Abstract
Mounting evidence shows that species interactions may mediate how individual species respond to climate change. However, long-term anthropogenic effects on species interactions are poorly characterized owing to a lack of data. Insect herbivory is a major ecological process that represents the interaction between insect herbivores and their host plants, but historical data on insect damage to plants is particularly sparse. Here, we suggest that museum collections of insects and plants can fill key gaps in our knowledge on changing trophic interactions, including proximate mechanisms and the net outcomes of multiple global change drivers across diverse insect herbivore–plant associations. We outline theory on how global change may affect herbivores and their host plants and highlight the unique data that could be extracted from museum specimens to explore their shifting interactions. We aim to provide a framework for using museum specimens to explore how some of the most diverse co-evolved relationships are responding to climate and land use change.
This article is part of the theme issue ‘Biological collections for understanding biodiversity in the Anthropocene’.
Keywords: species interactions, plant, insect, herbivore, global change, climate change
1. Introduction
Species interactions have received less attention in global change biology than individual species' responses [1]. In large part, this is because long-term data on species interactions spanning the period of intense anthropogenic environmental change are rare. For example, first flower dates of Japanese cherry blossoms (Prunus jamasakura) have been recorded in diaries since the ninth century [2], but we have no equivalent long-term records of cherry tree pollination, leaf microbial communities, or disease incidence. Data describing species interactions are laborious to collect and, in many cases, require technology, such as electron microscopy or DNA sequencing, that was not available until recent years. The lack of long-term data inhibits assessment of how species interactions are impacted by global change and limits our ability to determine how these effects mediate individual species' distributions, abundances, and ecologies.
Variation in species responses to global change has generated concern that interactions which were tightly coupled historically might become decoupled owing to phenological asynchronies. Phenological asynchrony arises when interacting species respond differently to global change—for example, if earlier flowering as a consequence of global warming is not matched by earlier pollinator emergence. Recent meta-analyses have suggested that phenological sensitivity to climate change differs among trophic levels, with lower trophic levels advancing more than higher trophic levels [3,4]. In one well-documented example, great tit (Parus major) reproduction did not advance in sync with peak food availability for young, leading to potential fitness costs [5]. Similar changes in interactions between trophic levels may happen as a consequence of other differential responses to global change, such as spatial mismatches between species whose ranges expand poleward or upward in elevation to different extents. While predictions for phenological and spatial asynchronies are clear, empirical data are sparse, and there is still no consensus on whether asynchrony is common or rare, or which traits regulate when asynchronies arise [6].
In the absence of long-term observational data, global change biologists increasingly mine museum collections to investigate how species interactions have shifted over time [7,8]. Diverse types of data are available only in natural history collections [7–9], and they could therefore have wide applicability in global change biology. While natural history specimens are not collected systematically—and their use in ecological and evolutionary research can present challenges [10,11]—they represent time-series data across much of the globe, span the tree of life, and may be able to fill gaps in species interactions data [12]. Importantly, a large proportion of specimens were collected prior to the intensification of anthropogenic change and therefore may serve as baselines for studying consequences of, for example, invasive species spread [13,14], pollution [15,16] and habitat alteration [17,18].
Here, we explore the potential for museum specimens to provide insights into interactions between insect herbivores and their host plants. Insects have been eating plants for nearly 400 million years, and these interactions have given rise to much of macroscopic diversity [19–21]. Herbivores co-evolved with plants, tracking plant speciation [22] or defensive profiles [23] and are frequently specialized [24]. Over the past 12 000 years or so, humans have altered these relationships by domesticating plants and moving them beyond their natural ranges, spraying pesticides, building cities, and changing the global climate. Effects of these global changes on herbivores and their host plants is of critical importance to ecosystem functioning and the provision of ecosystem services. Insect damage—‘herbivory’—drives ecosystem processes, including decomposition and primary productivity [25,26]. Herbivory also influences ecosystem properties that are of direct importance to people, including food production [27] and tree cover [28], which are linked to human physical [29] and mental health [30].
In general, warming is expected to increase insect herbivore abundance where insects are living below their thermal optima [31,32]. While most insects in temperate and boreal climates probably occupy niches well below their thermal optima—and thus may benefit from warming—warming may cause temperatures to exceed insect thermal optima in areas that are already relatively warm, including the tropics [33]. Insect fitness is not, however, a simple function of mean annual temperature, especially at the local scale. For example, many species have thermoregulatory behaviours that decouple body and air temperatures [34]. In some cases, warming may also have negative effects on insect fitness by reducing snowpack or disrupting diapause [35]. Furthermore, and contrary to the prediction that warming should increase temperate insect herbivore abundance, recent evidence suggests that insect biomass has declined precipitously in Germany since the 1980s [36]. The extent of these declines across space and the mechanism(s) driving them remain unknown, as does whether these declines have occurred in other continents, where comparable long-term insect data were not recorded.
While shifts in the global climate will undoubtedly shape species interactions, the local drivers of global change may have complex and nonlinear impacts. Urbanization is a more localized form of global change that is accelerating, with important consequences for plant–insect interactions [37], and has thus attracted much recent attention in ecology and evolution research [38,39]. Most people now live in urban areas for the first time in history, and the proportion of the world human population living in urban areas continues to grow. Impacts of urbanization on plant–herbivore interactions could thus have increasing consequence for society via its effects on urban greenery and agriculture. However, the key mechanisms driving insect and plant responses to urbanization remain debated because: (i) urban development, like climate change, has multiple, concurrent effects that are difficult to tease apart experimentally, and (ii) we lack long-term observational data to determine effects of urbanization over time. Despite these data challenges, studies in the past few decades consistently show that urban development can have profound impacts on plants and insect herbivores [37].
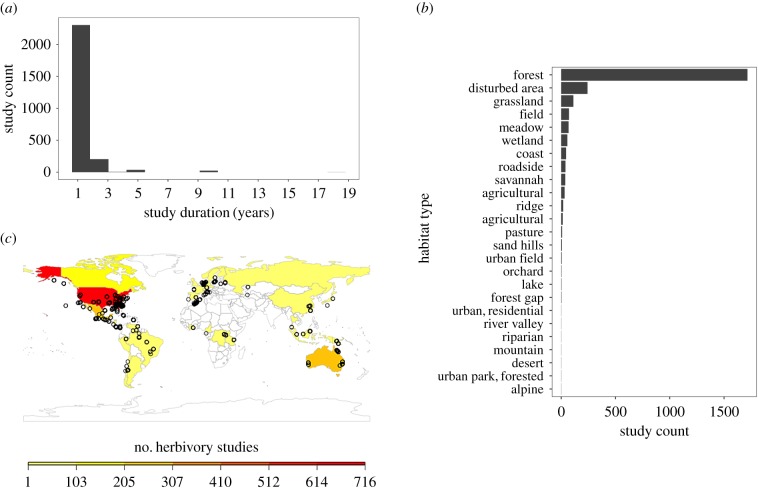
The suite of competing predictions of how insects might respond to global change, and the multitude of mechanisms linking insect herbivore abundance and fitness to herbivory, makes predicting changes in insect damage to plants difficult in the absence of long-term data on historical responses. Further, herbivory data for assessing competing hypotheses are currently sparse and thus ill-suited for making projections about herbivory change into the future. According to a recent compilation of studies [40], most short-term herbivory studies include just one year of data (figure 1a), focus on forest plants (figure 1b), are biased toward the northern hemisphere (figure 1c), and record only chewing damage [40], which represents a fraction of the damage types made by insects. Though short-term studies over space have highlighted that disturbance can have profound effects on plants and herbivores [37], only few studies focus on disturbed habitats, including cities and farms, areas where plant–herbivore relationships are likely to affect human wellbeing.
Figure 1.
Limitations of insect herbivore damage data. (a) Typical herbivory studies only last 1 year, (b) are biased spatially, with the vast majority located within the United States, (c) and cover a limited number of habitat types, with few studies in disturbed habitats. (Online version in colour.)
Here, we propose that biological collections could contribute data that would allow us to test major hypotheses on how diverse plant–herbivore relationships respond to global change. In contrast to observational or experimental studies of insect herbivory, data from museum collections span decades or sometimes centuries, include diverse growth forms and taxa from across the globe, and capture multiple types of herbivore damage [12]. We focus on hypotheses that may be particularly well served by the 380 million vascular pressed plant specimens [41]—herbarium specimens—and the more than 500 million insect herbivore specimens [42] held in museums worldwide that are increasingly available online in the form of images and metadata. In general, we focus on aboveground, not belowground herbivory, because intact roots required for assessing belowground herbivory are rarely available on herbarium specimens. Digitization of insect specimens has lagged behind plants owing to the difficulty of capturing three-dimensional specimens and the information from their labels. However, enormous efforts to digitize both plant and insect specimens are underway [43]. Digital collections are also increasingly aggregated in online databases, e.g. the Chinese Virtual Herbarium (http://www.cvh.ac.cn/news/8), LepNet (http://www.lep-net.org/), Symbiota (http://symbiota.org/docs/), GBIF (https://www.gbif.org/), and iDigBio (https://www.idigbio.org/). These databases facilitate ‘big’ data analysis, but are equally as important in helping focus data collection efforts when physical specimens need to be examined. As we discuss below, these specimens can provide a wealth of ecological data that is difficult or impossible to collect using more traditional approaches.
In the following sections, we demonstrate how natural history collections may provide unique insights into changing plant–insect herbivore interactions. We focus on species shifts in time and space as a response to recent anthropogenic climate change, and impacts of urbanization, representing one facet of habitat transformation, which is a major driver of current global change. In subsequent sections, we discuss how collections can also reveal species' rapid adaptive responses to recent global changes, an application that may be particularly consequential for agriculture. Finally, we review the challenges of natural history collections as sources of long-term data and suggest approaches to some of these challenges, with the goal of removing barriers that have prevented collections from becoming a standard source of data for twenty-first century ecology.
