/microbiolspec.VMBF-0002-2014. [DOI] [PubMed] [Google Scholar]
- 5.Friedrich N, Hagedorn M, Soldati-Favre D, Soldati T. Prison break: pathogens strategies to egress from host cells. Microbiol Mol Biol Rev. 2012;76(4):707–720. doi: 10.1128/MMBR.00024-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wirth CC, Pradel G. Molecular mechanisms of host cell egress by malaria parasites. Int J Med Microbiol. 2012;302(4-5):172–178. doi: 10.1016/j.ijmm.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Traven A, Naderer T. Microbial egress: a hitchhiker's guide to freedom. PLoS Pathog. 2014;10(7):e1004201. doi: 10.1371/journal.ppat.1004201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasamu AS, Glushakova S, Russo I, Vaupel B, Oksman A, Kim AS, Fremont DH, Tolia N, Beck JR, Meyers MJ, Niles JC, Zimmerberg J, Goldberg DE. Plasmepsins IX and X are essential and druggable mediators of malaria parasite egress and invasion. Science. 2017;358(6362):518–522. doi: 10.1126/science.aan1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pino P, Caldelari R, Mukherjee B, Vahokoski J, Klages N, Maco B, Collins CR, Blackman MJ, Kursula I, Heussler V, Brochet M, Soldati-Favre D. A multistage antimalarial targets the plasmepsins IX and X essential for invasion and egress. Science. 2017;358(6362):522–528. doi: 10.1126/science.aaf8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D'Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25(3):486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galluzzi L, Brenner C, Morselli E, Touat Z, Kroemer G. Viral control of mitochondrial apoptosis. PLoS Pathog. 2008;4(5):e1000018. doi: 10.1371/journal.ppat.1000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin CJ, Booty MG, Rosebrock TR, Nunes-Alves C, Desjardins DM, Keren I, Fortune SM, Remold HG, Behar SM. Efferocytosis is an innate antibacterial mechanism. Cell Host Microbe. 2012;12(3):289–300. doi: 10.1016/j.chom.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Zandbergen G, Klinger M, Mueller A, Dannenberg S, Gebert A, Solbach W, Laskay T. Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J Immunol. 2004;173(11):6521–6525. doi: 10.4049/jimmunol.173.11.6521. [DOI] [PubMed] [Google Scholar]
- 14.Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, Kamhawi S, Lawyer P, Fay MP, Germain RN, Sacks D. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321(5891):970–974. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Leon-Rodriguez CM, Rossi DCP, Fu MS, Dragotakes Q, Coelho C, Guerrero Ros I, Caballero B, Nolan SJ, Casadevall A. The outcome of the Cryptococcus neoformans-macrophage interaction depends on phagolysosomal membrane integrity. J Immunol. 2018;201(2):583–603. doi: 10.4049/jimmunol.1700958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11(10):700–14. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 17.Czabotar PE, Murphy JM. A tale of two domains - a structural perspective of the pseudokinase, MLKL. FEBS J. 2015;282(22):4268–78. doi: 10.1111/febs.13504. [DOI] [PubMed] [Google Scholar]
- 18.Lindgren SW, Stojiljkovic I, Heffron F. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1996;93(9):4197–4201. doi: 10.1073/pnas.93.9.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson N, McComb S, Mulligan R, Dudani R, Krishnan L, Sad S. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol. 2012;13(10):954–962. doi: 10.1038/ni.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dallenga T, Repnik U, Corleis B, Eich J, Reimer R, Griffiths GW, Schaible UE. M. tuberculosis-induced necrosis of infected neutrophils promotes bacterial growth following phagocytosis by macrophages. Cell Host Microbe. 2017;22(4):519–530.e3. doi: 10.1016/j.chom.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Maelfait J, Liverpool L, Bridgeman A, Ragan KB, Upton JW, Rehwinkel J. Sensing of viral and endogenous RNA by ZBP1/DAI induces necroptosis. EMBO J. 2017;36(17):2529–2543. doi: 10.15252/embj.201796476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Upton JW, Kaiser WJ. DAI another way: necroptotic control of viral infection. Cell Host Microbe. 2017;21(3):290–293. doi: 10.1016/j.chom.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Stutz MD, Ojaimi S, Allison C, Preston S, Arandjelovic P, Hildebrand JM, Sandow JJ, Webb AI, Silke J, Alexander WS, Pellegrini M. Necroptotic signaling is primed in Mycobacterium tuberculosis- infected macrophages, but its pathophysiological consequence in disease is restricted. Cell Death Differ. 2018;25(5):951–965. doi: 10.1038/s41418-017-0031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479(7371):117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 25.Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. 2017;8:14128. doi: 10.1038/ncomms14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661):99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 27.Prochnicki T, Latz E. Inflammasomes on the crossroads of innate immune recognition and metabolic control. Cell Metab. 2017;26(1):71–93. doi: 10.1016/j.cmet.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 28.Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265(1):130–142. doi: 10.1111/imr.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knodler LA, Crowley SM, Sham HP, Yang H, Wrande M, Ma C, Ernst RK, Steele-Mortimer O, Celli J, Vallance BA. Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens. Cell Host Microbe. 2014;6(2):249–256. doi: 10.1016/j.chom.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akhter A, Caution K, Abu Khweek A, Tazi M, Abdulrahman BA, Abdelaziz DH, Voss OH, Doseff AI, Hassan H, Azad AK, Schlesinger LS, Wewers MD, Gavrilin MA, Amer AO. Caspase-11 promotes the fusion of phagosomes harboring pathogenic bacteria with lysosomes by modulating actin polymerization. Immunity. 2012;37(1):35–47. doi: 10.1016/j.immuni.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uwamahoro N, Verma-Gaur J, Shen HH, Qu Y, Lewis R, Lu J, Bambery K, Masters SL, Vince JE, Naderer T, Traven A. The pathogen Candida albicans hijacks pyroptosis for escape from macrophages. MBio. 2014;5(2):e00003–14. doi: 10.1128/mBio.00003-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zitvogel L, Kepp O, Kroemer G. Decoding cell death signals in inflammation and immunity. Cell. 2010;140(6):798–804. doi: 10.1016/j.cell.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Collins CR, Hackett F, Strath M, Penzo M, Withers-Martinez C, Baker DA, Blackman MJ. Malaria parasite cGMP-dependent protein kinase regulates blood stage merozoite secretory organelle discharge and egress. PLoS Pathog. 2013;9(5):e1003344. doi: 10.1371/journal.ppat.1003344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dvorin JD, Martyn DC, Patel SD, Grimley JS, Collins CR, Hopp CS, Bright AT, Westenberger S, Winzeler E, Blackman MJ, Baker DA, Wandless TJ, Duraisingh MT. A plant-like kinase in Plasmodium falciparum regulates parasite egress from erythrocytes. Science. 2010;328(5980):910–912. doi: 10.1126/science.1188191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Absalon S, Blomqvist K, Rudlaff RM, DeLano TJ, Pollastri MP, Dvorin JD. Calcium-dependent protein kinase 5 is required for release of egress-specific organelles in Plasmodium falciparum. MBio. 2018;9(1):e00130–18. doi: 10.1128/mBio.00130-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeoh S, O'Donnell RA, Koussis K, Dluzewski AR, Ansell KH, Osborne SA, Hackett F, Withers-Martinez C, Mitchell GH, Bannister LH, Bryans JS, Kettleborough CA, Blackman MJ. Subcellular discharge of a serine protease mediates release of invasive malaria parasites from host erythrocytes. Cell. 2007;131(6):1072–1083. doi: 10.1016/j.cell.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 37.Arastu-Kapur S, Ponder EL, Fonović UP, Yeoh S, Yuan F, Fonović M, Grainger M, Phillips CI, Powers JC, Bogyo M. Identification of proteases that regulate erythrocyte rupture by the malaria parasite Plasmodium falciparum. Nat Chem Biol. 2008;4(3):203–213. doi: 10.1038/nchembio.70. [DOI] [PubMed] [Google Scholar]
