Abstract
Background:
Upper extremity (UE) impairment is common with primary progressive multiple sclerosis (PPMS).
Objective:
This exploratory analysis examined the effects of ocrelizumab on confirmed progression (CP) and confirmed improvement (CI) in UE impairment in patients from ORATORIO.
Methods:
Patients with PPMS received ocrelizumab 600 mg or placebo every 24 weeks for ⩾120 weeks. The Nine-Hole Peg Test (9HPT) was administered at baseline (BL) and every 12 weeks thereafter. Prespecified exploratory endpoints included change in 9HPT time and proportion of patients with CP of ⩾20% in 9HPT. Analysis populations included intention-to-treat (ITT) patients and subgroups stratified by BL 9HPT time and Expanded Disability Status Scale. Post hoc analyses included the proportion of patients achieving more severe thresholds of CP and the proportion achieving CI in 9HPT.
Results:
Among ITT patients, ocrelizumab significantly reduced the change in 9HPT time over 120 weeks, the risk of CP of ⩾20% in 9HPT time for both hands and the risk of more severe 9HPT progression versus placebo. Numerical trends also favoured ocrelizumab versus placebo with respect to achieving CI. Consistent directional trends were observed in subgroup analyses.
Conclusion:
Ocrelizumab reduces the risk of UE disability progression and may increase the possibility of improvement versus placebo in PPMS.
Keywords: Multiple sclerosis, progressive, disease-modifying therapies, ocrelizumab, disease progression, upper extremity impairment
Introduction
Primary progressive multiple sclerosis (PPMS) is characterized by gradually worsening, multifaceted neurological disability that routinely includes motor, sensory, coordination and cognitive dysfunction.1 Impaired upper extremity (UE) function, caused by sensory, coordination and motor deficits, is widely reported by patients across all multiple sclerosis (MS) types, although patients with progressive disease may have higher prevalence of UE dysfunction and greater impairment of manual dexterity compared with patients with less severe disease.2,3 UE impairment impacts patients’ ability to perform activities of daily living (ADL), affecting their independence and quality of life.4 Moreover, the association of UE dysfunction and unemployment in patients with MS highlights the economic impact of compromised hand/arm function in MS.5 Therefore, objective quantitative assessment of UE functionality is critical for monitoring overall MS disease progression and evaluating the benefit of MS therapies.
The Expanded Disability Status Scale (EDSS) remains the most widely used tool to measure disability in the clinic and in MS drug trials. However, the EDSS has been criticized for its emphasis on ambulation, high inter-rater variability and questionable ability to adequately detect critical aspects of disability progression such as UE dysfunction, especially in progressive MS.6,7 The Multiple Sclerosis Functional Composite (MSFC), which comprises three quantitative assessments to detect changes in ambulation, UE function and cognition, was proposed to address the limitations of the EDSS.8 The Nine-Hole Peg Test (9HPT), a component of the MSFC, is frequently used in MS clinical research and practice and has adequate sensitivity to detect differences in UE function across various levels of impairment.9 The 9HPT has high inter-rater and test–retest reliability,10 and although it does not assess all essential aspects of forelimb movement, it correlates with other measures of UE function encompassing a range of hand/arm manipulations and movements.9 Performance on the 9HPT is also a significant predictor of MS-related costs, further exemplifying its relevance in evaluating therapeutic efficacy.11 In studies, clinically meaningful change is typically defined as an increase of ⩾20% in 9HPT time.9,12
Ocrelizumab, a recombinant humanized monoclonal antibody that selectively depletes CD20-expressing B cells while preserving the capacity for B-cell reconstitution and pre-existing humoral immunity, has demonstrated consistent efficacy for the treatment of both relapsing MS and PPMS.13,14 In the Phase III ORATORIO trial (ClinicalTrials.gov: NCT01194570) in PPMS, ocrelizumab-treated patients had significantly lower rates of clinical and magnetic resonance imaging (MRI)-measured progression as assessed by 12- and 24-week confirmed disability progression on the EDSS, change in timed 25-foot walk, change in T2-weighted brain lesion volume and total brain volume loss.14 In an exploratory analysis of ORATORIO, ocrelizumab significantly reduced the risk of 12- and 24-week confirmed progression (CP) of ⩾20% in 9HPT time compared with placebo.14 The effects of ocrelizumab treatment on UE dysfunction in patients with PPMS, including measures of confirmed improvement (CI), were further evaluated in a series of exploratory and post hoc analyses.
Materials and methods
Study design and patient population
ORATORIO was a randomized, Phase III, double-blind, placebo-controlled study of ocrelizumab in patients with PPMS. Patients were randomized (2:1) to receive ocrelizumab 600 mg, given as two 300 mg intravenous infusions 14 days apart, or corresponding placebo every 24 weeks for at least 120 weeks and until approximately 253 events of 12-week confirmed disability progression occurred. Key eligibility criteria and prespecified endpoints subject to hierarchical testing have been published.14 This study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice. Local ethics committees approved the protocol. All patients provided written informed consent.
