Highlights
-
•
Patients who received BT experienced acute urinary morbidity 3 months after BT of the prostate.
-
•
Acute urinary symptoms gradually improved with time and returned to BL at 36 months.
-
•
Storage symptoms take longer to return to BL compared with voiding symptoms.
Abbreviations: BT, brachytherapy; BL, baseline; EBRT, external beam radiation therapy; GS, Gleason score; IMRT, intensity modulated radiation therapy; IQR, interquartile; LUTS, lower urinary tract symptoms; NADT, neoadjuvant androgen deprivation therapy; PV, prostate volume; QOL, quality of life
Keywords: Brachytherapy, Prostate cancer, Lower urinary tract symptom, Quality of life, Urinary symptom flare
Abstract
Purpose
To investigate chronological changes in lower urinary tract symptoms (LUTS) in patients who received iodine-125 brachytherapy (BT) for prostate cancer.
Methods
We enrolled 706 patients who received BT. Of these, 265 (38%) received BT combined with external beam radiation therapy (EBRT). An International Prostate Symptom Score (IPSS), IPSS quality of life (IPSS-QOL) score, and overactive bladder symptom score (OABSS) were recorded before BT (baseline, BL), and 1, 3, 6, 12, 24, 36, 48, and 60 months after BT. The sum of frequency (2), urgency (4) and nocturia (7) of the IPSS questionnaire was defined as the storage symptoms score, whereas the sum of emptying (1), intermittency (3), weak stream (5), and hesitancy (6) was defined as the voiding symptom score.
Results
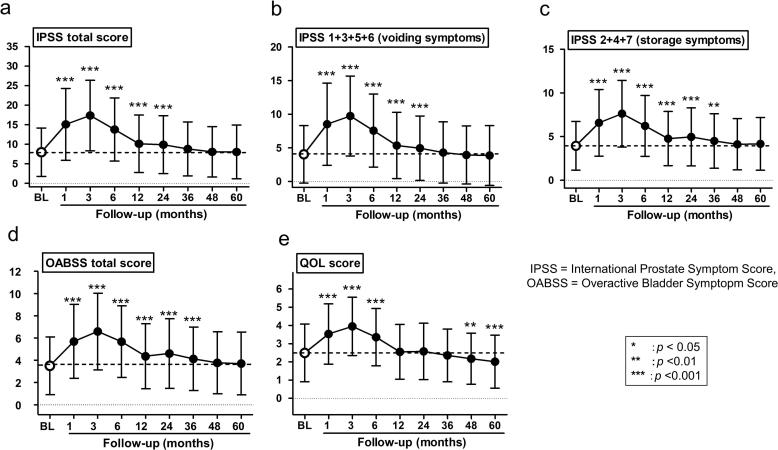
Total IPSS significantly increased at 3 months following BT compared with BL (mean score: 17.1 vs. 7.99, P < 0.001) and returned to BL by 36 months. The storage symptom score did not return to BL 36 months after BT. Total OABSS significantly increased 3 months after BT compared with BL (mean score: 6.52 vs. 3.45, P < 0.001), and returned to BL 48 months after BT. The IPSS-QOL score was the highest score (mean score: 2.46 vs. 3.9, P < 0.001) 3 months after BT and returned to BL 48 months after BT, however the IPSS-QOL score was lower than BL (mean score: 2.01 vs 2.46, P < 0.001) at 60 months. The risk factors for LUTS within 1 year after BT were BL IPSS (P < 0.001) and PV (P < 0.001). Patients who received combined EBRT experienced transient storage symptoms 24 and 36 months after BT, whereas those who received BT alone did not. However, the storage symptom score of the patients who received combined EBRT was improving 48 months after BT and eventually showed no significant difference compared with those treated with BT alone.
Conclusion
Three months after BT, LUTS, including storage symptoms, deteriorated the most but improved with time. The urinary symptom in patients who received combined EBRT can potentially flare again in 24 and 36 months after BT. Knowledge of changes in LUTS associated with BT may influence treatment recommendations and enable patients to make better-informed decisions.
1. Introduction
The use of iodine-125 brachytherapy (BT) for prostate cancer has increased in the United States since the 1990s. Many studies have shown the efficacy and safety of BT, and it has become an established treatment modality for localized prostate cancer [1], [2], [3], [4]. According to the NCCN (National Comprehensive Cancer Network) guidelines, BT with/without external beam radiation therapy (EBRT) is one of the first-line treatment modalities for low- and intermediate-risk patients because the oncologic outcomes are reported to be similar to those with radical prostatectomy (RP) and EBRT [5], [6]. The occurrence of adverse events varies depending on the treatment selected. Currently, selection of a treatment modality, consideration of the quality of life (QOL), and characteristics of adverse events are important since clinical outcomes are similar.
Chen et al. reported that 54.5% of patients who received BT had complications within 2 years, and 14.1% required an invasive procedure for obstruction, incontinence, bleeding, and fistula [7]. Particularly, supplemental EBRT showed higher incidence rates of both GU (genitourinary) and GI (gastrointestinal) toxicities [8]. However, the urinary and bowel domains among patients who received BT significantly improved after long-term follow-up [9].