- 38.Silmon de Monerri NC, Flynn HR, Campos MG, Hackett F, Koussis K, Withers-Martinez C, Skehel JM, Blackman MJ. Global identification of multiple substrates for Plasmodium falciparum SUB1, an essential malarial processing protease. Infect Immun. 2011;79(3):1086–1097. doi: 10.1128/IAI.00902-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruecker A, Shea M, Hackett F, Suarez C, Hirst EM, Milutinovic K, Withers-Martinez C, Blackman MJ. Proteolytic activation of the essential parasitophorous vacuole cysteine protease SERA6 accompanies malaria parasite egress from its host erythrocyte. J Biol Chem. 2012;287(45):37949–37963. doi: 10.1074/jbc.M112.400820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stallmach R, Kavishwar M, Withers-Martinez C, Hackett F, Collins CR, Howell SA, Yeoh S, Knuepfer E, Atid AJ, Holder AA, Blackman MJ. Plasmodium falciparum SERA5 plays a non-enzymatic role in the malarial asexual blood-stage lifecycle. Mol Microbiol. 2015;96(2):368–387. doi: 10.1111/mmi.12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins CR, Hackett F, Atid J, Tan MSY, Blackman MJ. The Plasmodium falciparum pseudoprotease SERA5 regulates the kinetics and efficiency of malaria parasite egress from host erythrocytes. PLoS Pathog. 2017;13(7):e1006453. doi: 10.1371/journal.ppat.1006453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas JA, Tan MSY, Bisson C, Borg A, Umrekar TR, Hackett F, Hale VL, Vizcay-Barrena G, Fleck RA, Snijders AP, Saibil HR, Blackman MJ. A protease cascade regulates release of the human malaria parasite Plasmodium falciparum from host red blood cells. Nat Microbiol. 2018;3(4):447–455. doi: 10.1038/s41564-018-0111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glushakova S, Mazar J, Hohmann-Marriott MF, Hama E, Zimmerberg J. Irreversible effect of cysteine protease inhibitors on the release of malaria parasites from infected erythrocytes. Cell Microbiol. 2009;11(1):95–105. doi: 10.1111/j.1462-5822.2008.01242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glushakova S, Humphrey G, Leikina E, Balaban A, Miller J, Zimmerberg J. New stages in the program of malaria parasite egress imaged in normal and sickle erythrocytes. Curr Biol. 2010;20(12):1117–1121. doi: 10.1016/j.cub.2010.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glushakova S, Beck JR, Garten M, Busse BL, Nasamu AS, Tenkova-Heuser T, Heuser J, Goldberg D, Zimmerberg J. Rounding precedes rupture and breakdown of vacuolar membranes minutes before malaria parasite egress from erythrocytes. Cell Microbiol. 2018:e12868. doi: 10.1111/cmi.12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hale VL, Watermeyer JM, Hackett F, Vizcay-Barrena G, van Ooij C, Thomas JA, Spink MC, Harkiolaki M, Duke E, Fleck RA, Blackman MJ, Saibil HR. Parasitophorous vacuole poration precedes its rupture and rapid host erythrocyte cytoskeleton collapse in Plasmodium falciparum egress. Proc Natl Acad Sci U S A. 2017;114(13):3439–3444. doi: 10.1073/pnas.1619441114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glushakova S, Yin D, Li T, Zimmerberg J. Membrane transformation during malaria parasite release from human red blood cells. Curr Biol. 2005;15(18):1645–1650. doi: 10.1016/j.cub.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 48.Abkarian M, Massiera G, Berry L, Roques M, Braun-Breton C. A novel mechanism for egress of malarial parasites from red blood cells. Blood. 2011;117(15):4118–4124. doi: 10.1182/blood-2010-08-299883. [DOI] [PubMed] [Google Scholar]
- 49.Perrin AJ, Collins CR, Russell MRG, Collinson LM, Baker DA, Blackman MJ. The actinomyosin motor drives malaria parasite red blood cell invasion but not egress. MBio. 2018;9(4):pii: e00905–18. doi: 10.1128/mBio.00905-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pradel G. Proteins of the malaria parasite sexual stages: expression, function and potential for transmission blocking strategies. Parasitology. 2007;134(Pt.14):1911–1929. doi: 10.1017/s0031182007003381. [DOI] [PubMed] [Google Scholar]
- 51.Kuehn A, Pradel G. The coming-out of malaria gametocytes. J Biomed Biotechnol. 2010;2010(2010):976827. doi: 10.1155/2010/976827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bennink S, Kiesow MJ, Pradel G. The development of malaria parasites in the mosquito midgut. Cell Microbiol. 2016;18(7):905–918. doi: 10.1111/cmi.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muhia DK, Swales CA, Deng W, Kelly JM, Baker DA. The gametocyte-activating factor xanthurenic acid stimulates an increase in membrane-associated guanylyl cyclase activity in the human malaria parasite Plasmodium falciparum. Mol Microbiol. 2001;42(2):553–560. doi: 10.1046/j.1365-2958.2001.02665.x. [DOI] [PubMed] [Google Scholar]
- 54.McRobert L, Taylor CJ, Deng W, Fivelman QL, Cummings RM, Polley SD, Billker O, Baker DA. Gametogenesis in malaria parasites is mediated by the cGMP-dependent protein kinase. PLoS Biol. 2008;6(6):e139. doi: 10.1371/journal.pbio.0060139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brochet M, Collins MO, Smith TK, Thompson E, Sebastian S, Volkmann K, Schwach F, Chappell L, Gomes AR, Berriman M, Rayner JC, Baker DA, Choudhary J, Billker O. Phosphoinositide metabolism links cGMP-dependent protein kinase G to essential Ca²⁺ signals at key decision points in the life cycle of malaria parasites. PLoS Biol. 2014;12(3):e1001806. doi: 10.1371/journal.pbio.1001806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raabe AC, Wengelnik K, Billker O, Vial HJ. Multiple roles for Plasmodium berghei phosphoinositide-specific phospholipase C in regulating gametocyte activation and differentiation. Cell Microbiol. 2011;13(7):955–966. doi: 10.1111/j.1462-5822.2011.01591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell. 2004;117(4):503–514. doi: 10.1016/s0092-8674(04)00449-0. [DOI] [PubMed] [Google Scholar]
- 58.Sebastian S, Brochet M, Collins MO, Schwach F, Jones ML, Goulding D, Rayner JC, Choudhary JS, Billker O. A Plasmodium calcium-dependent protein kinase controls zygote development and transmission by translationally activating repressed mRNAs. Cell Host Microbe. 2012;12(1):9–19. doi: 10.1016/j.chom.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alano P, Read D, Bruce M, Aikawa M, Kaido T, Tegoshi T, Bhatti S, Smith DK, Luo C, Hansra S, Carter R, Elliott JF. COS cell expression cloning of Pfg377, a Plasmodium falciparum gametocyte antigen associated with osmiophilic bodies. Mol Biochem Parasitol. 1995;74(2):143–156. doi: 10.1016/0166-6851(95)02491-3. [DOI] [PubMed] [Google Scholar]
- 60.Severini C, Silvestrini F, Sannella A, Barca S, Gradoni L, Alano P. The production of the osmiophilic body protein Pfg377 is associated with stage of maturation and sex in Plasmodium falciparum gametocytes. Mol Biochem Parasitol. 1999;100(2):247–252. doi: 10.1016/s0166-6851(99)00050-x. [DOI] [PubMed] [Google Scholar]
- 61.Ponzi M, Sidén-Kiamos I, Bertuccini L, Currà C, Kroeze H, Camarda G, Pace T, Franke-Fayard B, Laurentino EC, Louis C, Waters AP, Janse CJ, Alano P. Egress of Plasmodium berghei gametes from their host erythrocyte is mediated by the MDV-1/PEG3 protein. Cell Microbiol. 2009;11(8):1272–1288. doi: 10.1111/j.1462-5822.2009.01331.x. [DOI] [PubMed] [Google Scholar]
- 62.Talman AM, Lacroix C, Marques SR, Blagborough AM, Carzaniga R, Ménard R, Sinden RE. PbGEST mediates malaria transmission to both mosquito and vertebrate host. Mol Microbiol. 2011;82(2):462–474. doi: 10.1111/j.1365-2958.2011.07823.x. [DOI] [PubMed] [Google Scholar]