Randomisation and masking
Details on randomisation and masking have been previously published.14
9HPT
The 9HPT was administered at baseline (BL) and every 12 weeks until the end of the study. Both hands were tested twice – in two consecutive trials of the dominant hand, followed by two consecutive trials of the nondominant hand – to determine the time taken to complete the test. There was a 300-second time limit per trial. The hand with the shorter time at BL based on the average of two trials was designated as the ‘better hand’; the other hand was designated as the ‘worse hand’. The average of all four trials was considered the time for ‘both hands’. This represents the conventional paradigm of 9HPT administration and scoring in MS.15
An alternative method of calculating 9HPT time was also used whereby the best time of two trials was used as the score for each hand; the hand with the lower time was the better hand, and the hand with the higher time was the worse hand. The time for both hands was calculated as the average of the best trials for each hand.
Statistical analysis
A Cox proportional hazards model stratified by region (USA vs rest of world) and age (⩽45, >45 years) was used to assess time to CP in UE impairment, defined as an increase in 9HPT time across rising thresholds of progression (i.e. ⩾20% (prespecified) ⩾25%, ⩾30% and ⩾35%), confirmed after 12 and 24 weeks, as well as CI of ⩾15% and ⩾20%. Change in 9HPT time from BL to Week 120 was evaluated with a mixed-effects model of repeated measures with all post-randomisation data incorporated using an unstructured variance-covariance matrix: change = BL 9HPT time + geographical region (USA vs rest of world) + age (⩽45, >45 years) + week + treatment + treatment × week + BL 9HPT time × week. Missing values were treated as follows: if a trial result was not available owing to a ‘physical limitation’, it was imputed as the maximum possible value (300 seconds). Missing trial data for reasons other than ‘physical limitation’ were imputed using the time from the second trial of the same hand, or, if not available, the average score from the opposite hand (or the score from a single trial if only one trial is available). All analyses described herein are exploratory in nature.
Analysis populations
Analysis groups included the intention-to-treat (ITT) population as well as patient subgroups defined by BL 9HPT and EDSS. The 9HPT groups included patients with abnormal (>25 seconds) versus normal (⩽25 seconds) 9HPT time at BL, with the 25-second threshold determined using a reference population of patients aged 18–59 years (consistent with the age of the ORATORIO population) from a large-scale normative database (N = 4319).16 Specifically, the times for the dominant and nondominant hands were averaged within the sex- and age-specific subgroups in the reference population. These values were then averaged to give the mean time across all subgroups (i.e. 19·8 seconds). The standard deviation for each subgroup was calculated assuming a between-hand correlation of 0.95; pooled variance was then used to calculate the standard deviation for the reference population overall (i.e. 2·7 seconds). Assuming a normal distribution and a 95% prediction interval, the upper limit of 9HPT times (i.e. 97.5th percentile) was calculated to be 25.1 seconds.
An analysis of patients by BL EDSS scores was performed to assess the effects of treatment in patients with significant walking impairment (BL EDSS ⩾ 6.0), who are at higher risk of becoming wheelchair-confined. In this specific subgroup of severely ambulation-restricted patients, the preservation of UE function is of utmost importance for all ADL including the use of aids such as a cane or crutch or self-wheeling.
Results
Patient disposition
The disposition of ORATORIO patients has been published.14
BL UE function in the analysis populations
The means of BL 9HPT times for both hands, better hand and worse hand were mostly comparable in all analysis populations across the two treatment groups; however, in the subgroup of patients with BL EDSS ⩾ 6, BL 9HPT times were higher in the ocrelizumab treatment group compared with the placebo group (Table 1). A slightly higher mean number of T1 gadolinium-enhancing lesions was observed in the ocrelizumab group compared with placebo, which could come from some outliers (note standard deviations and ranges); the proportion of patients with ⩾1 T1 gadolinium-enhancing lesion was similar between groups (Tables S1 and S2). Other BL demographic and disease characteristics are available in the supplementary material (Tables S1 and S2).
Table 1.
Baseline 9HPT times in the analysis populations.
| Placebo |
Ocrelizumab |
|||
|---|---|---|---|---|
| Number of patients | BL 9HPT time mean (SD), seconds | Number of patients | BL 9HPT time mean (SD), seconds | |
| ITT population | ||||
| Both hands | 244 | 30.6 (13.36) | 488 | 31.86 (23.31) |
| Better hand | 27.33 (11.45) | 28.43 (20.87) | ||
| Worse hand | 41.56 (43.90) | 42.31 (49.98) | ||
| Patients with abnormal BL 9HPT time | ||||
| Both hands | 137 | 37.57 (14.19) | 297 | 38.51 (27.87) |
| Better hand | 112 | 34.90 (13.08) | 232 | 36.44 (28.06) |
| Worse hand | 168 | 50.54 (50.40) | 345 | 50.85 (57.31) |
| Patients with normal BL 9HPT time | ||||
| Both hands | 107 | 21.69 (2.82) | 191 | 21.53 (2.50) |
| Better hand | 132 | 20.90 (2.75) | 256 | 21.17 (2.63) |
| Worse hand | 76 | 21.72 (2.81) | 143 | 21.70 (2.51) |
| Patients with BL EDSS <6 | ||||
| Both hands | 163 | 27.31 (9.40) | 348 | 27.76 (10.03) |
| Better hand | 24.68 (7.39) | 25.18 (7.49) | ||
| Worse hand | 36.63 (41.05) | 34.92 (35.99) | ||
| Patients with BL EDSS ⩾6 | ||||
| Both hands | 81 | 37.22 (17.23) | 139 | 42.17 (38.92) |
| Better hand | 32.65 (15.64) | 36.59 (36.08) | ||
| Worse hand | 51.48 (47.86) | 60.90 (71.21) | ||
9HPT: Nine-Hole Peg Test; BL: baseline; EDSS: Expanded Disability Status Scale; ITT: intention-to-treat; SD: standard deviation.
