Abstract
There is increasing concern about the decline of pollinators worldwide. However, despite reports that pollinator declines are widespread, data are scarce and often geographically and taxonomically biased. These biases limit robust inference about any potential pollinator crisis. Non-structured and opportunistic historical specimen collection data provide the only source of historical information which can serve as a baseline for identifying pollinator declines. Specimens historically collected and preserved in museums not only provide information on where and when species were collected, but also contain other ecological information such as species interactions and morphological traits. Here, we provide a synthesis of how researchers have used historical data to identify long-term changes in biodiversity, species abundances, morphology and pollination services. Despite recent advances, we show that information on the status and trends of most pollinators is absent. We highlight opportunities and limitations to progress the assessment of pollinator declines globally. Finally, we demonstrate different approaches to analysing museum collection data using two contrasting case studies from distinct geographical regions (New Zealand and Spain) for which long-term pollinator declines have never been assessed. There is immense potential for museum specimens to play a central role in assessing the extent of the global pollination crisis.
This article is part of the theme issue ‘Biological collections for understanding biodiversity in the Anthropocene’.
Keywords: museums, biodiversity, global change, bees, syrphid flies, butterflies
1. Introduction
Animal pollinators are a critical component of both natural and agricultural ecosystems worldwide, given their role in plant reproduction [1] and food security [2]. As with many other taxa, pollinators are vulnerable to a range of anthropogenic disturbances, which can cause local and regional population declines or even extinctions. The vulnerability of pollinators was identified several decades ago and was popularized in 1996 by the influential book ‘The forgotten pollinators' [3]. However, early accounts of pollinator declines were somewhat anecdotal, given the lack of pollinator population data at that time. These initial claims triggered the first efforts to assess this potential issue and included the formation of a US National Academy of Science (NAS) panel in 2006, which was commissioned to assess the extent of pollinator declines. The NAS report concluded that ‘For most pollinator species […] the paucity of long-term population data and the incomplete knowledge of even basic taxonomy and ecology make definitive assessment of status exceedingly difficult’ [4, p. 8]. Since then, studies on pollinator responses to various global change drivers have multiplied rapidly. Researchers have now developed strong consensus that disturbances such as habitat destruction, land-use intensification, chemical exposure, exotic species and climate change are causing pollinator declines and often act synergistically [5,6]. Yet, the current status and population trends of most pollinator species worldwide remain unknown. For example, a recent IUCN report concluded that even for Europe's comparatively well-studied bee fauna, more than 55% of bee species fell into the ‘data deficient’ category [7]. For countries outside of Europe and the USA, data on pollinator populations are almost non-existent.
One of the main barriers to identifying long-term pollinator population trends is that pollinators are incredibly taxonomically diverse and include bees, flies, butterflies, beetles, birds, bats and lizards [8]. Additionally, many pollinators are highly mobile, short-lived and small in size, which make monitoring their populations difficult. Bees are generally regarded as the most important pollinator group due to their abundance, pollination efficiency and widespread distribution [9]. However, bees are diverse, with more than 20 000 species currently described worldwide [10], and often require expert taxonomists for identification. Furthermore, the uneven global distribution of research studies has resulted in geographical biases in bee decline research [11], as well as taxonomic biases towards species that are easier to identify, such as bumblebees [12,13].
One solution to overcoming these barriers is the use of space-for-time substitutions, where researchers compare pollinator populations across environmental gradients. Despite critiques on the robustness of this approach [14,15], these studies currently provide the most extensive source of pollinator population data. For example, researchers have recently estimated bee richness declines for every country in Europe using predictions from models of pollinator associations with different land-use types [16]. A second important method is the use of data collected from pollinator monitoring programmes, which are often driven by citizen scientists. This approach was inspired by successful butterfly monitoring programmes [17] and is currently being extended to other pollinator taxa. However, these programmes require significant time to generate long-term datasets and cannot be used to assess historical pollinator populations. Finally, the most practical approach for assessing long-term historical pollinator population trends is to use historical information on species occurrences, which is often archived in museum collections [18].
In this review, we first assess current evidence for pollinator richness declines and present a roadmap outlining a strategy for using historical collection data to fill current knowledge gaps. We highlight the major technical difficulties involved in using historical collection data and demonstrate several approaches for analysing different types of collection data to assess long-term pollinator population trends. Finally, we highlight the need to move beyond simple biological diversity descriptors and unleash the power of historical data to assess changes in species interactions, ecosystem functioning and evolutionary changes through time.
2. Current evidence on pollinator declines
At a global scale, current evidence of pollinator declines is highly limited with most data restricted to the USA and Europe. It is unsurprising that studies on pollinator declines are biased towards developed Western countries, which have also been subject to extensive anthropogenic disturbance. For example, in the UK and The Netherlands, a citizen science-based study using both observations and museum collection data detected strong richness declines for bees, hoverflies and flowering plants [19]. In The Netherlands, museum data have also revealed coupled plant and pollinator declines [20]. Specifically, bee species with the strongest host plant preferences (i.e. specialists) tend to display the strongest declines and thus are most threatened with extinction. However, it is important to note that even for these two countries, local estimates of pollinator richness are biased towards large cities and regions dominated by agriculture and thus lack data for areas subject to less human activity. Further exploration of this dataset revealed that for declining pollinator taxa, the declining species richness trend has attenuated in recent decades [21].