- 63.Olivieri A, Bertuccini L, Deligianni E, Franke-Fayard B, Currà C, Siden-Kiamos I, Hanssen E, Grasso F, Superti F, Pace T, Fratini F, Janse CJ, Ponzi M. Distinct properties of the egress-related osmiophilic bodies in male and female gametocytes of the rodent malaria parasite Plasmodium berghei. Cell Microbiol. 2015;17(3):355–368. doi: 10.1111/cmi.12370. [DOI] [PubMed] [Google Scholar]
- 64.Kehrer J, Frischknecht F, Mair GR. Proteomic analysis of the Plasmodium berghei pametocyte egressome and vesicular bioID of osmiophilic body proteins identifies merozoite TRAP-like protein (MTRAP) as an essential factor for parasite transmission. Mol Cell Proteomics. 2016;15(9):2852–2862. doi: 10.1074/mcp.M116.058263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kehrer J, Singer M, Lemgruber L, Silva PA, Frischknecht F, Mair GR. A putative small solute transporter is responsible for the secretion of G377 and TRAP-containing secretory vesicles during Plasmodium gamete egress and sporozoite motility. PLoS Pathog. 2016;12(7):e1005734. doi: 10.1371/journal.ppat.1005734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suárez-Cortés P, Sharma V, Bertuccini L, Costa G, Bannerman NL, Sannella AR, Williamson K, Klemba M, Levashina EA, Lasonder E, Alano P. Comparative proteomics and functional analysis reveal a role of Plasmodium falciparum osmiophilic bodies in malaria parasite transmission. Mol Cell Proteomics. 2016;15(10):3243–3255. doi: 10.1074/mcp.M116.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weißbach T, Golzmann A, Bennink S, Pradel G, Julius Ngwa C. Transcript and protein expression analysis of proteases in the blood stages of Plasmodium falciparum. Exp Parasitol. 2017;180:33–44. doi: 10.1016/j.exppara.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 68.Bargieri DY, Thiberge S, Tay CL, Carey AF, Rantz A, Hischen F, Lorthiois A, Straschil U, Singh P, Singh S, Triglia T, Tsuboi T, Cowman A, Chitnis C, Alano P, Baum J, Pradel G, Lavazec C, Menard R. Plasmodium merozoite TRAP family protein is essential for vacuole membrane disruption and gamete egress from erythrocytes. Cell Host Microbe. 2016;20(5):618–630. doi: 10.1016/j.chom.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baum J, Richard D, Healer J, Rug M, Krnajski Z, Gilberger TW, Green JL, Holder AA, Cowman AF. A conserved molecular motor drives cell invasion and gliding motility across malaria life cycle stages and other apicomplexan parasites. J Biol Chem. 2006;281(8):5197–5208. doi: 10.1074/jbc.M509807200. [DOI] [PubMed] [Google Scholar]
- 70.Deligianni E, Morgan RN, Bertuccini L, Wirth CC, Silmon de Monerri NC, Spanos L, Blackman MJ, Louis C, Pradel G, Siden-Kiamos I. A perforin-like protein mediates disruption of the erythrocyte membrane during egress of Plasmodium berghei male gametocytes. Cell Microbiol. 2013;15(8):1438–1455. doi: 10.1111/cmi.12131. [DOI] [PubMed] [Google Scholar]
- 71.Wirth CC, Glushakova S, Scheuermayer M, Repnik U, Garg S, Schaack D, Kachman MM, Weißbach T, Zimmerberg J, Dandekar T, Griffiths G, Chitnis CE, Singh S, Fischer R, Pradel G. Perforin-like protein PPLP2 permeabilizes the red blood cell membrane during egress of Plasmodium falciparum gametocytes. Cell Microbiol. 2014;16(5):709–733. doi: 10.1111/cmi.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sologub L, Kuehn A, Kern S, Przyborski J, Schillig R, Pradel G. Malaria proteases mediate inside-out egress of gametocytes from red blood cells following parasite transmission to the mosquito. Cell Microbiol. 2011;13(6):897–912. doi: 10.1111/j.1462-5822.2011.01588.x. [DOI] [PubMed] [Google Scholar]
- 73.Andreadaki M, Hanssen E, Deligianni E, Claudet C, Wengelnik K, Mollard V, McFadden GI, Abkarian M, Braun-Breton C, Siden-Kiamos I. Sequential membrane rupture and vesiculation during Plasmodium berghei gametocyte egress from the red blood cell. Sci Rep. 2018;8(1):3543. doi: 10.1038/s41598-018-21801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rupp I, Bosse R, Schirmeister T, Pradel G. Effect of protease inhibitors on exflagellation in Plasmodium falciparum. Mol Biochem Parasitol. 2008;158(2):208–212. doi: 10.1016/j.molbiopara.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 75.Pszenny V, Ehrenman K, Romano JD, Kennard A, Schultz A, Roos DS, Grigg ME, Carruthers VB, Coppens I. A lipolytic lecithin:c holesterol acyltransferase secreted by Toxoplasma facilitates parasite replication and egress. J Biol Chem. 2016;291(8):3725–3746. doi: 10.1074/jbc.M115.671974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schultz AJ, Carruthers VB. Toxoplasma gondii LCAT primarily contributes to tachyzoite egress. mSphere. 2018;3(1):pii: e00073-18. doi: 10.1128/mSphereDirect.00073-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kafsack BF, Pena JD, Coppens I, Ravindran S, Boothroyd JC, Carruthers VB. Rapid membrane disruption by a perforin-like protein facilitates parasite exit from host cells. Science. 2009;323(5913):530–533. doi: 10.1126/science.1165740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roiko MS, Carruthers VB. Functional dissection of Toxoplasma gondii perforin-like protein 1 reveals a dual domain mode of membrane binding for cytolysis and parasite egress. J Biol Chem. 2013;288(12):871287–25. doi: 10.1074/jbc.M113.450932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ni T, Williams SI, Rezelj S, Anderluh G, Harlos K, Stansfeld PJ, Gilbert RJC. Structures of monomeric and oligomeric forms of the Toxoplasma gondii perforin-like protein 1. Sci Adv. 2018;4(3):eaaq0762. doi: 10.1126/sciadv.aaq0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dogga SK, Mukherjee B, Jacot D, Kockmann T, Molino L, Hammoudi PM, Hartkoorn RC, Hehl AB, Soldati-Favre D. A druggable secretory protein maturase of Toxoplasma essential for invasion and egress. Elife. 2017;6:pii: e27480. doi: 10.7554/eLife.27480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gras S, Jackson A, Woods S, Pall G, Whitelaw J, Leung JM, Ward GE, Roberts CW, Meissner M. Parasites lacking the micronemal protein MIC2 are deficient in surface attachment and host cell egress, but remain virulent in vivo. Wellcome Open Res. 2017;2:32. doi: 10.12688/wellcomeopenres.11594.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saha S, Coleman BI, Dubey R, Blader IJ, Gubbels MJ. Two phosphoglucomutase paralogs facilitate ionophore-triggered secretion of the Toxoplasma micronemes. mSphere. 2017;2(6):pii: e00521-17. doi: 10.1128/mSphere.00521-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moudy R, Manning TJ, Beckers CJ. The loss of cytoplasmic potassium upon host cell breakdown triggers egress of Toxoplasma gondii. J Biol Chem. 2001;276(44):41492–41501. doi: 10.1074/jbc.M106154200. [DOI] [PubMed] [Google Scholar]
- 84.Roiko MS, Svezhova N, Carruthers VB. Acidification activates Toxoplasma gondii motility and egress by enhancing protein secretion and cytolytic activity. PLoS Pathog. 2014;10(11):e1004488. doi: 10.1371/journal.ppat.1004488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brown KM, Lourido S, Sibley LD. Serum albumin stimulates protein kinase G-dependent microneme secretion in Toxoplasma gondii. J Biol Chem. 2016;291(18):9554–9565. doi: 10.1074/jbc.M115.700518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stewart RJ, Whitehead L, Nijagal B, Sleebs BE, Lessene G, McConville MJ, Rogers KL, Tonkin CJ. Analysis of Ca2+ mediated signaling regulating Toxoplasma infectivity reveals complex relationships between key molecules. Cell Microbiol. 2017;19(4) doi: 10.1111/cmi.12685. [DOI] [PubMed] [Google Scholar]