Time to CP (⩾20% increase) in 9HPT time
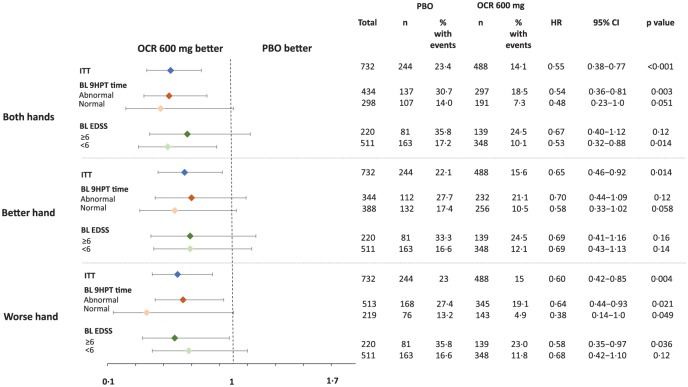
As previously reported, findings in the ITT population showed that ocrelizumab significantly reduced the risk of 12- and 24-week CP of ⩾20% in 9HPT time versus placebo for both hands (hazard ratio (HR) = 0.56, p < 0.001 and HR = 0.55, p < 0.001).14 Reductions were also observed for the better hand (HR = 0.72, p = 0.046 and HR = 0.65, p = 0.014) and worse hand (HR = 0.63, p = 0.005 and HR = 0.60, p = 0.004) (Figures 1 and S1), although the effects were less pronounced. Additional results based on the alternative best performance method of calculating 9HPT were consistent with these findings (Figure S2).
Figure 1.
Time to 24-week CP (⩾20% increase) in 9HPT time in the ITT population and subgroups of patients with abnormal/normal 9HPT times at baseline, and patients with baseline EDSS <6 and ⩾6.
HR derived from a Cox proportional hazards model stratified by region (USA vs rest of world) and age (⩽45, >45 years).
9HPT: Nine-Hole Peg Test; BL: baseline; CP: confirmed progression; EDSS: Expanded Disability Status Scale; HR: hazard ratio; ITT: intention-to-treat; OCR: ocrelizumab; PBO: placebo.
Among patients with abnormal BL 9HPT times, ocrelizumab significantly reduced the risk of 24-week CP of ⩾20% versus placebo for both hands (HR = 0.54, p = 0.003) and worse hand (HR = 0.64, p = 0·021); for better hand, the risk was numerically reduced but not significant (HR = 0.70, p = 0.12) (Figure 1). Similar results were observed on 12-week CP of ⩾20% (both hands: HR = 0.56, p = 0.003; worse hand: HR = 0.61, p = 0.006; better hand: HR = 0.83, p = 0.38) (Figure S1). Consistent directional trends favouring ocrelizumab were observed in other patient subgroups, including those with normal BL 9HPT times and patients with BL EDSS scores of <6 and ⩾6 (Figures 1 and S1). Kaplan–Meier estimates of the proportion of patients achieving CP of ⩾20% in 9HPT time at Week 120 are included in Table S3.
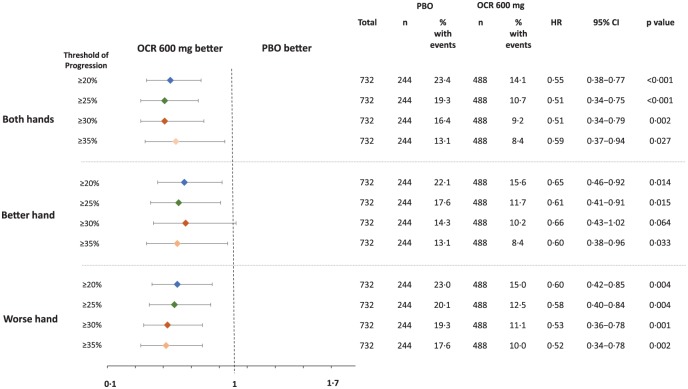
Time to more severe CP (⩾25%, ⩾30% and ⩾35% increase) in 9HPT time in the ITT population
Post hoc assessments of more severe progression in UE impairment included increases in 9HPT time of ⩾25%, ⩾30% and ⩾35%. In the placebo arm, the proportion of patients achieving 24-week CP in 9HPT time for both hands using these progressively higher thresholds was 19.3%, 16.4% and 13.1%, respectively, compared with 23.4% of patients using a threshold of ⩾20% (Figure 2). The risk of 24-week CP of ⩾25% in 9HPT time was significantly reduced with ocrelizumab compared with placebo for both hands (HR = 0.51, p < 0.001), better hand (HR = 0.61, p = 0.015) and worse hand (HR = 0.58, p = 0.004) (Figure 2). At higher thresholds of progression (⩾30% and ⩾35% increases), the reduction in risk with ocrelizumab versus placebo was significant for both hands and worse hand; for better hand, the risk was numerically reduced but not significant (Figure 2). Similar patterns were observed in both placebo- and ocrelizumab-treated patients with increasing thresholds using the 12-week CP endpoint (Figure S3).