Although studies of local native pollinator communities often detect richness declines, regional native richness may remain relatively stable. For example, regional estimates for bee species richness changes in the eastern USA show moderate declines [18] and very few regional extinctions [22]. This is a pattern also detected in the UK, where relatively few regional bee extinctions have been reported [23]. These regional findings contrast with the local extinctions reported in other studies. For example, Burkle et al. [24] compared historical observations of bee species' occurrences in a large forested ecosystem with remaining forest remnants and reported several local extinctions. However, it is important to note that there is strong concordance between local extinctions and regional declines [25], suggesting that local extinctions are indicators of regional population declines.
Reported declines for bumblebees are the most severe of all pollinator taxa. For example, declines of up to 18% in local bumblebee richness have been reported for Belgium and The Netherlands [21]. In other parts of Europe, local richness declines range from 5% in Great Britain [21] to 42% in Denmark [26] and 70% of bumblebee species are classified as threatened or with declining population trends by the IUCN [7]. In the USA, reported bumble declines are also severe with estimates ranging between 25% [27] and 30% [18].
Studies on species richness changes for other pollinator taxa are both scarce and geographically restricted. For butterflies, the only evidence of richness declines comes from Europe. Butterfly species richness has declined substantially in The Netherlands and Belgium since the 1950s, although declines in Great Britain have been less severe [21]. In Belgium, another study [28] found that butterfly richness declines have been severe (approx. 30%), although this study assessed richness changes over a longer time period (early 1900s to 2000) compared with the previous study [21] (1950–1969 versus 1970–1980 and 1970–1989 versus 1990–2009). In parts of Germany, up to 70% declines in local butterfly richness have been reported [29]. Compared with other insect pollinator taxa, there are very few studies on hoverfly species richness changes, of which all are restricted to Europe. Specifically, in Belgium, Great Britain and The Netherlands, hoverfly richness changes have been modest [21]. In The Netherlands, moderate increases in hoverfly species richness have been shown, whereas in Great Britain no significant directional changes were detected [19]. Furthermore, directionality (richness increase or decrease) varies depending on the time period assessed. For example, hoverfly richness decreased in Belgium by approximately 6% from 1950–1969 to 1970–1980, but increased by approximately 10% between 1970–1989 and 1990–2009 [21].
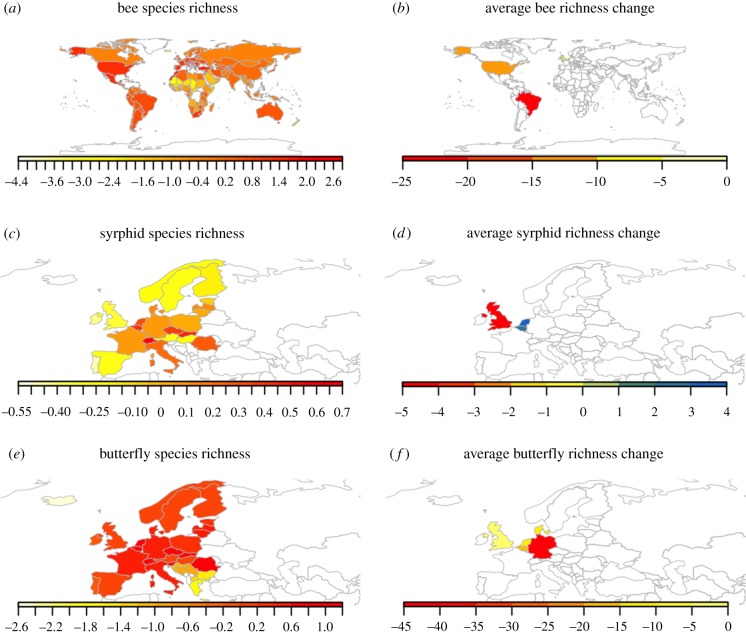
For illustrative purposes, we mapped the findings of these studies (figure 1) to show the large differences in bee species richness worldwide, with bee diversity hotspots in Mediterranean countries, against the paucity of countries for which we have any local or regional data on bee, syrphid or butterfly declines (raw data: electronic supplementary material, 1). Except for outside of Europe and the USA and for non-insect taxa, there are very few or no studies on pollinator declines that use historical records. However, there are species-specific examples of historical losses from different parts of the world (e.g. Bombus dalbhomi; [30]).
Figure 1.
Global map showing bee (a), syrphid (c) and butterfly (e) species richness per area (data from http://www.discoverlife.org, IUCN and http://www.syrphidae.com/). All richness levels are calculated as the residuals of the log–log regression between bee species richness per country and country size. This correction accounts for the species–area relationship. Warmer colours indicate higher bee diversity. Note that some African countries may have incomplete listed faunas and that Alaska is included with US values. Countries with available historical changes in (b) bee, (d) syrphid and (f) butterfly richness within the last 100 years. Warmer colours indicate steeper average declines. Countries without data are coloured in white. (Online version in colour.)
3. Using historical collection specimen records to fill knowledge gaps
Estimates of pollinator declines are lacking for most countries worldwide (figure 1). The use of historical collection data may be the most effective tool for filling these gaps. The core aim of museums is to conserve and curate historical collections. Thus, they serve as a precious repository for specimens, and at the same time, often ensure high quality taxonomic identification. Yet, the major bottleneck for researchers wanting to use these data is the lack of digitization. Digitizing old collection specimens is not a trivial task and requires expertize to (i) ensure proper taxonomic identification [31–33], (ii) geo-locate the coordinates of collection events (e.g. http://www.geonames.org) and (iii) store the data in a properly curated database [34]. Undertaking this process for tens or hundreds of thousands of museum collection specimens can be a daunting task and requires specialized personnel. While some tasks can only be undertaken by people with specialist skills (e.g. taxonomists), new technologies and citizen science can speed up the collection digitization process. High resolution photos of specimens and associated labels can be uploaded to the Internet, where the task of image transcription can be distributed across hundreds or thousands of volunteers (e.g. https://www.zooniverse.org/). In addition, new algorithms have been created that allow location geo-referencing based on vernacular names (e.g. https://geoparser.io). However, achieving this requires adequate funding [35].
