Abstract
In a large-scale radiation event, thousands may be exposed to unknown amounts of radiation, some of which may be life-threatening without immediate attention. In such situations, a method to quickly and reliably estimate dose would help medical responders triage victims to receive life-saving care. We developed such a method using electron paramagnetic resonance (EPR) to make in vivo measurements of the maxillary incisors. This report provides evidence that the use of in vitro studies can provide data that are fully representative of the measurements made in vivo. This is necessary because, in order to systematically test and improve the reliability and accuracy of the dose estimates made with our EPR dosimetry system, it is important to conduct controlled studies in vitro using irradiated human teeth. Therefore, it is imperative to validate whether our in vitro models adequately simulate the measurements made in vivo, which are intended to help guide decisions on triage after a radiation event.
Using a healthy volunteer with a dentition gap that allows using a partial denture, human teeth were serially irradiated in vitro and then, using a partial denture, placed in the volunteer’s mouth for measurements. We compared dose estimates made using in vivo measurements made in the volunteer’s mouth to measurements made on the same teeth in our complex mouth model that simulates electromagnetic and anatomic properties of the mouth. Our results demonstrate that this mouth model can be used in in vitro studies to develop the system because these measurements appropriately model in vivo conditions.
Keywords: biodosimetry, triage, electron paramagnetic resonance, tooth dosimetry, mouth model
1. Introduction
Unexpected and uncontrolled large-scale radiation events such as nuclear reactor accidents, the detonation of an improvised nuclear device, or terrorist attacks using nuclear weapons in large urban areas could result in thousands of people being potentially exposed to unknown levels of ionizing radiation, only some of whom will have received life-threatening doses requiring immediate treatment (Coleman et al 2009). Two gray (2 Gy) is a generally accepted threshold dose to identify those individuals whose level of exposure is sufficiently predictive of acute radiation syndrome to warrant medical intervention (Grace et al 2010, Sullivan et al 2013). Particularly in large-scale events where the healthcare system will itself be potentially compromised by the event and in any case will be unable to treat such a large number of victims, it is very important to have methods to quickly identify and differentiate those whose dose warrants immediate lifesaving treatment so that a triage system can direct limited resources to them rather than to those whose treatment can be delayed or foregone (Alexander et al 2007, Ainsbury et al 2011, Coleman and Koerner 2016). Nevertheless, at present there are very few approved methods available for use in such events, and even these have had little or no evaluation within the context of triaging hundreds of thousands of people with compromised healthcare and other systems (Flood et al 2014, Flood et al 2016a).
Dartmouth has developed in vivo electron paramagnetic resonance (EPR) tooth dosimetry to help meet this need (Swartz et al 2012, Williams et al 2014, Flood et al 2016b). The physical process by which tooth enamel reacts to ionizing radiation such that it is detectable by EPR is well known. Briefly, radiation generates stable carbonate anion radicals within the hydroxyapatite matrix of tooth enamel (Callens et al 1998) and the relative density of these radicals can be measured using EPR and related to absorbed dose via an empirical calibration.
The reaction is virtually instantaneous, and very stable. The stability of carbonate radicals, especially the CO2− radical, ranges from 108 to 1011 years (Skinner et al 2000) and this stability has become a foundation of EPR tooth dosimetry (Ikeya 1993, Fattibene and Callens 2010). This stability makes it suitable for long term retrospective dosimetry of survivors of radiation events. For example, the estimated dose of individual Hiroshima atomic-bomb survivors using in vitro EPR tooth dosimetry on extracted teeth, even a half a century after the event, was almost the same as that from conventional translocation data using lymphocytes (Nakamura et al 1998). More recently, tooth biodosimetry using in vivo EPR with low frequency (1.2 GHz) (Miyake et al 2000) allows measuring radiation-induced signals (RIS) in vivo and noninvasively, i.e. without requiring tooth extraction or biopsy.
In addition, the RIS in exposed teeth becomes stable within a few hours after exposure, i.e. prior to the time-frame that emergency triage would likely be carried out, making in vivo EPR tooth dosimetry well-suited for immediate assessment of individuals with unknown exposure associated with large scale events (Williams et al 2014). As previously reported (Flood et al 2016b), in order to make the in vivo EPR tooth dosimetry system suitable for use in the field during triage, the technology must also satisfy several practical, logistical requirements including: is easy to transport to provide point-of-care assessments; is simple to use, so that non-experts can operate the system after only a brief (~15 min) training; and provides accurate and timely information to support a triage decision, i.e. with a discrimination threshold of 2 Gy, made in vivo within 5 min of measurement time, and with a precision of 0.5 Gy.
In the process of developing and improving the instrument and measurement techniques to obtain reliable and accurate dose estimates, it is important to conduct systematic and controlled studies of irradiated teeth under conditions that accurately simulate the conditions expected in measurements in vivo in the mouth. Since it is not possible to irradiate volunteers for purposes of testing our dosimeter, it is necessary to develop suitable alternatives. One alternative, also used but not detailed here, is to use computer simulations using finite element methods to inform our technological developments.