Figure 2.
Time to more severe 24-week CP (⩾25%, ⩾30% and ⩾35% increase) in 9HPT time in the ITT population.
HR derived from a Cox proportional hazards model stratified by region (USA vs rest of world) and age (⩽45, >45 years). No adjustments were made to account for multiplicity of testing.
9HPT: Nine-Hole Peg Test; BL: baseline; CP: confirmed progression; EDSS: Expanded Disability Status Scale; HR: hazard ratio; ITT: intention-to-treat; OCR: ocrelizumab; PBO: placebo.
Time to CI (⩾15%, ⩾20%) in 9HPT time
Consistent trends directionally favoured ocrelizumab versus placebo in an exploratory analysis of the time to first event of 12- and 24-week CI in UE function, as measured by decreases in 9HPT time (both hands) of ⩾15% and ⩾20% in the ITT population (Table S4). The effect of ocrelizumab, although not reaching significance, was generally similar in patients with BL 9HPT time >25 seconds (Table S4).
Change in 9HPT time from BL to week 120
In the ITT population, the change in 9HPT time from BL to Week 120 was significantly improved with ocrelizumab compared with placebo across analyses of both hands (difference in adjusted means (standard error (SE)): –5.749 (1.720), p < 0.001) and worse hand (–7.572 (3.686), p = 0.041), with a numerically consistent trend for better hand (–3.671 (1.911), p = 0.056; Table 2). In patients with abnormal BL 9HPT times, the change in 9HPT time from BL to Week 120 was significantly improved with ocrelizumab compared with placebo across analyses of both hands (–10.765 (3.137), p < 0.001), worse hand (–11.900 (5.396), p = 0.028) and better hand (–15.674 (6.576), p = 0.021). In patients with normal BL 9HPT times, patients with BL EDSS <6 and patients with BL EDSS ⩾6, the change in 9HPT time from BL to Week 120 was directionally consistent and favoured ocrelizumab across all analyses but reached statistical significance only in the analysis of better hand for patients with normal BL 9HPT time (–1.072 (0.486), p = 0.029) and both hands for patients with BL EDSS <6 (–3.027 (1.053), p = 0.004).
Table 2.
Change in 9HPT time from BL to Week 120 in the analysis populations.
| Both hands | Better hand | Worse hand | ||||
|---|---|---|---|---|---|---|
| PBO N = 244 |
OCR 600 mg N = 488 |
PBO N = 244 |
OCR 600 mg N = 488 |
PBO N = 244 |
OCR 600 mg N = 488 |
|
| Change from BL to Week 120 in 9HPT time in the ITT population | ||||||
| n | 172 | 400 | 172 | 400 | 171 | 398 |
| Adjusted mean (SE) | 9.245 (1.464) | 3.496 (1.047) | 8.300 (1.641) | 4.628 (1.171) | 14.692 (3.072) | 7.120 (2.140) |
| Difference (OCR vs PBO) in adjusted means (SE), seconds | −5.749 (1.720) | −3.671 (1.911) | −7.572 (3.686) | |||
| p value | <0.001 | 0.056 | 0.041 | |||
| Change from BL to Week 120 in 9HPT time in patients with abnormal BL 9HPT | ||||||
| n | 86 | 240 | 70 | 185 | 110 | 280 |
| Adjusted mean (SE) | 16.730 (2.655) | 5.965 (1.778) | 26.793 (5.511) | 11.118 (3.667) | 22.022 (4.525) | 10.122 (3.053) |
| Difference (OCR vs PBO) in adjusted means (SE), seconds | −10.765 (3.137) | −15.674 (6.576) | −11.900 (5.396) | |||
| p value | <0.001 | 0.021 | 0.028 | |||
| Change from BL to Week 120 in 9HPT time in patients with normal BL 9HPT | ||||||
| n | 86 | 160 | 102 | 215 | 61 | 118 |
| Adjusted mean (SE) | 1.774 (0.990) | 1.468 (0.739) | 2.125 (0.451) | 1.053 (0.336) | 1.169 (0.577) | 0.118 (0.432) |
| Difference (OCR vs PBO) in adjusted means (SE), seconds | −0.306 (1.219) | −1.072 (0.486) | −1.051 (0.664) | |||
| p value | 0.80 | 0.029 | 0.12 | |||
| Change from BL to Week 120 in 9HPT time in patients with BL EDSS ⩾6 | ||||||
| n | 51 | 104 | 51 | 104 | 51 | 104 |
| Adjusted mean (SE) | 20.343 (4.783) | 9.904 (3.667) | 20.443 (5.478) | 11.569 (4.187) | 32.636 (7.775) | 14.195 (5.877) |
| Difference (OCR vs PBO) in adjusted means (SE), seconds | −10.440 (5.948) | −8.874 (6.793) | −18.442 (9.690) | |||
| p value | 0.085 | 0.20 | 0.059 | |||
| Change from BL to Week 120 in 9HPT time in patients with BL EDSS <6 | ||||||
| n | 121 | 296 | 121 | 296 | 120 | 294 |
| Adjusted mean (SE) | 4.097 (0.918) | 1.070 (0.633) | 4.536 (1.394) | 2.415 (0.958) | 6.116 (2.895) | 4.195 (1.934) |
| Difference (OCR vs PBO) in adjusted means (SE), seconds | −3.027 (1.053) | −2.121 (1.556) | −1.921 (3.433) | |||
| p value | 0.004 | 0.17 | 0.58 | |||
9HPT: Nine-Hole Peg Test; BL: baseline; EDSS: Expanded Disability Status Scale; ITT: intention-to-treat; OCR: ocrelizumab; PBO: placebo; SE: standard error.