The ability to predict complications may help in selecting the appropriate patients for BT. This study aimed to evaluate chronological changes in lower urinary tract symptoms (LUTS) after iodine-125 BT of the prostate.
2. Materials and methods
Between July 2004 and January 2014, 706 patients with prostate cancer received iodine-125 BT at our hospital. Low-risk patients (cT2a, Gleason score 6, and Prostate Specific Antigen (PSA) ≤ 10 ng/mL) and intermediate-risk patients (cT2a and PSA ≤ 10 ng/mL with a Gleason score of 3 + 4 and a positive biopsy core of<50%) were treated by seed implantation alone with the prescribed dose of 145 Gy or 160 Gy. The other patients received combination treatment including EBRT with a prescribed dose of 110 Gy. The target for EBRT was determined 1 month after seed implantation, and the patients received 45 Gy (in 25 fractions of 1.8 Gy per fraction) using 10 MV photon energy. The clinical target volume included both the whole prostate and one third of the proximal seminal vesicle. With respect to selection criteria for brachytherapy, we excluded patients with severe dysuria such as cases with IPSS > 20 points or cases of large amounts of post-voided residual for selection criteria of brachytherapy, and patients with prostate volume (PV) > 40 mL who have performed neoadjuvant androgen deprivation therapy (NADT) for 4 months for cytoreduction in principle. The median follow-up period was 48 months (range 12–126 months). Of these patients, 441 (62%) received BT alone (monotherapy group), and 265 (38%) were treated with combined EBRT using a three-dimensional conformal technique (boost group). And 306 (44%) received NADT. (Table 1).
Table 1.
This study included 706 patients who received BT in our hospital from July 2004 to January 2014. Of 706 patients, 265 (38%) were treated with combined BT and external beam radiation therapy (EBRT). Half of the patients were classified as clinical T1c, and 340 patients (48%) had an intermediate risk based on the D’Amico risk classification.
| BT as monotherapy | 441 (62%) |
| BT + EBRT | 265 (38%) |
| None | 390 (55%) |
| Neoadjuvant ADT | 243 (35%) |
| Adjuvant ADT | 10 (1%) |
| Neo ADT/ adjuvant ADT | 63 (9%) |
| Clinical stage | |
| T1c | 351 (50%) |
| T2a | 239 (34%) |
| T2b | 58 (8%) |
| T2c | 30 (4%) |
| T3a | 28 (4%) |
| D‘Amico risk classification | |
| Low risk | 248 (35%) |
| Intermediate risk | 340 (48%) |
| High risk (including cT3a) | 118 (17%) |
| Gleason Score | |
| −6 | 322 (45%) |
| 7 | 323 (46%) |
| 8- | 61 (9%) |
| Median age (years) | 70 (range 48–84) |
| Median PSA (ng/mL) | 7.16 (range 1.17–113) |
| Median PV (mL) | 24.6 (range 7.7–61.9) |
| Mean total IPSS | 7.99 (range 0–33) |
| Mean IPSS-QOL score | 3.9 (range 0–6) |
| Mean total OABSS | 3.45 (range 0–14) |
BT = brachytherapy, EBRT = external beam radiation therapy, ADT = androgen deprivation therapy, PSA = prostate-specific antigen, PV = prostate volume, IPSS = International Prostate Symptom Score, OABSS = Overactive Bladder Symptom Score.
We evaluated urinary symptoms using the International Prostate Symptom Score (IPSS), IPSS-QOL Score, and Overactive Bladder Symptom Score (OABSS), and we examined the prostate volume and urination condition by transrectal ultrasound (TRUS) and uroflowmetry (Qmax [maximal voiding rate], voided volume, post-voided residual). The sum of frequency (2), urgency (4), and nocturia (7) of IPSS questionnaire was defined as the storage symptoms score, and the sum of emptying (1), intermittency (3), weak stream (5), and hesitancy (6) was defined as the voiding symptom score.
The protocol for the research project was approved by the Institutional Review Board for clinical studies (Medical Ethics Committee), and all patients agreed to participate in the present study and signed an informed consent form. We evaluated before seed implantation (BL) and 1, 3, 6, 12, 24, 36, 48, and 60 months after treatment.
Almost all patients experienced an increase from their BL IPSS after BT. The highest increase in IPSS was classified as the “initial peak” in symptom. We defined an increase of 11 or more points from the BL within 1 year as “early LUTS.” The lowest IPSS after the initial peak was classified as “IPSS nadir.” Patients who experienced a second exacerbation in urinary symptom, called “urinary symptom flare.” We defined an increase of 10 or more points from IPSS nadir as urinary symptom flare. All patients started α-1 adrenergic antagonist immediately after BT. This medication was continued until subjective symptom or IPSS improved.
