Abstract
The potential use of herbarium specimens to detect herbivory trends is enormous but largely untapped. The objective of this study was to reconstruct the long-term herbivory pressure on the Eurasian invasive plant, purple loosestrife (Lythrum salicaria), by evaluating leaf damage over 1323 specimens from southern Québec (Canada). The hypothesis tested is that that the prevalence of herbivory damage on purple loosestrife is low during the invasion phase and increases throughout the saturation phase. Historical trends suggest a gradual increase in hole feeding and margin feeding damage from 1883 to around 1940, followed by a period of relative stability. The percentage of specimens with window feeding damage did not begin to increase until the end of the twentieth century, from 3% (2–6%) in 1990 to 45% (14–81%) in 2015. Temporal changes in the frequency of window feeding damage support the hypothesis of an increasing herbivory pressure by recently introduced insects. This study shows that leaf damage made by insects introduced for the biocontrol of purple loosestrife, such as coleopterans of the Neogalerucella genus, can be assessed from voucher specimens. Herbaria are a rich source in information that can be used to answer questions related to plant-insect interactions in the context of biological invasions and biodiversity changes.
This article is part of the theme issue ‘Biological collections for understanding biodiversity in the Anthropocene’.
Keywords: biocontrol, enemy release hypothesis, Lythraceae, Neogalerucella, voucher specimens
1. Introduction
The ecological and economic concerns raised by biological invasions have spurred research aimed at understanding the mechanisms underlying the success of exotic invasive plants. Several hypotheses have been advanced in this regard, one of the best-known being the enemy release hypothesis [1]. Although there are several variants of this hypothesis (see [2]), they all generally state that if invaders proliferate in their introduced range it is because they are not confronted to enemies (herbivores, pathogens) from their native range. Nevertheless, this absence of enemies may only be temporary. Following an initial invasion phase during which the population of the introduced plant expands, the invader may suffer increasing damage from various native or exotic consumer guilds, or from a pathogen pressure that builds up over time.
Several studies have found that herbivory, or the prevalence of enemy-induced damage, increases with residence time of the introduced plant [3–7]. According to this principle, the probability of encountering enemies increases as the plant distribution range expands and the population densifies during the invasion process [5]. Nevertheless, this trend is not systematically observed [8,9]. Exotic plants also represent a new food source for the native entomofauna, which may develop the ability to locate and exploit it [10]. The impact on plant's fitness varies not only as a function of the type of damage [11], but also according to the time of the year when it occurs [12]. Moreover, damage frequency is influenced by insect density, which varies across time and space [13]. The ultimate consequence of this process is the decline of the plant invader, a phenomenon increasingly reported in the literature [14], though the exact causes are still unknown.
Purple loosestrife (Lythraceae: Lythrum salicaria L.) is an invasive exotic plant native to Eurasia that was introduced to North America in the early ninteenth century, probably for ornamental purposes [15,16], or accidentally in the ballast of ships sailing between continents [17]. In the early stages of the invasion, purple loosestrife was mainly observed along the eastern coast of the USA, but then spread towards the interior of the continent following corridors such as railways, roads or waterways [16,17]. In 1980, purple loosestrife was found throughout the North American continent south of the boreal forest, with particularly high densities in the northeastern United States, southern Ontario and Quebec, Canada [17]. Owing to its rapid expansion and its increasing presence in wetlands, purple loosestrife is perceived as a serious threat to the integrity of marsh ecosystems [18].
Despite the lack of quantitative data documenting the suspected negative impacts on the flora and fauna of North American wetlands [19,20], purple loosestrife has been and still is the target of a multinational (Canada, USA) biological control campaign, which began in the early 1990s. During this campaign, two leaf beetles, Neogalerucella calmariensis L. and Neogalerucella pusilla Duftschmid (Chrysomelidae), were released by the millions to control purple loosestrife because of their host-specificity, their broad geographical distribution in Europe and their reported effect on the growth and vigor of purple loosestrife [21]. Biological control was justified by the absence of natural enemies able to cause significant damage to the plant [21,22]. These biocontrol agents were first introduced in eight American states and one Canadian province, before being released in over 30 states and nine provinces [23–25]. Several North American studies have evaluated the impact of released leaf beetles on purple loosestrife (e.g. [26–29]); yet, few of the above were long-term studies that considered temporal dynamics at the population level and none have quantified the herbivory pressure that prevailed long before the introduction of insects.
Since its introduction, purple loosestrife has been the subject of a large sampling effort of herbarium specimens throughout its distribution. The potential use of herbarium specimens to detect spatio-temporal trends in herbivory is enormous but untapped [6,30–34]. For example, they allowed the detection of geographical differences in herbivory pressure [30], or the study of the historical spread of insect pests [31]. However, studies are complicated by biases associated with collecting, which is never random nor systematic, and amplified by the fact that botanists do not usually sample specimens highly damaged by insects [35,36]. On the other hand, the fact that insects likely to cause significant damage were intentionally introduced, and that the introduction years and locations are accurately known, provide precise benchmarks for assessing the long-term effects of herbivores on purple loosestrife with herbarium specimens.
The main objective of this study was to determine whether, using herbarium specimens, we could reconstruct herbivory trends in an invasive plant, namely purple loosestrife. This study focused on specimens collected in Quebec for four reasons: (i) the province is among the oldest invaded areas in North America, and massive invasions were recorded as early as the 1940s; (ii) herbarium specimens for this species are numerous in Quebec and easily accessible; (iii) the sites and years (1996–1998) where biological control was attempted in the province are well known; and (iv) there are indications that the plant is locally declining [16,20,37,38]. The hypothesis tested was that insect damage was absent or rare in the early stages of the invasion, followed by an increase in the frequency and intensity of damage over time, particularly following the release of insects under the biocontrol programme of the 1990s.