Where digitization has been completed, the data provide a rich source of information, allowing assessment of the current status and long-term trends of pollinator populations [18,20,36]. This is despite the fact that museum collections often have a number of biases, including unknown sampling effort, personal interests of collectors and the curatorial techniques used. For example, collectors tend to target rare or unusual over common taxa, discard damaged individuals or only accession a certain number of individuals. In addition, collections are often undertaken opportunistically, which may lead to spatial bias where difficult to access areas are under-sampled or conversely, where samples are biased towards easily accessed locations (e.g. towns, cities and roadsides). Furthermore, museum collection data can only be used to determine where species are present, not where they are absent. However, given adequate sample sizes and appropriate statistical techniques, most biases can be accounted for [18,37,38].
4. The way forward: prioritizing the low hanging fruit
As we have shown, there is a paucity of countries for which historical collection data are available (figure 1), and hence can be used as a baseline for assessing pollinator population declines. While ideally one would aim to digitize all museum collection records, this is unlikely in the near future, predominantly due to funding constraints. Here, we show how researchers can optimize the use of historical collection data to assess long-term pollinator population changes.
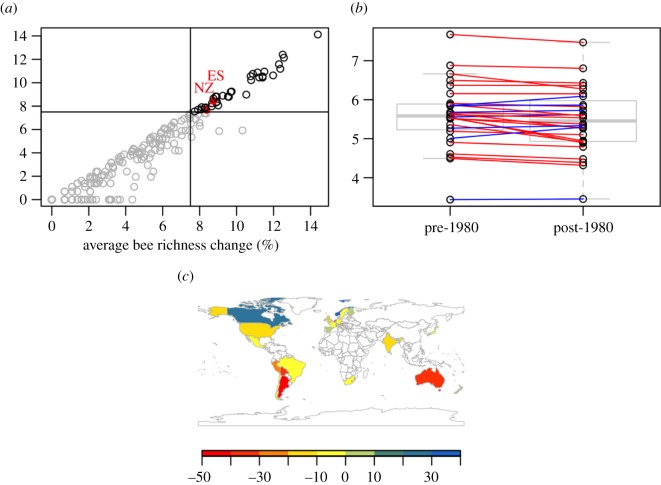
The Global Biodiversity Information Facility (GBIF) (https://www.gbif.org/) is a central repository for global species occurrence data. Much of these data come from museums, private collections and government research institutes, but several other sources are also integrated. In combination with the popular statistical language R [39], GBIF can be directly queried into your computer [40] and data availability can be checked for the region of interest. Focusing on bee taxa, we show here the number of modern and historical bee records currently available for different countries (figure 2a; electronic supplementary material, 2). We selected 37 countries that have more than 1800 records in each time period, making these data potentially analysable without further data collection effort (see figure 2b,c for an initial exploration). We selected this arbitrary threshold as a compromise between sample size and the number of countries that can be included in a general analysis. However, proper analysis of this dataset, including the determination of the minimum sample size needed per country, would require careful inspection of the data. We outline how this can be achieved below using two examples (Spain and New Zealand). We show that most countries fall short in one or both axes. For example, a variety of countries located in different continents such as Switzerland, Sri Lanka, Nicaragua or Zimbabwe have a decent number of recent records, but lack historical collections. Thus, researchers should prioritize the digitalization of old material before embarking on data analyses for these countries. It is also important to note that historical records are not always vouchered in local museums (i.e. many European and US museums contain large collections of pollinators from other countries). By contrast, more than 192 countries have less than 1000 records for both time periods, making them poor candidates for analysing long-term pollinator population trends. We focus here on bees, but similar exploratory analyses can easily be conducted for other taxa.
Figure 2.
Exploration of available data for bee records showing (a) the number of bee occurrences before 1980 and after 1980 in GBIF for each country. The upper right quadrat (records in black) contains well-covered countries with New Zealand (NZ) and Spain (ES) marked in red. For those countries, we show a tentative comparison of the rarefied number of species in both time periods and show that for most countries, the number of species recorded is slightly lower (red lines) in recent time periods (b). A more careful analysis of these data would help complete the map of global declines (c). In the map, we plot that % change as species recorded in GBIF for the available countries to show the geographical coverage. Note that these data are likely to contain strong undetected biases, as we explore below. (Online version in colour.)
As stated above, once historical datasets are made available, researchers must identify any potential biases. We explore this process with two contrasting dataset examples (Spain and New Zealand). In the Spanish dataset, most of the data comes from a few specific locations and was collected by a few specific teams. Hence, the geographical coverage is not representative. Even worse, historical and modern collections do not overlap spatially, making any inference impossible to interpret. In this case, we contacted the original collectors of the historical data to define their sampling protocols. We then resurveyed the same sites (30 years after the original surveys) using the same sampling protocols. By contrast, the New Zealand dataset includes a wide suite of collectors and collection locations and shows no obvious biases in geographical or taxonomic coverage through time. We complemented GBIF data with further museum collections for bees and flies, and analyse the regional richness changes through time. For these two case studies, we provide annotated R scripts as examples of analysis for different dataset types (electronic supplementary material, 3). These different analytical approaches allow us to reveal long-term trends in pollinator populations for regions with contrasting sampling histories. We hope that this resource will encourage researchers to analyse data for regions where current information on pollinator declines is lacking.