We use three complementary approaches to obtain experimental results in measuring dose in human teeth: (1) in vivo measurements in patients whose teeth are irradiated as part of radiation therapy (Williams et al 2014, Bahar et al 2015); (2) in vitro measurements of teeth placed in mouth models; as reported elsewhere, we developed a variety types of mouth models that differ in the degree to which they try to simulate the human oral environment (Kobayashi et al 2016); and (3) as reported here, a hybrid between these two approaches that measures extracted teeth in vivo, using a tooth placed in a partial denture, which is positioned in a suitable gap in the mouth of a volunteer.
Each approach has strengths and limitations. Briefly, in the first approach, the use of patients who are undergoing radiation therapy involving their natural teeth offers many advantages for simulating the whole body exposures of victims of radiation events. However, it usually involves using very ill volunteers, sometimes with very sensitive tissues in the mouth, in whom the dose rate, the number and amount of each fraction used to deliver the prescribed dose, and total dose prescribed, depend on the treatment planned for the patient. Such treatments are fairly rarely done at a given medical center, thereby limiting the number and variation of volunteers, as well as narrowly restricting the doses that can be examined to those doses and rates as prescribed by their doctor.
Regarding the second approach, the use of in vitro models enables carrying out systematic and controlled studies of dose-response, e.g. by serial irradiation of the teeth to known doses and at amounts that are pertinent to our intended use. It also facilitates studying the effects of anatomical variations and microwave properties of the mouth. However, these models depend upon our having correctly identified all of the electromagnetic and anatomical conditions that may impact the measurements.
1.1. Study design: to compare the measurements in vivo with teeth irradiated in vitro
The present article reports on the third approach: using a replaceable extracted tooth that can be placed in a volunteer whose mouth has a gap due to a missing incisor, thereby allowing measurement of the same teeth both in vitro and inside a human mouth. This makes it feasible to directly determine the relationship between the in vitro models and measurements in the human mouth comparing dose response curves.
Figure 1 shows the three types of systems in which the measurements were made: The simple mouth model (a single tooth secured in putty) (Figure 1a), the more complex model that includes neighboring teeth and secured in a rigid epoxy ‘mouth’ structure with lossy material added to simulate the tongue and lips (Figure 1b), and the technique for measuring extracted teeth in vivo (Figure 1c), i.e. in an in vivo surrogate model. (Note: we use the term ‘in vivo surrogate model’ because, unlike the situation in which the in vivo measurements would be made in the event of a real incident, the irradiation occurred in vitro and the rest of the teeth were not irradiated.)
Figure 1.
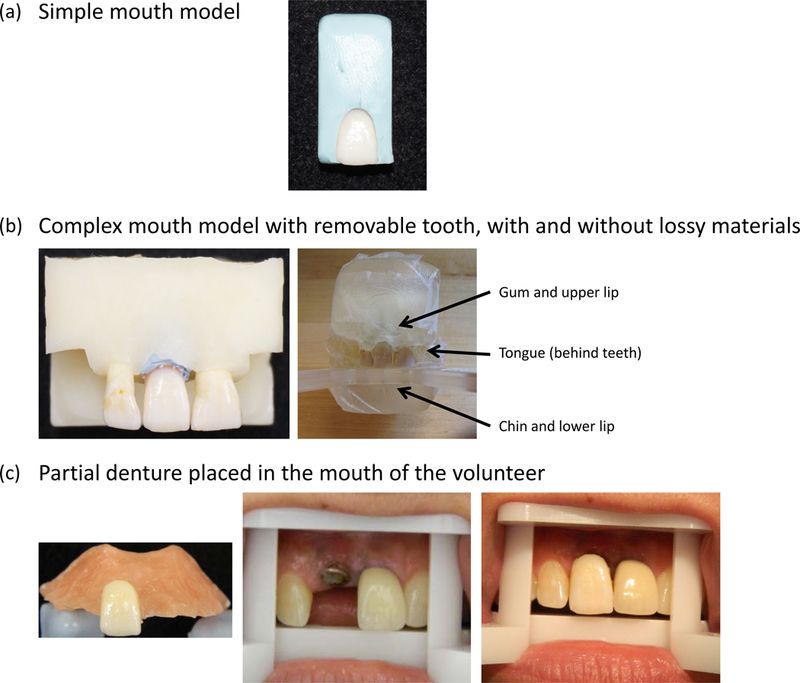
Three measurement conditions for the five experimental teeth: (a) in a simple mouth model (embedded in blue putty); (b) in a complex mouth model with anatomically correct positioning of teeth in an epoxy frame (b, left) and with lossy materials added to simulate gum, tongue, and chin (b, right); (c) in vivo using a partial denture to hold the experimental tooth (c, left); volunteer’s gap (with titanium implant), shown positioned on a ‘bite block’ to rest the mouth in the magnet and expose the tooth during in vivo EPR tooth measurements (c, center); experimental tooth in denture that is placed in volunteer’s mouth, ready to make in vivo EPR tooth measurements (c, right).
To prepare to use the in vivo surrogate model in EPR measurements, we selected five extracted maxillary incisors based on meeting our standard criteria for healthy incisors and for closely matching the size of the volunteer’s gap and otherwise having very similar properties. Figure 2 illustrates the basic steps to obtain the measurements.
Figure 2.

EPR measurements in the primary study plan: measurements are made on five teeth in the simple and complex mouth models and in vivo in the volunteer’s mouth.