Shading denotes significant results.
Discussion
In a chronic disease like PPMS that is typically diagnosed during the most productive years of the patient’s life span, preservation of UE function is an important therapeutic goal. In addition to its significant impact on performance of routine daily activities – limiting patient independence and quality of life4 – UE impairment is also associated with greater unemployment, resulting in a considerable economic burden.5 Findings from this analysis showed that ocrelizumab mitigated progression of UE impairment in patients with PPMS using the 9HPT.
The 9HPT is the most frequently used tool to assess UE function in MS clinical trials. Furthermore, changes in 9HPT performance are associated with patient-rated daily life disability, highlighting its significance as a patient-centred outcome.12 Various approaches have been used to define thresholds for UE dysfunction using the 9HPT.9 In this exploratory analysis of ORATORIO, impaired UE function was defined as a 9HPT time of >25 seconds for both hands, better hand and worse hand and was derived from normative data in a population with demographic characteristics similar to those of the trial population. More than 50% of ORATORIO participants met this criterion at study entry, suggesting a high prevalence of UE dysfunction in patients with PPMS.
Current evidence supports an increase of ⩾20% as the minimal threshold for detecting clinically meaningful change on the 9HPT. Multiple studies have shown that increases in 9HPT time of 15%–20% correlate with clinically meaningful changes on other disability measures, including the EDSS, Guys Neurological Disability Scale, Multiple Sclerosis Impact Scale and patient perception of disability.9,12 A 15%–20% threshold is also robust in differentiating patients with disability improvement or worsening from stable patients, although a 20% cut-off is associated with a better signal-to-noise ratio and therefore preferred in clinical studies.9,12 In this study, ocrelizumab significantly reduced the risk of CP of ⩾20% on the 9HPT in the ITT population based on the times for both hands, worse hand, and better hand, with optimal performance observed using the both hands method. Results across patient subgroups with compromised UE function or walking impairment (EDSS ⩾ 6) at BL were directionally consistent with the ITT population. Patients in these subgroups may stand to benefit the most from preserved or improved UE function. Specifically, impairment in the upper limbs is associated with considerable limitations on performance of essential ADL, such as eating, personal hygiene and getting dressed;4 furthermore, hand function measured by the 9HPT has been strongly correlated with measures of social engagement and quality of life.4,17 In patients with restricted walking ability, maintaining or improving UE function is particularly important as this can affect the ability to use walking aids.18 Indeed, preservation of UE function has been noted as one of the most important treatment benefits in patients with MS, and potentially more desirable than functional improvements in the lower limbs.19 Although significance was not reached across all subgroups, the numerical trends consistently favoured ocrelizumab over placebo.
Post hoc analyses showed that ocrelizumab also reduced more severe patterns of deterioration of UE function, measured as 9HPT progression above increasing thresholds of change (⩾25%, ⩾30% and ⩾35%). Compared with the ⩾20% threshold, ocrelizumab generally demonstrated a stronger treatment effect for the more severe levels of progression; however, the event rates drop considerably with increasing thresholds of change, reducing statistical power and limiting interpretation of the results. Finally, results for the change in 9HPT time from BL to Week 120 demonstrated a consistent beneficial effect of ocrelizumab versus placebo, particularly in patients who had abnormal 9HPT time at BL. These observations further support the other findings of this analysis and highlight improved preservation of UE function with ocrelizumab.
These results should be considered within some limitations. All analyses were exploratory in nature, and no adjustments were introduced for multiplicity of testing. The subgroup analyses in patients with normal or abnormal UE function or in patients with more advanced disability status at EDSS ⩾6.0 should be considered hypothesis generating at best. The comprehensive benefit of ocrelizumab treatment in preventing progression of UE impairment in patients with PPMS needs to be further investigated in patients who are wheelchair confined at an EDSS ⩾7.0, where maintenance of hand-arm function is of critical importance.