5. Case study 1: Spain
Spain provides an interesting study system because its natural habitats have been transformed extensively by humans over a long time period, but land use is not as intensive compared with many other European countries. In addition, Spain is a bee diversity hotspot (figure 1a) and maintains a relatively heterogeneous landscape. Spain has already digitalized a large amount of pollinator occurrence data for both historical and recent periods (figure 2a). However, visual inspection of the data revealed clustering around a few localities. Furthermore, historical records did not spatially match recent records, making comparisons difficult. For this dataset, most of the historical records were located around Valladolid and were collected by Enrique Asensio and collaborators. There has been no recent sampling of bees in this area. However, we found that Enrique systematically sampled six independent locations and that additional historical data were available at the ‘Museo de Historia Nacional’ and other minor collections (E. Asensio, personal communication). Digitization of these records, along with a resurvey of the original sampling locations, provided an excellent dataset for a before and after comparison of bee communities (figure 3).
Figure 3.
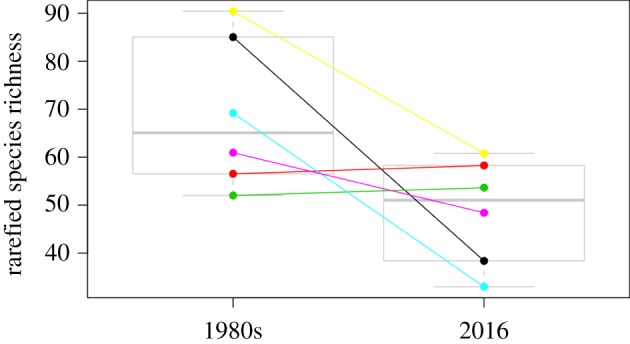
Comparison of historical collections (1980s) and modern re-surveys (2016) of the rarefied richness of bees at six Spanish localities. (Online version in colour.)
In brief, after cleaning taxonomic names for errata and synonyms using the taxize package [41], we checked for sampling completeness for both time periods and compared rarefied species richness for each site before and after 1980 with a paired t-test (mean sample size pre-1980 per site: 16 376 specimens; mean sample size pre-1980 per site: 2006 specimens; rarefication at 1000 specimens per site). We found that there were a reduced number of species at sites after 1980 (mean difference 20.27 species; 95% confidence interval: −1.03, 41.58; t = 2.44, d.f. = 5, p = 0.06). However, this trend was highly dependent on site identity, as two out of three sites showed no richness declines. Interestingly, these two localities have both experienced less dramatic land-use changes (both are natural areas embedded into agro-ecosystems). By contrast, the other four localities experienced substantial urban or agricultural intensification. In addition, species not recorded in the resurvey are not a random selection of species, but are clustered in a few genera. For example, Andrenids and their parasites (e.g. Nomada) showed the strongest declines, whereas Halictids tend to be more stable (electronic supplementary material, 4). This pattern of winners and losers of land-use intensification is in accordance with findings elsewhere [18], indicating that some clades are more sensitive to disturbance than others.
6. Case study 2: New Zealand
In contrast to Spain, New Zealand is an isolated oceanic archipelago, with a distinctive pollinator biota and a unique history of human occupation. Much of New Zealand's pollinator fauna is also relatively depauperate. For example, New Zealand has only 28 native bee species [42], which is a fraction of nearby Australia's approximately 1600 species [10]. However, New Zealand has a surprisingly high diversity of flies (Diptera), which are important pollinators in many ecosystems [43]. Thus, New Zealand provides a unique system to study long-term changes in pollinator communities, and is unlike continental Europe and the USA, which have been the focus of an overwhelming majority of pollinator decline studies.
In global terms, human colonization of New Zealand was relatively recent (approx. 740 years) [44]. Before human arrival, New Zealand was predominately forested, but has since been dramatically altered by people. Therefore, we can use museum records to identify trends in pollinator communities during New Zealand's more recent history. We used New Zealand bee collection records gathered from multiple sources, including university, research institute, museum and private collections. Collection records from the New Zealand Arthropod Collection (NZAC) are freely available online (https://scd.landcareresearch.co.nz/). Fly pollinator data were obtained from three participating New Zealand museums and covers two families (Calliphoridae and Syrphidae) that contain important fly pollinators. Collections for the bee and fly datasets span over 100 years (early 1900s to late 2000s).
We followed protocols outlined in Bartomeus et al. [18] to analyse the New Zealand data. First, we filtered our original datasets so that data used for analyses only included independent collection events. To do this, we removed specimens collected at the same location, on the same date, and by the same collector. We found our data had reasonable coverage along time periods and across space, although there was a peak in collection occurrences from 1960 to 1980. Further exploration of the New Zealand native bee data raised doubts on collection completeness in records prior to 1970. For example, some genera are not represented in old records. Hence, we removed these records from further analyses. We accounted for differences in collection effort through binning collection records by time so that each bin had a similar number of records but a different number of years. We then estimated species richness for each time period bin by rarefying all bins to an equal number of specimens and calculated the rarefied species richness ±s.e. for each bin. Finally, we estimated the significance of change in richness using a permutation test that randomly reordered time periods and calculated the correlation between time period and species richness. Thus, reported p-values were the proportion of permutations that had higher or lower correlations compared with the correlation between richness and the actual chronological time period sequence.