All statistical analyses were performed using PASW Statistics 17.0 (SPSS Inc., Chicago, IL, USA) and Prism software 5.00 (Graph pad software San Diego, CA, USA). Comparisons between time points for individual data were made using χ2-test and the Mann–Whitney U test, and Wilcoxon signed rank test with P < 0.05 was considered statistically significant. The Cox proportional hazard methods were used for univariate and multivariate analyses to identify risk factors for early LUTS after BT. For multivariate analysis, variables were selected based on P < 0.05 in the univariate analysis. The discontinuation rate of the use of α-1 adrenergic antagonist was determined using Kaplan–Meier curves with log-rank test.
3. Results
The median age at baseline (BL), prostate-specific antigen (PSA) at diagnosis, and PV were 70 years (range 48–83 years), 7.16 ng/mL (range 1.17–113 ng/mL), and 24.6 mL (range 7.7–61.9 mL), respectively. The patients with a GS of 6, 7, and 8–10 were 322 (45.6%), 323 (45.8%), and 61 (8.6%), respectively. The mean of the total IPSS, IPSS-QOL, and total OABSS was 7.99 (range 0–33), 3.9 (range 0–6), and 3.45 (range 0–14), respectively. NADT was administered to 306 patients (43.3%). Almost half of the patients were classified as clinical T1c. Approximately 45.6%, 45.8%, and 8.6% of patients were low risk (PSA ≦ 10 ng/mL and GS ≦ 6 or T1–T2a), intermediate risk (10 < PSA≦20 ng/mL or GS 7 or T2b), and high risk (20 ng/mL < PSA or GS 8–10 or T2c), respectively, based on modified D’Amico risk classification (T3a was included in high risk) [10].
3.1. Dosimetric parameters
The dosimetry data of the minimal dose (Gy) received by 30% of the urethra (UD30), the minimal dose (Gy) received by 90% of the urethra (UD90), the percentage of the prostate volume receiving 100% of the prescribed minimal peripheral dose (V100) and the percentage of the prostate volume receiving 150% of the prescribed minimal peripheral dose (V150) are as follows. The median UD30 of patients in the monotherapy group was 207.2 Gy (interquartile (IQR) 190.9–225.1) and the median UD30 of patients in the boost group was 149.2 Gy (IQR 139.3–163.6). The median UD90 of patients in the monotherapy group was 146.8 Gy (IQR 134.6–159) and the median UD90 of patients in the boost group was 106.7 Gy (IQR 97.1–116.3). The median V100 of patients in the monotherapy group was 95.5% (IQR 92.4–97.4) and the median V100 of patients in the boost group was 97.9% (IQR 96–98.9). The median V150 of patients in the monotherapy group was 59.8% (IQR 51.8–68.2) and the median V150 of patients in the boost group was 63% (IQR 52.8–71.9). The values of UD30, UD90, V100 and V150 of patients in boost group were significantly higher than those of patients in monotherapy group (P < 0.001, P < 0.001, P < 0.001, P = 0.017, respectively).
3.2. Chronological changes of subjective data
In this study, IPSS, OABSS and IPSS-QOL scores became worse immediately after treatment, lasting for 6–36 months compared with BL. However, these symptoms improved with time and returned to BL by 12–36 months. Eventually, according to the IPSS-QOL score, urinary symptoms were better than BL after 48 months (Table 2).
Table 2.
Chronological changes of mean score in total IPSS, IPSS-QOL, and total OABSS compared with baseline.
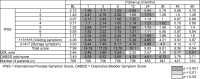 |
Total IPSS was significantly increased at 3 months following BT compared with BL (mean score, 17.1 vs. 7.99, P < 0.001) and returned to BL by 36 months (Fig. 1a). The voiding symptom score of IPSS was significantly increased at 3 months after BT (mean, 4.06 vs. 9.58, P < 0.001) and returned to BL by 36 months (Fig. 1b). Similarly, the storage symptom score was significantly increased at 3 months after BT (mean, 3.93 vs. 7.49, P < 0.001) but did not return to BL at 36 months after BT (mean, 3.93 vs. 4.5, P < 0.01) and returned to BL at 48 months (Fig. 1c). Total OABSS was significantly increased 3 months after BT compared with BL (mean score, 6.52 vs. 3.45, P < 0.001) and returned 48 months after BT (Fig. 1-d). IPSS-QOL score at 3 months after BT showed the highest score (mean score, 3.89 vs. 2.46, P < 0.001) and gradually returned to BL 12 months after BT, and eventually the IPSS-QOL score at 60 months was lower than BL (mean score, 2.01 vs. 2.46, P < 0.001) (Fig. 1-e).
Fig. 1.
a: Total IPSS was significantly increased at 3 months following BT compared with BL (mean score, 17.1 vs. 7.99, P < 0.001), and returned to BL by 36 months. b: The voiding symptom score of IPSS was significantly increased at 3 months after BT (mean: 4.06 vs. 9.58, P < 0.001) and returned to BL by 36 months. c: The storage symptom score was significantly increased at 3 months after BT (mean: 3.93 vs. 7.49, P < 0.001), but did not return to BL by 36 months after BT (mean: 3.93 vs. 4.5, P < 0.01) and returned to BL at 48 months. d: Total OABSS was significantly increased 3 months after BT compared with BL (mean score, 6.52 vs. 3.45, P < 0.001) and returned 48 months after BT. e: IPSS-QOL score showed the highest score at 3 months after BT (mean score, 3.89 vs. 2.46, P < 0.001) and gradually returned to BL 12 months after BT, and eventually the IPSS-QOL score at 60 months was lower than BL (mean score, 2.01 vs. 2.46, P < 0.001).