2. Drivers of shifting interactions
(a). Phenological change
Phenology—the seasonal timing of life-history events such as flowering and leaf-out in plants—is both a response to and an indicator of global change. Phenological models, such as the Spring Indices [44–46], allow us to map with increasing accuracy the transition from winter to spring across the northern hemisphere [47]. These models integrate daily climate variables from meteorological records to predict day of year of first leaf and first bloom. However, the Spring Indices are calibrated using an extensive network of phenological observations on a single cloned lilac cultivar (Syringa) and two honeysuckle cultivars (Lonicera) across the temperate United States (US). While meta-analyses reveal a consistent fingerprint of climate change on plant phenology [48,49], they also reveal large interspecific variation in plant responses [50]. Thus, responses to climate change remain poorly characterized for the majority of plant species. Herbarium specimens can serve as phenological records of flowering or leaf-out for these species [51–54]. The vast wealth of vouchers within herbaria greatly expand the spatial, taxonomic, and temporal extent of phenological observations and, as a consequence, the inference we can draw across climate space, even for species for which phenology has been documented in long-term observations [55].
Animals are also shifting their phenology with climate change; many species are migrating sooner, advancing seasonal breeding times, and insects are emerging earlier [56]. Natural history collections have been valuable in demonstrating how animal species respond to climate warming [57,58]. Thanks to the biases of early Victorian naturalists and their attractiveness to contemporary collectors, the Lepidoptera—butterflies and moths, which in their immature stages are herbivorous—have been collected more comprehensively than many other groups and are thus the subject of a large proportion of collections research on animals [59]. Butterfly collections document occurrences of species in time and space and, importantly, the seasonal timing of butterfly flight. Using data from approximately 48 000 collection records of Canadian butterflies, Kharouba et al. [60] were able to show that timing of flight season predictably responded to temperature, and that species with early flight seasons and low dispersal ability appear most sensitive. However, in one recent study, Brooks et al. [61] collected data from 83 500 specimens of British butterflies spanning 100 years of climate change which suggested that early flying species might be approaching the limits of their phenological advancement. If advances in butterfly phenology are slowing but their host plants continue to leaf-out and flower earlier, we might observe phenological asynchrony between them.
In collections research the potential for phenological synchrony between plants and their pollinators has tended to attract most attention [62], and collections data have been less frequently used to explore plant–herbivore interactions. However, the few studies that have considered phenological asynchronies between plants and insect herbivores demonstrate the potential for collections to inform such analyses. For example, Kharouba et al. [63] showed that flowering time was more sensitive to temperature than the timing of nectar-feeding butterfly flight, suggesting that caterpillars or adult butterflies of these species might become phenologically mismatched with their host plants if warming continues.
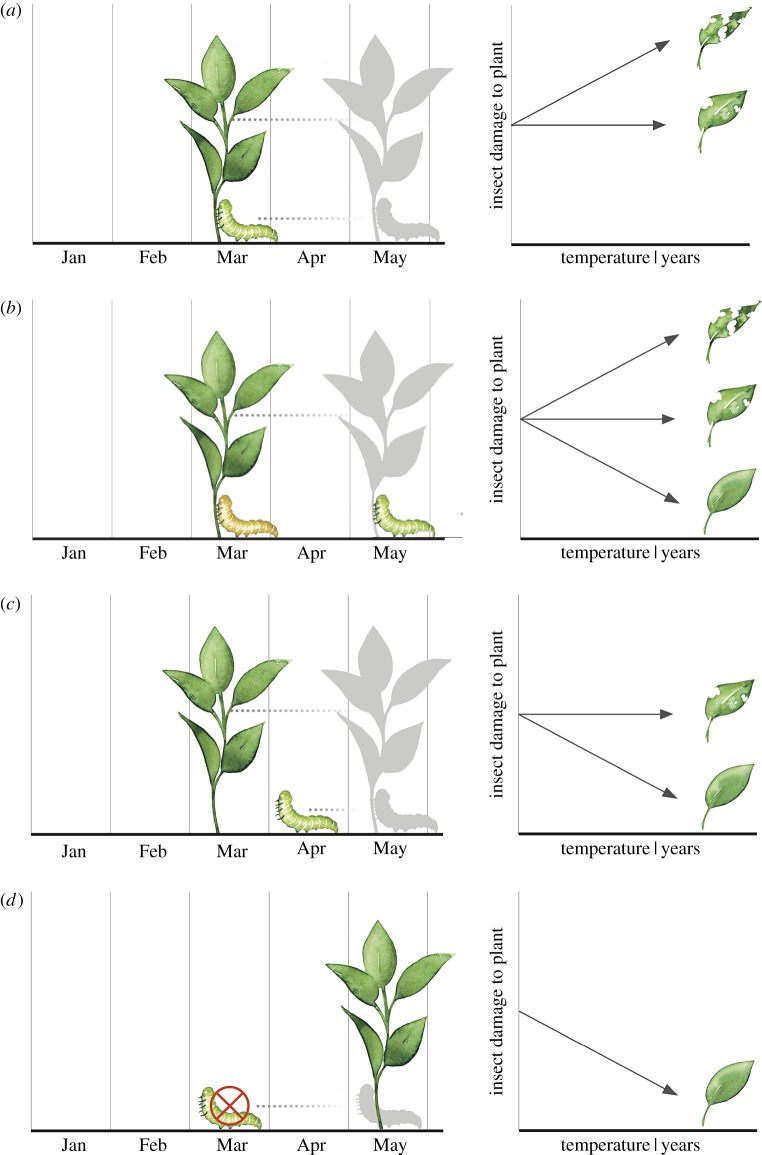
While collections data can be extensive for particular taxonomic groups, it is nonetheless rare for collections to capture temporally and spatially matching data on interacting species, such as on both butterflies and their host plants. However, herbarium collections can offer data on phenological sensitivity of plant species (flowering and leaf-out dates [53,55]) and, indirectly, data on their herbivores as captured by the amount of leaf area removed by herbivory [12,64,65]. Herbaria may thus offer a unique opportunity to explore how shifts in plant phenology have affected herbivory since the onset of climate change. In figure 2, we outline some possible scenarios describing how warming could affect plant and insect phenology, and how these responses might translate to changes in herbivore damage to plants. For simplicity, we focus on spring phenology and specialized herbivores, though for plants that are commonly eaten primarily by generalists, we could derive an additional set of predictions. Additional factors, such as herbivore developmental plasticity [66], host plant nutritional quality (as reviewed in [67]), relationships with natural enemies [68], and differential responses among herbivores of a single host plant, might add complexity to the predictions described in figure 2, but could be placed within this general framework.
Figure 2.
Some theoretical expectations for how phenological change in spring may affect synchrony between specialized insect herbivores and their host plants. (a) If a plant leafs out earlier with warming, and its native herbivores also emerge early as plant-eating larvae (caterpillars), herbivory rates may remain unchanged. (b) If a plant leafs out earlier owing to climate change and, as a consequence, is exposed to novel herbivores, herbivory may increase if the plant has no natural defences to the novel herbivore; however, herbivory may decrease if the plant is not recognized as a host by the novel herbivore. (c) If a plant leafs out earlier, and its key insect herbivore(s) do not emerge earlier, it may experience an ‘enemy-free’ period early in the year. Herbivory may then decrease if young leaves with fewer herbivore defences are able to develop rapidly and increase defensive compound concentrations prior to herbivore emergence. (d) If herbivores emerge before leaf-out, herbivore populations may crash, resulting in reduced herbivory (although there should be strong selection for host-tracking, and herbivory may be expected to return to historical levels within a relatively short time period). These hypotheses are testable with herbarium specimens, which provide data on plant phenology (timing of leaf-out and first flower) and insect damage (herbivory), and museum data for insects on time of year of first flight and, in cases where larval collections are available, data on phenological timing of larval stages. (Online version in colour.)
The alternative hypotheses we present in figure 2 could be addressed with herbarium specimens for plant species with long-term herbarium records and which vary in their phenological sensitivities to climate change. While collections may not always allow us to differentiate between alternative scenarios, they could reveal how herbivory changes with warming for plants across a range of phenological sensitivities, and inform field experiments to tease apart mechanisms. In some cases, it may be possible to test for herbivory by novel herbivores by quantifying types of damage that can be traced back to particular insect genera or species, such as galls and leaf mines (see below), or chewing damage that is characteristic of certain insect orders, e.g. margin feeding, circular hole feeding, and skeletonization. Butterfly collections might also help in resolving alternative scenarios, although we suspect that larvae, responsible for most herbivore damage, may be under-represented in collections compared to adult Lepidoptera, and flight phenology may not be correlated with larval phenology (e.g. [69]).
The mismatch between adult and larval butterfly life-histories is a challenge for using butterfly collections to explore phenological asynchronies. However, there are also scenarios in which phenological change at the adult stage may affect herbivory, which may offer opportunities to use the extensive collections of butterflies and moths that are available. For example, some Lepidoptera species may develop ‘lost generations’, in which warmer temperatures signal caterpillars to develop into adults rather than entering diapause. The adults of the last generation may suffer high mortality rates at the onset of winter; for a more thorough discussion of this topic, see [66]. Museum specimens of moths and butterflies could inform how common it is for Lepidoptera species to add another generation in response to climate change, and contrasting herbarium specimens of their host plants could reveal how herbivory is differentially impacted by species that have and have not added generations with climate change.