Second, to determine if the probability of finding a species in the collection changed over time, we used a general linear model with a binomial distribution and a logit link. For species that showed overdispersion, we used a quasi-binomial distribution. Furthermore, we only included species in this analysis for which we had 30 or more independent records [18]. Preliminary analyses using random subsampling of specimens from species with large sample sizes indicated that trends became too variable where there were fewer than 30 records. To account for differences in sampling effort between years, we weighted each year by the total number of samples collected that year.
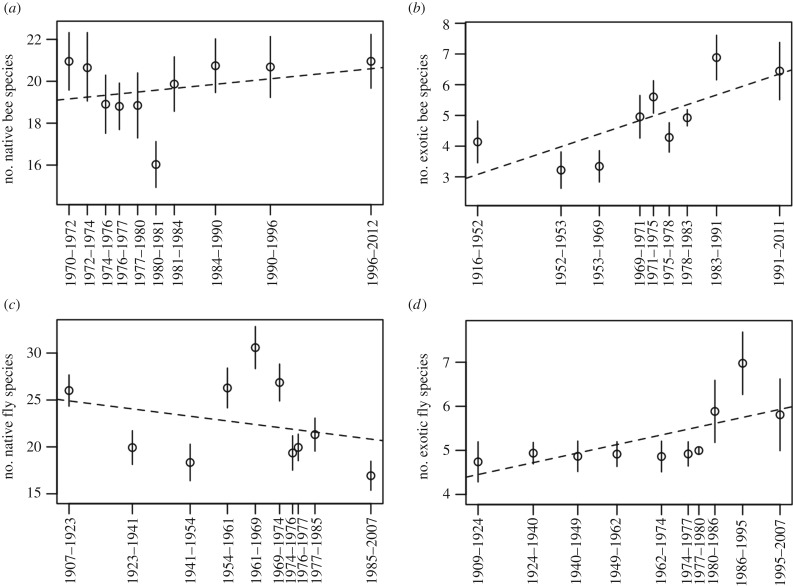
We found that rarefied richness for native bees was stable through time (figure 4). Exotic bees showed an increase in rarefied richness, but this trend was non-significant (p-value for both native and exotic bees greater than 0.05). By contrast, native fly richness declined, whereas exotic fly richness increased, although results for these groups were also non-significant (p-values for both groups greater than 0.05). Note that rarefied richness is sensitive to species evenness, so increases in rarefied richness over time may actually indicate increased species evenness and vice versa for decreased richness [45].
Figure 4.
Changes in rarefied species richness for different pollinator groups in New Zealand over time. All trends were non-significant (α = 0.05).
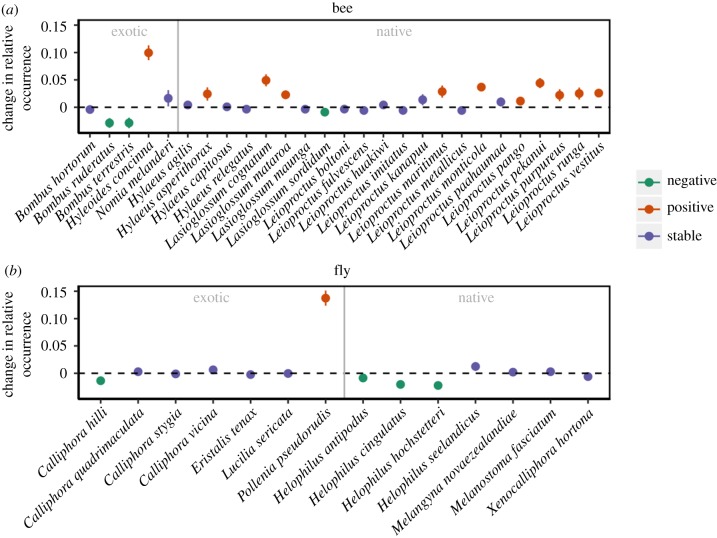
However, at the species level, we found that 11 out of 27 bee species increased in relative occurrence over time (10 native and one exotic) and three bee species declined in relative occurrence (one native and two exotic) (figure 5). Interestingly, the two exotic bee species that declined in relative occurrence were both in the genus Bombus, which were intentionally introduced into New Zealand for the pollination of crops. Native bees that increased in relative occurrence were mostly from the genus Leioproctus, which are medium-sized, ground-nesting, solitary bees. Only one out of 14 fly species increased in relative occurrence, which was exotic, whereas four species decreased in occurrence (three native and one exotic). Native flies that decreased in relative occurrence were all Syrphidae in the genus Helophilus.
Figure 5.
Model estimated changes (±1 s.e.) in the relative occurrence frequency of different New Zealand bee and fly species in museum collections over time. (Online version in colour.)
7. Beyond species occurrences
A recent study found that more than 90% of the papers investigating pollinator responses to land-use change focused solely on richness and abundance descriptors [9]. However, in addition to local (α) diversity and regional (γ) diversity, researchers need to assess changes in turnover between sites (β diversity). Environmental changes often result in a few ‘winner’ species and many ‘loser’ species [18]. Identifying winners and losers is critical as the few winners are often exotic and represent a subset of traits that facilitate survival in highly modified environments [46]. These changes can have important effects for pollination of native plant species and crops [47].
In addition, digitalized museum specimen collections can provide much more information besides species occurrence records, given that such information is recorded when digitizing collections. This is particularly important for identifying mechanisms of decline and adaptation. For example, recording the date of collection is particularly important for tracking of phenological advances congruent with contemporary climate change [48]. In addition, pollinator specimen labels often include information about the host plant on which the specimen was collected. This information is critical for understanding past and present species interactions [49]. Aside from this information, bee specimens often contain pollen loads trapped on hairs, from which past visitation events can be identified [50]. Museum specimens can be measured to track evolutionary changes by measuring the traits of specimens. This approach has already been used to investigate tongue length [51] and body size [52,53] in response to climate and land-use change. Finally, plant specimens stored in herbaria may contain indirect evidence of pollination declines [54], thus linking pollinator declines with consequences for ecosystem functioning.