3.3. Chronological changes of objective data
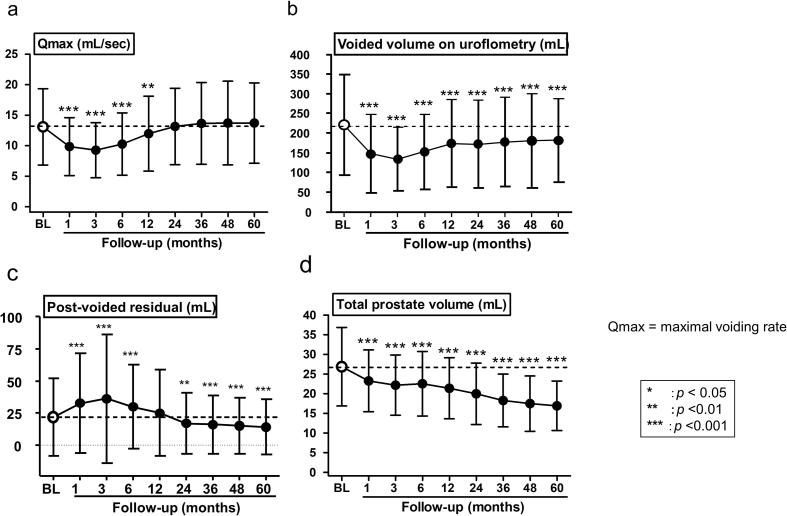
At 3 months after treatment, patients experienced transient deterioration of Qmax (mean, 13.1 mL/s vs. 9.3 mL/s, P < 0.001) (Fig. 2a), voided volume (mean, 220.9 mL vs. 134 mL, P < 0.001) (Fig. 2b), and post-voided residual (mean, 21.8 mL vs. 36.1 mL, P < 0.001) (Fig. 2c) of uroflowmetry. However, these values gradually improved with time. Qmax and post-voided residual returned to BL at 24 months and 12 months, respectively, and residual urine at 60 months was less than BL (mean, 21.8 mL vs. 14 mL, P < 0.001). However, voided volume did not return to BL by 60 months (mean, 220.9 mL vs. 182.1 mL, P < 0.001). In contrast, PV decreased with time, and at 60 months a 10 mL decrease (37.2%) was found compared to BL (mean, 26.9 mL vs. 16.9 mL, P < 0.001) (Fig. 2d). Of the 706 patients, the 390 patients who did not undergo neoadjuvant hormonal therapy (NHT) showed that PV gradually decreased with time after BT. The PV of the patients who did not undergo NHT was 25.23 mL at BL and decreased to 15.96 mL in 5 years. They had a 9.1-mL (IQR 5.3–14.3) decrease in PV (36.4%) in five years. In contrast, the PV of 316 patients who received NHT rapidly decreased immediately after administration of hormone therapy. From before BT (at the time of pre-plan) to 3 months after BT, the PV of patients with NHT significantly decreased compared with patients who did not undergo NHT. However, after 6 months, no significant difference was found in the changes of PV, regardless of the use of NHT (Table 3).
Fig. 2.
a: Qmax of uroflowmetry was worst at 3 months after treatment (mean: 13.1 mL/s vs. 9.3 mL/s, P < 0.001) and gradually improved. b: The voided volume of uroflowmetry was least at 3 months after treatment (mean: 220.9 mL vs. 134 mL, P < 0.001). c: Post-voided residual of uroflowmetry became transiently worse at 3 months after treatment (mean: 21.8 mL vs. 36.1 mL, P < 0.001). d: Prostate volume decreased with time, and a significant volume reduction was found between BL and at 60 months (mean: 26.9 mL vs. 16.9 mL, P < 0.001).
Table 3.
Chronological changes in PV after BT in groups with and without neoadjuvant hormonal therapy (NHT). The PV of patients with NHT significantly decreased compared with patients without NHT before BT (at the time of pre-plan) to 3 months after BT. After 6 months, no significant difference was found in the changes of PV, regardless of the use of NHT.
SD = standard deviation, NHT = neoadjuvant hormone therapy, PV = prostate volume, BL = baseline, PP = pre-plan just before BT (After neoadjuvant therapy).
Baseline versus †p < 0.05, ‡p < 0.01. Between-group difference *p < 0.05 , **p < 0.01.
3.4. Acute urinary retention
In this study, 15 patients (2.1%) suffered from acute urinary retention. They required invasive procedural interventions. Of these 15 patients, 5 patients needed catheter placement and 10 patients required self-catheterization. However, they were eventually free from these interventions due to improving of urinary conditions.