2. Material and methods
(a). Phyllophagous insects associated with purple loosestrife in Quebec
We reviewed the studies that inventoried insects associated with purple loosestrife in North America [22,39,40] to identify guilds of phyllophagous (leaf eating) insects potentially responsible for damage to this plant. Given that these studies were conducted in the USA and Manitoba, the presence of these insects in Quebec was verified using the species list of the Quebec Insect Collection [41–43]. A beetle species (Cyrtepistomus castaneus Roelofs), though absent from this list, was included owing to a Quebec mention recorded by Douglas et al. [44]. Species names were harmonized between studies using the Integrated Taxonomic Information System [45]. We searched the literature to document the types of damage associated with each of the phyllophagous insects reported to feed on purple loosestrife. However, we did not consider insects of the Hemiptera order, as these are piercing-sucking insects that cause leaf distortion or rolling, stippling and necrotic spots [46], which are rarely encountered on herbarium specimens.
(b). Data collection
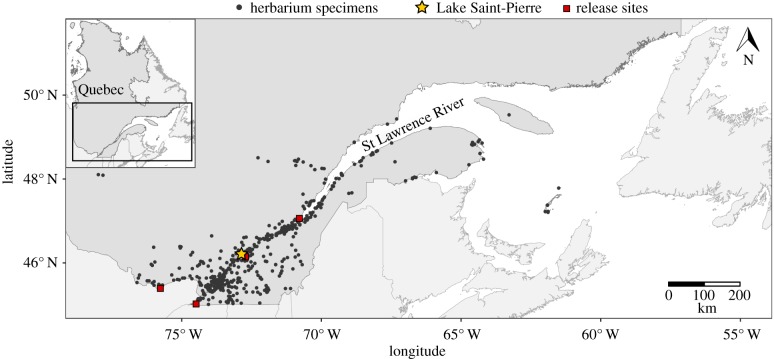
To detect evidence of herbivory in the province of Quebec, 1323 purple loosestrife specimens from four Quebec herbaria (MT: Université de Montréal Herbarium; QFA: Université Laval Herbarium; QUE: Québec Herbarium; UQTR: Université du Québec à Trois-Rivières Herbarium) and two federal herbaria (CAN: Canadian Museum of Nature Herbarium; DAO: Agriculture and Agri-Food Canada Herbarium) were examined (figure 1). The collection date of the specimens was noted, and the geographical coordinates were obtained by referring to a list of official Quebec place names [47]. We favoured the latter approach to avoid introducing uncertainties related to the positioning, transcription or conversion of geographical coordinates. Specimens without a collection year were excluded from the analysis. Those for which the year was not specified, but where information regarding the collection period was available (for example, around 1900), were included in the analysis. Where a time interval was indicated, the mid-point of the interval was considered the collection year.
Figure 1.
Spatial distribution of the purple loosestrife (Lythrum salicaria) herbarium specimens collected in Quebec (Canada). The star indicates the location of Lake Saint-Pierre, where purple loosestrife was sampled in 2016. The squares indicate the four sectors where insects were released (1996–1998) in the province as part of a biological control programme.
(c). Damage evaluation
In the literature, different types of leaf blade damage are attributed to phyllophagous insects, namely hole feeding, margin feeding, surface feeding and skeletonization [48]. The first three types of damage (figure 2) were distinguished in this study, as they were the only ones observed on herbarium specimens. Hole feeding damage was considered a hole of variable shape and size completely piercing the blade. Margin feeding damage was identified by an incomplete blade margin resulting from insect feeding rather than poor specimen preservation. Surface feeding, henceforth called window feeding damage, was characterized by a thin residual membrane not consumed by the insect. For each specimen and type of damage, the number of affected leaves was noted. Only leaves positioned outside of the inflorescence and thus easy to distinguish from the bracts were considered.
Figure 2.
Different types of damage found on purple loosestrife herbarium specimens (Lythrum salicaria) in Quebec (Canada): hole, margin or window.
(d). Estimation of leaf area
As the number of leaves damaged by insect feeding activity is dependent of plant size, the leaf area of each specimen was estimated. This area was used in the statistical models to account for the effect of plant size on the number of damaged leaves. This was accomplished using photographs of the mounted herbarium specimens taken with an EOS Digital Rebel T5i camera (Canon Inc., Tokyo, Japan) equipped with a lens (EF-S 18-135 mm f/3.5–5.6 IS STM). A 0.5 cm grid was overlain on each photograph using the image editing program GIMP, v. 2.8.16 [49]. The number of 0.25 cm2 squares more than half covered by leaves was counted; leaf area was estimated from this number.
(e). Historical reconstruction of herbivory pressure
Generalized additive models (GAMs) were used to estimate changes in herbivory pressure over time from the introduction of the species in Quebec. GAMs are used to describe a temporal demographic response without imposing a predetermined parametric shape [50]. The GAMs were adjusted using the library mgcv [51], where the degree of smoothness of the function was determined using a generalized cross-validation method [52].
As a first step, the probability of observing damaged specimens was modelled from the presence–absence data of damage types observed on specimens. For each type of damage, the probability of observing a damaged specimen (presence or absence of a type of damage) was estimated in relation to the collection year, with the leaf area as a covariate. This was accomplished using a logistic regression model with a smooth term for the collection year and a linear term for the leaf area. The GAM relationships were visualized using partial residual plots (visreg R package; [53]), where an inverse logistic transformation was applied to the regression estimates and confidence intervals. The confidence interval corresponded to ±2 s.e. around the regression estimate. In a second step, only the damaged specimens were considered to determine if the number of damaged leaves increased over time. A regression model (negative binomial distribution) was used, again with a smooth term for the collection year and a linear term for the leaf area of the specimens.
To detect a possible violation of model independence, GAM residuals were visualized as a function of their spatial coordinates [54]. Geary's C was then calculated to evaluate spatial autocorrelation of model residuals [55]. This was accomplished by generating a spatial weight matrix using the geographical coordinates of the specimens with the library spdep [56]. Temporal autocorrelation was also evaluated using correlograms. Statistical analyses were performed using R software, v. 3.3.3 [57].