8. Conclusion
Unleashing the power of museum collection data to answer pressing ecological and evolutionary questions is in our hands, but requires the coordinated effort of many actors. Using two case studies, we show that strong collaboration between museum curators and ecologists is key to understanding data and treating it appropriately. To advance our understanding of the global pollination crisis, researchers and curators must aim to digitize museum collection data and make it readily available in a format that is widely accessible. Centralization of regional and national museum collection data in existing global platforms, such as GBIF, would facilitate free and widespread access. However, datasets could also be stored in alternative webpages or database repositories (e.g. university and museum webpages or Dryad), providing they are thoroughly documented and easy to retrieve, and combined with other datasets using open science tools [55].
We must revolutionize the way that researchers collaborate with museums in order to foster healthy bidirectional relationships. For example, ecological researchers collect massive amounts of specimens, but these are often inappropriately vouchered [56,57], rendering them less useless for future research. To improve this process, strong communication between museums and researchers is required. However, this can only be achieved with adequate funding and recognition that accurate data recording and long-term preservation are critical for research [58].
To identify global trends in pollinator declines, we require robust data, collected from diverse geographical regions. It is also crucial that these data are analysed appropriately. This requires researches to identify biases and to fill any taxonomic and geographical gaps where possible. We need to place increased emphasis on quantifying pollinator declines in regions outside of the USA and Europe, and for pollinator groups other than bees. For most other pollinator taxa and regions throughout the world, we know almost nothing. Moving forward, the first step for many taxa will be to identify and describe species [31]. Only then can we begin to document pollinator declines.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Curro Molina, Carola Warner, Patrick McQuinn and Crona McMonagle for data entry and Gregorio Aguado for carrying out the Spanish re-sampling. We thank Barry Donovan for providing New Zealand bee collection records and E. Asensio for sharing his historical data and knowledge. We thank the ‘Museo Nacional de Ciencias Naturales', specially Mercedes Paris, ITACyL (Instituto Tecnológico Agrario de Castilla y León), Canterbury Museum, the New Zealand Arthropod Collection and the Museum of New Zealand Te Papa Tongarewa for access to historical collections. I.B. was funded by the Fundación Banco Bilbao Vizcaya Argentaria (FBBVA) project. D.W. was funded through Landcare Research within the Characterizing New Zealand's Land Biota Portfolio.
Data accessibility
All data used can be found at: https://doi.org/10.5281/zenodo.1326310.
Authors' contributions
I.B. conceived the idea. I.B. and J.R.S. analysed the data. D.W., J.R.S. and O.A. provided data. I.B. wrote the first draft of the manuscript and all authors edited and commented on it.
Competing interests
We have no competing interests.
Funding
I.B. was funded by ‘Fundación Banco Bilbao Vizcaya Argentaria (FBBVA)’.
References
- 1.Ollerton J, Winfree R, Tarrant S. 2011. How many flowering plants are pollinated by animals? Oikos 120, 321–326. ( 10.1111/j.1600-0706.2010.18644.x) [DOI] [Google Scholar]
- 2.Klein AM, Vaissiere BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T. 2007. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 274, 303–313. ( 10.1098/rspb.2006.3721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchmann SL, Nabhan GP.. 1996. The forgotten pollinators. Washington, DC: Island Press. [Google Scholar]
- 4.National Research Council. 2007. Status of Pollinators in North America. Washington, DC: The National Academies Press. [Google Scholar]
- 5.Goulson D, Nicholls E, Botías C, Rotheray EL. 2015. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957 ( 10.1126/science.1255957) [DOI] [PubMed] [Google Scholar]
- 6.Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. 2010. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353. ( 10.1016/j.tree.2010.01.007) [DOI] [PubMed] [Google Scholar]
- 7.Nieto A, et al. 2014. European red list of bees. Luxembourg: Publication Office of the European Union. [Google Scholar]
- 8.Ollerton J. 2017. Pollinator diversity: distribution, ecological function, and conservation. Annu. Rev. Ecol. Evol. Syst. 48, 353–376. ( 10.1146/annurev-ecolsys-110316-022919) [DOI] [Google Scholar]
- 9.Winfree R, Bartomeus I, Cariveau DP. 2011. Native pollinators in anthropogenic systems. Annu. Rev. Ecol. Syst. 42, 1–21. ( 10.1146/annurev-ecolsys-102710-145042) [DOI] [Google Scholar]
- 10.Ascher JS, Pickering J. 2018. Discover Life bee species guide and world checklist (Hymenoptera: Apoidea: Anthophila). See http://www.discoverlife.org/mp/20q?guide=Apoidea_species.