(b). Distributional shifts
One of the most supported predictions in global change biology is that species' ranges will shift poleward and upward in elevation as the climate warms [48,70]. For many insect species, poleward range expansion may be explained by increased over-winter survival and/or feeding owing to warmer winters [71]. For multivoltine insects, longer growing seasons can also increase the number of generations completed per year [72], leading to population growth that might facilitate range expansion if host plants are available [31]. Most predictions on plant species' range shifts are predicated on the assumption that abiotic factors determine range edges; however, biotic factors can also contribute to range limits [73]. There is also growing evidence that biotic factors, such as herbivores and disease can interact with abiotic factors to determine the trailing (more tropical) range edges of some plant species [74,75]. However, the factors that drive range limits at leading and trailing edges remain unknown for most species.
Biological collections typically have associated metadata describing when and where collections were made, and therefore provide rich data on species distributions and distributional shifts over time [70,76–80]. Species distribution models are commonly used to map past and present distributions, but they are intrinsically limited by the number and representation of input records, and, in the case of global change research, the number of records available from before and after global change. The extensive digitization efforts currently underway for insect and plant specimens will improve our predictions and ability to track changing distributions. For well sampled plant species, we might also be able to investigate changes in herbivory at poleward range edges to determine if it has declined over time as plant ranges expand into novel habitats—an extension of the enemy release hypothesis associated with species invasions, discussed below.
Larger digital collections of insect herbivores will provide the opportunity to compare range shifts across insect clades and to identify traits that govern range expansion and contraction. For example, we might expect that warmer winters will disrupt winter diapause for many insect species, leading to range contraction and decline, while those that do not have diapause will benefit from higher rates of winter survival [31]. However, it is also possible that insects with diapause are more likely to maintain phenological synchrony with their hosts [81]. We might then predict that plant–herbivore interactions would vary not only with warming temperatures, but also with the relative proportion of insect herbivores with winter diapause. Currently, widespread data on which insect species diapause is not available. Information deposited in centralized databases on diapause along with other life-history traits that might mediate insect herbivore range shifts would allow us to make better predictions on how herbivore damage will change over time. Species traits might also determine whether species shift over time (phenologically) or space, and how these two responses trade-off (see box 1).
Box 1. Changing interactions across space and time.
We have discussed some of the most studied aspects of climate change—shifts in space (distributions) and time (phenology)—and how they might affect interactions between plants and insect herbivores. These changes are concurrent and thus will probably have complex, interactive effects on insect communities and herbivory. Collections may allow us to test hypotheses about what traits mediate whether species are more likely to experience shifts in phenology, distributions, or both simultaneously. For example, we might predict that plants and insects that are good dispersers will be quicker to expand their distributions poleward. Body size is a good proxy for dispersal ability in animals, in plants many species have traits that facilitate long-distance dispersal (e.g. zoochorous, anemochorous, and hydrochorous species), and we might thus predict these species show the strongest geographical shifts in response to climate change. By contrast, we might predict that more sessile animals, and plants with no long-distance dispersal strategy, are more likely to respond by adjusting the timing of their seasonal life-history events (phenology). Collections data on plant and herbivore distributions and phenology may allow us to examine responses in space and time, revealing whether this potential trade-off has consequences for herbivore damage.
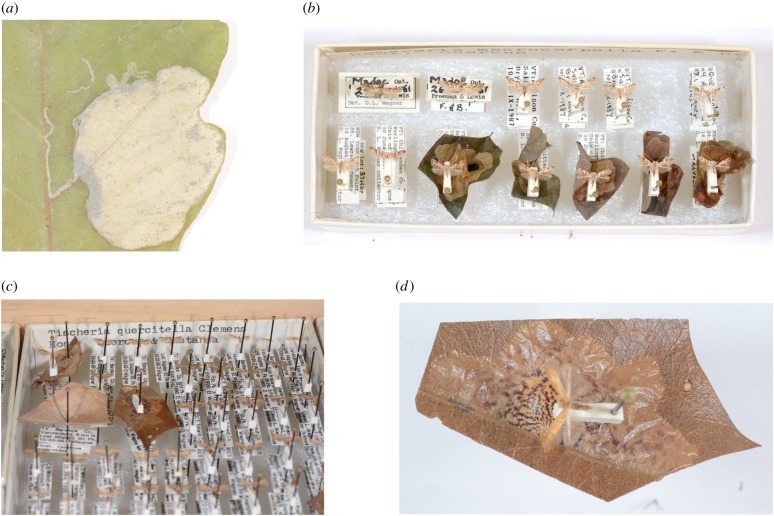
The ability of insect herbivores to switch host plants may be another factor that constrains or facilitates herbivore range expansion, and thus plant–herbivore interaction strengths. Specialized insects that do not feed on newly encountered plant species may be limited in their geographical spread, whereas more generalist herbivores would be less constrained. Herbaria may capture such switches to novel hosts, showing up as new types of herbivore damage on specimens as host plants and their insect herbivores shift their distributions and provide the opportunity for novel plant–herbivore interactions. For example, leaf mines and galls—which are preserved on herbarium specimens—are made by a wide variety of insect herbivore taxa, including some of the most diverse groups of insects—Lepidoptera, Coleoptera (beetles), and Diptera (flies)—and are often specific to insect genera or species (figure 3). The Lepidoptera that make leaf mines are not well represented in long-term citizen science data because leaf miners are typically micromoths, which are not the focus of long-term observations, and leaf mining and galling damage are only rarely included in herbivory studies, which tend to focus on chewing damage [40]. Thus, herbarium specimens provide a record of a unique insect herbivore fauna not represented in long-term herbivore monitoring or herbivory studies.
Figure 3.
Unique signatures of insect herbivore damage on herbarium specimens that can be linked to herbivore taxa. (a) This image shows a leaf mine on a Quercus alba herbarium specimen from the northeastern US made by Acrocercops strigosus (Lepidoptera: Gracillariidae). Several aspects of this leaf mine are diagnostic, such as its presence on the upper surface of a young leaf and its unique shape. (b–d) Leaf miners can be reared and are thus represented in insect collections, allowing researchers to link mines to insect species. (d) This Tischeria quercitella (Lepidoptera: Tischeriidae) specimen was reared from its mine. The purplish streaks and circular refugium in the centre of the mine are diagnostic for this species. (Online version in colour.)
Herbarium specimens may also provide data on a key hypothesis in global change biology that is based on theory which dates back to Darwin: the role of natural enemy release in species invasions. The enemy release hypothesis describes the escape from native predators and parasites when species are introduced into novel habitats. While there is evidence that introduced plants escape their native herbivores [82], it is unclear how long this ‘release’ persists. Herbarium specimens can provide rare long-term data on herbivory and disease pressure [12,83,84] that allows us to resolve this question. In a well-documented example, Schilthuizen et al. [64] used herbarium specimens to show that the non-native cherry tree, Prunus serotina, acquired higher rates of herbivory over time after its introduction to Europe, while its native congener, Prunus padus, had stable herbivory levels over the same time period. This led to field investigations into the contemporary herbivore communities for these congeners, which revealed that, surprisingly, P. serotina had a richer herbivore community than the native P. padus, and that P. serotina had acquired specialized herbivores from other native host genera. This supports the hypothesis that non-native plants accumulate herbivore taxa over time in their novel habitats, which might have significant implications for plants that shift in their geographical distributions.
(c). Urbanization
Urbanization affects insect herbivores via a variety of mechanisms, including habitat fragmentation, habitat and host plant loss, and introduction of novel host plants that attract and support non-native herbivore communities. Given these concurrent pressures, the effects of urbanization on plant–herbivore relationships are complex and varied (as reviewed in [37]). However, in recent years, it has become increasingly clear that a key aspect of urbanization, the urban heat island effect, can drive relationships between plants and herbivores and may uniquely inform climate change predictions. The urban heat-island effect—the local warming of urban areas relative to surrounding countryside—increases urban temperatures 1–12°C higher than rural temperatures [85]. Thus, local warming caused by urban development is similar in magnitude to warming expected globally over the next 100+ years [86], and it has therefore been suggested that cities may provide insights into the future effects of climate change [87,88]. Like global warming, urban warming drives phenological advance in plants and insect herbivores. For example, plants leaf out and flower earlier in cities than in nearby rural areas [89,90], and urban heat is associated with earlier egg production for certain insect herbivore species [91]. While the effects of warming global temperatures on the synchrony of plant–herbivore interactions is still generally unresolved owing to a lack of data, these relationships can be studied across urban temperature gradients, and there is some evidence for reduced synchrony between insect herbivores and their natural enemies as a result of urban warming [91].
Because of the parallels between the abiotic and biotic effects of urban and global warming, natural history collections from urban areas may allow us to more broadly predict how global climate warming will affect interactions between plants and their insect herbivores. Phenology data from specimens—e.g. flowering, leaf-out, insect flight—paired with data on urbanization intensity in the areas where specimens were collected could inform predictions on phenological change and synchrony for a broad range of plant and herbivore species. Specimen data from urban areas are, perhaps surprisingly, plentiful. A recent study shows that across three areas with large digitized herbarium collections—the US, South Africa and Australia—plant specimens are often collected close to natural history museums or roads [11]. Thus, specimens could be used to explore urban-natural gradients. Like temperature, urbanization can be easily assigned to historical specimens via contemporary measurements or existing data. As a proxy for urbanization, we can use human population density data from censuses, which many countries have been collecting since the early 1900s, and in some places urbanization can be translated from historical maps or as impervious surface derived from satellite imagery. One novel approach might be to derive markers of urbanization from the herbarium specimens themselves, for example, signature pollutants [15,16,92], although disentangling the contributions of different drivers would then present additional challenges.