(f). Evaluation of contemporary herbivory pressure
To estimate herbivory pressure without collection bias, and to have a more current picture of the invasion, 150 purple loosestrife specimens were collected in the wetlands of Lake Saint-Pierre (figure 1), a widening of the St Lawrence River, which has the greatest concentration of freshwater marshes and swamps (16 762 ha) of the province of Quebec [38]. This area was selected because it represents the largest (1375 ha) and oldest (since at least the 1930s) documented purple loosestrife invasion in North America. Evidence suggests that this population has been declining since at least the early 2000s [20,38], possibly owing to increased herbivory pressure. Moreover, biocontrol agents (N. calmariensis and N. pusilla) were introduced to Lake Saint-Pierre from 1996 to 1998 [37]. Exploratory fieldwork in 2015 revealed that the beetles were present, and that some purple loosestrife plants were severely damaged. A total of 150 purple loosestrife specimens were collected from 8 to 10 August 2016 in five sectors on the north shore of the lake. This shore is the most accessible and the least disturbed by agricultural activities. The sectors were: (i) Maskinongé (46°11′29″ N; 73°00′07″ E), (ii) Louiseville (46°13′07″ N; 72°55′12″ E), (iii) Porte de la Mauricie (46°15′02″ N; 72°51′49″ E), (iv) Yamachiche (46°15′42″ N; 72°48′47″ E), and (v) Pointe-du-Lac (46°17′26″ N; 72°43′54″ E). In each sector, 30 geographical coordinates were randomly drawn from a 1m spaced grid, and the purple loosestrife specimen closest to each coordinate set was located in the field with a geographical positioning system and then sampled. Specimens were harvested near the end of the summer to detect insect damage incurred over the purple loosestrife growing season. Specimens were pressed and dried. Damage was evaluated using the method previously described for herbarium specimens. The proportion of damaged specimens, as well as the average number of damaged leaves were calculated for each type of damage.
3. Results
(a). Phyllophagous insects associated with purple loosestrife in Quebec
Most of the phyllophagous insects found on purple loosestrife in the United States and in Manitoba are also present in Quebec. A total of 36 phyllophagous insects could damage leaf blades and consequently leave hole, margin or window feeding damage on purple loosestrife herbarium specimens (table 1; electronic supplementary material, table S1). Among these insects, 18 taxa belong to the order of Coleoptera and 16 to the order of Lepidoptera. The two other taxa belong to the orders of Hymenoptera and Orthoptera. Apart from the biological control agents, the majority of these insects are generalists and only occasionally feed on purple loosestrife. About 10 taxa seem to more frequently use purple loosestrife as they are more abundant on this plant than the other insects.
Table 1.
Phyllophagous insects most frequently associated with purple loosestrife (Lythrum salicaria) in North America. (These taxa were identified based on abundance criteria (greater than five specimens sampled per hour) from Hight [22], as well as the number of collected specimens (greater than 50 specimens) from Diehl et al. [40]. The insect development stages are: adult (A), larva (L) or nymph (N). The different types of leaf damage are: hole (H), margin (M) or window (W).)
| taxon | development stage | damage type | reference |
|---|---|---|---|
| Coleoptera: | |||
| Chrysomelidae | |||
| Altica sp. (Geoffroy) | A | W, H | [58] |
| Crepidodera nana (Say) | A | W, H | [59] |
| Galerucella nymphaeae (L.) | L, A | W | [60] |
| Neogalerucella calmariensis (L.)a | L, A | W, H | [61] |
| Neogalerucella pusilla (Duftschmid)a | L, A | W, H | [61] |
| Scarabaeidae | |||
| Popillia japonica (Newman) | A | H | [62] |
| Hymenoptera: | |||
| Tenthredinidae | |||
| Ametastegia glabrata (Fallen) | L | H | [63] |
| Lepidoptera: | |||
| Arctiidae | |||
| Spilosoma virginica (Fabricius) | L | W, H | [64] |
| Noctuidae | |||
| Eudryas unio (Hübner) | L | M | personal observations (2016) |
| Orthoptera: | |||
| Acrididae | |||
| Melanoplus femurrubrum (De Geer) | N, A | M, H | [65] |
aSpecies introduced for the biological control programme of purple loosestrife were added and noted.
It was not possible to find references specifying the types of leaf damage associated with certain insects. In fact, information on the feeding activities of many generalist insects which are not considered pests or nuisances is fragmentary. In addition, some insects cause more than one type of damage depending on their developmental stage. Within our list of phyllophagous insects feeding on purple loosestrife, Coleopterans mainly cause hole feeding damage (65% of the Coleopteran taxa in our list), whereas Lepidopterans, which often cause more than one type of damage, are more likely to yield margin or window feeding damage (64% of the Lepidopteran taxa in our list).