- 87.Chini EN, Nagamune K, Wetzel DM, Sibley LD. Evidence that the cADPR signalling pathway controls calcium-mediated microneme secretion in Toxoplasma gondii. Biochem J. 2005;389(Pt 2):269–277. doi: 10.1042/BJ20041971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jia Y, Marq JB, Bisio H, Jacot D, Mueller C, Yu L, Choudhary J, Brochet M, Soldati-Favre D. Crosstalk between PKA and PKG controls pH-dependent host cell egress of Toxoplasma gondii. EMBO J. 2017;36(21):3250–3267. doi: 10.15252/embj.201796794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nagamune K, Hicks LM, Fux B, Brossier F, Chini EN, Sibley LD. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii. Nature. 2008;451(7175):207–210. doi: 10.1038/nature06478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arroyo-Olarte RD, Brouwers JF, Kuchipudi A, Helms JB, Biswas A, Dunay IR, Lucius R, Gupta N. Phosphatidylthreonine and lipid-mediated control of parasite virulence. PLoS Biol. 2015;13(11):e1002288. doi: 10.1371/journal.pbio.1002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kuchipudi A, Arroyo-Olarte RD, Hoffmann F, Brinkmann V, Gupta N. Optogenetic monitoring identifies phosphatidylthreonine-regulated calcium homeostasis in Toxoplasma gondii. Microb Cell. 2016;3(5):215–223. doi: 10.15698/mic2016.05.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kong P, Ufermann CM, Zimmermann DLM, Yin Q, Suo X, Helms JB, Brouwers JF, Gupta N. Two phylogenetically and compartmentally distinct CDP-diacylglycerol synthases cooperate for lipid biogenesis in Toxoplasma gondii. J Biol Chem. 2017;292(17):7145–7159. doi: 10.1074/jbc.M116.765487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lourido S, Shuman J, Zhang C, Shokat KM, Hui R, Sibley LD. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature. 2010;465(7296):359–362. doi: 10.1038/nature09022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McCoy JM, Stewart RJ, Uboldi AD, Li D, Schröder J, Scott NE, Papenfuss AT, Lehane AM, Foster LJ, Tonkin CJ. A forward genetic screen identifies a negative regulator of rapid Ca2+-dependent cell egress (MS1) in the intracellular parasite Toxoplasma gondii. J Biol Chem. 2017;292(18):7662–7674. doi: 10.1074/jbc.M117.775114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McCoy JM, Whitehead L, van Dooren GG, Tonkin CJ. TgCDPK3 regulates calcium-dependent egress of Toxoplasma gondii from host cells. PLoS Pathog. 2012;8(12):e1003066. doi: 10.1371/journal.ppat.1003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gaji RY, Johnson DE, Treeck M, Wang M, Hudmon A, Arrizabalaga G. Phosphorylation of a motor by TgCDPK3 facilitates rapid initiation of motility during Toxoplasma gondii egress. PLoS Pathog. 2015;11(11):e1005268. doi: 10.1371/journal.ppat.1005268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bullen HE, Jia Y, Yamaryo-Botté Y, Bisio H, Zhang O, Jemelin NK, Marq JB, Carruthers V, Botté CY, Soldati-Favre D. Phosphatidic acid-mediated signaling regulates microneme secretion in Toxoplasma. Cell Host Microbe. 2016;19(3):349–360. doi: 10.1016/j.chom.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 98.Burda PC, Roelli MA, Schaffner M, Khan SM, Janse CJ, Heussler VT. A Plasmodium phospholipase is involved in disruption of the liver stage parasitophorous vacuole membrane. PLoS Pathog. 2015;11(3):e1004760. doi: 10.1371/journal.ppat.1004760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Risco-Castillo V, Topçu S, Marinach C, Manzoni G, Bigorgne AE, Briquet S, Baudin X, Lebrun M, Dubremetz JF, Silvie O. Malaria sporozoites traverse host cells within transient vacuoles. Cell Host Microbe. 2015;18(5):593–603. doi: 10.1016/j.chom.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 100.Yang ASP, O'Neill MT, Jennison C, Lopaticki S, Allison CC, Armistead JS, Erickson SM, Rogers KL, Ellisdon AM, Whisstock JC, Tweedell RE, Dinglasan RR, Douglas DN, Kneteman NM, Boddey JA. Cell traversal activity is important for Plasmodium falciparum liver infection in humanized mice. Cell Rep. 2017;18(13):3105–3116. doi: 10.1016/j.celrep.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 101.Noronha FS, Cruz JS, Beirão PS, Horta MF. Macrophage damage by Leishmania amazonensis cytolysin: evidence of pore formation on cell membrane. Infect Immun. 2000;68(8):4578–4584. doi: 10.1128/iai.68.8.4578-4584.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Castro-Gomes T, Almeida-Campos FR, Calzavara-Silva CE, da Silva RA, Frézard F, Horta MF. Membrane binding requirements for the cytolytic activity of Leishmania amazonensis leishporin. FEBS Lett. 2009;583(19):3209–3214. doi: 10.1016/j.febslet.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 103.Almeida-Campos FR, Castro-Gomes T, Machado-Silva A, de Oliveira JS, Santoro MM, Frézard F, Horta MF. Activation of Leishmania spp. leishporin: evidence that dissociation of an inhibitor not only improves its lipid-binding efficiency but also endows it with the ability to form pores. Parasitol Res. 2013;112(9):3305–3314. doi: 10.1007/s00436-013-3510-4. [DOI] [PubMed] [Google Scholar]
- 104.Rubin-de-Celis SS, Uemura H, Yoshida N, Schenkman S. Expression of trypomastigote trans-sialidase in metacyclic forms of Trypanosoma cruzi increases parasite escape from its parasitophorous vacuole. Cell Microbiol. 2006;8(12):1888–1898. doi: 10.1111/j.1462-5822.2006.00755.x. [DOI] [PubMed] [Google Scholar]
- 105.Freire-de-Lima L, da Fonseca LM, da Silva VA, da Costa KM, Morrot A, Freire-de-Lima CG, Previato JO, Mendonça-Previato L. Modulation of cell sialoglycophenotype: A stylish mechanism adopted by Trypanosoma cruzi to ensure its persistence in the infected host. Front Microbiol. 2016;7:698. doi: 10.3389/fmicb.2016.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Andrews NW, Abrams CK, Slatin SL, Griffiths G. A T. cruzi-secreted protein immunologically related to the complement component C9: evidence for membrane pore-forming activity at low pH. Cell. 1990;61(7):1277–1287. doi: 10.1016/0092-8674(90)90692-8. [DOI] [PubMed] [Google Scholar]
- 107.Hall BF, Webster P, Ma AK, Joiner KA, Andrews NW. Desialylation of lysosomal membrane glycoproteins by Trypanosoma cruzi: a role for the surface neuraminidase in facilitating parasite entry into the host cell cytoplasm. J Exp Med. 1992;176(2):313–325. doi: 10.1084/jem.176.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Di Russo Case E, Samuel JE. Contrasting lifestyles within the host cell. Microbiol Spectr. 2016;4(1) doi: 10.1128/microbiolspec.vmbf-0014-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Portnoy DA, Jacks PS, Hinrichs DJ. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167(4):1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cheng LW, Portnoy DA. Drosophila S2 cells: an alternative infection model for Listeria monocytogenes. Cell Microbiol. 2003;5(12):875–885. doi: 10.1046/j.1462-5822.2003.00327.x. [DOI] [PubMed] [Google Scholar]
- 111.Birmingham CL, Canadien V, Kaniuk NA, Steinberg BE, Higgins DE, Brumell JH. Listeriolysin O allows Listeria monocytogenes replication in macrophage vacuoles. Nature. 2008;451(7176):350–354. doi: 10.1038/nature06479. [DOI] [PubMed] [Google Scholar]