- 11.Martin LJ, Blossey B, Ellis E. 2012. Mapping where ecologists work: biases in the global distribution of terrestrial ecological observations. Front. Ecol. Environ. 10, 195–201. ( 10.1890/110154) [DOI] [Google Scholar]
- 12.Archer CR, Pirk CWW, Carvalheiro LG, Nicolson SW. 2014. Economic and ecological implications of geographic bias in pollinator ecology in the light of pollinator declines. Oikos 123, 401–407. ( 10.1111/j.1600-0706.2013.00949.x) [DOI] [Google Scholar]
- 13.De Palma A, et al. 2016. Predicting bee community responses to land-use changes: effects of geographic and taxonomic biases. Sci. Rep. 6, 31153 ( 10.1038/srep31153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franca F, Louzada J, Korasaki V, Griffiths H, Silveira JM, Barlow J. 2016. Do space-for-time assessments underestimate the impacts of logging on tropical biodiversity? An Amazonian case study using dung beetles. J. Appl. Ecol. 53, 1098–1105. ( 10.1111/1365-2664.12657) [DOI] [Google Scholar]
- 15.De Palma A, et al. 2018. Challenges with inferring how land-use affects terrestrial biodiversity: study design, time, space and synthesis. Next Gener. Biomonitor. 58, 163–199. ( 10.1016/bs.aecr.2017.12.004) [DOI] [Google Scholar]
- 16.De Palma A, Kuhlmann M, Bugter R, Ferrier S, Hoskins AJ, Potts SG, Roberts SP, Schweiger O, Purvis A. 2017. Dimensions of biodiversity loss: spatial mismatch in land-use impacts on species, functional and phylogenetic diversity of European bees. Divers. Distrib. 23, 1435–1446. ( 10.1111/ddi.12638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Swaay CA, Nowicki P, Settele J, van Strien AJ. 2008. Butterfly monitoring in Europe: methods, applications and perspectives. Biodivers. Conserv. 17, 3455–3469. ( 10.1007/s10531-008-9491-4) [DOI] [Google Scholar]
- 18.Bartomeus I, Ascher JS, Gibbs J, Danforth BN, Wagner DL, Hedtke SM, Winfree R. 2013. Historical changes in northeastern US bee pollinators related to shared ecological traits. Proc. Natl Acad. Sci. USA 110, 4656–4660. ( 10.1073/pnas.1218503110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biesmeijer JC, et al. 2006. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313, 351–354. ( 10.1126/science.1127863) [DOI] [PubMed] [Google Scholar]
- 20.Scheper J, Reemer M, van Kats R, Ozinga WA, van der Linden GT, Schaminée JH, Siepel H, Kleijn D. 2014. Museum specimens reveal loss of pollen host plants as key factor driving wild bee decline in The Netherlands. Proc. Natl Acad. Sci. 111, 17 552–17 557. ( 10.1073/pnas.1412973111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carvalheiro LG, et al. 2013. Species richness declines and biotic homogenisation have slowed down for NW-European pollinators and plants. Ecol. Lett. 16, 870–878. ( 10.1111/ele.12121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colla SR, et al. 2012. Documenting persistence of most Eastern North American bee species (Hymenoptera: Apoidea: Anthophila) to 1990–2009. J. Kans. Entomol. Soc. 85, 14–22. ( 10.2317/JKES110726.1) [DOI] [Google Scholar]
- 23.Ollerton J, Erenler H, Edwards M, Crockett R. 2014. Extinctions of aculeate pollinators in Britain and the role of large-scale agricultural changes. Science 346, 1360–1362. ( 10.1126/science.1257259) [DOI] [PubMed] [Google Scholar]
- 24.Burkle LA, Marlin JC, Knight TM. 2013. Plant-pollinator interactions over 120 years: loss of species, co-occurrence, and function. Science 339, 1611–1615. ( 10.1126/science.1232728) [DOI] [PubMed] [Google Scholar]
- 25.Bartomeus I, Winfree R. 2013. Pollinator declines: reconciling scales and implications for ecosystem services. F1000Research 2, 146 ( 10.12688/f1000research.2-146.v1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dupont YL, Damgaard C, Simonsen V. 2011. Quantitative historical change in bumblebee (Bombus spp.) assemblages of red clover fields. PLoS ONE 6, e25172 ( 10.1371/journal.pone.0025172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grixti JC, Wong LT, Cameron SA, Favret C. 2009. Decline of bumble bees (Bombus) in the North American Midwest. Biol. Conserv. 142, 75–84. ( 10.1016/j.biocon.2008.09.027) [DOI] [Google Scholar]
- 28.Maes D, Van Dyck H. 2001. Butterfly diversity loss in Flanders (north Belgium): Europe's worst case scenario? Biol. Conserv. 99, 263–276.( 10.1016/S0006-3207(00)00182-8) [DOI] [Google Scholar]
- 29.Wenzel M, Schmitt T, Weitzel M, Seitz A. 2006. The severe decline of butterflies on western German calcareous grasslands during the last 30 years: a conservation problem. Biol. Conserv. 128, 542–552. ( 10.1016/j.biocon.2005.10.022) [DOI] [Google Scholar]
- 30.Morales CL, Arbetman MP, Cameron SA, Aizen MA. 2013. Rapid ecological replacement of a native bumble bee by invasive species. Front. Ecol. Environ. 11, 529–534. ( 10.1890/120321) [DOI] [Google Scholar]
- 31.Meier R, Dikow T. 2004. Significance of specimen databases from taxonomic revisions for estimating and mapping the global species diversity of invertebrates and repatriating reliable specimen data. Conserv. Biol. 18, 478–488. ( 10.1111/j.1523-1739.2004.00233.x) [DOI] [Google Scholar]
- 32.Huber JT. 1998. The importance of voucher specimens, with practical guidelines for preserving specimens of the major invertebrate phyla for identification. J. Nat. Hist. 32, 367–385. ( 10.1080/00222939800770191) [DOI] [Google Scholar]
- 33.Thomson SA, et al. 2018. Taxonomy based on science is necessary for global conservation. PLoS Biol. 16, e2005075 ( 10.1371/journal.pbio.2005075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hart EM, et al. 2016. Ten simple rules for digital data storage. PLoS Comput. Biol. 12, e1005097 ( 10.1371/journal.pcbi.1005097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiggins GB, Marshall SA, Downes JA. 1991. Importance of research collections of terrestrial arthropods. A brief prepared by the Biological Survey of Canada (Terrestrial Arthropods). Bull. Entomol. Soc. Canada 23, 16. [Google Scholar]
- 36.Young BE, Auer S, Ormes M, Rapacciuolo G, Schweitzer D, Sears N. 2017. Are pollinating hawk moths declining in the Northeastern United States? An analysis of collection records. PLoS ONE 12, e0185683 ( 10.1371/journal.pone.0185683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keil P, Biesmeijer JC, Barendregt A, Reemer M, Kunin WE. 2011. Biodiversity change is scale-dependent: an example from Dutch and UK hoverflies (Diptera, Syrphidae). Ecography 34, 392–401. ( 10.1111/j.1600-0587.2010.06554.x) [DOI] [Google Scholar]
- 38.Pearce JL, Boyce MS. 2006. Modelling distribution and abundance with presence-only data. J. Appl. Ecol. 43, 405–412. ( 10.1111/j.1365-2664.2005.01112.x) [DOI] [Google Scholar]
- 39.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 40.Chamberlain S, Ram K, Barve V, Mcglinn D. 2014. rgbif: interface to the Global Biodiversity Information Facility API. R package version 0.77.