The findings presented further support the need to use the 9HPT in routine clinical practice, particularly for patients with progressive MS, as a fit-for-purpose treat-to-target instrument to complete assessment of the target disability picture. Along these lines, the NEDA (no evidence of disease activity) outcome was recently proposed to be expanded to NEPAD (no evidence of progression and active disease), which integrates measures of hand/arm function (9HPT) and ambulation (timed 25-foot walk). Ocrelizumab was shown to enhance the proportion of PPMS patients achieving NEPAD by threefold compared with placebo.20
Understanding the association between progressive worsening of UE function as measured by 9HPT and MRI measures of tissue damage/preservation in the central nervous system (CNS) requires further investigation. Based on cross-sectional analyses of patients with progressive MS, worse performance on the 9HPT correlated with cortical grey matter volume (cGMV) atrophy in Brodmann cortical area 44,21 T2-weighted lesion volume and measures of tissue integrity within T2 lesions, and fractional anisotropy in the normal-appearing white matter (NAWM).22 However, one may not draw causal inference from cross-sectional findings.
We have previously shown that ocrelizumab reduced the progression of hand/arm impairment as measured by 12-week and 24-week CP ⩾20%, both in PPMS patients with and without BL MRI features of acute inflammatory disease activity (T1 Gd-enhancing lesions), by 58%–36% and 61%–37%, respectively.14,23 In this analysis, the between-group difference in magnitude of ocrelizumab treatment did not reach significance based on treatment by subgroup interaction p-values,23 which suggests that the mechanism of action of ocrelizumab in preventing UE deterioration in progressive MS might be independent, at least in part, from its potent effect to silence accumulation of acute demyelinating lesions. Future analyses of long-term outcomes are needed to elucidate the relative importance of longitudinal change in regional cGMV versus chronic CNS axonal/myelin tissue loss in NAWM, change in meningeal inflammation or acute versus chronic white matter, and/or cortical lesion activity to predict progressive worsening of UE function as measured by 9HPT.
Supplemental Material
Supplemental material, MSJ808189_CONSORT_checklist for Ocrelizumab reduces progression of upper extremity impairment in patients with primary progressive multiple sclerosis: Findings from the phase III randomized ORATORIO trial by Edward J Fox, Clyde Markowitz, Angela Applebee, Xavier Montalban, Jerry S Wolinsky, Shibeshih Belachew, Damian Fiore, Jinglan Pei, Bruno Musch and Gavin Giovannoni in Multiple Sclerosis Journal
Supplemental Material
Supplemental material, MSJ808189_supplementary_figures for Ocrelizumab reduces progression of upper extremity impairment in patients with primary progressive multiple sclerosis: Findings from the phase III randomized ORATORIO trial by Edward J Fox, Clyde Markowitz, Angela Applebee, Xavier Montalban, Jerry S Wolinsky, Shibeshih Belachew, Damian Fiore, Jinglan Pei, Bruno Musch and Gavin Giovannoni in Multiple Sclerosis Journal
Supplemental Material
Supplemental material, MSJ808189_supplementary_tables for Ocrelizumab reduces progression of upper extremity impairment in patients with primary progressive multiple sclerosis: Findings from the phase III randomized ORATORIO trial by Edward J Fox, Clyde Markowitz, Angela Applebee, Xavier Montalban, Jerry S Wolinsky, Shibeshih Belachew, Damian Fiore, Jinglan Pei, Bruno Musch and Gavin Giovannoni in Multiple Sclerosis Journal
Acknowledgments
We would like to thank all patients and the investigators for their participation in this trial. Writing and editorial assistance was provided by Karishma Amin and Liz LaFlamme of Health Interactions, Inc., and funded by Genentech, Inc., South San Francisco, CA, USA. All authors had full access to all data in the study and participated in writing the manuscript, and the corresponding author had final responsibility for the decision to submit for publication.