A key mechanism by which urbanization could influence herbivory is via insect herbivore abundance. In recent years, growing evidence shows that urban warming may increase abundance of certain herbivores, notably sap-feeders [93–95], potentially leading to more insect damage on urban than on rural plants, a pattern that has been documented by entomologists for over a century [96]. Sap-feeding herbivores, such as scale insects and aphids, are often preserved on leaves and branches and thus may provide insights into changing herbivore pressure in response to urbanization. In a recent study, Youngsteadt et al. [88] counted armoured scale insects (Hemiptera: Diaspididae) on branches of herbarium specimens of red maple Acer rubrum and on branches of live trees across an urban warming gradient. Using these data, they showed that inter-annual warming and urban warming may have surprisingly congruent effects on scale insect prevalence. In box 2, we discuss how herbarium specimens might be used to investigate more complicated interactions between multiple trophic levels, relationships that could inform biological control efforts and management of urban plants.
Box 2. Herbarium specimens for tracking multitrophic interactions: an example.
The complex interaction between drivers of global change is magnified by the complexity of ecological interactions within ecological communities. The extent of herbivore damage experienced by any particular host plant is mediated by multiple trophic levels, including the natural enemies of insect herbivores, and their parasites and parasitoids. Herbarium specimens could provide information on these multitrophic responses to global change for certain taxa. For example, armoured scale insects (Hemiptera: Diaspididae) create waxy covers above their bodies that are preserved on herbarium specimens [88]. When parasitoids emerge from the scale insects, they chew exit holes in these waxy covers. Thus, scale insects that are preserved on herbarium specimens can also harbour data on parasitism rates. If lower trophic levels are shifting phenology more with climate change [3,4], then we may expect parasitism rates to decrease with both urban heat and climate change, and this may, in turn, influence herbivore damage; herbaria provide the opportunity to test this hypothesis. Here, we show data on another tritrophic interaction that is indirectly captured on herbarium specimens, that between scale insects, host plants, and sooty mould. Scale insects and other phloem-feeders produce honeydew—frass that is mostly composed of sugar water—that contacts leaves, where it is colonized by sooty mould fungi that inhibit photosynthesis [97]. Sooty moulds are preserved on herbarium specimens, perhaps more frequently than are the aphids and scale insects whose honeydew promotes sooty mould. The prevalence of sooty mould could thus be used as a proxy for damage from sap feeding insect herbivores.
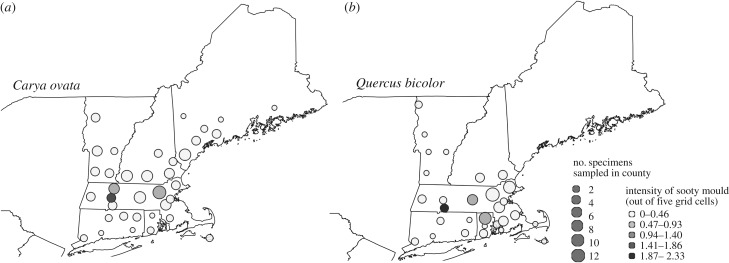
We documented patterns of sooty mould on two canopy tree species across the northeastern US, Quercus bicolor, the swamp white oak, and Carya ovata, shagbark hickory. We examined a total of 217 specimens from the Harvard University Herbaria. A grid with 5×5 cm cells was placed over herbarium specimens, and we scored presence or absence of sooty mould on five randomly selected cells with at least one-fourth leaf cover (for a more thorough description of this method, see [12]). For each specimen, we derived mean May temperatures from PRISM Climate Group gridded data (http://prism.oregonstate.edu) in the county, month, and year where a specimen was collected [98], and modelled predictors of sooty mould prevalence among specimens using generalized linear models with a logit link function in the lme4 package [99] in R [100]. In the first model, we included the following predictors (all as fixed effects): specimen latitude, longitude, May temperature—the month when many phloem-feeders develop and produce honeydew in the area—and day of year, as specimens collected later in the year may be more likely to have acquired sooty mould. In a second model, we assessed effects of urbanization as human population density estimated from the US Census (as in [65]) and day of year as predictors; we assessed urbanization separately because it was highly correlated with latitude. Though sooty mould was only present on few specimens, it showed strong responses to environmental predictors. Sooty mould was more likely to occur on specimens from lower latitudes (Z = −0.76, p = 0.033) and warmer areas (Z = 0.73, p < 0.0001). Sooty mould was also more likely to be on specimens from areas of high human population density (Z = 0.39, p = 0.004) and, as expected, on specimens collected later in the year (from first model described above: Z = 1.80, p < 0.0001).
Our analysis is only preliminary, but indicates that sooty mould may increase in this region with uncertain effects on plant physiology. Future studies could expand this analysis to more plant species and explore whether sooty mould is associated with markers of plant function that can also be measured in herbarium specimens, such as water use efficiency [101]. Perhaps more significantly, if sooty moulds are indeed good markers for sap feeders, our analyses also suggest that sap feeders will increase in prevalence in the northeastern US as the climate warms and as urbanization increases. In addition to creating substrate for sooty mould, sap feeding insects directly reduce plant growth and reproduction as they feed [102].
While the urban heat island effect benefits certain herbivores that survive within the urban matrix by advancing their phenology [91] and increasing their abundances [93–95], urbanization also excludes some insect species—a pattern which has been documented with insect museum specimens [103]—making the effects of urbanization on herbivore damage to plants difficult to predict. Long-term records of butterfly flight from Britain showed that habitat loss is associated with butterfly decline, especially for species that are less mobile and are habitat specialists [104]. Relatedly, a recent study across 16 European cities showed that leaf chewing damage was lower in cities relative to nearby rural areas, perhaps driven in part by higher rates of bird and ant predation on insect herbivores in cities than in rural areas [105]. Thus, a pattern that might be emerging from the literature is that certain sap-feeding insects benefit from urban heat (see above), while leaf chewing and the insects that cause this type of damage, notably Lepidoptera, decline in response to loss of habitat and host plants caused by urbanization. This finding suggests that the effects of bottom-up versus top-down forces driving insect herbivore fitness might differ among feeding guilds (sap-feeders versus leaf chewers). Measurements of broad-scale chewing herbivory (as in [12]), presence of sap-feeders, and incidence of sooty mould as a proxy (see box 2) from herbarium specimens, along with insect herbivore occurrence data, could be used to test this hypothesis (figure 4).
Figure 4.
Distribution of sooty mould on herbarium specimens across the northeastern US. Herbarium specimens in more southern locations were significantly more likely to have sooty mould than those at more northern locations, indicating that sooty moulds, which inhibit plant photosynthesis, and sap-feeding insects may become more prevalent in more northern locations owing to climate warming.
In addition to describing the effects of urbanization at the local scale, museum specimens may also reveal how urbanization affects species distributions at broader spatial scales. For example, while urbanization may disrupt poleward range expansion for some species, it is possible that cities serve as warm habitat stepping stones for species with long-distance dispersal mechanisms, facilitating their poleward expansion. The insects that create leaf mines have been described as ‘aerial plankton’ because they tend to disperse long distances. Herbarium specimens might capture this rapid northern expansion of leaf mining insects and provide a record of shifting interactions with native plants that may be more likely to respond in time (shifting their phenology) than space (shifting their geographical distributions)—see box 1. Similarly, a comparison of herbivory damage on herbarium specimens of non-native plants in urban versus rural environments might provide insights into one pathway towards species invasion. Many non-native plant species are introduced into urban areas [106], and urbanization may provide warm, enemy-free space where they can establish and subsequently expand. Though the role of urbanization in natural enemy release and subsequent invasions is not well characterized, we might predict that non-native plants escape their natural enemies in urban areas and experience increased herbivory rates when they move into natural areas where they encounter a higher diversity of herbivores.
3. Adaptation and agriculture
Responses to global change, such as those in space and time, discussed above, encompass plasticity in behaviour or physiology and distributional shifts, which may be rapid [107]. However, there is growing evidence that evolutionary responses might also be rapid [108], assuming there is sufficient standing genetic variation for selection to act upon [109]. While fluctuating selection can maintain this standing variation [110], providing the raw material for future adaptive responses, strong directional selection, such as that imposed by anthropogenic climate warming, can erode genetic variation and potentially impede evolutionary adaptation, elevating population extinction risk [111]. Insect herbivores and the plants they feed upon are locked in an evolutionary arms race, and insect herbivory drives contemporary plant evolution, changing plant allele frequencies within a few generations [112]. It is likely that climate-induced shifts in herbivory will impose additional selection on both plants and insect herbivores already under pressure from direct effects of climate change.
Natural history collections that span multiple generations can provide a record of evolutionary changes and constraints [58]. It can be difficult, however, to disentangle plastic and evolutionary responses [113]. Evolutionary responses can be predicted from the breeder's equation, but this requires extensive long-term population data [114]. For species that can be stored in a dormant state, such as plants, it is possible to contrast ancestral and descendent genotypes grown under common conditions, and Franks et al. [115] were able to demonstrate evolution to earlier flowering in Brassica using stored seeds. Seedbanks and other collections that hold propagules, intentionally or incidentally, could thus provide important data for exploring evolutionary responses [116] and testing whether species might be approaching limits in their adaptive responses [117]. Sequencing of archived tissue of plants and animals already allows for the signature of selection to be sought directly in their DNA [118–120]. New collections could systematically sample seeds (or tissue) through time or across populations, providing the potential to resurrect past populations (or their genotypes) and examine micro-evolutionary change [121].
Evolutionary insights from herbarium specimens might be particularly useful for adapting agricultural practices with global change. Alongside the insights that collections data can provide on ecological and impacts of global change in natural systems, herbaria are additionally repositories of crop wild relatives (CWR). CWR are important sources of phenotypic and genetic information on pest and disease resistance that may be introgressed into crops [122]. For example, comparative analyses of CWR might provide an opportunity to identify herbivore-resistance traits relevant to agricultural and ornamental species, such as glandular trichomes that act as physical defences against insects and can be detected on herbarium specimens with a microscope. Herbaria provide a record of this genetic diversity even when it is no longer present in the wild. In addition, herbivore damage on CWR herbarium specimens might help predict increases in pest pressure on crops, because closely related host species tend to be vulnerable to similar suites of pests and pathogens [123].