These comparisons of the surrogate in vivo measurements to the simple and complex models are reported as the primary results. The series of added doses (0, 1, 2, 4, 6, and 10 Gy) was chosen for the primary study because it encompasses the range of greatest interest for purposes of using biodosimetry for triage, including the standard cutoff of 2 Gy for initiating medical treatment following incidental exposures.
Secondary but important for the validity of the comparisons of the in vivo surrogate to the mouth models, we needed to ensure that the lossy material used in the complex mouth model would remain stable throughout the days or weeks needed to complete studies. Results from these preliminary investigations regarding physical stability as well as suitability of electromagnetic properties, necessary for validating our method, are also briefly reported.
2. Material and methods
2.1. Materials
Gelatin, from porcine skin type A, and ethylene glycol were obtained from Sigma-Aldrich (St. Louis, MO, USA). Sodium chloride (NaCl) was purchased from Fisher Scientific (Pittsburgh, PA, USA). Henry Schein® self-curing chairside reline material was purchased from Henry Schein (Melville, NY, USA). The dental die stone was purchased from Jade Stone (Whip Mix Corp, Louisville, KY, USA). Vinyl polysiloxane impression material (Exaflex® Putty) was purchased from GC America Inc. (Alsip, IL, USA). Fast-Setting Non-Sag 2 (Double/Bubble® Epoxy) were purchased from Hardman®, Royal Adhesives and Sealants, LLC (South Bend, IN, USA). The alginate impression material (Kromatica) was purchased from Matech, Inc. (Sylmar, CA, USA).
2.2. Tissue equivalent lossy material
A mixture of ethylene glycol, ultrapure water, NaCl and gelatin was used as a tissue equivalent lossy material (Robinson et al 1991, Lazebnik et al 2005, Ibrahim et al 2008) to simulate electromagnetic properties of the oral cavity and their impact on the radio frequency (RF) and quality factor (Q factor) of the EPR resonators used in this study. (Note: the frequency used by our resonator is 1.15 GHz. While this is technically within the microwave range, we use the more general term RF (‘radio frequency’) to describe the resonant frequency of the electromagnetic waves.) This lossy material was wrapped with plastic wrap and installed on the mouth models described below. Standard methods for short and long-term storage of the lossy materials between measurement sessions and for optimizing the ingredients to simulate the electromagnetic properties of the human mouth were developed after performing several preliminary studies as described below. Briefly, the lossy materials are preserved in a plastic bag and refrigerated at 15 °C for short experiments (<14 d) and use a 50% concentration of water, based on the evidence of its being the best simulator of in vivo Q factor (data not shown).
2.3. Fabrication of the mouth models
All teeth used in this study were natural, healthy, whole human teeth. Most were obtained from cadaver donations, made available through the National Disease Research Interchange (Philadelphia, PA, USA) or Science Care (Phoenix, AZ, USA).
As shown in Figure 1, two types of mouth models were used for the EPR studies. The simple mouth model (Figure 1a) consisted of using a maxillary incisor held in position for EPR measurements using a non-paramagnetic putty shaped to fit in a non-paramagnetic plastic holder that could be placed on a platform, to allow accurate positioning in the magnet for EPR measurements. Figure 1b illustrates one of the complex mouth models using three maxillary incisors, designed to simulate anatomical features of the upper jaw and electromagnetic conditions in the mouth. A rigid structure that holds the incisors in a natural anatomical position was used. This structure could be affixed to a platform that both stabilizes the mouth model during measurements and allows the mouth model to be positioned in the center of the magnet for EPR measurements (Kobayashi et al 2016).
In the complex mouth model used in the primary studies reported here, two maxillary incisors were embedded in the epoxy as described above; see Figure 1b. Because we wanted to measure each study tooth at no added radiation dose (0 Gy) and after adding 1, 2, 4, 6 or 10 Gy, we needed to be able to insert and remove the tooth from a gap of the mouth model (simulating the gap in the human volunteer). Therefore, the gap in the mouth model was designed to accommodate the width of the experimental teeth to be used in the volunteer. This mouth model was also designed to allow the placement of the lossy material that simulated the electromagnetic conditions of the oral cavity.
Two versions of the complex mouth model were used in the preliminary studies, depending on the type of test. The electromagnetic properties (RF and Q factor) were tested with the resonator placed on the maxillary incisor surface in a model with 12 teeth, using a network analyzer (HP 8753A Network Analyzer, Hewlett-Packard). Second, storage conditions were tested in a complex model with 4 teeth, which were mounted to a plastic disposable articulator (W.O.W. Full Arch Articulator, Premier® Dental Products, a division of Premier Products Co., Plymouth Meeting, PA, USA) with an oral cavity cover (Kilgore International Inc., Coldwater, MI, USA) to simulate the anatomy of an enclosed mouth.
2.4. Preparing the dentures
We recruited a subject with a missing maxillary incisor tooth (‘gap’) who gave informed consent to participate in EPR measurements of experimental teeth that would be inserted one at a time into the gap via custom-made dentures. Briefly, a local dentist had extracted the volunteer’s right maxillary central incisor tooth due to a periapical lesion. The dentist’s care plan was to perform dental implant surgery then restore the missing tooth with a permanent artificial tooth after allowing approximately three to four months for the tissue to heal. During healing, in order to protect the gap and prevent the surrounding teeth from shifting into the gap, the volunteer was to use a temporary denture.