(b). Historical reconstruction of herbivory pressure
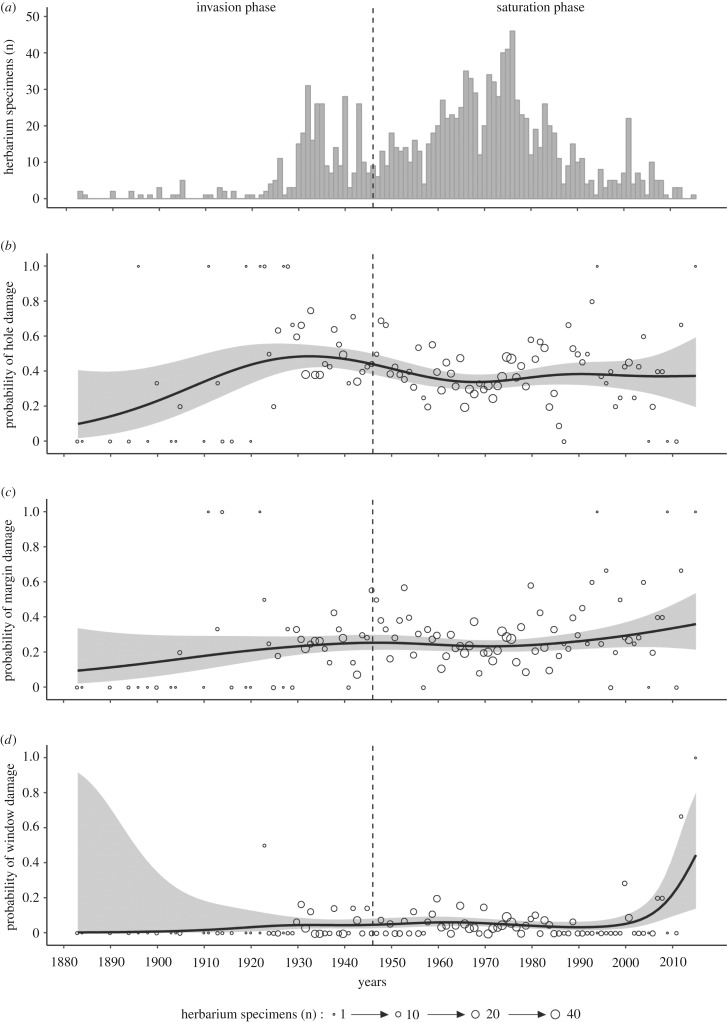
The spatial distribution of purple loosestrife herbarium specimens collected in Quebec covered the entire St Lawrence River corridor, from Lake Saint-François in the south to the Magdalen Islands in eastern Quebec (figure 1). The number of specimens collected varied strongly over the years (figure 3a). Although purple loosestrife has been sampled for more than 125 years, relatively few specimens were collected from the presumed naturalization year, i.e. 1865 at the latest [66] until 1930, then from 1990 until 2017. The sampling effort was higher during two periods, i.e. from 1930 to 1940 and from 1960 to 1985, which correspond to numerous floristic surveys undertaken in the province [16,67].
Figure 3.
Historical reconstruction of the number of purple loosestrife (Lythrum salicaria) specimens collected in Quebec (Canada) and leaf damage exerted by phyllophagous insects between 1880 and 2015. (a) Number of purple loosestrife specimens sampled in the herbaria (n = 1323). Predicted values and 95% confidence intervals of the probability that a purple loosestrife specimen will show signs of (b) hole, (c) margin or (d) window feeding damage. The predicted values are from a generalized additive model (GAM) and include a smooth term on the collection year and a linear term on the leaf area. The invasion phases of purple loosestrife in Quebec are indicated (see text for details). The circles in (b–d) represent the relative proportion of specimens observed with damage signs in a given year and their size indicates the number of herbarium specimens.
Half (50%) of the herbarium specimens were damaged by insects. Many specimens (20%) had more than one type of damage. Hole (41%) and margin (26%) feeding damage were the most frequent damage types, while window feeding damage (5%) was more rarely observed. On average, a herbarium specimen with evidence of leaf damage had three to five damaged leaves per damage type.
No spatial pattern was detected in the distribution of specimens damaged by phyllophagous insects. No spatial or temporal autocorrelation was detected in the residuals of the statistical models. The Geary's C indices were all close to 1 (hole: 0.969; margin: 0.953; window: 0.976), indicating the absence of a spatial structure in the residuals.
Historical reconstructions of herbivory pressure exerted by insects showed a trend towards increased probability of encountering damaged specimens over time (figure 3b–d). For the two most common types of feeding damage (hole and margin), an increased probability of encountering damaged specimens was observed between 1883 and 1930, reaching a plateau of around 30% affected specimens. The percentage of specimens with window feeding damage followed a different temporal trend: the increased probability of grazed membranes did not begin until the end of the twentieth century, and increased from 3% (2–6%) in 1990 to 45% (14–81%) in 2015 (figure 3d). Finally, although a general trend towards an increased number of grazed specimens was observed over time, the number of affected leaves per specimen increased only slightly for each type of damage (electronic supplementary material, figure S1).
(c). Contemporary damage evaluation
The percentage of damaged purple loosestrife specimens in 2016 on the north shore of Lake Saint-Pierre was much higher than that determined from the herbarium specimens. In fact, 97% of the collected plants had hole feeding damage, 91% had margin feeding damage and 51% had window feeding damage. The percentage of specimens with perforation and margin feeding damage varied between sectors, but was always high (75–100% of specimens damaged). Differences were observed between the sectors for window feeding damage: the percentage of affected plants ranged from 17 to 93% (Yamachiche: 17%; Maskinongé: 37%; Pointe-du-lac: 40%; Louiseville: 70%; Porte de la Mauricie: 93%). Moreover, when a plant was damaged, the average number of damaged leaves was higher than in the herbarium collections. On average, per specimen, 32 leaves had hole feeding damage, 21 had margin feeding damage and 17 had window feeding damage. None of the collected specimens was intact, though damage was sometimes minimal.
4. Discussion
Herbivory pressure inferred from herbarium specimens suggests a gradual increase of insect damage on purple loosestrife between 1883 and 1930, and in particular an increase in hole feeding and margin feeding damage from 1883 to around 1940. We can draw a parallel between this reconstruction and the periods of purple loosestrife range expansion in Quebec. There were two expansion phases, the first between 1890 and 1905, the second, more important, between 1923 and 1946 [16]. Herbivory pressure is not necessarily influenced by range expansion. However, we hypothesize that during these periods the density of purple loosestrife plants also strongly increased in the oldest infestation epicenters, i.e. along the St Lawrence River. Historical documents attest to this for Lake Saint-Pierre as early as the 1930s [20]. On the other hand, as the confidence intervals around the damage frequency estimates are particularly wide at the beginning of the time series, we cannot completely rule out that purple loosestrife was grazed by native phyllophagous insects as soon as it was introduced, and that there has been no increase of herbivory pressure until very recently. The stabilization of damage levels around 1940, which lasted until the end of the twentieth century, could be owing to a lack of specificity of native insects that feed on purple loosestrife in Quebec.