- 112.Tilney LG, Portnoy DA. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109(4 Pt 1):1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gaillard JL, Berche P, Mounier J, Richard S, Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987;55(11):2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Camilli A, Tilney LG, Portnoy DA. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993;8(1):143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smith GA, Marquis H, Jones S, Johnston NC, Portnoy DA, Goldfine H. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun. 1995;63(11):4231–4237. doi: 10.1128/iai.63.11.4231-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Seveau S. Multifaceted activity of listeriolysin O, the cholesterol-dependent cytolysin of Listeria monocytogenes. Subcell Biochem. 2014;80:161–195. doi: 10.1007/978-94-017-8881-6_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tattoli I, Sorbara MT, Yang C, Tooze SA, Philpott DJ, Girardin SE. Listeria phospholipases subvert host autophagic defenses by stalling pre-autophagosomal structures. EMBO J. 2013;32(23):3066–3078. doi: 10.1038/emboj.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mitchell G, Ge L, Huang Q, Chen C, Kianian S, Roberts MF, Schekman R, Portnoy DA. Avoidance of autophagy mediated by PlcA or ActA is required for Listeria monocytogenes growth in macrophages. Infect Immun. 2015;83(5):2175–2184. doi: 10.1128/IAI.00110-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Beauregard KE, Lee KD, Collier RJ, Swanson JA. pH-dependent perforation of macrophage phagosomes by listeriolysin O from Listeria monocytogenes. J Exp Med. 1997;186(7):1159–1163. doi: 10.1084/jem.186.7.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Glomski IJ, Gedde MM, Tsang AW, Swanson JA, Portnoy DA. The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells. J Cell Biol. 2002;156(6):1029–1038. doi: 10.1083/jcb.200201081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shaughnessy LM, Hoppe AD, Christensen KA, Swanson JA. Membrane perforations inhibit lysosome fusion by altering pH and calcium in Listeria monocytogenes vacuoles. Cell Microbiol. 2006;8(5):781–792. doi: 10.1111/j.1462-5822.2005.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.High N, Mounier J, Prévost MC, Sansonetti PJ. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 1992;11(5):1991–1999. doi: 10.1002/j.1460-2075.1992.tb05253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Page AL, Ohayon H, Sansonetti PJ, Parsot C. The secreted IpaB and IpaC invasins and their cytoplasmic chaperone IpgC are required for intercellular dissemination of Shigella flexneri. Cell Microbiol. 1999;1(2):183–193. doi: 10.1046/j.1462-5822.1999.00019.x. [DOI] [PubMed] [Google Scholar]
- 124.Hayward RD, Cain RJ, McGhie EJ, Phillips N, Garner MJ, Koronakis V. Cholesterol binding by the bacterial type III translocon is essential for virulence effector delivery into mammalian cells. Mol Microbiol. 2005;56(3):590–603. doi: 10.1111/j.1365-2958.2005.04568.x. [DOI] [PubMed] [Google Scholar]
- 125.Picking WL, Nishioka H, Hearn PD, Baxter MA, Harrington AT, Blocker A, Picking WD. IpaD of Shigella flexneri is independently required for regulation of Ipa protein secretion and efficient insertion of IpaB and IpaC into host membranes. Infect Immun. 2005;73(3):1432–1440. doi: 10.1128/IAI.73.3.1432-1440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stamm LM, Morisaki JH, Gao LY, Jeng RL, McDonald KL, Roth R, Takeshita S, Heuser J, Welch MD, Brown EJ. Mycobacterium marinum escapes from phagosomes and is propelled by actin-based motility. J Exp Med. 2003;198(9):1361–1368. doi: 10.1084/jem.20031072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van der Wel N, Hava D, Houben D, Fluitsma D, van Zon M, Pierson J, Brenner M, Peters PJ. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129(7):1287–1298. doi: 10.1016/j.cell.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 128.Gao LY, Guo S, McLaughlin B, Morisaki H, Engel JN, Brown EJ. A mycobacterial virulence gene cluster extending RD1 is required for cytolysis, bacterial spreading and ESAT-6 secretion. Mol Microbiol. 2004;53(6):1677–1693. doi: 10.1111/j.1365-2958.2004.04261.x. [DOI] [PubMed] [Google Scholar]
- 129.de Jonge MI, Pehau-Arnaudet G, Fretz MM, Romain F, Bottai D, Brodin P, Honoré N, Marchal G, Jiskoot W, England P, Cole ST, Brosch R. ESAT-6 from Mycobacterium tuberculosis dissociates from its putative chaperone CFP-10 under acidic conditions and exhibits membrane-lysing activity. J Bacteriol. 2007;189(16):6028–6034. doi: 10.1128/JB.00469-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Houben D, Demangel C, van Ingen J, Perez J, Baldeón L, Abdallah AM, Caleechurn L, Bottai D, van Zon M, de Punder K, van der Laan T, Kant A, Bossers-de Vries R, Willemsen P, Bitter W, van Soolingen D, Brosch R, van der Wel N, Peters PJ. ESX-1-mediated translocation to the cytosol controls virulence of mycobacteria. Cell Microbiol. 2012;14(8):1287–1298. doi: 10.1111/j.1462-5822.2012.01799.x. [DOI] [PubMed] [Google Scholar]
- 131.De Leon J, Jiang G, Ma Y, Rubin E, Fortune S, Sun J. Mycobacterium tuberculosis ESAT-6 exhibits a unique membrane-interacting activity that is not found in its ortholog from non-pathogenic Mycobacterium smegmatis. J Biol Chem. 2012;287(53):44184–44191. doi: 10.1074/jbc.M112.420869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Peng X, Jiang G, Liu W, Zhang Q, Qian W, Sun J. Characterization of differential pore-forming activities of ESAT-6 proteins from Mycobacterium tuberculosis and Mycobacterium smegmatis. FEBS Lett. 2016;590(4):509–519. doi: 10.1002/1873-3468.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Simeone R, Bottai D, Frigui W, Majlessi L, Brosch R. ESX/type VII secretion systems of mycobacteria: Insights into evolution, pathogenicity and protection. Tuberculosis (Edinb) 2015;95(Suppl 1):S150–154. doi: 10.1016/j.tube.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 134.Simeone R, Majlessi L, Enninga J, Brosch R. Perspectives on mycobacterial vacuole-to-cytosol translocation: the importance of cytosolic access. Cell Microbiol. 2016;18(8):1070–1077. doi: 10.1111/cmi.12622. [DOI] [PubMed] [Google Scholar]
- 135.Simeone R, Bobard A, Lippmann J, Bitter W, Majlessi L, Brosch R, Enninga J. Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death. PLoS Pathog. 2012;8(2):e1002507. doi: 10.1371/journal.ppat.1002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Simeone R, Sayes F, Song O, Gröschel MI, Brodin P, Brosch R, Majlessi L. Cytosolic access of Mycobacterium tuberculosis: critical impact of phagosomal acidification control and demonstration of occurrence in vivo. PLoS Pathog. 2015;11(2):e1004650. doi: 10.1371/journal.ppat.1004650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhong G, Fan P, Ji H, Dong F, Huang Y. Identification of a chlamydial protease-like activity factor responsible for the degradation of host transcription factors. J Exp Med. 2001;193(8):935–942. doi: 10.1084/jem.193.8.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bavoil PM, Byrne GI. Analysis of CPAF mutants: new functions, new questions (the ins and outs of a chlamydial protease). Pathog Dis. 2014;71(3):287–291. doi: 10.1111/2049-632X.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang C, Starr T, Song L, Carlson JH, Sturdevant GL, Beare PA, Whitmire WM, Caldwell HD. Chlamydial lytic exit from host cells is plasmid regulated. MBio. 2015;6(6):e01648-15. doi: 10.1128/mBio.01648-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hybiske K, Stephens RS. Mechanisms of host cell exit by the intracellular bacterium Chlamydia. Proc Natl Acad Sci U S A. 2007;104(27):11430–11435. doi: 10.1073/pnas.0703218104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Steele-Mortimer O, Méresse S, Gorvel JP, Toh BH, Finlay BB. Biogenesis of Salmonella typhimurium-containing vacuoles in epithelial cells involves interactions with the early endocytic pathway. Cell Microbiol. 1999;1(1):33–49. doi: 10.1046/j.1462-5822.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- 142.Zhang Y, Higashide W, Dai S, Sherman DM, Zhou D. Recognition and ubiquitination of Salmonella type III effector SopA by a ubiquitin E3 ligase, HsRMA1. J Biol Chem. 2005;280(46):38682–38688. doi: 10.1074/jbc.M506309200. [DOI] [PubMed] [Google Scholar]
- 143.Drecktrah D, Knodler LA, Howe D, Steele-Mortimer O. Salmonella trafficking is defined by continuous dynamic interactions with the endolysosomal system. Traffic. 2007;8(3):212–225. doi: 10.1111/j.1600-0854.2006.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bakowski MA, Braun V, Brumell JH. Salmonella- containing vacuoles: directing traffic and nesting to grow. Traffic. 2008;9(12):2022–2031. doi: 10.1111/j.1600-0854.2008.00827.x. [DOI] [PubMed] [Google Scholar]