- 41.Chamberlain S, Szoecs E. 2013. taxize - taxonomic search and retrieval in R. F1000Research 2, 191 See http://f1000research.com/articles/2-191/v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donovan BJ. 2007. Fauna of New Zealand 57: Apoidea (Insecta: Hymenoptera). Lincoln, New Zealand: Manaaki Whenua Press. [Google Scholar]
- 43.Newstrom L, Robertson AW. 2005. Progress in understanding pollination systems in New Zealand. N. Z. J. Bot. 43, 1–59. ( 10.1080/0028825X.2005.9512943) [DOI] [Google Scholar]
- 44.Wilmshurst JM, Anderson AJ, Higham TF, Worthy TH. 2008. Dating the late prehistoric dispersal of Polynesians to New Zealand using the commensal Pacific rat. Proc. Natl Acad. Sci. USA 105, 7676–7680.( 10.1073/pnas.0801507105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gotelli NJ, Colwell RK. 2001. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 4, 379–391.( 10.1046/j.1461-0248.2001.00230.x) [DOI] [Google Scholar]
- 46.Stavert JR, Pattemore DE, Gaskett AC, Beggs JR, Bartomeus I. 2017. Exotic species enhance response diversity to land-use change but modify functional composition. Proc. R. Soc. B 284, 20170788 ( 10.1098/rspb.2017.0788) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stavert JR, Pattemore DE, Bartomeus I, Gaskett AC, Beggs JR. 2018. Exotic flies maintain pollination services as native pollinators decline with agricultural expansion. J. Appl. Ecol. 55, 1737–1746. ( 10.1111/1365-2664.13103) [DOI] [Google Scholar]
- 48.Bartomeus I, Ascher JS, Wagner D, Danforth BN, Colla S, Kornbluth S, Winfree R. 2011. Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proc. Natl Acad. Sci. USA 108, 20 645–20 649. ( 10.1073/pnas.1115559108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tylianakis JM, Didham RK, Bascompte J, Wardle DA. 2008. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 11, 1351–1363. ( 10.1111/j.1461-0248.2008.01250.x) [DOI] [PubMed] [Google Scholar]
- 50.Kleijn D, Raemakers I. 2008. A retrospective analysis of pollen host plant use by stable and declining bumble bee species. Ecology 89, 1811–1823. ( 10.1890/07-1275.1) [DOI] [PubMed] [Google Scholar]
- 51.Miller-Struttmann NE, et al. 2015. Functional mismatch in a bumble bee pollination mutualism under climate change. Science 349, 1541–1544.( 10.1126/science.aab0868) [DOI] [PubMed] [Google Scholar]
- 52.Oliveira MO, Freitas BM, Scheper J, Kleijn D. 2016. Size and sex-dependent shrinkage of Dutch bees during one-and-a-half centuries of land-use change. PLoS ONE 11, e0148983 ( 10.1371/journal.pone.0148983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Renauld M, Hutchinson A, Loeb G, Poveda K, Connelly H. 2016. Landscape simplification constrains adult size in a native ground-nesting bee. PLoS ONE 11, e0150946 ( 10.1371/journal.pone.0150946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pauw A, Hawkins JA. 2011. Reconstruction of historical pollination rates reveals linked declines of pollinators and plants. Oikos 120, 344–349. ( 10.1111/j.1600-0706.2010.19039.x) [DOI] [Google Scholar]
- 55.Dallas T. 2016. helminthR: an R interface to the London Natural History Museum's host–parasite database. Ecography 39, 391–393.( 10.1111/ecog.02131) [DOI] [Google Scholar]
- 56.Packer L, Monckton SK, Onuferko TM, Ferrari RR. 2018. Validating taxonomic identifications in entomological research. Insect Conserv. Divers. 11, 1–12. ( 10.1111/icad.12284) [DOI] [Google Scholar]
- 57.Wheeler TA. 2003. The role of voucher specimens in validating faunistic and ecological research: a brief. Biological Survey of Canada (Terrestrial Arthropods) Document Series 9.
- 58.Ward DF, Leschen RA, Buckley TR. 2015. More from ecologists to support natural history museums. Trends Ecol. Evol. 30, 373–374. ( 10.1016/j.tree.2015.04.015) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used can be found at: https://doi.org/10.5281/zenodo.1326310.