With the subject’s and dentist’s permission, we borrowed the volunteer’s dental cast surrounding the gap in order to prepare five identical partial dentures. An alginate impression of the dental cast was taken and dental stone was poured into the impression to fabricate the study cast. Then self-curing chairside reline material was poured onto the hard palate portion of the cast to form a denture around one of the experimental teeth (Figure 1c). Five dentures were manufactured, one for each of the five experimental teeth. Each experimental tooth was a healthy maxillary central incisor, selected to have a width that would fit tightly but comfortably into the subject’s gap and be approximately the same height as the neighboring central incisor. Because the tooth embedded in the denture needed to be placed on top of the volunteer’s gums, we removed the root before placing the tooth in the denture.
2.5. EPR dosimetry instrumentation
The EPR instrument was a prototype of an instrument capable of being deployed to a site near a large-scale radiation disaster to perform rapid, noninvasive in vivo tooth biodosimetry for medical triage decision-making. The instrument, developed at Dartmouth, was a combination of commercial and custom-built components. In this study, all in vitro and in vivo EPR dosimetry measurements were taken using continuous-wave EPR with an operating frequency of approximately 1.15 GHz (L-band) and an associated magnetic field strength of 41 mT. See (Flood et al 2016b) for details regarding how we place and stabilize humans for making rapid and effective in vivo measurements. Briefly, each volunteer uses a sterile ‘bite block’ fastened in place in the middle of the magnet; the bite block allows comfortable exposure of the subject’s upper incisors and their rapid placement in the magnet, while a head strap and forehead-rest gently holds the head stable during the 5 min measurements. This system, developed and tested for use during the intended point-of-care in vivo measurements, was used for the volunteer in this study. It is also easily adapted for holding the mouth models for comparable measurements as we have done for this study.
Dartmouth also developed a surface loop resonant coil, i.e. a resonator suitable for in vivo tooth measurements (Williams et al 2011a, Williams et al 2014). These resonators are of conventional design, but the detection loops were tailored specifically for dosimetric measurements of adult maxillary incisor teeth (Salikhov et al 2003, Walczak et al 2005, Williams et al 2011a). The detection loops were constructed of high-purity silver wire, with a wire thickness of 1.0 mm and loop diameter of 9.0 mm.
The resonator was centered on the labial surface of the tooth by a suitably trained operator using a non-magnetic and lockable articulating arm (MJR Medical Supply Inc., Huntington, NY, USA). A spring-loading mechanism was incorporated to ensure consistent pressure of the loop against the tooth throughout the measurement, to minimize the possibility of changing positions on the tooth due to subject movements during measurements (Williams et al 2011a).
Prior to starting a day’s acquisition of experimental data, a control standard consisting of a sample without any irradiation signal was measured to check for instrumental problems such as high background signal. The sample was a white Delrin® Acetal Resin Ball (McMaster-Carr, Princeton, NJ, USA).
In the primary in vivo study reported here, operators followed the standard methods for performing experimental EPR measurements on teeth. Data for a given measurement were acquired in multiple sets, where each set was comprised of 20 3 s scans (i.e. 1 min/set); the resonator was repositioned between sets. A total of 18 sets was taken for in vitro conditions and 5 sets for in vivo conditions. A reference standard, containing 15N-perdeuterated tempone (15N-PDT) (Sigma-Aldrich, St Louis, MO, USA), was fixed to the resonator near the loop and measured simultaneously with each scan, providing direct verification that the proper instrumental parameters were used and that data acquisition performance was within tolerances (Williams et al 2011a, Williams et al 2011b). RIS amplitudes were then estimated using spectral fitting (Williams et al 2014).
2.6. Statistical analysis
Statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Inc., Cary, NC, USA). In the preliminary studies to evaluate the physical properties of lossy material and electromagnetic properties of the prototype of complex mouth model, generalized linear models were used to assess stability over time (Figures 3 and 4). A generalized linear mixed model with repeated measurements (using SAS Proc MIXED) was used to assess the effect of the change in temperature on electromagnetic properties (Figure 5). Student’s t-tests were used to compare EPR signal amplitudes on the controls, materials, and tooth (Figure 6). For the main study, a regression line was fitted to the plots obtained from the dose response study (Figure 7). A p value <0.05 was considered to be statistically significant.
Figure 3.
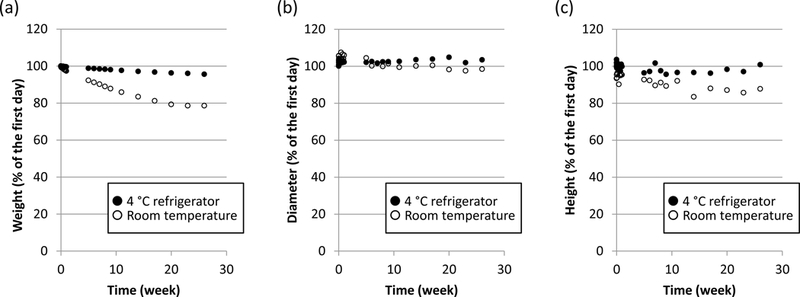
Comparing three physical changes in (a) weight, (b) diameter and (c) height (expressed as % change from day 1) of lossy materials stored in two conditions for 26 weeks. Legend: black circle = stored in 4 °C refrigerator; white circle = stored at room temperature; (lossy material was stored in a plastic bag in both conditions).