The low number of grazed leaves on herbarium specimens contrasts sharply with observations at Lake Saint-Pierre in 2016. A plausible explanation is collection bias by botanists, who select visually intact plants. While it may have been possible to select intact plants in the twentieth century without much effort, this would have been difficult in 2016 at Lake Saint-Pierre as the purple loosestrife plants were frequently and heavily grazed. Unfortunately, there is no direct historical data of herbivory pressure exerted by insects on purple loosestrife in Quebec. It is not possible to calibrate field damage intensity as a function of what is observed on herbarium specimens. The percentage of damaged herbarium specimens is ultimately no more than the lower limit of the true prevalence in a region [30,32,34]. Said otherwise, the proportion of damaged specimens found in herbaria is a lower-bound estimate, assuming no collection bias.
Our data nevertheless allow us to obtain an estimate of the collection bias. The probability of selecting a damaged plant from a population of purple loosestrife (P) is:
| 4.1 |
where o is the probability of finding damaged specimens in herbaria and b the probability that a collector will avoid selecting a damaged plant. By isolating the variable b, we obtain an estimation of collection bias. The prevalence of hole feeding damage on loosestrife specimens in herbaria has remained relatively stable, around 40%, since the 1940s in Quebec (figure 3b). However, the percentage of purple loosestrife specimens presenting hole feeding damage in Lake Saint-Pierre in 2016 was never less than 75% in the five sampled sectors. By isolating b in the above equation (p = 0.75 and o = 0.40), we obtain a collection bias of 47%. The collection bias may be interpreted as the percentage of botanists who systematically avoid collecting damaged specimens.
The above estimation of collection bias is based on two unverified but plausible assumptions: (i) the probability of encountering a specimen with hole feeding damage in the natural populations of Lake Saint-Pierre in 2016 is representative of all of southern Quebec, and (ii) collection bias by botanists has remained constant since the 1940s. Nonetheless, this exercise illustrates the potential importance of collection bias and the difficulties that may arise from it. For example, if the frequency of grazed herbarium specimens is 40% (o = 0.40) and the harvest bias is 50% (one in two collectors systematically avoid damaged specimens; p = 0.50), then the prevalence of herbivory in natural populations is 80%. To detect a 15% increase in damage prevalence in the above population (from 80 to 95%), a 7% increase in damaged herbarium specimens is required (the percentage of damaged specimens will increase from 40 to 47%). Thus, the high percentage of hole feeding damage in herbarium specimens since 1940 could, in theory, mask a contemporary increase in herbivory pressure. At high level of herbivory pressure, the collection bias suggests that the percentage of specimens presenting hole feeding damage in the wild must increase by 15% to yield a small relative increase of 7% in herbarium specimens. This could be the case for hole feeding damage caused by newly introduced insects such as Popillia japonica Newman (Scarabaeidae) and species of the genus Neogalerucella (table 1).
The increased window feeding leaf damage observed in the twenty-first century herbarium specimens supports the hypothesis that insects introduced for biological control increase herbivory pressure. This recent increase in herbivory pressure is probably owing to insects released during the biological control programme of purple loosestrife (Neogalerucella calmariensis and N. pusilla), because the increase was observed soon after the insects were released in Quebec between 1996 and 1998. Moreover, the impacts of these insects are never immediate, and a time lag of at least 5 years is often observed [29]; this is also what the specimens suggest. In addition, Neogalerucella larvae do indeed cause this type of damage, which is characteristic of the species [61]. In 2012, two herbarium specimens collected 26–36 km from the beetle release sites had Neogalerucella eggs in addition to window feeding damage. Given that window feeding damage is distinct from other types of leaf damage [48], and that the prevalence changed little over more than 100 years, the contemporary increase is probably related to the introduction of exotic beetles.
Other insects frequently found on purple loosestrife plants can also cause window feeding damage. Among these is Galerucella nymphaeae L. (Chrysomelidae), a native beetle related to the biocontrol agents used to fight purple loosestrife. This insect mainly feeds on aquatic plants such as Nuphar Sm. (Nymphaeaceae) and Polygonum L. (Polygonaceae), but occasionally uses members of the Lythraceae family to which purple loosestrife belongs [68]. Flea beetles (Altica sp. (Geoffroy) and Crepidodera nana (Say)), two Coleoptera, can also cause window feeding damage to purple loosestrife by leaving the epidermal layer intact during feeding [59]. In addition to these Coleoptera, the larvae of the Lepidoptera Spilosoma viriginica (Fabricius) may also cause window damage in the early stages of its development [64]. All these insects are generalists and do not specifically nor significantly attack purple loosestrife, contrary to the beetles of the genus Neogalerucella. Therefore, the contemporary increase in damage is most probably owing to the introduction of biological control agents.
5. Conclusion
This study shows the usefulness of herbarium specimens for reconstructing temporal trends of herbivory by phyllophagous insects of an invasive plant. The stability of the frequency and intensity of leaf damage sustained by purple loosestrife over time, particularly following the invasion phase (after 1946 in Quebec), indicates that purple loosestrife is part of the diet of a few native species, but does not significantly contribute to their nutrition. The herbivory pressure release hypothesis is therefore entirely plausible for purple loosestrife in North America. This study also shows that the impacts of voluntarily introduced biocontrol insects can be detected on herbarium specimens, which can be very useful for reconstructing their effects over large areas in the absence of extensive field sampling. On the other hand, collection bias may hinder the ability to detect temporal changes when the damage frequency is high in natural populations. In this case, the method used seems to be more effective for detecting less frequently observed damage types, or those caused by a limited number of phyllophagous insects.
Herbarium specimens are a rich source in information that may be used to answer questions related to global changes and biodiversity loss [34]. They make it possible to tackle problems associated with exotic invasive species, including the enemy release hypothesis. The present study focused on herbivory pressure on an invasive plant in one of the introduced ranges of the plant, but the use of herbarium specimens is far from limited to this particular setting. Indeed, it would be possible to study the enemy release hypothesis from different angles [69] using a multi-species approach (for example, by evaluating herbivory of native and exotic species in the host community) or multi-region (by assessing herbivory of an invasive plant in both its native and introduced ranges). Herbarium specimens not only help to reconstruct the invasion history of an invasive species. They also facilitate the identification of the mechanisms that favour successful invasion in the introduced range.