Specimens in herbaria can also serve as records of past biotic threats and inform how we can avoid these threats in the future. For example, Yoshida et al. [124] sequenced the genomes of Phytophthora infestans, the cause of potato late blight, infamous for its role in the Irish Potato Famine, from herbarium collections of infected potatoes and tomatoes. Using genomic tools, they found one strain of P. infestans linked to the potato blight in the nineteenth century, but that multiple strains moved globally in the twentieth century [124,125]. Specimens may also offer insights into more recent effects of global change on crop species. In a recent study [65], we quantified historical insect damage on a crop species, the lowbush blueberry, Vaccinium angustifolium, growing in the wild to determine how pest pressure has changed with recent climate change. The lowbush blueberry is an ecologically and economically important endemic species in northeastern North America, whose production has seen recent increases owing to awareness of the health benefits of blueberries [126]. Collection records from the Harvard University Herbaria suggest that herbivore damage has increased in recent years, with evidence that increased herbivory is a result of winter climate warming [65]. This highlights the need for increased monitoring of herbivore species on V. angustifolium and allows the development of proactive pest management practices that could be implemented before economic impacts are felt.
4. Challenges associated with research on natural history collections
(a). Biases, gaps and uncertainty in museum data
Given the millions of plants and insect specimens that are becoming available online, it will increasingly be possible to assess changes in phenological synchrony, distributions, and occurrence over time across diverse taxa and large spatial areas. The sampling of species within museums and herbaria, however, is non-random and often sparse [10], which can present distinct challenges depending on the response variable of interest and how robust the data are for answering particular questions. However, the depth of sampling within natural history collections is difficult to assess because natural history collections data are often dark—without searchable databases—despite efforts to rapidly digitize. Another obstacle is that data associated with museum specimens can have large uncertainty; for example, specimens collected before the advent of geographical information system technology often have only coarse scale location data that may prohibit local-scale analyses.
When assessing phenological change, the most important challenges arise because of biases in collecting. Herbarium specimens are more likely to be collected near roads; rare species are, perhaps unsurprisingly, collected less frequently, and collections are more likely to be made in spring or summer months [11]. Such biases can make specimen data difficult to work with. For example, roads might be warmer than the surrounding countryside, and observations of shifts in phenology through time might, in part, also reflect the increasing extent of the road network. Finally, sampling frequency may bias estimates of first (or last) events, because we are more likely to observe earlier (or later) events with greater sampling intensity [127]. Such sampling biases can make it difficult to compare across species when sampling effort varies, for example, between common and rare species. New methods offer a solution to such challenges. For example, methods have been established for calibrating species distribution models according to known biases in presence-only data [128], and newly constructed statistical models allow robust estimates of the tail of a distribution—in the context of phenology, first flower, for example—even when sampling is uneven [129].
Shifting collection practices may also introduce biases. Herbivory measurements derived from herbarium specimens are probably underestimates in most cases because collectors try to avoid collecting damaged specimens. Even so, herbivory is prevalent on specimens and matches patterns derived from theory and observations from living plants [12]. Importantly, biases introduced by collectors are not necessarily problematic if they do not vary across axes of interest. For example, if collectors are equally likely to collect relatively undamaged specimens across latitude, herbarium specimens might still provide insights into how herbivory varies with latitude. When there are concerns that collecting practices may have influenced observations—for example, perhaps collectors are more or less likely to collect damaged specimens over time—collector identity may be added to statistical models to partially control for such biases.
A unique challenge to using herbarium specimens is that they are eaten by a suite of insects within museums. This loss of material can reduce the use of insect specimens for morphological and genetic analyses. For plants, chewing herbivory created indoors, after a plant was collected, can be confused with damage created while plants were alive. We developed protocols that allowed us to reliably discriminate chewing damage created pre- and post- collection described in [12,65]. Such approaches, however, require careful examination of specimens with a microscope and entomological knowledge to recognize diagnostic features of damage to plants, which is a barrier to large scale, rapid scoring of chewing damage on digitized specimens. Other types of insect damage, such as leaf mines, skeletonization and galls, are almost never a product of insects eating plants within museums, and might be scored more reliably, although their prevalence is lower [12].
(b). The future of collections-based research on species interactions
We have previously argued that herbaria should be repositioned as hubs for ecological research, and we provided suggestions for how to manage collections to promote ecological research on global change [12]. Because the vast majority of research in collections has historically been on taxonomy and systematics, collections are rarely (but increasingly) faced with providing data for ecologists and evolutionary biologists. Stronger relationships between researchers in the field, collections managers, and digital data providers would help ecologists to better address the challenging ecological questions of global change, and, importantly, could also increase funding opportunities for maintaining and building natural history collections, which are often under-funded and threatened by institutional priorities. Here, we provide three suggestions for how natural history collections and ecologists can work together to support global change research on species interactions.
First, it would be helpful to detail the sampling protocol used to collect specimens. In many cases, collecting practices are haphazard, but if, for example, a curator collects a specimen expressly to document a gall, this would change the inferences we can make from this specimen (we discuss this further in [12]). Second, ecologists could engage with curators in projects that involve resampling areas and taxa that have long historical records, ideally at the same time of year and with the same research effort involved in previous collections. Third, specimens are most useful when researchers can associate collections with important predictor variables representing species traits or abiotic data related to global change. New opportunities exist to link specimens to the published literature and trait databases (e.g. TRY Plant Trait Database (https://www.try-db.org/), BIEN (http://bien.nceas.ucsb.edu/bien/)) as well as non-traditional data sources, such as written records [130] and historical photographs [131]. The better integration of new bioinformatics tools and digital databases within biological collections will help transition museums and herbaria into ecological data centres.
Acknowledgements
We thank Anthony Brach and Charles Davis for assistance in the Harvard University Herbaria, and Robert Capers and David Wagner for assistance in the University of Connecticut's George Stafford Torrey Herbarium, and access to his personal leaf miner collection. Two anonymous reviewers provided valuable feedback that improved this manuscript.
Data accessibility
The dataset supporting this article is available from the Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.cq2k7sq [132].
Authors' contributions
Both authors wrote the paper and gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This project was supported by a Discovery Grant from The Natural Sciences and Engineering Research Council of Canada (http://www.nserc-crsng.gc.ca/index_eng.asp) to T.J.D. This material is based upon work supported by the National Science Foundation Postdoctoral Research Fellowship in Biology under Grant no. (1611880) to E.K.M. We thank the Harvard University Herbaria, McGill University, and the University of Connecticut for institutional support.