- 145.Knodler LA. Salmonella enterica: living a double life in epithelial cells. Curr Opin Microbiol. 2015;23:23–31. doi: 10.1016/j.mib.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 146.Knodler LA, Vallance BA, Celli J, Winfree S, Hansen B, Montero M, Steele-Mortimer O. Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proc Natl Acad Sci U S A. 2010;107(41):17733–17738. doi: 10.1073/pnas.1006098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Miki T, Okada N, Shimada Y, Danbara H. Characterization of Salmonella pathogenicity island 1 type III secretion-dependent hemolytic activity in Salmonella enterica serovar Typhimurium. Microb Pathog. 2004;37(2):65–72. doi: 10.1016/j.micpath.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 148.Roy D, Liston DR, Idone VJ, Di A, Nelson DJ, Pujol C, Bliska JB, Chakrabarti S, Andrews NW. A process for controlling intracellular bacterial infections induced by membrane injury. Science. 2004;304(5676):1515–1518. doi: 10.1126/science.1098371. [DOI] [PubMed] [Google Scholar]
- 149.Birmingham CL, Smith AC, Bakowski MA, Yoshimori T, Brumell JH. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J Biol Chem. 2006;281(16):11374–11383. doi: 10.1074/jbc.M509157200. [DOI] [PubMed] [Google Scholar]
- 150.Yu HB, Croxen MA, Marchiando AM, Ferreira RB, Cadwell K, Foster LJ, Finlay BB. Autophagy facilitates Salmonella replication in HeLa cells. MBio. 2014;5(2):e00865-14. doi: 10.1128/mBio.00865-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Beuzón CR, Méresse S, Unsworth KE, Ruíz-Albert J, Garvis S, Waterman SR, Ryder TA, Boucrot E, Holden DW. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 2000;19(13):3235–3249. doi: 10.1093/emboj/19.13.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ruiz-Albert J, Yu XJ, Beuzón CR, Blakey AN, Galyov EE, Holden DW. Complementary activities of SseJ and SifA regulate dynamics of the Salmonella typhimurium vacuolar membrane. Mol Microbiol. 2002;44(3):645–661. doi: 10.1046/j.1365-2958.2002.02912.x. [DOI] [PubMed] [Google Scholar]
- 153.Lossi NS, Rolhion N, Magee AI, Boyle C, Holden DW. The Salmonella SPI-2 effector SseJ exhibits eukaryotic activator-dependent phospholipase A and glycerophospholipid: cholesterol acyltransferase activity. Microbiology. 2008;154(Pt 9):2680–2688. doi: 10.1099/mic.0.2008/019075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Boucrot E, Henry T, Borg JP, Gorvel JP, Méresse S. The intracellular fate of Salmonella depends on the recruitment of kinesin. Science. 2005;308(5725):1174–1178. doi: 10.1126/science.1110225. [DOI] [PubMed] [Google Scholar]
- 155.Henry T, Couillault C, Rockenfeller P, Boucrot E, Dumont A, Schroeder N, Hermant A, Knodler LA, Lecine P, Steele-Mortimer O, Borg JP, Gorvel JP, Méresse S. The Salmonella effector protein PipB2 is a linker for kinesin-1. Proc Natl Acad Sci U S A. 2006;103(36):13497–1502. doi: 10.1073/pnas.0605443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dumont A, Boucrot E, Drevensek S, Daire V, Gorvel JP, Poüs C, Holden DW, Méresse S. SKIP, the host target of the Salmonella virulence factor SifA, promotes kinesin-1-dependent vacuolar membrane exchanges. Traffic. 2010;11(7):899–911. doi: 10.1111/j.1600-0854.2010.01069.x. [DOI] [PubMed] [Google Scholar]
- 157.Newton HJ, Ang DK, van Driel IR, Hartland EL. Molecular pathogenesis of infections caused by Legionella pneumophila. Clin Microbiol Rev. 2010;23(2):274–298. doi: 10.1128/CMR.00052-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kirby JE, Vogel JP, Andrews HL, Isberg RR. Evidence for pore-forming ability by Legionella pneumophila. Mol Microbiol. 1998;27(2):323–336. doi: 10.1046/j.1365-2958.1998.00680.x. [DOI] [PubMed] [Google Scholar]
- 159.Molmeret M, Alli OA, Radulic M, Susa M, Doric M, Kwaik YA. The C-terminus of IcmT is essential for pore formation and for intracellular trafficking of Legionella pneumophila within Acanthamoeba polyphaga. Mol Microbiol. 2002;43(5):1139–1150. doi: 10.1046/j.1365-2958.2002.02842.x. [DOI] [PubMed] [Google Scholar]
- 160.Molmeret M, Bitar DM, Han L, Kwaik YA. Disruption of the phagosomal membrane and egress of Legionella pneumophila into the cytoplasm during the last stages of intracellular infection of macrophages and Acanthamoeba polyphaga. Infect Immun. 2004;72(7):4040–4051. doi: 10.1128/iai.72.7.4040-4051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Silveira TN, Zamboni DS. Pore formation triggered by Legionella spp. is an Nlrc4 inflammasome-dependent host cell response that precedes pyroptosis. Infect Immun. 2010;78(3):1403–1413. doi: 10.1128/IAI.00905-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hiller M, Lang C, Michel W, Flieger A. Secreted phospholipases of the lung pathogen Legionella pneumophila. Int J Med Microbiol. 2017;S1438-4221(17):30290–4. doi: 10.1016/j.ijmm.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 163.Creasey EA, Isberg RR. The protein SdhA maintains the integrity of the Legionella-containing vacuole. Proc Natl Acad Sci U S A. 2012;109(9):3481–3486. doi: 10.1073/pnas.1121286109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lang C, Hiller M, Flieger A. Disulfide loop cleavage of Legionella pneumophila PlaA boosts lysophospholipase A activity. Sci Rep. 2017;7(1):16313. doi: 10.1038/s41598-017-12796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Aly AS, Matuschewski K. A malarial cysteine protease is necessary for Plasmodium sporozoite egress from oocysts. J Exp Med. 2005;202(2):225–230. doi: 10.1084/jem.20050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mastan BS, Narwal SK, Dey S, Kumar KA, Mishra S. Plasmodium berghei plasmepsin VIII is essential for sporozoite gliding motility. Int J Parasitol. 2017;47(5):239–245. doi: 10.1016/j.ijpara.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 167.Pradel G, Hayton K, Aravind L, Iyer LM, Abrahamsen MS, Bonawitz A, Mejia C, Templeton TJ. A multidomain adhesion protein family expressed in Plasmodium falciparum is essential for transmission to the mosquito. J Exp Med. 2004;199(11):1533–1544. doi: 10.1084/jem.20031274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Raine JD, Ecker A, Mendoza J, Tewari R, Stanway RR, Sinden RE. Female inheritance of malarial lap genes is essential for mosquito transmission. PLoS Pathog. 2007;3(3):e30. doi: 10.1371/journal.ppat.0030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lavazec C, Moreira CK, Mair GR, Waters AP, Janse CJ, Templeton TJ. Analysis of mutant Plasmodium berghei parasites lacking expression of multiple PbCCp genes. Mol Biochem Parasitol. 2009;163(1):1–7. doi: 10.1016/j.molbiopara.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 170.Klug D, Frischknecht F. Motility precedes egress of malaria parasites from oocysts. Elife. 2017;6:pii: e19157. doi: 10.7554/eLife.19157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Welch MD, Way M. Arp2/3-mediated actin-based motility: a tail of pathogen abuse. Cell Host Microbe. 2013;14(3):242–255. doi: 10.1016/j.chom.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Stevens JM, Galyov EE, Stevens MP. Actindependent movement of bacterial pathogens. Nature Rev Microbiol. 2006;4(2):91–101. doi: 10.1038/nrmicro1320. [DOI] [PubMed] [Google Scholar]
- 173.Rafelski SM, Theriot JA. Mechanism of polarization of Listeria monocytogenes surface protein ActA. Mol Microbiol. 2006;9(4):1262–1279. doi: 10.1111/j.1365-2958.2006.05025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Boujemaa-Paterski R, Gouin E, Hansen G, Samarin S, Le Clainche C, Didry D, Dehoux P, Cossart P, Kocks C, Carlier MF, Pantaloni D. Listeria protein ActA mimics WASp family proteins: it activates filament barbed end branching by Arp2/3 complex. Biochemistry. 2001;40(38):11390–11404. doi: 10.1021/bi010486b. [DOI] [PubMed] [Google Scholar]