3.5. Early LUTS
Most patients experienced transient deterioration of urinary symptom immediately after treatment. Early LUTS occurred in 50.3% of patients (355 patients). After the peak, the scores subsequently returned to approximate BL scores. Using a univariate analysis, factors associated with early LUTS were BL IPSS (P = 0.002), PV (P < 0.001), and number of needles (P = 0.042). Furthermore, using multivariate analysis, BL IPSS (P < 0.001) and PV (P < 0.001) were predictive factors for early LUTS (Table 4).
Table 4.
Risk factors for early LUTS using univariate and multivariate analyses. The factors associated with early LUTS were BL IPSS (P = 0.002), PV (P < 0.001), and number of needles (P = 0.042) on univariate analysis. BL IPSS (P < 0.001) and PV (P < 0.001) were independent predictive factors for early LUTS on multivariate analysis.
| Univariate |
Multivariate |
||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age (y) | <72 | 1 | |||||
| ≧72 | 0.8 | 0.59–1.07 | 0.13 | ||||
| iPSA (ng/mL) | < 6.5 | 1 | |||||
| ≧6.5 | 0.91 | 0.67–1.22 | 0.52 | ||||
| baseline IPSS | <8 | 1 | 1 | ||||
| ≧8 | 0.62 | 0.46–0.84 | 0.002 | 0.6 | 0.44–0.81 | < 0.001 | |
| clinical T | cT1c | 1 | |||||
| cT2a- | 0.99 | 0.74–1.33 | 0.94 | ||||
| Gleason score sum | 6 | 1 | |||||
| ≧ 7 | 1.07 | 0.79–1.44 | 0.66 | ||||
| D'Amico risk classification | low | 1 | |||||
| intermediate/high | 1.07 | 0.79–1.46 | 0.67 | ||||
| Prostate volume (cm3) | < 27 | 1 | 1 | ||||
| ≧ 27 | 1.76 | 1.3–2.38 | < 0.001 | 1.78 | 1.29–2.44 | < 0.001 | |
| ADT(neo and/or adjuvant) | No | 1 | |||||
| Yes | 0.99 | 0.71–1.38 | 0.95 | ||||
| EBRT | No | 1 | |||||
| Yes | 0.96 | 0.62–1.49 | 0.87 | ||||
| BED (Gy2) | < 200 | 1 | |||||
| ≧ 200 | 1.13 | 0.84–1.52 | 0.41 | ||||
| Number of Needles | < 23 | 1 | 1 | ||||
| ≧ 23 | 1.37 | 1.01–1.84 | 0.042 | 1.16 | 0.84–1.58 | 0.37 | |
| Number of Seeds | < 65 | 1 | |||||
| ≧ 65 | 1.24 | 0.92–1.67 | 0.15 | ||||
| α-1 adrenergic antagonist | No | 1 | |||||
| Yes | 0.99 | 0.66–1.48 | 0.95 | ||||
| Hypertension | No | 1 | |||||
| Yes | 0.95 | 0.7–1.28 | 0.72 | ||||
| Diabetes Mellitus | No | 1 | |||||
| Yes | 1.39 | 0.9–2.14 | 0.14 | ||||
HR = harzard ratio, CI = Confidence interval, iPSA = initial prostate-specific antigen, ADT = androgen-deprivation therapy, EBRT = external beam radiotherapy, BED = biological effective dose.
3.6. Duration of α-1 adrenergic antagonist administration
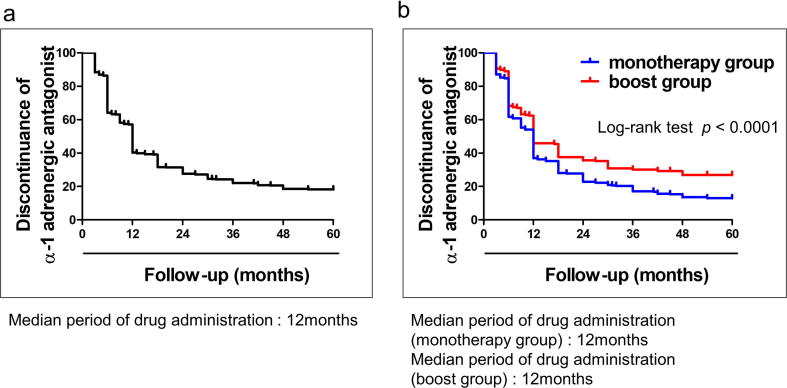
All patients started α-1 adrenergic antagonist treatment immediately after BT. Before BT, 84 patients (15.5%) had already been administered α-1 adrenergic antagonist, and they continued after BT until subjective symptom or IPSS improved. Fig. 3a represents the discontinuation rate of α-1 adrenergic antagonist treatment using the Kaplan–Meier method. The median period of drug administration was 12 months (range, 3–60 months). Within 1 year after BT, 310 patients (43.9%) had stopped taking the medication, and 620 patients (87.8%) had stopped within 5 years. Fig. 3b represents the comparison of discontinuation rates of α-1 adrenergic antagonist between the monotherapy and boost groups using the Kaplan–Meier method. The median period of drug administration in the monotherapy and boost groups was 12 months, but the discontinuation rate of drug administration in the boost group was worse than that in the monotherapy group. Stopping the use of α-1 adrenergic antagonist tended to be difficult in patients with EBRT.