References
- 1.Magurran AE, Baillie SR, Buckland ST, Dick JM, Elston DA, Scott EM, Smith RI, Somerfield PJ, Watt AD. 2010. Long-term datasets in biodiversity research and monitoring: assessing change in ecological communities through time. Trends Ecol. Evol. 25, 574–582. ( 10.1016/j.tree.2010.06.016) [DOI] [PubMed] [Google Scholar]
- 2.Aono Y, Kazui K. 2008. Phenological data series of cherry tree flowering in Kyoto, Japan, and its application to reconstruction of springtime temperatures since the 9th century. Int. J. Climatol. 28, 905–914. ( 10.1002/joc.1594) [DOI] [Google Scholar]
- 3.Thackeray SJ, et al. 2010. Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Glob. Change Biol. 16, 3304–3313. ( 10.1111/j.1365-2486.2010.02165.x) [DOI] [Google Scholar]
- 4.Thackeray SJ, et al. 2016. Phenological sensitivity to climate across taxa and trophic levels. Nature 535, 241–245. ( 10.1038/nature18608) [DOI] [PubMed] [Google Scholar]
- 5.Visser M, Van Noordwijk A, Tinbergen J, Lessells C. 1998. Warmer springs lead to mistimed reproduction in great tits (Parus major). Proc. R. Soc. Lond. B 265, 1867–1870. ( 10.1098/rspb.1998.0514) [DOI] [Google Scholar]
- 6.Kharouba HM, Ehrlén J, Gelman A, Bolmgren K, Allen JM, Travers SE, Wolkovich EM. 2018. Global shifts in the phenological synchrony of species interactions over recent decades. Proc. Natl Acad. Sci. USA 115, 5211–5216. ( 10.1073/pnas.1714511115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLean BS, et al. 2015. Natural history collections-based research: progress, promise, and best practices. J. Mammal. 97, 287–297. ( 10.1093/jmammal/gyv178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavoie C. 2013. Biological collections in an ever changing world: herbaria as tools for biogeographical and environmental studies. Perspect. Plant Ecol. Evol. Syst. 15, 68–76. ( 10.1016/j.ppees.2012.10.002) [DOI] [Google Scholar]
- 9.Heberling JM, Isaac BL. 2017. Herbarium specimens as exaptations: new uses for old collections. Am. J. Bot. 104, 963–965. ( 10.3732/ajb.1700125) [DOI] [PubMed] [Google Scholar]
- 10.Meyer C, Weigelt P, Kreft H. 2016. Multidimensional biases, gaps and uncertainties in global plant occurrence information. Ecol. Lett. 19, 992–1006. ( 10.1111/ele.12624) [DOI] [PubMed] [Google Scholar]
- 11.Daru BH, et al. 2018. Widespread sampling biases in herbaria revealed from large-scale digitization. New Phytol. 217, 939–955. ( 10.1111/nph.14855) [DOI] [PubMed] [Google Scholar]
- 12.Meineke EK, Davis CC, Davies TJ. 2018. The unrealized potential of herbaria in global change biology. Ecol. Monogr. 00, 1–21. ( 10.1002/ecm.1307) [DOI] [Google Scholar]
- 13.Lees DC, Lack HW, Rougerie R, Hernandez-Lopez A, Raus T, Avtzis ND, Augustin S, Lopez-Vaamonde C. 2011. Tracking origins of invasive herbivores through herbaria and archival DNA: the case of the horse-chestnut leaf miner. Front. Ecol. Environ. 9, 322–328. ( 10.1890/100098) [DOI] [Google Scholar]
- 14.Crawford PHC, Hoagland BW. 2009. Can herbarium records be used to map alien species invasion and native species expansion over the past 100 years? J. Biogeogr. 36, 651–661. ( 10.1111/j.1365-2699.2008.02043.x) [DOI] [Google Scholar]
- 15.Stewart GR, Aidar MPM, Joly CA, Schmidt S. 2002. Impact of point source pollution on nitrogen isotope signatures (delta N-15) of vegetation in SE Brazil. Oecologia 131, 468–472. ( 10.1007/s00442-002-0906-8) [DOI] [PubMed] [Google Scholar]
- 16.Sigal LL, Nash TH. 1983. Lichen communities on conifers in southern California mountains: an ecological survey relative to oxidant air pollution. Ecology 64, 1343–1354. ( 10.2307/1937489) [DOI] [Google Scholar]
- 17.Bertin RI. 2002. Losses of native plant species from Worcester, Massachusetts. Rhodora 104, 325–349. [Google Scholar]
- 18.Dolan RW, Moore ME, Stephens JD. 2011. Documenting effects of urbanization on flora using herbarium records. J. Ecol. 99, 1055–1062. ( 10.1111/j.1365-2745.2011.01820.x) [DOI] [Google Scholar]
- 19.Southwood T. 1961. The number of species of insect associated with various trees. J. Anim. Ecol. 30, 1–8. ( 10.2307/2109) [DOI] [Google Scholar]
- 20.Janzen DH. 1968. Host plants as islands in evolutionary and contemporary time. Am. Nat. 102, 592–595. ( 10.1086/282574) [DOI] [Google Scholar]
- 21.Ehrlich PR, Raven PH. 1964. Butterflies and plants: a study in coevolution. Evolution 18, 586–608. ( 10.1111/j.1558-5646.1964.tb01674.x) [DOI] [Google Scholar]
- 22.Futuyma DJ, Agrawal AA. 2009. Macroevolution and the biological diversity of plants and herbivores. Proc. Natl Acad. Sci. USA 106, 18 054–18 061. ( 10.1073/pnas.0904106106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Endara M-J, Coley PD, Ghabash G, Nicholls JA, Dexter KG, Donoso DA, Stone GN, Pennington RT, Kursar TA. 2017. Coevolutionary arms race versus host defense chase in a tropical herbivore–plant system. Proc. Natl Acad. Sci. USA 114, E7499–E7505. ( 10.1073/pnas.1707727114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forister ML, et al. 2015. The global distribution of diet breadth in insect herbivores. Proc. Natl Acad. Sci. USA 112, 442–447. ( 10.1073/pnas.1423042112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belovsky G, Slade J. 2000. Insect herbivory accelerates nutrient cycling and increases plant production. Proc. Natl Acad. Sci. USA 97, 14 412–14 417. ( 10.1073/pnas.250483797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Classen AT, Chapman SK, Whitham TG, Hart SC, Koch GW. 2007. Genetic-based plant resistance and susceptibility traits to herbivory influence needle and root litter nutrient dynamics. J. Ecol. 95, 1181–1194. ( 10.1111/j.1365-2745.2007.01297.x) [DOI] [Google Scholar]
- 27.Zhang W, Ricketts TH, Kremen C, Carney K, Swinton SM. 2007. Ecosystem services and dis-services to agriculture. Ecol. Econ. 64, 253–260. ( 10.1016/j.ecolecon.2007.02.024) [DOI] [Google Scholar]
- 28.Flower CE, Knight KS, Gonzalez-Meler MA. 2013. Impacts of the emerald ash borer (Agrilus planipennis Fairmaire) induced ash (Fraxinus spp.) mortality on forest carbon cycling and successional dynamics in the eastern United States. Biol. Invasions 15, 931–944. ( 10.1007/s10530-012-0341-7) [DOI] [Google Scholar]
- 29.Donovan GH, Butry DT, Michael YL, Prestemon JP, Liebhold AM, Gatziolis D, Mao MY. 2013. The relationship between trees and human health: evidence from the spread of the emerald ash borer. Am. J. Prev. Med. 44, 139–145. ( 10.1016/j.amepre.2012.09.066) [DOI] [PubMed] [Google Scholar]
- 30.Sarkar C, Webster C, Gallacher J. 2018. Residential greenness and prevalence of major depressive disorders: a cross-sectional, observational, associational study of 94 879 adult UK Biobank participants. Lancet Planet. Health 2, e162–e173. ( 10.1016/S2542-5196(18)30051-2) [DOI] [PubMed] [Google Scholar]
- 31.Bale JS, et al. 2002. Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob. Change Biol. 8, 1–16. ( 10.1046/j.1365-2486.2002.00451.x) [DOI] [Google Scholar]
- 32.Kingsolver JG, Diamond SE, Buckley LB. 2013. Heat stress and the fitness consequences of climate change for terrestrial ectotherms. Funct. Ecol. 27, 1415–1423. ( 10.1111/1365-2435.12145) [DOI] [Google Scholar]
- 33.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kearney M, Shine R, Porter WP. 2009. The potential for behavioral thermoregulation to buffer ‘cold-blooded’ animals against climate warming. Proc. Natl Acad. Sci. USA 106, 3835–3840. ( 10.1073/pnas.0808913106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bale J, Hayward S. 2010. Insect overwintering in a changing climate. J. Exp. Biol. 213, 980–994. ( 10.1242/jeb.037911) [DOI] [PubMed] [Google Scholar]
- 36.Hallmann CA, et al. 2017. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12, e0185809 ( 10.1371/journal.pone.0185809) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raupp MJ, Shrewsbury PM, Herms DA. 2010. Ecology of herbivorous arthropods in urban landscapes. Annu. Rev. Entomol. 55, 19–38. ( 10.1146/annurev-ento-112408-085351) [DOI] [PubMed] [Google Scholar]
- 38.Johnson MTJ, Munshi-South J. 2017. Evolution of life in urban environments. Science 358, eaam8327 ( 10.1126/science.aam8327) [DOI] [PubMed] [Google Scholar]
- 39.Grimm NB, Faeth SH, Golubiewski NE, Redman CL, Wu J, Bai X, Briggs JM. 2008. Global change and the ecology of cities. Science 319, 756–760. ( 10.1126/science.1150195) [DOI] [PubMed] [Google Scholar]
- 40.Turcotte MM, Thomsen CJ, Broadhead GT, Fine PV, Godfrey RM, Lamarre GP, Meyer ST, Richards LA, Johnson MT. 2014. Percentage leaf herbivory across vascular plant species. Ecology 95, 788 ( 10.1890/13-1741.1) [DOI] [Google Scholar]
- 41.Thiers B. Continuously updated. Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium, accessed January 2018.