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: E.J.F. has received speaker honoraria and travel expense reimbursement from, participated in clinical research or speakers bureaus for, or served on advisory/consulting/steering committees for AbbVie, Acorda, Allergan, Bayer, Biogen, Celgene, Chugai, EMD Serono, F. Hoffmann-La Roche Ltd, Mallinckrodt, MedDay, Novartis, Sanofi Genzyme, Teva Pharmaceuticals, and TG Therapeutics. C.M. has served as a consultant for Bayer HealthCare, Biogen, F. Hoffmann-La Roche Ltd and Genentech, Inc., Mallinckrodt, Merck Serono, Mylan, Novartis, Sanofi Genzyme, and Teva Pharmaceuticals. A.A. has served on advisory boards, received speaker honoraria, or participated in clinical trials for Acorda Therapeutics, Actelion Pharmaceuticals, Biogen, F. Hoffmann-La Roche Ltd and Genentech, Inc., Novartis Pharmaceuticals, Opexa Therapeutics, Sanofi Genzyme, and Teva Pharmaceuticals. X.M. has received speaker honoraria and travel expense reimbursement for participation in scientific meetings and has been a steering committee member of clinical trials or served on advisory boards of clinical trials for Actelion, Almirall, Bayer, Biogen, F. Hoffmann-La Roche Ltd, Genzyme, Merck, Novartis, Octapharma, Receptos, Sanofi, Teva Pharmaceuticals, and Trophos. J.S.W. has served on advisory boards and data monitoring or steering committees, has consulting agreements, or received speaker honoraria from the following entities: AbbVie, Actelion, Alkermes, Bayer HealthCare, Biogen, Bionest, Celgene, Clene Nanomedicine, EMD Serono, Forward Pharma A/S, MedDay Pharmaceuticals, Novartis Pharmaceuticals, Otsuka, PTC Therapeutics, Roche Genentech, Sanofi Genzyme, Strategic Consultants International, Takeda, and Teva Pharmaceuticals; royalties are received for out licenced monoclonal antibodies through UTHealth from Millipore Corporation. S.B. is an employee and shareholder of F. Hoffmann-La Roche Ltd. D.F. is an employee of Genentech, Inc., and a shareholder of F. Hoffmann-La Roche Ltd. J.P. is an employee of Genentech, Inc. B.M. is an employee of Genentech, Inc., and a shareholder of F. Hoffmann-La Roche Ltd. G.G. has received honoraria from AbbVie, Atara Biotherapeutics, Bayer HealthCare, Biogen, Canbex Therapeutics, F. Hoffmann-La Roche Ltd, Five Prime Therapeutics, Genzyme, GlaxoSmithKline, GW Pharmaceuticals, Merck, Merck Serono, Novartis, Protein Discovery Laboratories, Synthon, Teva Pharmaceuticals, UCB, and Vertex; has received research grant support from Biogen, Ironwood, Merck Serono, Merz, and Novartis; and has received compensation from Elsevier.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by F. Hoffmann La-Roche Ltd, Basel, Switzerland, which together with a steering committee was responsible for the design of the trial and the data analysis and contributed to data interpretation. A statistician employed by the study sponsor was a member of the steering committee and critically reviewed all results. The funder had no role in data collection.
ORCID iD: Jerry S Wolinsky  https://orcid.org/0000-0002-8197-2762
https://orcid.org/0000-0002-8197-2762
Contributor Information
Edward J Fox, Central Texas Neurology Consultants and Dell Medical School, The University of Texas at Austin, Round Rock, TX, USA.
Clyde Markowitz, Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Angela Applebee, Department of Neurology, St. Peter’s Health Partners, Albany, NY, USA.
Xavier Montalban, Division of Neurology, St. Michael’s Hospital, University of Toronto, Toronto, ON, Canada/ Department of Neurology/Neuroimmunology, Centre d’Esclerosi Múltiple de Catalunya (CEMCAT), Hospital Universitari Vall d’Hebron, Barcelona, Spain.
Jerry S Wolinsky, McGovern Medical School, The University of Texas Health Science Center at Houston (UTHealth), Houston, TX, USA.
Shibeshih Belachew, F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Damian Fiore, Genentech, Inc., South San Francisco, CA, USA.
Jinglan Pei, Genentech, Inc., South San Francisco, CA, USA.
Bruno Musch, Genentech, Inc., South San Francisco, CA, USA.
Gavin Giovannoni, Department of Neurology, Queen Mary University of London, London, UK.
References
- 1. Ytterberg C, Johansson S, Andersson M, et al. Variations in functioning and disability in multiple sclerosis. A two-year prospective study. J Neurol 2008; 255(7): 967–973. [DOI] [PubMed] [Google Scholar]
- 2. Poole JL, Nakamoto T, McNulty T, et al. Dexterity, visual perception, and activities of daily living in persons with multiple sclerosis. Occup Ther Health Care 2010; 24(2): 159–170. [DOI] [PubMed] [Google Scholar]
- 3. Holper L, Coenen M, Weise A, et al. Characterization of functioning in multiple sclerosis using the ICF. J Neurol 2010; 257(1): 103–113. [DOI] [PubMed] [Google Scholar]
- 4. Yozbatiran N, Baskurt F, Baskurt Z, et al. Motor assessment of upper extremity function and its relation with fatigue, cognitive function and quality of life in multiple sclerosis patients. J Neurol Sci 2006; 246(1–2): 117–122. [DOI] [PubMed] [Google Scholar]
- 5. Marrie RA, Cutter GR, Tyry T, et al. Upper limb impairment is associated with use of assistive devices and unemployment in multiple sclerosis. Mult Scler Relat Disord 2017; 13: 87–92. [DOI] [PubMed] [Google Scholar]
- 6. Noseworthy JH, Vandervoort MK, Wong CJ, et al. Interrater variability with the expanded disability status scale (EDSS) and functional systems (FS) in a multiple sclerosis clinical trial. The Canadian Cooperation MS Study Group. Neurology 1990; 40(6): 971–975. [DOI] [PubMed] [Google Scholar]