Fig. 3.
a Discontinuation rates of the use of α-1 adrenergic antagonist using the Kaplan–Meier method. The median length of drug administration was 12 months (range, 3–60 months). Within 1 year after BT, 310 patients (43.9%) stopped taking the medication, and 620 patients (87.8%) stopped within 5 years. b: Discontinuation rates of the use of α-1 adrenergic antagonist between the monotherapy and boost groups using the Kaplan–Meier method. Stopping the use of α-1 adrenergic antagonist tended to be difficult in patients with EBRT on log-rank Test (P < 0.0001).
3.7. Urinary symptom flare
Urinary symptom flare occurred in 35.1% (248 patients) of patients. The proportion of urinary symptom flare in monotherapy group and boost group were 33.1% (146 patients) and 38.5% (102 patients), respectively. There was no significant difference in the occurrence of urinary symptom flare between two groups (P = 0.147).
Table 5 shows the chronological changes in the mean values (SD) of IPSS, OABSS and IPSS-QOL score. Patients in the boost group experienced transient storage symptoms at 24 and 36 months after BT, whereas those in the monotherapy group did not (P < 0.05). However, the storage symptom score in the boost group had been improving by 48 months after BT and eventually showed no significant difference compared with the patients in the monotherapy group. Similarly, the storage symptom score of patients in the boost group was significantly increased at 3 months after BT, but had transient worsening at 24 and 36 months compared with the monotherapy group (P < 0.05). In contrast, the voiding symptom score had transient worsening only at 36 months compared with the monotherapy group (P < 0.05). Likewise, with regard to OABSS and IPSS-QOL score, patients treated with EBRT experienced transient storage symptoms at 24 and 36 months after BT, whereas patients who did not undergo EBRT did not.
Table 5.
Patients in the boost group experienced transient storage symptoms at 24 and 36 months after BT, whereas those in the monotherapy group did not (P < 0.05). The storage symptom score of patients in the boost group significantly increased at 3 months after BT, and had a transient worsening at 24 and 36 months, compared with the monotherapy group (P < 0.05). The voiding symptom score of patients in the boost group had a transient worsening only at 36 months compared with the monotherapy group (P < 0.05). With the OABSS and IPSS-QOL scores, patients treated with EBRT experienced transient storage symptoms at 24 and 36 months after BT, whereas those who did not undergo EBRT did not.
 |
SD = standard deviation, IPSS = International Prostate Symptom Score, OABSS = Overactive Bladder Symptom Score.
Baseline versus †p < 0.05, ‡p < 0.01. Between-group difference *p < 0.05, **p < 0.01.
Urinary symptoms of patients in the boost group worsened transiently at 24 and 36 months after BT, though there was no significant difference in the occurrence of urinary symptom flare between two groups.
4. Discussion
BT provides excellent long-term oncological outcome by delivering a high-radiation dose to the prostate, however, most patients who undergo BT develop some degree of urinary morbidity [1]. We previously reported the serial changes of urinary symptoms during the first 12 months after BT by using IPSS and objective parameters [11].
Generally, acute urinary morbidity occurs mostly within the first 1 to 3 months after BT, and these symptoms are improved 12–36 months after BT [12], [13], [14], [15], [16], [17], [18], [19]. Acute urinary morbidity is a relatively common complication following BT, experienced by almost 70% of patients [20]. Symptoms occur mostly from the first 1 to 3 months following implantation [12], [13], [14], [15], [16], and symptoms can improve at 12–36 months after treatment [12], [13], [17], [18], [19]. Decreased prostate volume after BT may be associated with improvement of urinary symptom. Previous studies have shown that after radiation therapy, prostate volume tends to decrease with time because of the damage to endothelial cells, which cause ischemia that leads to atrophy [21], [22]. Furthermore, Bruce et al. reported that irritative symptoms take longer to return to BL when compared with obstructive symptoms [23], which was similar to the result in our study. The reason for prolonged resolution in storage symptoms may be urethral stricture or urethral length. Earley et al. reported that doses to the apex or the bulbomembranous urethra are an important factor for prostate BT-related urethral stricture [24]. We should be careful to irradiate the apical and peri-apical urethra. Marigliano et al. also reported that magnetic resonance imaging showed urethral shortening and increased the signal intensities of the urethral wall and pelvic muscles in substantial percentages of patients after radiation therapy for prostate cancer [25]. In addition, patients after BT may have higher incidence of detrusor overactivity (DO). Blaivas et al. reported the comparison of urodynamic findings in men with unselected causes of LUTS vs LUTS due to BT [26]. Patients who received BT had a significantly higher rate of DO than patients with unselected causes of LUTS. In contrast, the incidence of urethral obstruction showed no significant differences in two groups. For these reasons, irritative symptoms may take longer time to resolve. We should take measures not only against obstructive symptoms, but also irritative symptoms. We may consider prescribing drugs for irritative symptoms, such as anticholinergic agents or β-3 stimulant agents. In addition, Yu et al. reported that α-1 receptor antagonist plus low-dose sildenafil combination therapy was a beneficial treatment for post-implantation progression of LUTS [27]. These procedures may improve the QOL of patients.