- 42.Short AEZ, Dikow T, Moreau CS. 2018. Entomological collections in the age of big data. Annu. Rev. Entomol. 63, 513–530. ( 10.1146/annrev-ento-031616035536) [DOI] [PubMed] [Google Scholar]
- 43.Nelson G, Ellis S. 2018. The history and impact of digitization and digital data mobilization on biodiversity research in the Anthropocene. Phil. Trans. R. Soc. B 373, 20170391 ( 10.1098/rstb.2017.0391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz MD. 1998. Green-wave phenology. Nature 394, 839 ( 10.1038/29670) [DOI] [Google Scholar]
- 45.Schwartz MD, Ault TR, Betancourt JL. 2013. Spring onset variations and trends in the continental United States: past and regional assessment using temperature-based indices Int. J. Climatol. 33, 2917–2922. ( 10.1002/joc.3625) [DOI] [Google Scholar]
- 46.Schwartz MD, Reiter BE. 2000. Changes in North American spring. Int. J. Climatol. 20, 929–932. ( 10.1002/1097-0088(20000630)20:8%3C929::AID-JOC557%3E3.0.CO;2-5) [DOI] [Google Scholar]
- 47.Ault TR, Zurita-Milla R, Schwartz MD. 2015. A Matlab© toolbox for calculating spring indices from daily meteorological data. Comput. Geosci. 83, 46–53. ( 10.1016/j.cageo.2015.06.015) [DOI] [Google Scholar]
- 48.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42. ( 10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 49.Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA. 2003. Fingerprints of global warming on wild animals and plants. Nature 421, 57–60. ( 10.1038/nature01333) [DOI] [PubMed] [Google Scholar]
- 50.Wolkovich EM, et al. 2012. Warming experiments underpredict plant phenological responses to climate change. Nature 485, 494–497. ( 10.1038/nature11014) [DOI] [PubMed] [Google Scholar]
- 51.Willis CG, et al. 2017. Old plants, new tricks: phenological research using herbarium specimens. Trends Ecol. Evol. 32, 532–546. ( 10.1016/j.tree.2017.03.015) [DOI] [PubMed] [Google Scholar]
- 52.Primack D, Imbres C, Primack RB, Miller-Rushing AJ, Del Tredici P. 2004. Herbarium specimens demonstrate earlier flowering times in response to warming in Boston. Am. J. Bot. 91, 1260–1264. ( 10.3732/ajb.91.8.1260) [DOI] [PubMed] [Google Scholar]
- 53.Everill PH, Primack RB, Ellwood ER, Melaas EK. 2014. Determining past leaf-out times of New England's deciduous forests from herbarium specimens. Am. J. Bot. 101, 1293–1300. ( 10.3732/ajb.1400045) [DOI] [PubMed] [Google Scholar]
- 54.Park DS, Williams AC, Law E, Ellison AM, Davis CC. 2018. Herbarium specimens reveal substantial and unexpected variation in phenological sensitivity across the easterm United States. Phil. Trans. R. Soc. B 374, 20170394 ( 10.1098/rstb.20170394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis CC, Willis CG, Connolly B, Kelly C, Ellison AM. 2015. Herbarium records are reliable sources of phenological change driven by climate and provide novel insights into species’ phenological cueing mechanisms. Am. J. Bot. 102, 1599–1609. ( 10.3732/ajb.1500237) [DOI] [PubMed] [Google Scholar]
- 56.Peñuelas J, Filella I, Comas P. 2002. Changed plant and animal life cycles from 1952 to 2000 in the Mediterranean region. Glob. Change Biol. 8, 531–544. ( 10.1046/j.1365-2486.2002.00489.x) [DOI] [Google Scholar]
- 57.Sheridan JA, Caruso NM, Apodaca JJ, Rissler LJ. 2018. Shifts in frog size and phenology: testing predictions of climate change on a widespread anuran using data from prior to rapid climate warming. Ecol. Evol. 8, 1316–1327. ( 10.1002/ece3.3636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holmes MW, et al. 2016. Natural history collections as windows on evolutionary processes. Mol. Ecol. 25, 864–881. ( 10.1111/mec.13529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kharouba HM, Lewthwaite JMM, Guralnick R, Kerr JT, Vellend M. 2018. Using insect natural history collections to study global change impacts: challenges and opportunities. Phil. Trans. R. Soc. B 373, 20170405 ( 10.1098/rstb.2017.0405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vellend M, Brown CD, Kharouba HM, McCune JL, Myers-Smith IH. 2013. Historical ecology: using unconventional data sources to test for effects of global environmental change. Am. J. Bot. 100, 1294–1305. ( 10.3732/ajb.1200503) [DOI] [PubMed] [Google Scholar]
- 61.Brooks SJ, Self A, Powney GD, Pearse WD, Penn M, Paterson GL. 2017. The influence of life history traits on the phenological response of British butterflies to climate variability since the late-19th century. Ecography 40, 1152–1165. ( 10.1111/ecog.02658) [DOI] [Google Scholar]
- 62.Bartomeus I, Ascher JS, Wagner D, Danforth BN, Colla S, Kornbluth S, Winfree R. 2011. Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proc. Natl Acad. Sci. USA 108, 20 645–20 649. ( 10.1073/pnas.1115559108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kharouba HM, Vellend M. 2015. Flowering time of butterfly nectar food plants is more sensitive to temperature than the timing of butterfly adult flight. J. Anim. Ecol. 84, 1311–1321. ( 10.1111/1365-2656.12373) [DOI] [PubMed] [Google Scholar]
- 64.Schilthuizen M, et al. 2016. Incorporation of an invasive plant into a native insect herbivore food web. PeerJ 4, e1954 ( 10.7717/peerj.1954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meineke EK, Classen AT, Sanders NJ, Davies TJ. 2018. Herbarium specimens reveal increasing herbivory over the past century. J. Ecol. 00, 1–13. [Google Scholar]
- 66.Van Dyck H, Bonte D, Puls R, Gotthard K, Maes D. 2015. The lost generation hypothesis: could climate change drive ectotherms into a developmental trap? Oikos 124, 54–61. ( 10.1111/oik.02066) [DOI] [Google Scholar]
- 67.Netherer S, Schopf A. 2010. Potential effects of climate change on insect herbivores in European forests—general aspects and the pine processionary moth as specific example. Forest Ecol. Manage. 259, 831–838. ( 10.1016/j.foreco.2009.07.034) [DOI] [Google Scholar]
- 68.Evans E, Carlile N, Innes M, Pitigala N. 2013. Warm springs reduce parasitism of the cereal leaf beetle through phenological mismatch. J. Appl. Entomol. 137, 383–391. ( 10.1111/jen.12028) [DOI] [Google Scholar]
- 69.Salis L, van den Hoorn E, Beersma DG, Hut RA, Visser ME. 2018. Photoperiodic cues regulate phenological carry-over effects in an herbivorous insect. Funct. Ecol. 32, 171–180. ( 10.1111/1365-2435.12953) [DOI] [Google Scholar]
- 70.Parmesan C, et al. 1999. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399, 579–583. ( 10.1038/21181) [DOI] [Google Scholar]
- 71.Battisti A, Stastny M, Netherer S, Robinet C, Schopf A, Roques A, Larsson S. 2005. Expansion of geographic range in the pine processionary moth caused by increased winter temperatures. Ecol. Appl. 15, 2084–2096. ( 10.1890/04-1903) [DOI] [Google Scholar]
- 72.Altermatt F. 2010. Climatic warming increases voltinism in European butterflies and moths. Proc. R. Soc. B 277, 1281–1287. ( 10.1098/rspb.2009.1910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sexton JP, McIntyre PJ, Angert AL, Rice KJ. 2009. Evolution and ecology of species range limits. Annu. Rev. Ecol. Evol. Syst. 40, 415–436. ( 10.1146/annurev.ecolsys.110308.120317) [DOI] [Google Scholar]
- 74.Harley CD. 2003. Abiotic stress and herbivory interact to set range limits across a two-dimensional stress gradient. Ecology 84, 1477–1488. ( 10.1890/0012-9658(2003)084%5B1477:ASAHIT%5D2.0.CO;2) [DOI] [Google Scholar]
- 75.Hargreaves AL, Samis KE, Eckert CG. 2013. Are species’ range limits simply niche limits writ large? A review of transplant experiments beyond the range. Am. Nat. 183, 157–173. ( 10.1086/674525) [DOI] [PubMed] [Google Scholar]
- 76.Feeley KJ. 2012. Distributional migrations, expansions, and contractions of tropical plant species as revealed in dated herbarium records. Glob. Change Biol. 18, 1335–1341. ( 10.1111/j.1365-2486.2011.02602.x) [DOI] [Google Scholar]
- 77.Calinger KM. 2015. A functional group analysis of change in the abundance and distribution of 207 plant species across 115 years in north-central North America. Biodivers. Conserv. 24, 2439–2457. ( 10.1007/s10531-015-0936-2) [DOI] [Google Scholar]
- 78.D'Andrea L, Broennimann O, Kozlowski G, Guisan A, Morin X, Keller-Senften J, Felber F. 2009. Climate change, anthropogenic disturbance and the northward range expansion of Lactuca serriola (Asteraceae). J. Biogeogr. 36, 1573–1587. ( 10.1111/j.1365-2699.2008.02060.x) [DOI] [Google Scholar]
- 79.Riera R, Sangil C, Sanson M. 2015. Long-term herbarium data reveal the decline of a temperate-water algae at its southern range. Estuar. Coast. Shelf Sci. 165, 159–165. ( 10.1016/j.ecss.2015.05.008) [DOI] [Google Scholar]
- 80.Wernberg T, Russell BD, Thomsen MS, Gurgel CFD, Bradshaw CJA, Poloczanska ES, Connell SD. 2011. Seaweed communities in retreat from ocean warming. Curr. Biol. 21, 1828–1832. ( 10.1016/j.cub.2011.09.028) [DOI] [PubMed] [Google Scholar]
- 81.van Asch M, Visser ME. 2007. Phenology of forest caterpillars and their host trees: the importance of synchrony. Annu. Rev. Entomol. 52, 37–55. ( 10.1146/annurev.ento.52.110405.091418) [DOI] [PubMed] [Google Scholar]
- 82.Liu H, Stiling P. 2006. Testing the enemy release hypothesis: a review and meta-analysis. Biol. Invasions 8, 1535–1545. ( 10.1007/s10530-005-5845-y) [DOI] [Google Scholar]
- 83.Malmstrom CM, Shu R, Linton EW, Newton LA, Cook MA. 2007. Barley yellow dwarf viruses (BYDVs) preserved in herbarium specimens illuminate historical disease ecology of invasive and native grasses. J. Ecol. 95, 1153–1166. ( 10.1111/j.1365-2745.2007.01307.x) [DOI] [Google Scholar]
- 84.Antonovics J, Hood ME, Thrall H, Abrams JY, Duthie GM. 2003. Herbarium studies on the distribution of anther-smut fungus (Microbotryum violaceum) and Silene species (Caryophyllaceae) in the eastern United States. Am. J. Bot. 90, 1522–1531. ( 10.3732/ajb.90.10.1522) [DOI] [PubMed] [Google Scholar]
- 85.Oke TR. 1973. City size and the urban heat island. Atmos. Environ. 7, 769–779. ( 10.1016/0004-6981(73)90140-6) [DOI] [Google Scholar]
- 86.Pachauri RK, Meyer L, Plattner GK, Stocker T. 2015. IPCC, 2014: Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, IPCC.