Supplementary Material
Acknowledgements
We thank Louis Desrochers, Guillaume Rheault and Charles Martin for their help in the laboratory and with statistical analyses, and two reviewers for helpful comments on an earlier draft. Elisabeth Groeneveld helped with English editing of the manuscript.
Data accessibility
Additional data available as part of the electronic supplementary material.
Authors' contributions
C.B., R.P. and C.L. designed the study. C.B collected data and carried out the statistical analyses. All authors helped draft the manuscript and approved the final version of the article.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) to R.P. and C.L., and a scholarship to C.B. (NSERC and Fonds de recherche du Québec – Nature et technologies (FRQNT)).
References
- 1.Keane RM, Crawley MJ. 2002. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 17, 164–170. ( 10.1016/S0169-5347(02)02499-0) [DOI] [Google Scholar]
- 2.Catford JA, Jansson R, Nilsson C. 2009. Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Divers. Distrib. 15, 22–40. ( 10.1111/j.1472-4642.2008.00521.x) [DOI] [Google Scholar]
- 3.Siemann E, Rogers WE, Dewalt SJ. 2006. Rapid adaptation of insect herbivores to an invasive plant. Proc. R. Soc. B 273, 2763–2769. ( 10.1098/rspb.2006.3644) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell CE, Blumenthal D, Jarošík V, Puckett EE, Pyšek P. 2010. Controls on pathogen species richness in plants' introduced and native ranges: roles of residence time, range size and host traits. Ecol. Lett. 13, 1525–1535. ( 10.1111/j.1461-0248.2010.01543.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultheis EH, Berardi AE, Lau JA. 2015. No release for the wicked: enemy release is dynamic and not associated with invasiveness. Ecology 96, 2446–2457. ( 10.1890/14-2158.1) [DOI] [PubMed] [Google Scholar]
- 6.Schilthuizen M, et al. 2016. Incorporation of an invasive plant into a native insect herbivore food web. PeerJ 4, e1954 ( 10.7717/peerj.1954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stricker KB, Harmon PF, Goss EM, Clay K, Flory SL. 2016. Emergence and accumulation of novel pathogens suppress an invasive species. Ecol. Lett. 19, 469–477. ( 10.1111/ele.12583) [DOI] [PubMed] [Google Scholar]
- 8.Carpenter D, Cappuccino N. 2005. Herbivory, time since introduction and the invasiveness of exotic plants. J. Ecol. 93, 315–321. ( 10.1111/j.1365-2745.2005.00973.x) [DOI] [Google Scholar]
- 9.Day NJ, Dunfield KE, Antunes PM. 2015. Temporal dynamics of plant-soil feedback and root-associated fungal communities over 100 years of invasion by a non-native plant. J. Ecol. 103, 1557–1569. ( 10.1111/1365-2745.12459) [DOI] [Google Scholar]
- 10.Vellend M, Harmon LJ, Lockwood JL, Mayfield MM, Hughes AR, Wares JP, Sax DF. 2007. Effects of exotic species on evolutionary diversification. Trends Ecol. Evol. 22, 481–488. ( 10.1016/j.tree.2007.02.017) [DOI] [PubMed] [Google Scholar]
- 11.Taylor WE, Bardner R. 1968. Effects of feeding by larvae of Phaedon cochleariae (F.) and Plutella maculipennis (Curt.) on the yield of radish and turnip plants. Ann. Appl. Biol. 62, 249–254. ( 10.1111/j.1744-7348.1968.tb02820.x) [DOI] [Google Scholar]
- 12.Trumble JT, Kolodny-Hirsch DM, Ting IP. 1993. Plant compensation for arthropod herbivory. Annu. Rev. Entomol. 38, 93–119. ( 10.1146/annurev.en.38.010193.000521) [DOI] [Google Scholar]
- 13.Root RB, Cappuccino N. 1992. Patterns in population change and the organization of the insect community associated with goldenrod. Ecol. Monogr. 62, 393–420. ( 10.2307/2937117) [DOI] [Google Scholar]
- 14.Simberloff D, Gibbons L. 2004. Now you see them, now you don't! Population crashes of established introduced species. Biol. Invasions 6, 161–172. ( 10.1023/B:BINV.0000022133.49752.46) [DOI] [Google Scholar]
- 15.Mack RN. 1991. The commercial seed trade: an early disperser of weeds in the United States. Econ. Bot. 45, 257–273. ( 10.1007/BF02862053) [DOI] [Google Scholar]
- 16.Delisle F, Lavoie C, Jean M, Lachance D. 2003. Reconstructing the spread of invasive plants: taking into account biases associated with herbarium specimens. J. Biogeogr. 30, 1033–1042. ( 10.1046/j.1365-2699.2003.00897.x) [DOI] [Google Scholar]
- 17.Stuckey RL. 1980. Distributional history of Lythrum salicaria (purple loosestrife) in North America. Bartonia 47, 3–20. [Google Scholar]
- 18.Thompson DQ, Stuckey RL, Thompson EB. 1987. Spread, impact, and control of purple loosestrife (Lythrum salicaria) in North American wetlands. Washington, DC: United States Fish and Wildlife Service. [Google Scholar]
- 19.Hager HA, McCoy KD. 1998. The implications of accepting untested hypotheses: a review of the effects of purple loosestrife (Lythrum salicaria) in North America. Biodivers. Conserv. 7, 1069–1079. ( 10.1023/A:1008861115557) [DOI] [Google Scholar]
- 20.Lavoie C. 2010. Should we care about purple loosestrife? The history of an invasive plant in North America. Biol. Invasions 12, 1967–1999. ( 10.1007/s10530-009-9600-7) [DOI] [Google Scholar]