Urinary symptom flare is defined as transient recurrence of urinary symptoms after an asymptomatic period, first reported by Cesaretti et al. 2002 [13]. We previously reported that 51.5% of patients experienced flare after BT (definition of an IPSS of ≥ 6 points greater than the postimplant nadir) [28]. In other study, this occurred in 35.5%–58% of patients 16–24 months after BT [13], [14], [20]. Its etiology is unclear, but the occurrence of late radiation prostatitis and/or urethritis may be possible. The urinary symptom in patients who received combined EBRT worsened again in 24 and 36 months after BT, although we could not show the causal relationship between urinary symptom flare and supplemental EBRT in this study. Further study regarding urinary symptom flare should be conducted.
Our study has some limitations. First, the long-term outcome is unknown because of a medium follow-up period (48 months) in this study, so longer-term follow-up observations are desired. Second, this report focused exclusively on patients who received BT. Comparison with other modalities of treatment is necessary to appropriately choose the best treatment for patients diagnosed with localized prostate cancer. However, we believe that because of the large cohort (706 patients), this research is significant to elucidate the time course changes of urinary symptom after BT.
5. Conclusion
Patients experienced acute urinary morbidity three months after iodine-125 BT treatment of the prostate, and they gradually improved with time, returning to BL at 36 months. Storage symptoms take longer to return to BL compared with voiding symptoms. The urinary symptom in patients who received combined EBRT can potentially flare again in 24 and 36 months after BT.
There is no evidence for effective treatment of patients with storage symptom after BT. To prevent the occurrence of urinary symptoms, we should consider using prophylactic drugs, such as not only α-1 receptor antagonist, but also anticholinergic agents, β-3 stimulant agents, and/or low-dose phosphodiesterase inhibitor, depending on symptoms of patients. Further study regarding the treatment for storage symptom after BT should be conducted.
Knowledge of changes in LUTS associated with BT may influence treatment recommendations and enable patients to make better-informed decisions.
Conflicts of interest
The authors declare no conflicts of interest associated with this manuscript.
Acknowledgement
We would like to thank Editage (www.editage.jp) for English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2018.11.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Tanaka N., Asakawa I., Nakai Y. Comparison of PSA value at last follow-up of patients who underwent low-dose rate brachytherapy and intensity-modulated radiation therapy for prostate cancer. BMC Cancer. 2017;17:573. doi: 10.1186/s12885-017-3565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grimm P., Billiet I., Bostwick D. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the prostate cancer results study group. BJU Int. 2012;109:22–29. doi: 10.1111/j.1464-410X.2011.10827.x. [DOI] [PubMed] [Google Scholar]
- 3.Taira A.V., Merrick G.S., Butler W.M. Long-term outcome for clinically localized prostate cancer treated with permanent interstitial brachytherapy. Int J Radiat Oncol Biol Phys. 2011;79:1336–1342. doi: 10.1016/j.ijrobp.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Hinnen K.A., Battermann J.J., van Roermund J.G. Long-term biochemical and survival outcome of 921 patients treated with I-125 permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2010;76:1433–1438. doi: 10.1016/j.ijrobp.2009.03.049. [DOI] [PubMed] [Google Scholar]
- 5.D'Amico A.V., Whittington R., Malkowicz S.B. Biochemical outcome after radical prostatectomy or external beam radiation therapy for patients with clinically localized prostate carcinoma in the prostate specific antigen era. Cancer. 2002;95:281–286. doi: 10.1002/cncr.10657. [DOI] [PubMed] [Google Scholar]
- 6.Potters L., Klein E.A., Kattan M.W. Monotherapy for stage T1–T2 prostate cancer: radical prostatectomy, external beam radiotherapy, or permanent seed implantation. Radiother Oncol. 2004;71:29–33. doi: 10.1016/j.radonc.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Chen A.B., D'Amico A.V., Neville B.A. Patient and treatment factors associated with complications after prostate brachytherapy. J Clin Oncol. 2006;24:5298–5304. doi: 10.1200/JCO.2006.07.9954. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka N., Asakawa I., Anai S. Periodical assessment of genitourinary and gastrointestinal toxicity in patients who underwent prostate low-dose-rate brachytherapy. Radiat Oncol. 2013;8:25. doi: 10.1186/1748-717X-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller D.C., Sanda M.G., Dunn R.L. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23(12):2772–2780. doi: 10.1200/JCO.2005.07.116. [DOI] [PubMed] [Google Scholar]