- 87.Lahr EC, Dunn RR, Frank SD. 2018. Getting ahead of the curve: cities as surrogates for global change. Proc. R. Soc. B 285, 20180643 ( 10.1098/rspb.2018.0643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Youngsteadt E, Dale AG, Terando AJ, Dunn RR, Frank SD. 2015. Do cities simulate climate change? A comparison of herbivore response to urban and global warming. Glob. Change Biol. 21, 97–105. ( 10.1111/gcb.12692) [DOI] [PubMed] [Google Scholar]
- 89.Neil K, Wu J. 2006. Effects of urbanization on plant flowering phenology: a review. Urban Ecosyst. 9, 243–257. ( 10.1007/s11252-006-9354-2) [DOI] [Google Scholar]
- 90.Polgar CA, Primack RB. 2011. Leaf-out phenology of temperate woody plants: from trees to ecosystems. New Phytol. 191, 926–941. ( 10.1111/j.1469-8137.2011.03803.x) [DOI] [PubMed] [Google Scholar]
- 91.Meineke EK, Dunn RR, Frank SD. 2014. Early pest development and loss of biological control are associated with urban warming. Biol. Lett. 10, 20140586 ( 10.1098/rsbl.2014.0586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weiss D, Shotyk W, Kramers JD, Gloor M. 1999. Sphagnum mosses as archives of recent and past atmospheric lead deposition in Switzerland. Atmos. Environ. 33, 3751–3763. ( 10.1016/S1352-2310(99)00093-X) [DOI] [Google Scholar]
- 93.Dale AG, Frank SD. 2014. Urban warming trumps natural enemy regulation of herbivorous pests. Ecol. Appl. 24, 1596–1607. ( 10.1890/13-1961.1) [DOI] [PubMed] [Google Scholar]
- 94.Meineke EK, Dunn RR, Sexton JO, Frank SD. 2013. Urban warming drives insect pest abundance on street trees. PLoS ONE 8, e59687 ( 10.1371/journal.pone.0059687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meineke E, Youngsteadt E, Dunn RR, Frank SD. 2016. Urban warming reduces aboveground carbon storage. Proc. R. Soc. B 283, 20161574 ( 10.1098/rspb.2016.1574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Putnam JD. 1880. Biological and other notes on Coccidae, Gazette Company, Printers.
- 97.Wood BW, Tedders WL, Reilly CC. 1988. Sooty mold fungus on pecan foliage suppresses light penetration and net photosynthesis. Hortic. Sci. 23, 851–853. [Google Scholar]
- 98.Park DS, Davis CC. 2017. Implications and alternatives of assigning climate data to geographical centroids. J. Biogeogr. 44, 2188–2198. [Google Scholar]
- 99.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 48 ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 100.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 101.Miller-Rushing AJ, Primack RB, Templer PH, Rathbone S, Mukunda S. 2009. Long-term relationships among atmospheric CO2, stomata, and intrinsic water use efficiency in individual trees. Am. J. Bot. 96, 1779–1786. ( 10.3732/ajb.0800410) [DOI] [PubMed] [Google Scholar]
- 102.Zvereva EL, Lanta V, Kozlov MV. 2010. Effects of sap-feeding insect herbivores on growth and reproduction of woody plants: a meta-analysis of experimental studies. Oecologia 163, 949–960. ( 10.1007/s00442-010-1633-1) [DOI] [PubMed] [Google Scholar]
- 103.Fattorini S. 2011. Insect extinction by urbanization: a long term study in Rome. Biol. Conserv. 144, 370–375. ( 10.1016/j.biocon.2010.09.014) [DOI] [Google Scholar]
- 104.Warren MS, et al. 2001. Rapid responses of British butterflies to opposing forces of climate and habitat change. Nature 414, 65–69. ( 10.1038/35102054) [DOI] [PubMed] [Google Scholar]
- 105.Kozlov MV, Lanta V, Zverev V, Rainio K, Kunavin MA, Zvereva EL. 2017. Decreased losses of woody plant foliage to insects in large urban areas are explained by bird predation. Glob. Change Biol. 23, 4354–4364. [DOI] [PubMed] [Google Scholar]
- 106.Reichard SE. 1997. Prevention of invasive plant introductions on national and local levels. In Assessment and management of plant invasions, pp. 215–227. New York, NY: Springer. [Google Scholar]
- 107.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669. ( 10.2307/annurev.ecolsys.37.091305.30000024) [DOI] [Google Scholar]
- 108.Hoffmann AA, Sgrò CM. 2011. Climate change and evolutionary adaptation. Nature 470, 479–485. ( 10.1038/nature09670) [DOI] [PubMed] [Google Scholar]
- 109.Barrett RD, Schluter D. 2008. Adaptation from standing genetic variation. Trends Ecol. Evol. 23, 38–44. ( 10.1016/j.tree.2007.09.008) [DOI] [PubMed] [Google Scholar]
- 110.Bell G. 2010. Fluctuating selection: the perpetual renewal of adaptation in variable environments. Phil. Trans. R. Soc. B 365, 87–97. ( 10.1098/rstb.2009.0150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jump AS, Penuelas J. 2005. Running to stand still: adaptation and the response of plants to rapid climate change. Ecol. Lett. 8, 1010–1020. ( 10.1111/j.1461-0248.2005.00796.x) [DOI] [PubMed] [Google Scholar]
- 112.Agrawal AA, Hastings AP, Johnson MT, Maron JL, Salminen J-P. 2012. Insect herbivores drive real-time ecological and evolutionary change in plant populations. Science 338, 113–116. ( 10.1126/science.1225977) [DOI] [PubMed] [Google Scholar]
- 113.Merilä J. 2012. Evolution in response to climate change: in pursuit of the missing evidence. Bioessays 34, 811–818. ( 10.1002/bies.201200054) [DOI] [PubMed] [Google Scholar]
- 114.Réale D, McAdam AG, Boutin S, Berteaux D. 2003. Genetic and plastic responses of a northern mammal to climate change. Proc. R. Soc. Lond. B 270, 591–596. ( 10.1098/rspb.2002.2224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Franks SJ, Sim S, Weis AE. 2007. Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proc. Natl Acad. Sci. USA 104, 1278–1282. ( 10.1073/pnas.0608379104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Beaton LL, Van Zandt PA, Esselman EJ, Knight TM. 2011. Comparison of the herbivore defense and competitive ability of ancestral and modern genotypes of an invasive plant, Lespedeza cuneata. Oikos 120, 1413–1419. ( 10.1111/j.1600-0706.2011.18893.x) [DOI] [Google Scholar]
- 117.Anderson JT, Inouye DW, McKinney AM, Colautti RI, Mitchell-Olds T. 2012. Phenotypic plasticity and adaptive evolution contribute to advancing flowering phenology in response to climate change. Proc. R. Soc. B 20121051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wandeler P, Hoeck PE, Keller LF. 2007. Back to the future: museum specimens in population genetics. Trends Ecol. Evol. 22, 634–642. ( 10.1016/j.tree.2007.08.017) [DOI] [PubMed] [Google Scholar]
- 119.Lozier JD, Cameron SA. 2009. Comparative genetic analyses of historical and contemporary collections highlight contrasting demographic histories for the bumble bees Bombus pensylvanicus and B. impatiens in Illinois. Mol. Ecol. 18, 1875–1886. ( 10.1111/j.1365-294X.2009.04160.x) [DOI] [PubMed] [Google Scholar]
- 120.Exposito-Alonso M, et al. 2018. The rate and potential relevance of new mutations in a colonizing plant lineage. PLoS Genet. 14, e1007155 ( 10.1371/journal.pgen.1007155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Franks SJ, Avise JC, Bradshaw WE, Conner JK, Etterson JR, Mazer SJ, Shaw RG, Weis AE. 2008. The resurrection initiative: storing ancestral genotypes to capture evolution in action. Bioscience 58, 870–873. [Google Scholar]
- 122.Hodgkin T, Hajjar R. 2007. Using crop wild relatives for crop improvement: trends and perspectives. In Crop wild relative conservation and use (eds Maxted N, Ford-Lloyd BV, Kell SP, Iriondo JM, Dulloo ME, Turok J), pp. 535–548. Rome, Italy: Biodiversity International. [Google Scholar]
- 123.Gilbert GS, Webb CO. 2007. Phylogenetic signal in plant pathogen–host range. Proc. Natl Acad. Sci. USA 104, 4979–4983. ( 10.1073/pnas.0607968104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yoshida K, et al. 2013. The rise and fall of the Phytophthora infestans lineage that triggered the Irish potato famine. Elife 2, e00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Martin MD, et al. 2013. Reconstructing genome evolution in historic samples of the Irish potato famine pathogen. Nat. comm. 4, 2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kaur J, Percival D, Hainstock LJ, Privé J-P. 2012. Seasonal growth dynamics and carbon allocation of the wild blueberry plant (Vaccinium angustifolium Ait.). Can. J. Plant Sci. 92, 1145–1154. ( 10.4141/cjps2011-204) [DOI] [Google Scholar]
- 127.Miller-Rushing AJ, Primack RB. 2008. Global warming and flowering times in Thoreau's Concord: a community perspective. Ecology 89, 332–341. ( 10.1890/07-0068.1) [DOI] [PubMed] [Google Scholar]
- 128.Fithian W, Elith J, Hastie T, Keith DA. 2015. Bias correction in species distribution models: pooling survey and collection data for multiple species. Methods Ecol. Evol. 6, 424–438. ( 10.1111/2041-210X.12242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pearse WD, Davis CC, Inouye DW, Primack RB, Davies TJ. 2017. A statistical estimator for determining the limits of contemporary and historic phenology. Nat. Ecol. Evol. 1, 1876 ( 10.1038/s41559-017-0350-0) [DOI] [PubMed] [Google Scholar]
- 130.Primack RB, Miller-Rushing AJ. 2012. Uncovering, collecting, and analyzing records to investigate the ecological impacts of climate change: a template from Thoreau's Concord. Bioscience 62, 170–181. ( 10.1525/bio.2012.62.2.10) [DOI] [Google Scholar]
- 131.De Frenne P, Van Langenhove L, Van Driessche A, Bertrand C, Verheyen K, Vangansbeke P. 2018. Using archived television video footage to quantify phenology responses to climate change. Methods Ecol. Evol. 9, 1–9. [Google Scholar]
- 132.Meineke EK, Davies TJ. 2018. Data from: Museum specimens provide novel insights into changing plant–herbivore interactions Dryad Digital Repository. ( 10.5061/dryad) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Meineke EK, Davies TJ. 2018. Data from: Museum specimens provide novel insights into changing plant–herbivore interactions Dryad Digital Repository. ( 10.5061/dryad) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The dataset supporting this article is available from the Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.cq2k7sq [132].