- 175.Fattouh R, Kwon H, Czuczman MA, Copeland JW, Pelletier L, Quinlan ME, Muise AM, Higgins DE, Brumell JH. The diapha- nous-related formins promote protrusion formation and cell-to-cell spread of Listeria monocytogenes. J Infect Dis. 2014;211(7):1185–1195. doi: 10.1093/infdis/jiu546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rajabian T, Gavicherla B, Heisig M, Müller-Altrock S, Goebel W, Gray-Owen SD, Ireton K. The bacterial virulence factor InlC perturbs apical cell junctions and promotes cell-to-cell spread of Listeria. Nat Cell Biol. 2009;11(10):1212–1218. doi: 10.1038/ncb1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pust S, Morrison H, Wehland J, Sechi AS, Herrlich P. Listeria monocytogenes exploits ERM protein functions to efficiently spread from cell to cell. EMBO J. 2005;24(6):1287–1300. doi: 10.1038/sj.emboj.7600595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Robbins JR, Barth AI, Marquis H, de Hostos EL, Nelson WJ, Theriot JA. Listeria monocytogenes exploits normal host cell processes to spread from cell to cell. J Cell Biol. 1999;146(6):1333–1350. doi: 10.1083/jcb.146.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chong R, Squires R, Swiss R, Agaisse H. RNAi screen reveals host cell kinases specifically involved in Listeria monocytogenes spread from cell to cell. PLoS One. 2011;6(8):e23399. doi: 10.1371/journal.pone.0023399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vazquez-Boland JA, Kocks C, Dramsi S, Ohayon H, Geoffroy C, Mengaud J, Cossart P. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect Immun. 1992;60(1):219–230. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gedde MM, Higgins DE, Tilney LG, Portnoy DA. Role of listeriolysin O in cell-to-cell spread of Listeria monocytogenes. Infect Immun. 2000;68(2):999–1003. doi: 10.1128/iai.68.2.999-1003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gründling A, Gonzalez MD, Higgins DE. Requirement of the Listeria monocytogenes broad-range phospholipase PC-PLC during infection of human epithelial cells. J Bacteriol. 2003;185(21):6295–6307. doi: 10.1128/jb.185.21.6295-6307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Alberti-Segui C, Goeden KR, Higgins DE. Differential function of Listeria monocytogenes listeriolysin O and phospholipases C in vacuolar dissolution following cell-to-cell spread. Cell Microbiol. 2007;9(1):179–195. doi: 10.1111/j.1462-5822.2006.00780.x. [DOI] [PubMed] [Google Scholar]
- 184.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;68(3):521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- 185.Portnoy DA, Auerbuch V, Glomski IJ. The cell biology of Listeria monocytogenes infection: the intersection of bacterial pathogenesis and cell-mediated immunity. J Cell Biol. 2002;158(3):409–414. doi: 10.1083/jcb.200205009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bernardini ML, Mounier J, d’Hauteville H, Coquis-Rondon M, Sansonetti PJ. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci USA. 1989;86(10):3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sansonetti PJ, Mounier J, Prévost MC, Mège RM. Cadherin expression is required for the spread of Shigella flexneri between epithelial cells. Cell. 1994;76(5):829–839. doi: 10.1016/0092-8674(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 188.Leung Y, Ally S, Goldberg MB. Bacterial actin assembly requires toca-1 to relieve N-wasp autoinhibition. Cell Host Microbe. 2008;3(1):39–47. doi: 10.1016/j.chom.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bishai EA, Sidhu GS, Li W, Dhillon J, Bohil AB, Cheney RE, Hartwig JH, Southwick FS. Myosin-X facilitates Shigella-induced membrane protrusions and cell-to-cell spread. Cell Microbiol. 2013;15(3):353–367. doi: 10.1111/cmi.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Heindl JE, Saran I, Yi CR, Lesser CF, Goldberg MB. Requirement for formin-induced actin polymerization during spread of Shigella flexneri. Infect Immun. 2010;78(1):193–203. doi: 10.1128/IAI.00252-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yoshida S, Handa Y, Suzuki T, Ogawa M, Suzuki M, Tamai A, Abe A, Katayama E, Sasakawa C. Microtubule-severing activity of Shigella is pivotal for intercellular spreading. Science. 2006;314(5801):985–989. doi: 10.1126/science.1133174. [DOI] [PubMed] [Google Scholar]
- 192.Germane KL, Ohi R, Goldberg MB, Spiller BW. Structural and functional studies indicate that Shigella VirA is not a protease and does not directly destabilize microtubules. Biochemistry. 2008;47(39):10241–10243. doi: 10.1021/bi801533k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Monack DM, Theriot JA. Actin-based motility is sufficient for bacterial membrane protrusion formation and host cell uptake. Cell Microbiol. 2001;3(9):633–647. doi: 10.1046/j.1462-5822.2001.00143.x. [DOI] [PubMed] [Google Scholar]
- 194.Fukumatsu M, Ogawa M, Arakawa S, Suzuki M, Nakayama K, Shimizu S, Kim M, Mimuro H, Sasakawa C. Shigella targets epithelial tricellular junctions and uses a noncanonical clathrin-dependent endocytic pathway to spread between cells. Cell Host Microbe. 2012;11(4):325–336. doi: 10.1016/j.chom.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 195.Reed SC, Lamason RL, Risca VI, Abernathy E, Welch MD. Rickettsia actin-based motility occurs in distinct phases mediated by different actin nucleators. Curr Biol. 2014;24(1):98–103. doi: 10.1016/j.cub.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Haglund CM, Choe JE, Skau CT, Kovar DR, Welch MD. Rickettsia Sca2 is a bacterial formin-like mediator of actin-based motility. Nat Cell Biol. 2010;12(11):1057–1063. doi: 10.1038/ncb2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kleba B, Clark TR, Lutter EI, Ellison DW, Hackstadt T. Disruption of the Rickettsia rickettsii Sca2 autotransporter inhibits actin-based motility. Infect Immun. 2010;78(5):2240–2247. doi: 10.1128/IAI.00100-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lamason RL, Bastounis E, Kafai NM, Serrano R, Del Álamo JC, Theriot JA, Welch MD. Rickettsia Sca4 reduces vinculin-mediated intercellular tension to promote spread. Cell. 2016;167(3):670–683.e10. doi: 10.1016/j.cell.2016.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Thomas S, Popov VL, Walker DH. Exit mechanisms of the intracellular bacterium Ehrlichia. PLoS One. 2010;5(12):e15775. doi: 10.1371/journal.pone.0015775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mohan Kumar D, Lin M, Xiong Q, Webber MJ, Kural C, Rikihisa Y. EtpE binding to DNase X induces ehrlichial entry via CD147 and hnRNP-K recruitment, followed by mobilization of N-WASP and actin. MBio. 2015;6(6):e01541-15. doi: 10.1128/mBio.01541-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kespichayawattana W, Rattanachetkul S, Wanun T, Utaisincharoen P, Sirisinha S. Burkholderia pseudomallei induces cell fusion and actin-associated membrane protrusion: a possible mechanism for cell-to-cell spreading. Infect Immun. 2000;68(9):5377–5384. doi: 10.1128/iai.68.9.5377-5384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Benanti EL, Nguyen CM, Welch MD. Virulent Burkholderia species mimic host actin polymerases to drive actin-based motility. Cell. 2015;161(2):348–360. doi: 10.1016/j.cell.2015.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.French CT, Toesca IJ, Wu TH, Teslaa T, Beaty SM, Wong W, Liu M, Schröder I, Chiou PY, Teitell MA, Miller JF. Dissection of the Burkholderia intracellular life cycle using a photothermal nanoblade. Proc Natl Acad Sci U S A. 2011;108(29):12095–12100. doi: 10.1073/pnas.1107183108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Stamm LM, Pak MA, Morisaki JH, Snapper SB, Rottner K, Lommel S, Brown EJ. Role of the WASP family proteins for Myco-bacterium marinum actin tail formation. Proc Natl Acad Sci U S A. 2005;102(41):14837–14842. doi: 10.1073/pnas.0504663102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Volceanov L, Herbst K, Biniossek M, Schilling O, Haller D, Nölke T, Subbarayal P, Rudel T, Zieger B, Häcker G. Septins arrange F-actin-containing fibers on the Chlamydia trachomatis inclusion and are required for normal release of the inclusion by extrusion. MBio. 2014;5(5):e01802-14. doi: 10.1128/mBio.01802-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lutter EI, Barger AC, Nair V, Hackstadt T. Chlamydia trachomatis inclusion membrane protein CT228 recruits elements of the myosin phosphatase pathway to regulate release mechanisms. Cell Rep. 2013;3(6):1921–1931. doi: 10.1016/j.celrep.