Figure 4.
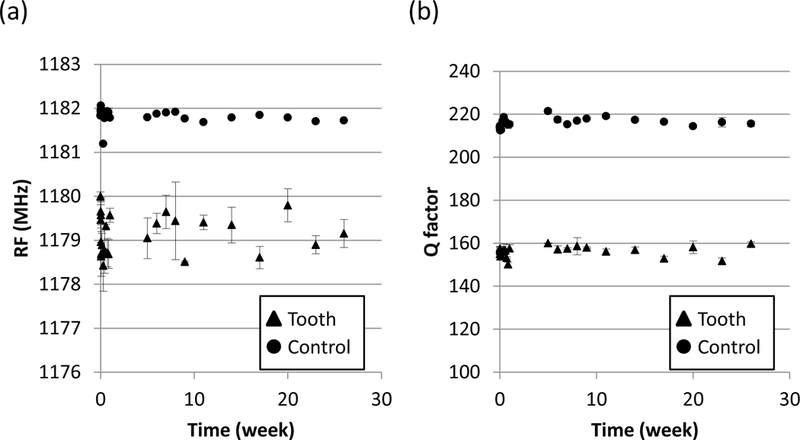
Time trends over 26 weeks of electromagnetic properties when lossy material was placed on a mouth model (mean ± SD, n = 3): (a) RF and (b) Q factor. Legend: circle = control (measurements made with no sample when the resonator was held in free space); note: SD is so small that it is not visible in the figure; triangle = tooth (measurements made on tooth embedded in a complex mouth model with lossy material in place).
Figure 5.
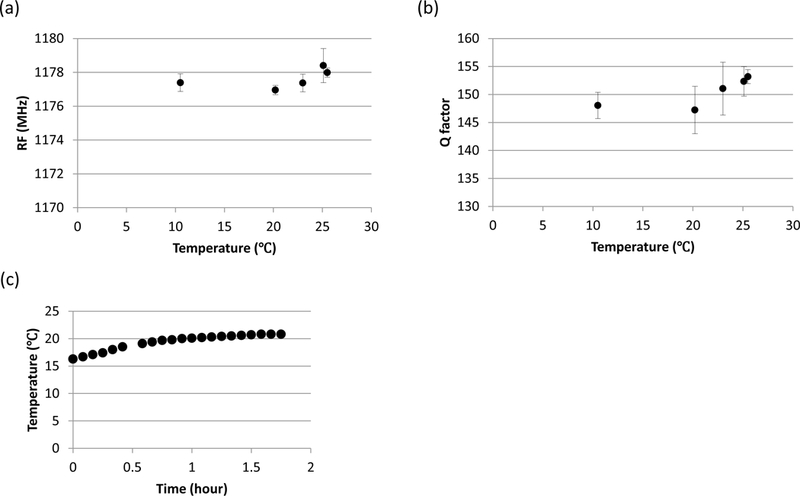
Temperature changes associated with warming lossy materials and their effects on EPR measurements (RF and Q factor). (a) The typical effects of change in temperature from ~10 °C to 25 °C on RF. Triplicate measurements were performed every hour, for the first 5 h after removing from the refrigerated storage (Shown is mean ± SD for measurements taken on same day; not shown: the test was repeated on two additional days with similar results.). (b) Same, on Q factor. (c) Hour to warm from 15 °C to room temperature (on 1 d).
Figure 6.

Testing whether dental reline material used in denture and artificial roots impacts the EPR signal amplitude (displayed in arbitrary voltage units). (a) Comparing 6 EPR measurements made on dental material 50 min and 16 h after preparation, to the resin ball as a control standard. (b) Comparing 18 EPR measurements made on each unirradiated experimental tooth with natural (‘real’) root versus artificial (‘not’) root. Mean ± SD is shown; Student’s t-test was used; NS = not significant.
Figure 7.

Comparing dose response curves of experimental teeth measured in simple and complex mouth models and in vivo in a volunteer using a denture. Y axis is the measured EPR amplitude in arbitrary units; X axis is the known added dose to each tooth, using a cesium irradiator. Mean ± SD is displayed for n = 18 measurements per tooth in each mouth model and n = 4–5 for each tooth measured in vivo in volunteer. Slope and intercept of the dose response curves were not significantly different between the complex mouth model and volunteer (p = 0.8498 and p = 0.5218, slope and intercept, respectively); in contrast, the slope and intercept between the simple mouth model and the volunteer were statistically significant at p < 0.001.
3. Preliminary results: tests to ensure the integrity of the materials and procedures in making the complex mouth model and denture for in vivo measurements
3.1. Preliminary study 1: physical stability of lossy material depends on storage temperature
As shown in Figure 3, the physical properties (weight, diameter, and height) of a cylinder of lossy material changed relative to their initial condition for both temperature storage conditions when stored over 26 weeks. The properties were more stable when the lossy material was refrigerated at 4 °C compared to room temperature (p < 0.0001 for weight and diameter and p = 0.0003 for height) and therefore 4 °C was used as the standard for long-term storage.