- 10.D'Amico A.V., Whittington R., Malkowicz S.B. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka N., Fujimoto K., Hirao Y. Variations in international prostate symptom scores, uroflowmetric parameters, and prostate volume after (125)I permanent brachytherapy for localized prostate cancer. Urology. 2009;74:407–411. doi: 10.1016/j.urology.2008.12.062. [DOI] [PubMed] [Google Scholar]
- 12.Ash D., Bottomley D., Al-Qaisieh B. A prospective analysis of long-term quality of life after permanent I-125 brachytherapy for localised prostate cancer. Radiother Oncol. 2007;84:135–139. doi: 10.1016/j.radonc.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Cesaretti J.A., Stone N.N., Stock R.G. Urinary symptom flare following I-125 prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2003;56:1085–1092. doi: 10.1016/s0360-3016(03)00210-4. [DOI] [PubMed] [Google Scholar]
- 14.Keyes M., Miller S., Moravan V. Urinary symptom flare in 712 125I prostate brachytherapy patients: long-term follow-up. Int J Radiat Oncol Biol Phys. 2009;75:649–655. doi: 10.1016/j.ijrobp.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 15.Tanimoto R., Bekku K., Katayama N. Predictive factors for acute and late urinary toxicity after permanent interstitial brachytherapy in Japanese patients. Int J Urol. 2013;20:812–817. doi: 10.1111/iju.12050. [DOI] [PubMed] [Google Scholar]
- 16.Ohashi T., Yorozu A., Saito S. Urinary and rectal toxicity profiles after permanent iodine-125 implant brachytherapy in Japanese Men: Nationwide J-POPS Multi-institutional Prospective Cohort Study. Int J Radiat Oncol Biol Phys. 2015;93:141–149. doi: 10.1016/j.ijrobp.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Williams S.G., Millar J.L., Duchesne G.M. Factors predicting for urinary morbidity following 125 iodine transperineal prostate brachytherapy. Radiother Oncol. 2004;73:33–38. doi: 10.1016/j.radonc.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 18.Keyes M., Miller S., Moravan V. Predictive factors for acute and late urinary toxicity after permanent prostate brachytherapy: long-term outcome in 712 consecutive patients. Int J Radiat Oncol Biol Phys. 2009;73:1023–1032. doi: 10.1016/j.ijrobp.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 19.Magi-Galluzzi C., Sanderson H., Epstein J.I. Atypia in nonneoplastic prostate glands after radiotherapy for prostate cancer: duration of atypia and relation to type of radiotherapy. Am J Surg Pathol. 2003;27:206–212. doi: 10.1097/00000478-200302000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Ohga S., Nakamura K., Shioyama Y. Acute urinary morbidity after a permanent 125I implantation for localized prostate cancer. J Radiat Res. 2014;55:1178–1183. doi: 10.1093/jrr/rru065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petraki C.D., Sfikas C.P. Histopathological changes induced by therapies in the benign prostate and prostate adenocarcinoma. Histol Histopathol. 2007;22:107–118. doi: 10.14670/HH-22.107. [DOI] [PubMed] [Google Scholar]
- 22.Teishima J., Iwamoto H., Miyamoto K. Impact of pre-implant lower urinary tract symptoms on postoperative urinary morbidity after permanent prostate brachytherapy. Int J Urol. 2012;19:1083–1089. doi: 10.1111/j.1442-2042.2012.03105.x. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs B.L., Smith R.P., Beriwal S. Changes in lower urinary tract symptoms after prostate brachytherapy. J Contemp Brachyther. 2011;3:115–120. doi: 10.5114/jcb.2011.24816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Earley J.J., Abdelbaky A.M., Cunningham M.J. Correlation between prostate brachytherapy-related urethral stricture and peri-apical urethral dosimetry: a matched case-control study. Radiother Oncol. 2012;104:187–191. doi: 10.1016/j.radonc.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Marigliano C., Donati O.F., Vargas H.A. MRI findings of radiation-induced changes in the urethra and periurethral tissues after treatment for prostate cancer. Eur J Radiol. 2013;82:775–781. doi: 10.1016/j.ejrad.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blaivas J.G., Weiss J.P., Jones M. The pathophysiology of lower urinary tract symptoms after brachytherapy for prostate cancer. BJU Int. 2006;98:1233–1237. doi: 10.1111/j.1464-410X.2006.06491.x. [DOI] [PubMed] [Google Scholar]
- 27.Yu Y.D., Kang M.H., Choi C.I. Clinical efficacy of combination therapy with an alpha blocker and low-dose sildenafil on post-therapy lower urinary tract symptoms after low-dose-rate brachytherapy for prostate cancer. World J Urol. 2016;34:1269–1274. doi: 10.1007/s00345-016-1777-7. [DOI] [PubMed] [Google Scholar]
- 28.Miyake M., Tanaka N., Asakawa I. Assessment of lower urinary symptom flare with overactive bladder symptom score and International Prostate Symptom Score in patients treated with iodine-125 implant brachytherapy: Long-term follow-up experience at a single institute. BMC Urol. 2017;17:62. doi: 10.1186/s12894-017-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.