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kim MJ, Kim MK, Kang JS. Involvement of lipid rafts in the budding-like exit of Orientia tsutsugamushi. Microb Pathog. 2013;63:37–43. doi: 10.1016/j.micpath.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 208.Salje J. Orientia tsutsugamushi: A neglected but fascinating obligate intracellular bacterial pathogen. PLoS Pathog. 2017;13(12):e1006657. doi: 10.1371/journal.ppat.1006657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sturm A, Amino R, van de Sand C, Regen T, Retzlaff S, Rennenberg A, Krueger A, Pollok JM, Menard R, Heussler VT. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science. 2006;313(5791):1287–1290. doi: 10.1126/science.1129720. [DOI] [PubMed] [Google Scholar]
- 210.Burda PC, Caldelari R, Heussler VT. Manipulation of the host cell membrane during Plasmodium liver stage egress. MBio. 2017;8(2):pii: e00139-17. doi: 10.1128/mBio.00139-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Graewe S, Rankin KE, Lehmann C, Deschermeier C, Hecht L, Froehlke U, Stanway RR, Heussler V. Hostile takeover by Plasmodium: reorganization of parasite and host cell membranes during liver stage egress. PLoS Pathog. 2011;7(9):e1002224. doi: 10.1371/journal.ppat.1002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Baer K, Klotz C, Kappe SH, Schnieder T, Frevert U. Release of hepatic Plasmodium yoelii merozoites into the pulmonary microvasculature. PLoS Pathog. 2007;3(11):e171. doi: 10.1371/journal.ppat.0030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Südhof TC, Rizo J. Synaptic vesicle exocytosis. Cold Spring Harb Perspect Biol. 2011;3(12):a005637. doi: 10.1101/cshperspect.a005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ireton K, Van Ngo H, Bhalla M. Interaction of microbial pathogens with host exocytic pathways. Cell Microbiol. 2018;24:e12861. doi: 10.1111/cmi.12861. [DOI] [PubMed] [Google Scholar]
- 215.Johnston SA, May RC. Cryptococcus interactions with macrophages: evasion and manipulation of the phagosome by a fungal pathogen. Cell Microbiol. 2013;15(3):403–411. doi: 10.1111/cmi.12067. [DOI] [PubMed] [Google Scholar]
- 216.Chen J, de Felipe KS, Clarke M, Lu H, Anderson OR, Segal G, Shuman HA. Legionella effectors that promote nonlytic release from protozoa. Science. 2004;303(5662):1358–1361. doi: 10.1126/science.1094226. [DOI] [PubMed] [Google Scholar]
- 217.de Felipe KS, Glover RT, Charpentier X, Anderson OR, Reyes M, Pericone CD, Shuman HA. Legionella eukaryotic-like type IV substrates interfere with organelle trafficking. PLoS Pathog. 2008;4(8):e1000117. doi: 10.1371/journal.ppat.1000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gomez-Valero L, Rusniok C, Cazalet C, Buchrieser C. Comparative and functional genomics of Legionella identified eukaryotic like proteins as key players in host-pathogen interactions. Front Microbiol. 2011;2:208. doi: 10.3389/fmicb.2011.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Shi X, Halder P, Yavuz H, Jahn R, Shuman HA. Direct targeting of membrane fusion by SNARE mimicry: Convergent evolution of Legionella effectors. Proc Natl Acad Sci U S A. 2016;113(31):8807–8812. doi: 10.1073/pnas.1608755113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Berk SG, Ting RS, Turner GW, Ashburn RJ. Production of respirable vesicles containing live Legionella pneumophila cells by two Acanthamoeba spp. Appl Environ Microbiol. 1998;64(1):279–286. doi: 10.1128/aem.64.1.279-286.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Nolan SJ, Fu MS, Coppens I, Casadevall A. Lipids affect the Cryptococcus neoformans-macrophage interaction and promote nonlytic exocytosis. Infect Immun. 2017;85(12):pii: e00564-17. doi: 10.1128/IAI.00564-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Santiago-Tirado FH, Onken MD, Cooper JA, Klein RS, Doering TL. Trojan horse transit contributes to blood-brain barrier crossing of a eukaryotic pathogen. MBio. 2017;8(1):pii: e02183-16. doi: 10.1128/mBio.02183-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Alvarez M, Casadevall A. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr Biol. 2006;16(21):2161–2165. doi: 10.1016/j.cub.2006.09.061. [DOI] [PubMed] [Google Scholar]
- 224.Ma H, Croudace JE, Lammas DA, May RC. Expulsion of live pathogenic yeast by macrophages. Curr Biol. 2006;16(21):2156–2160. doi: 10.1016/j.cub.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 225.Johnston SA, May RC. The human fungal pathogen Cryptococcus neoformans escapes macrophages by a phagosome emptying mechanism that is inhibited by Arp2/3 complex-mediated actin polymerisation. PLoS Pathog. 2010;6(8):e1001041. doi: 10.1371/journal.ppat.1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chayakulkeeree M, Johnston SA, Oei JB, Lev S, Williamson PR, Wilson CF, Zuo X, Leal AL, Vainstein MH, Meyer W, Sorrell TC, May RC, Djordjevic JT. SEC14 is a specific requirement for secretion of phospholipase B1 and pathogenicity of Cryptococcus neoformans. Mol Microbiol. 2011;80(4):1088–1101. doi: 10.1111/j.1365-2958.2011.07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Nicola AM, Robertson EJ, Albuquerque P, Derengowski Lda S, Casadevall A. Nonlytic exocytosis of Cryptococcus neoformans from macrophages occurs in vivo and is influenced by phagosomal pH. MBio. 2011;2(4):pii: e00167-11. doi: 10.1128/mBio.00167-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Gilbert AS, Seoane PI, Sephton-Clark P, Bojarczuk A, Hotham R, Giurisato E, Sarhan AR, Hillen A, Velde GV, Gray NS, Alessi DR, Cunningham DL, Tournier C, Johnston SA, May RC. Vomocytosis of live pathogens from macrophages is regulated by the atypical MAP kinase ERK5. Sci Adv. 2017;3(8):e1700898. doi: 10.1126/sciadv.1700898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hagedorn M, Rohde KH, Russell DG, Soldati T. Infection by tubercular mycobacteria is spread by nonlytic ejection from their amoeba hosts. Science. 2009;323(5922):1729–1733. doi: 10.1126/science.1169381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Smith J, Manoranjan J, Pan M, Bohsali A, Xu J, Liu J, McDonald KL, Szyk A, LaRonde-LeBlanc N, Gao LY. Evidence for pore formation in host cell membranes by ESX-1-secreted ESAT-6 and its role in Mycobacterium marinum escape from the vacuole. Infect Immun. 2008;76(12):5478–5487. doi: 10.1128/IAI.00614-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gerstenmaier L, Pilla R, Herrmann L, Herrmann H, Prado M, Villafano GJ, Kolonko M, Reimer R, Soldati T, King JS, Hagedorn M. The autophagic machinery ensures nonlytic transmission of mycobacteria. Proc Natl Acad Sci U S A. 2015;112(7):E687–E692. doi: 10.1073/pnas.1423318112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bijker EM, Borrmann S, Kappe SH, Mordmüller B, Sack BK, Khan SM. Novel approaches to whole sporozoite vaccination against malaria. Vaccine. 2015;33(52):7462–7468. doi: 10.1016/j.vaccine.2015.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kreutzfeld O, Müller K, Matuschewski K. Engineering of genetically arrested parasites (GAPs) for a precision malaria vaccine. Front Cell Infect Microbiol. 2017;7:198. doi: 10.3389/fcimb.2017.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Singer M, Frischknecht F. Time for genome editing: next-generation attenuated malaria parasites. Trends Parasitol. 2017;33(3):202–213. doi: 10.1016/j.pt.2016.09.012. [DOI] [PubMed] [Google Scholar]