- 21.Malecki RA, Blossey B, Hight SD, Schroeder D, Kok LT, Coulson JR. 1993. Biological control of purple loosestrife. BioScience 43, 680–686. ( 10.2307/1312339) [DOI] [Google Scholar]
- 22.Hight SD. 1990. Available feeding niches in populations of Lythrum salicaria (purple loosestrife) in the northeastern United States. In Proceedings of the VII Int. Symp. on the biological control of weeds (ed. Delfosse ES.), pp. 269–278. Rome, Italy: Istituto Sperimentale per la Patologia Vegetale, Ministero dell'Agricoltura e delle Foreste. [Google Scholar]
- 23.Hight SD, Blossey B, Laing J, De Clerck-Floate RA. 1995. Establishment of insect biological control agents from Europe against Lythrum salicaria in North America. Environ. Entomol. 24, 967–977. ( 10.1093/ee/24.4.967) [DOI] [Google Scholar]
- 24.Blossey B, Casagrande R, Tewksbury L, Landis DA, Wiedenmann RN, Ellis DR. 2001. Nontarget feeding of leaf-beetles introduced to control purple loosestrife (Lythrum salicaria L.). Nat. Areas J. 21, 368–377. [Google Scholar]
- 25.Lindgren CJ, Corrigan J, De Clerck-Floate RA. 2002. Lythrum salicaria L., purple loosestrife (Lythraceae). In Biological control programmes in Canada, 1981–2000 (eds Mason PG, Huber JT), pp. 383–390. Wallingford, UK: CAB International. [Google Scholar]
- 26.Dech JP, Nosko P. 2002. Population establishment, dispersal, and impact of Galerucella pusilla and G. calmariensis, introduced to control purple loosestrife in central Ontario. Biol. Control 23, 228–236. ( 10.1006/bcon.2001.1018) [DOI] [Google Scholar]
- 27.Denoth M, Myers JH. 2005. Variable success of biological control of Lythrum salicaria in British Columbia. Biol. Control 32, 269–279. ( 10.1016/j.biocontrol.2004.10.006) [DOI] [Google Scholar]
- 28.Grevstad FS. 2006. Ten-year impacts of the biological control agents Galerucella pusilla and G. calmariensis (Coleoptera: Chrysomelidae) on purple loosestrife (Lythrum salicaria) in Central New York State. Biol. Control 39, 1–8. ( 10.1016/j.biocontrol.2006.03.007) [DOI] [Google Scholar]
- 29.McAvoy TJ, Kok LT, Johnson N. 2016. A multiyear year study of three plant communities with purple loosestrife and biological control agents in Virginia. Biol. Control 94, 62–73. ( 10.1016/j.biocontrol.2015.12.007) [DOI] [Google Scholar]
- 30.Morrow PA, Fox LR. 1989. Estimates of pre-settlement insect damage in Australian and North American forests. Ecology 70, 1055–1060. ( 10.2307/1941374) [DOI] [Google Scholar]
- 31.Abbott I, Wills A, Burbidge T. 1999. Historical incidence of Perthida leafminer species (Lepidoptera) in southwest Western Australia based on herbarium specimens. Austral. Ecol. 24, 144–150. ( 10.1046/j.1442-9993.1999.241958.x) [DOI] [Google Scholar]
- 32.Zangerl R, Berenbaum MR. 2005. Increase in toxicity of an invasive weed after reassociation with its coevolved herbivore. Proc. Natl Acad. Sci. USA 102, 15 529–15 532. ( 10.1073/pnas.0507805102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lees DC, Lack HW, Rougerie R, Hernandez-Lopez A, Raus T, Avtzis ND, Augustin S, Lopez-Vaamonde C. 2011. Tracking origins of invasive herbivores through herbaria and archival DNA: the case of the horse-chestnut leaf miner. Front. Ecol. Environ. 9, 322–328. ( 10.1890/100098) [DOI] [Google Scholar]
- 34.Meineke EK, Davis CC, Davies TJ In press. The unrealized potential of herbaria for global change biology. Ecol. Monogr. ( 10.1002/ecm.1307) [DOI] [Google Scholar]
- 35.Meyer C, Weigelt P, Kreft H. 2016. Multidimensional biases, gaps and uncertainties in global plant occurrence information. Ecol. Lett. 19, 992–1006. ( 10.1111/ele.12624) [DOI] [PubMed] [Google Scholar]
- 36.Daru BH, et al. 2018. Widespread sampling biases in herbaria revealed from large-scale digitization. New Phytol. 217, 939–955. ( 10.1111/nph.14855) [DOI] [PubMed] [Google Scholar]
- 37.Templeton K. 1999. Biological control of purple loosestrife (Lythrum salicaria) in Quebec. Master thesis, McGill University, Montreal, Canada. [Google Scholar]
- 38.Lavoie C, Jean M, Delisle F, Létourneau G. 2003. Exotic plant species of the St Lawrence River wetlands: a spatial and historical analysis. J. Biogeogr. 30, 537–549. ( 10.1046/j.1365-2699.2003.00854.x) [DOI] [Google Scholar]
- 39.Anderson MG. 1995. Interactions between Lythrum salicaria and native organisms: a critical review. Environ. Manage. 19, 225–231. ( 10.1007/BF02471992) [DOI] [Google Scholar]
- 40.Diehl JK, Holliday NJ, Lindgren CJ, Roughley RE. 1997. Insects associated with purple loosestrife, Lythrum salicaria L., in southern Manitoba. Can. Entomol. 129, 937–948. ( 10.4039/Ent129937-5) [DOI] [Google Scholar]
- 41.Ministère des Forêts, de la Faune et des Parcs du Québec. 2015. Listes des coléoptères de la Collection d'insectes du Québec, pp. 1–100. See https://www.mffp.gouv.qc.ca/publications/forets/fimaq/collections/liste-coleopteres.pdf (accessed 25 April 2018).
- 42.Ministère des Forêts, de la Faune et des Parcs du Québec. 2015. Liste des hyménoptères de la Collection d'insectes du Québec, pp. 1–33. See https://www.mffp.gouv.qc.ca/publications/forets/fimaq/collections/liste-hymenopteres.pdf (accessed 25 April 2018).