3.2. Preliminary study 2: stability of electromagnetic properties associated with lossy material
To determine if the EPR measurements made on a complex mouth model might change over 26 weeks due to physical changes in the lossy material, we measured the stability of the RF and Q factor. The lossy material was installed in a complex mouth model and preserved in a plastic bag and refrigerated at 4 °C between measurements. The measurements were made by placing the resonator on a maxillary incisor tooth of the complex mouth model and using a network analyzer to assess RF and Q factor. As a control, measurements on the network analyzer were made on the same day with the resonator held in free space.
Figure 4 displays these measurements (mean ± SD, n = 3) for RF and Q factor. As expected, the control measurements were higher than measurements made in the presence of lossy material. In addition, the measurements made in the control setting appear to be less variable. This is mostly due to an artifact of the measurement technique, i.e. while measurements of the tooth required replacement of the loop against the tooth’s surface, which was not always exact, the loop was held in free space in the control setting. Important for this preliminary study, no time trends in RF and Q factor measurements made on the tooth or in the control setting were significant, i.e. neither RF (p = 0.79 and 0.26, tooth and control, respectively) nor Q factor (p = 0.56 and 0.187, tooth and control, respectively) changed over time. These results provide evidence that the electromagnetic properties of the lossy material in a complex mouth model can be assumed to be stable in an EPR study lasting for up to six months.
3.3. Preliminary study 3: the implications of warming the lossy material to room themperature
Even though we decided that it is desirable to refrigerate the complex mouth model between EPR measurements for a given study to preserve its physical properties, EPR measurements of the mouth models are best performed at room temperature. Therefore, we carried out a preliminary study to determine how quickly the lossy material could warm and whether the changes in temperature of the lossy material that occurred when moving between refrigeration and room temperature affected the electromagnetic properties.
Temperature of the complex mouth model was measured under the ‘tongue’, i.e. the thickest part that would most likely be the last area to warm up. It was observed that starting from refrigerated temperature to room temperature took approximately 5 h to reach equilibrium (data not shown). Since it would be inconvenient to wait several hours before using the mouth model for EPR measurements, it is important to be able to shorten the warm-up time (either by warming it quickly, starting measurements before reaching room temperature, or by storing at warmer temperatures for shorter experiments). We therefore investigated the temperature dependence of the EPR measurements in the complex mouth models to assess how critical it is to reach equilibrium.
Figures 5a and 5b display the typical result of RF and Q factor measurements (mean ± SD, n = 3) when the loop was placed on the tooth of the complex mouth model as the lossy material warmed up between ~10 and 25 °C. The measurements were repeated for 3 different days (data not shown). In this study, there were no significant effects of temperature on RF (p = 0.71) or the Q factor (p = 0.74) as the temperature warmed from 10 to 25 °C.
Figure 5c shows that, when stored at 15 °C, the lossy materials reached approximately 20 °C (room temperature) by ~45 min. Because the primary study took place over less than a week, these results together provided evidence that EPR measurement would not be affected by storing the complex mouth models at 15 °C and using wait times of less than 1 h before initiating EPR measurements.
3.4. Preliminary study 4: confirming the material used for the dentures and the artificial roots does not have an intrinisic EPR signal that could affect the measurements
Prior to fabrication of the denture or artificial roots, the effect of self-curing chairside reline material was prepared and measured by EPR after curing for 50 min or 16 h compared to the resin ball as control standard. As shown in Figure 6a, there were no significant differences in the EPR signals collected after curing for 50 min (p = 0.0667) or 16 h (p = 0.7851) compared to the control.
We also investigated the effect of self-curing chairside reline material on the tooth EPR signal when the artificial root was installed; see Figure 2, stage 1 and 2, for a photo of a tooth with natural and artificial roots respectively. Figure 6b compares the mean EPR signal intensity for each experimental tooth in two conditions: with its natural root and with its artificial root. There were no statistically significant differences, based on the p value of Student’s t-tests performed on each tooth. These findings provide that using this denture material in our mouth models would not have any significant impact on the EPR measurements of the teeth.
4. Primary study to compare EPR measurements made on irradiated teeth in a simple and a complex mouth model to those made in vivo in a volunteer with a denture to hold the same teeth: results and discussion
Figure 7 compares the dose response curves, i.e. between the measured EPR amplitude of each tooth relative to its known added dose, for each of the three measurement conditions, i.e. in both the simple and complex mouth models and in the volunteer’s dentition gap. The first result to note is, as expected, the dose response relationship between the measured EPR amplitude and the known dose added to the tooth is linear for all three measurement conditions.
The linearity of this relationship across both types of mouth models and the in vivo surrogate model is the basis for being able to create a calibration curve to convert EPR measurements made in a person’s teeth to estimates of his or her exposure to significant levels of radiation. The linear relationship to dose as assessed in vivo is fundamentally important for EPR to be useful for biodosimetry in radiation accidents or malicious events involving inadvertent exposure of people to ionizing radiation.