- 7. Cadavid D, Cohen JA, Freedman MS, et al. The EDSS-plus, an improved endpoint for disability progression in secondary progressive multiple sclerosis. Mult Scler 2017; 23(1): 94–105. [DOI] [PubMed] [Google Scholar]
- 8. Cutter GR, Baier ML, Rudick RA, et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain 1999; 122(Pt 5): 871–882. [DOI] [PubMed] [Google Scholar]
- 9. Feys P, Lamers I, Francis G, et al. The Nine-Hole Peg Test as a manual dexterity performance measure for multiple sclerosis. Mult Scler 2017; 23(5): 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohen JA, Fischer JS, Bolibrush DM, et al. Intrarater and interrater reliability of the MS functional composite outcome measure. Neurology 2000; 54(4): 802–806. [DOI] [PubMed] [Google Scholar]
- 11. Koch MW, Murray TJ, Fisk J, et al. Hand dexterity and direct disease related cost in multiple sclerosis. J Neurol Sci 2014; 341(1–2): 51–54. [DOI] [PubMed] [Google Scholar]
- 12. Bosma LV, Kragt JJ, Brieva L, et al. Progression on the multiple sclerosis functional composite in multiple sclerosis: What is the optimal cut-off for the three components? Mult Scler 2010; 16(7): 862–867. [DOI] [PubMed] [Google Scholar]
- 13. Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2017; 376(3): 221–234. [DOI] [PubMed] [Google Scholar]
- 14. Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med 2017; 376(3): 209–220. [DOI] [PubMed] [Google Scholar]
- 15. Fischer JS, Jack AJ, Kniker JE, et al. Multiple sclerosis functional composite (MSFC) administration and scoring manual, http://main.nationalmssociety.org/docs/HOM/MSFC_Manual_and_Forms.pdf
- 16. Wang YC, Bohannon RW, Kapellusch J, et al. Dexterity as measured with the 9-Hole Peg Test (9-HPT) across the age span. J Hand Ther 2015; 28(1): 53–59; quiz 60. [DOI] [PubMed] [Google Scholar]
- 17. Kierkegaard M, Einarsson U, Gottberg K, et al. The relationship between walking, manual dexterity, cognition and activity/participation in persons with multiple sclerosis. Mult Scler 2012; 18(5): 639–646. [DOI] [PubMed] [Google Scholar]
- 18. Kraft GH, Amtmann D, Bennett SE, et al. Assessment of upper extremity function in multiple sclerosis: Review and opinion. Postgrad Med 2014; 126(5): 102–108. [DOI] [PubMed] [Google Scholar]
- 19. Kapoor R, Ho PR, Campbell N, et al. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): A phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol 2018; 17(5): 405–415. [DOI] [PubMed] [Google Scholar]
- 20. Wolinsky JS, Montalban X, Hauser SL, et al. Evaluation of no evidence of progression or active disease (NEPAD) in patients with primary progressive multiple sclerosis in the ORATORIO trial. Ann Neurol. Epub ahead of print 29 August 2018. DOI: 10.1002/ana.25313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. MacKenzie-Graham A, Kurth F, Itoh Y, et al. Disability-specific atlases of gray matter loss in relapsing-remitting multiple sclerosis. JAMA Neurol 2016; 73(8): 944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ammitzboll C, Dyrby TB, Lyksborg M, et al. Disability in progressive MS is associated with T2 lesion changes. Mult Scler Relat Disord 2018; 20: 73–77. [DOI] [PubMed] [Google Scholar]
- 23. Wolinsky JS, Montalban X, Hauser SL, et al. Prespecified subgroup analyses of ocrelizumab efficacy in patients with primary progressive multiple sclerosis from the phase III ORATORIO study. Int J MS Care 2018; 20(Suppl 1): 1–128.29507537 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MSJ808189_CONSORT_checklist for Ocrelizumab reduces progression of upper extremity impairment in patients with primary progressive multiple sclerosis: Findings from the phase III randomized ORATORIO trial by Edward J Fox, Clyde Markowitz, Angela Applebee, Xavier Montalban, Jerry S Wolinsky, Shibeshih Belachew, Damian Fiore, Jinglan Pei, Bruno Musch and Gavin Giovannoni in Multiple Sclerosis Journal
Supplemental material, MSJ808189_supplementary_figures for Ocrelizumab reduces progression of upper extremity impairment in patients with primary progressive multiple sclerosis: Findings from the phase III randomized ORATORIO trial by Edward J Fox, Clyde Markowitz, Angela Applebee, Xavier Montalban, Jerry S Wolinsky, Shibeshih Belachew, Damian Fiore, Jinglan Pei, Bruno Musch and Gavin Giovannoni in Multiple Sclerosis Journal
Supplemental material, MSJ808189_supplementary_tables for Ocrelizumab reduces progression of upper extremity impairment in patients with primary progressive multiple sclerosis: Findings from the phase III randomized ORATORIO trial by Edward J Fox, Clyde Markowitz, Angela Applebee, Xavier Montalban, Jerry S Wolinsky, Shibeshih Belachew, Damian Fiore, Jinglan Pei, Bruno Musch and Gavin Giovannoni in Multiple Sclerosis Journal