- 43.Ministère des Forêts, de la Faune et des Parcs du Québec. 2015. Liste des lépidoptères de la Collection d'insectes du Québec, pp. 1–44. See https://www.mffp.gouv.qc.ca/publications/forets/fimaq/collections/liste-lepidopteres.pdf (accessed 25 April 2018).
- 44.Douglas H, Bouchard P, Anderson RS, De Tonnancour P, Vigneault R, Webster RP. 2013. New Curculionoidea (Coleoptera) records for Canadа. ZooKeys 309, 13–48. ( 10.3897/zookeys.309.4667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Integrated taxonomic information system. 2017. ITIS online database. See http://www.itis.gov (accessed 25 April 2018).
- 46.Hill DS. 1987. Agricultural insect pests of temperate regions and their control. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 47.Commission de toponymie du Québec. 2012. Topos sur le web. Noms et lieux du Québec. See http://www.toponymie.gouv.qc.ca/ct/ToposWeb/recherche.aspx?s (accessed 10 March 2017).
- 48.Labandeira CC, Wilf P, Johnson KR, Marsh F. 2007. Guide to insect (and other) damage types on compressed plant fossils. Version 3.0. Washington, DC: Smithsonian Institution. [Google Scholar]
- 49.Kimball S, Mattis P, GIMP Development Team. 2015. GIMP - The GNU image manipulation program. See http://www.gimp.org (accessed 20 June 2017).
- 50.Fewster RM, Buckland ST, Siriwardena GM, Baillie SR, Wilson JD. 2000. Analysis of population trends for farmland birds using generalized additive models. Ecology 81, 1970–1984. ( 10.1890/0012-9658(2000)081%5B1970:AOPTFF%5D2.0.CO;2) [DOI] [Google Scholar]
- 51.Wood SN. 2011. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. B 73, 3–36. ( 10.1111/j.1467-9868.2010.00749.x) [DOI] [Google Scholar]
- 52.Wood SN. 2006. Generalized additive models: an introduction with R. Boca Raton, FL: Chapman & Hall/CRC. [Google Scholar]
- 53.Breheny P, Burchett W. 2017. Visualization of regression models using visreg. R. J. 9, 56–71. [Google Scholar]
- 54.Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer. [Google Scholar]
- 55.Fortin MJ, Dale MRT. 2014. Spatial analysis: a guide for ecologists, 2nd edn Cambridge, UK: Cambridge University Press. [Google Scholar]
- 56.Bivand R, Hauke J, Kossowski T. 2013. Computing the Jacobian in Gaussian spatial autoregressive models: an illustrated comparison of available methods. Geogr. Anal. 45, 150–179. ( 10.1111/gean.12008) [DOI] [Google Scholar]
- 57.R Development Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 58.Heidel B, Tronstad L, Handley J. 2011. Flea beetle (Altica spp.) herbivory on a threatened plant, F.E. Warren Air Force Base, Wyoming. Nat. Areas J. 31, 283–287. ( 10.3375/043.031.0310) [DOI] [Google Scholar]
- 59.Parry RH. 1986. The systematics and biology of the flea beetle genus Crepidodera chevrolat (Coleoptera: Chrysomelidae) in America north of Mexico. Insecta Mundi 1, 156–196. [Google Scholar]
- 60.Kouki J. 1991. The effect of the water-lily beetle, Galerucella nymphaeae, on leaf production and leaf longevity of the yellow water-lily, Nuphar lutea. Freshwater Biol. 26, 347–353. ( 10.1111/j.1365-2427.1991.tb01402.x) [DOI] [Google Scholar]
- 61.Hight SD, Drea JJ. 1991. Prospects for a classical biological control project against purple loosestrife (Lythrum salicaria L.). Nat. Areas J. 11, 151–157. [Google Scholar]
- 62.Fleming WE. 1972. Biology of the Japanese beetle. Washington, DC: United States Department of Agriculture; Technical Bulletin 1449. [Google Scholar]
- 63.Eastman J. 2003. The book of field and roadside: open-country weeds, trees, and wildflowers of eastern North America. Mechanicsburg, PA: Stackpole Books. [Google Scholar]
- 64.Hagen RH, Chabot JF. 1986. Leaf anatomy of maples (Acer) and host use by Lepidoptera larvae. Oikos 47, 335–345. ( 10.2307/3565446) [DOI] [Google Scholar]
- 65.Hodgson E, Sisson A, Mueller D, Jesse L, Saalau-Rojas E, Duster A. 2012. Field crop insects. Ames, IA: Iowa State University Extension and Outreach. [Google Scholar]
- 66.Rousseau C. 1968. Histoire, habitat et distribution de 220 plantes introduites au Québec. Nat. Can. 95, 49–169. [Google Scholar]
- 67.Lavoie C, Saint-Louis A, Guay G, Groeneveld E, Villeneuve P. 2012. Naturalization of exotic plant species in north-eastern North America: trends and detection capacity. Divers. Distrib. 18, 180–190. ( 10.1111/j.1472-4642.2011.00826.x) [DOI] [Google Scholar]
- 68.Manguin S, White R, Blossey B, Hight SD. 1993. Genetics, taxonomy, and ecology of certain species of Galerucella (Coleoptera: Chrysomelidae). Ann. Entomol. Soc. Am. 86, 397–410. ( 10.1093/aesa/86.4.397) [DOI] [Google Scholar]
- 69.Meijer K, Zemel H, Chiba S, Smit C, Beukeboom LW, Schilthuizen M. 2015. Phytophagous insects on native and non-native host plants: combining the community approach and the biogeographical approach. PLoS ONE 10, e0125607 ( 10.1371/journal.pone.0125607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beaulieu C, Lavoie C, Proulx R. 2018. Data from: Bookkkeeping of insect herbivory trends in herbarium specimens of purple loosestrife (Lythrum salicaria). Figshare Digital Repository. ( 10.6084/m9.figshare.6213785) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional data available as part of the electronic supplementary material.