Furthermore, especially because of the need to conduct in vitro tests of the EPR instrument (since people cannot volunteer to receive life threatening levels of radiation), it is also necessary to develop in vitro models with properties that closely simulate in vivo conditions to test and improve the EPR biodosimetry technique. Therefore, it is important to determine how closely the measurements correspond across the three types of models (the simple and complex mouth models and the in vivo surrogate model), where the latter is used as the ‘gold standard’ for estimating the effect of including the electromagnetic properties of in vivo measurements. Thus, to examine the validity of our mouth models, we turn next to compare the intercepts and slopes of the regression lines of each mouth model to those of the in vivo surrogate.
The second result to note in Figure 7 involves comparing the slopes and intercepts of the dose response curves (regression) made on exactly the same teeth, irradiated to known added doses, in the three models. The slope and intercept assessed in a simple mouth model differed statistically significantly from those assessed in the human mouth (p < 0.001 for slope and intercept). In contrast, the slope and intercept of these teeth when measured in a complex mouth model cannot be distinguished from those made in the human mouth (p = 0.8498 and p = 0.5218, slope and intercept, respectively).
This result is not unexpected, in that the simple mouth model (a single tooth mounted in a non-paramagnetic putty) is known not to have any features that reflect the electromagnetic properties of the human mouth, while the lossy materials in the complex mouth model were deliberately developed to simulate the human oral conditions in regard to the electromagnetic properties, i.e. the RF and Q factor of the human mouth. In addition, beyond needing to establish the equivalence that can occur at the time of fabricating the lossy materials, it is important to establish the stability of the lossy material over time and to optimize the storage conditions so that studies to improve and test the EPR conditions can be carried out in complex mouth models over time, without deterioration of models themselves. In the current study, we succeeded in establishing most the equivalence of our complex model to in vivo measurements but also the stability of the model over the course of long-term testing.
Note that the lossy materials were developed and tested independently of the volunteer used in the in vivo surrogate study, i.e. we did not try to fabricate the mouth model with the lossy materials having the same RF or Q factor to match this particular volunteer’s mouth; instead we optimized the Q factor based on the average human data (data not shown) before making our EPR measurements of the teeth. Therefore, the results in Figure 7 are an indication of how well the complex mouth model generally simulates the human mouth in regard to the electromagnetic properties important to EPR spectroscopy. Hence, showing that the measurements made on the same teeth in the complex mouth model were indistinguishable from those made in the human mouth is an important validation of this in vitro model.
In addition, the novel use of a volunteer with a dentition gap allowed us to test the dose response of teeth measured in vivo across a series of doses. Because these doses are potentially lethal if the volunteer had been exposed directly, this allowed us to safely and ethically test the in vivo situation in a healthy volunteer.
5. Overall conclusions
In conclusion, we have demonstrated that our measurements in an appropriate in vitro model provide information that is consistent with that found from irradiated teeth measured in vivo. The versatility of the in vitro complex mouth model for simulating conditions in the human mouth and its suitability for extensive studies that cannot readily be made in human subjects (e.g. systematic consideration of factors such as age, race, and gender) indicate that this mouth model can be used as an effective pathway for the full development of in vivo EPR tooth dosimetry.
Acknowledgments
This research was supported by awards from the US Department of Health and Human Services: (1) grant U19-AI091173 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) and (2) contract HHSO100201100024C with the Biomedical Advanced Research and Development Authority (BARDA), Office of the Assistant Secretary for Preparedness and Response. We gratefully acknowledge the technical help of Ankit Gupta, Eugene Demidenko, Holly Boyle, Jean P. Lariviere, Kevin Rychert, Maciej Kmiec, Matthew Feldman, Sergey V. Petryakov, Shireen Geimer, Tim Raynolds, and Wilson Schreiber.
Footnotes
Disclosure: ABF and HMS are co-owners of Clin-EPR, LLC of Lyme, NH. This company manufactures and sells LiPc and EPR spectroscopy for in vivo clinical applications for investigational use only.
References
- Ainsbury EA et al. 2011. Review of retrospective dosimetry techniques for external ionizing radiation exposures Radiat. Prot. Dosim 147 573–92 [DOI] [PubMed] [Google Scholar]
- Alexander GA et al. 2007. BiodosEPR-2006 Meeting: acute dosimetry consensus committee recommendations on biodosimetry applications in events involving uses of radiation by terrorists and radiation accidents Radiat. Meas 42 972–96 [Google Scholar]
- Bahar N et al. 2015. SU-C-BRD-05: non-invasive in vivo biodosimetry in radiotherapy patients using electron paramagnetic resonance (EPR) spectroscopy Med. Phys 42 3192–3 [Google Scholar]
- Callens F, Vanhaelewyn G, Matthys P and Boesman E 1998. EPR of carbonate derived radicals: applications in dosimetry, dating and detection of irradiated food Appl. Magn. Reson 14 235–54 [Google Scholar]
- Coleman CN, Hrdina C, Bader JL, Norwood A, Hayhurst R, Forsha J, Yeskey K and Knebel A 2009. Medical response to a radiologic/nuclear event: integrated plan from the Office of the Assistant Secretary for Preparedness and Response, Department of Health and Human Services Ann. Emerg. Med 53 213–22 [DOI] [PubMed] [Google Scholar]
- Coleman CN and Koerner JF 2016. Biodosimetry: medicine, science, and systems to support the medical decision-maker following a large scale nuclear or radiation incident Radiat. Prot. Dosim 172 38–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattibene P and Callens F 2010. EPR dosimetry with tooth enamel: a review Appl. Radiat. Isot 68 2033–116 [DOI] [PubMed] [Google Scholar]
- Flood AB, Boyle HK, Du G, Demidenko E, Nicolalde RJ, Williams BB and Swartz HM 2014. Advances in a framework to compare bio-dosimetry methods for triage in large-scale radiation events Radiat. Prot. Dosim 159 77–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood AB, Ali AN, Boyle HK, Du G, Satinsky VA, Swarts SG, Williams BB, Demidenko E, Schreiber W and Swartz HM 2016a. Evaluating the special needs of the military for radiation biodosimetry for tactical warfare against deployed troops: comparing military to civilian needs for biodosimetry methods Health Phys 111 169–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood AB, Williams BB, Schreiber W, Du G, Wood V A, Kmiec MM, Petryakov SV, Demidenko E and Swartz HM 2016b. Advances in in vivo EPR tooth biodosimetry: meeting the targets for initial triage following a large-scale radiation event Radiat. Prot. Dosim 172 72–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace MB, Moyer BR, Prasher J, Cliffer KD, Ramakrishnan N, Kaminski J, Coleman CN, Manning RG, Maidment BW and Hatchett R 2010. Rapid radiation dose assessment for radiological public health emergencies: roles of NIAID and BARDA Health Phys 98 172–8 [DOI] [PubMed] [Google Scholar]
- Ibrahim WMA, Algabroun HM and Almaqtari MT 2008. Short review on the used recipes to simulate the bio-tissue at microwave frequencies 4th Kuala Lumpur Int. Conf. on Biomedical Engineering 2008 pp 234–7 [Google Scholar]
- Ikeya M 1993. New Applications of Electron Spin Resonance: Dating, Dosimetry and Microscopy (Singapore: World Scientific; ) [Google Scholar]
- Kobayashi K, Dong R, Nicolalde RJ, Williams BB, Du G, Swartz HM and Flood AB 2016. Evolution and optimization of tooth models for testing in vivo EPR tooth dosimetry Radiat. Prot. Dosim 172 152–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazebnik M, Madsen EL, Frank GR and Hagness SC 2005. Tissue-mimicking phantom materials for narrowband and ultrawideband microwave applications Phys. Med. Biol 50 4245–58 [DOI] [PubMed] [Google Scholar]
- Miyake M, Liu KJ, Walczak TM and Swartz HM 2000. In vivo EPR dosimetry of accidental exposures to radiation: experimental results indicating the feasibility of practical use in human subjects Appl. Radiat. Isot 52 1031–8 [DOI] [PubMed] [Google Scholar]
- Nakamura N, Miyazawa C, Sawada S, Akiyama M and Awa AA 1998. A close correlation between electron spin resonance (ESR) dosimetry from tooth enamel and cytogenetic dosimetry from lymphocytes of Hiroshima atomic-bomb survivors Int. J. Radiat. Biol 73 619–27 [DOI] [PubMed] [Google Scholar]
- Robinson MP, Richardson MJ, Green JL and Preece AW 1991. New materials for dielectric simulation of tissues Phys. Med. Biol 36 1565–71 [DOI] [PubMed] [Google Scholar]
- Salikhov I, Hirata H, Walczak T and Swartz HM 2003. An improved external loop resonator for in vivo L-band EPR spectroscopy J. Magn. Reson 164 54–9 [DOI] [PubMed] [Google Scholar]
- Skinner AR, Blackwell BA, Chasteen ND, Shao J and Min SS 2000. Improvements in dating tooth enamel by ESR Appl. Radiat. Isot 52 1337–44 [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Prasanna PG, Grace MB, Wathen LK, Wallace RL, Koerner JF and Coleman CN 2013. Assessment of biodosimetry methods for a mass-casualty radiological incident: medical response and management considerations Health Phys 105 540–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz HM et al. 2012. Electron paramagnetic resonance dosimetry for a large-scale radiation incident Health Phys 103 255–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak T, Leśniewski P, Salikhov I, Sucheta A, Szybiński K and Swartz HM 2005. L-band electron paramagnetic resonance spectrometer for use in vivo and in studies of aqueous biological samples Rev. Sci. Instrum 76 013107 [Google Scholar]
- Williams BB et al. 2011a. A deployable in vivo EPR tooth dosimeter for triage after a radiation event involving large populations Radiat. Meas 46 772–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BB, Dong R, Nicolalde RJ, Matthews TP, Gladstone DJ, Demidenko E, Zaki BI, Salikhov IK, Lesniewski PN and Swartz HM 2011b. Physically-based biodosimetry using in vivo EPR of teeth in patients undergoing total body irradiation Int. J. Radiat. Biol 87 766–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BB, Flood AB, Salikhov I, Kobayashi K, Dong R, Rychert K, Du G, Schreiber W and Swartz H M 2014. In vivo EPR tooth dosimetry for triage after a radiation event involving large populations Radiat. Environ. Biophys 53 335–46 [DOI] [PMC free article] [PubMed] [Google Scholar]


