Abstract
Background:
Demonstration of clinical benefits on disability progression measures is an important attribute of effective multiple sclerosis (MS) treatments.
Objective:
Examine efficacy of daclizumab beta versus intramuscular (IM) interferon beta-1a on measures of disability progression in patient subgroups from DECIDE.
Methods:
Twenty-four-week confirmed disability progression (CDP), 24-week sustained worsening on a modified Multiple Sclerosis Functional Composite (MSFCS) where 3-Second Paced Auditory Serial Addition Test was replaced by Symbol Digit Modalities Test, and proportion of patients with clinically meaningful worsening in 29-Item Multiple Sclerosis Impact Scale physical impact subscale (MSIS-29 PHYS) score from baseline to week 96 were examined in the overall population and subgroups defined by baseline demographic/disease characteristics.
Results:
Daclizumab beta significantly reduced risk of 24-week CDP (hazard ratio (HR), 0.73; 95% confidence interval (95% CI), 0.55–0.98), risk of 24-week sustained MSFCS progression (HR, 0.80; 95% CI, 0.67–0.95), and odds of clinically meaningful worsening in MSIS-29 PHYS (odds ratio, 0.76; 95% CI, 0.60–0.95) versus IM interferon beta-1a. Point estimates showed trends favoring daclizumab beta over IM interferon beta-1a across several patient subgroups for all three outcome measures.
Conclusion:
Daclizumab beta showed consistent benefit versus IM interferon beta-1a across measures assessing patient disability/function and across a range of clinical baseline characteristics in patients with relapsing-remitting MS.
Keywords: Daclizumab beta, disability progression, efficacy, interferon beta-1a, relapsing-remitting multiple sclerosis, subgroup analysis
Introduction
Delaying progression of disability is a key therapeutic goal of disease-modifying therapy (DMT) in patients with multiple sclerosis (MS).1,2 Multiple outcome measures have been developed to assess disease progression or patient function in clinical studies of patients with MS. The Expanded Disability Status Scale (EDSS) is the most established outcome measure in MS clinical trials.3,4 The EDSS has notable limitations, particularly with regard to insensitivity in upper extremity function and non-motor functions once ambulation is severely restricted.2,5–7 Additionally, it does not provide an adequate assessment of cognitive impairment related to MS.6
The Multiple Sclerosis Functional Composite (MSFC), developed to overcome some of the limitations of the EDSS, comprises three components that evaluate different patient functional outcomes: the Timed 25-Foot Walk (T25FW) for ambulation, the 9-Hole Peg Test (9HPT) for hand/arm dexterity, and the 3-Second Paced Auditory Serial Addition Test (PASAT-3) for cognition.7 Because clinical interpretation of the composite z-score methodology employed for the MSFC can be challenging, examining worsening of each MSFC component separately has been proposed as an alternative analytic measure.8 It has also been suggested that the MSFC is a more robust assessment if the Symbol Digit Modalities Test (SDMT) is used instead of the PASAT-3 for assessing cognition.6,9 The SDMT is easier and faster to administer,10 may be more reliable,6,9,11 and has demonstrated smaller practice effects,9 a known concern with the PASAT-3.12
DECIDE (NCT01064401) was a phase 3 study that evaluated the efficacy and safety of treatment with daclizumab beta 150 mg subcutaneous once every 4 weeks versus interferon (IFN) beta-1a 30 mcg intramuscular (IM) once weekly in patients with relapsing-remitting MS (RRMS).13 Daclizumab beta (formerly known as daclizumab high-yield process) was approved as ZINBRYTA®, which has a different form and structure than an earlier form of daclizumab. In the overall study population, daclizumab beta demonstrated greater benefit compared with IM IFN beta-1a on several outcome measures of disability. While 12-week confirmed disability progression (CDP) as assessed by EDSS did not differ significantly between the two treatment groups, 24-week CDP, a more robust outcome than 12-week CDP, was reduced by 27% in patients treated with daclizumab beta versus IM IFN beta-1a (p = 0.033).13 At week 96, patients in the daclizumab beta versus the IM IFN beta-1a group had greater median change from baseline (25th, 75th percentiles) in overall MSFC score (0.091 (−0.096 to 0.287) vs 0.055 (−0.136 to 0.240), respectively; p < 0.001), in each individual MSFC component score (all p < 0.05), and a greater mean change on the SDMT (p = 0.03).13 Additionally, patients in the daclizumab beta group had a 24% reduction in the odds of experiencing a clinically meaningful worsening in the patient-reported 29-Item Multiple Sclerosis Impact Scale physical impact subscale (MSIS-29 PHYS) score at week 96 (odds ratio (OR), 0.76; 95% confidence interval (95% CI), 0.60–0.95; nominal p = 0.0176).14
In addition to demonstrating efficacy of an MS therapy in the overall study population, subgroup analyses may inform on treatment effects across different demographic and clinical characteristics.15,16 This post hoc analysis examined treatment effects of daclizumab beta compared with IM IFN beta-1a on measures of patient disability or impairment across patient subgroups according to baseline demographic and disease characteristics in DECIDE. The measures included a modified MSFC, in which the PASAT-3 was replaced with the SDMT.
Methods
Full details of DECIDE have been reported.13 Briefly, patients of age 18–55 years with a confirmed diagnosis of RRMS were randomized (1:1) to daclizumab beta 150 mg subcutaneous every 4 weeks and IM placebo once weekly or IFN beta-1a 30 mcg IM once weekly and subcutaneous placebo every 4 weeks for a minimum of 96 weeks and up to 144 weeks. Magnetic resonance imaging (MRI) consistent with MS, baseline EDSS score of 0–5.0, and two or more relapses within the previous 3 years (one or more in year before study) or one or more relapse(s) and one or more new MRI lesion(s) within 2 years (one or more event(s) in year before study) constituted additional inclusion criteria. All patients provided written informed consent. Central and local ethics committee approvals were obtained, and the study was performed in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice.17
Assessments
Three outcome measures of patient disability/function were examined for the overall study population and by subgroup. These included 24-week CDP as measured by EDSS18 (tertiary endpoint in DECIDE), 24-week sustained worsening on the MSFCS (analysis performed post hoc), and the proportion of patients experiencing a clinically meaningful worsening in MSIS-29 PHYS score at week 96 (secondary endpoint in DECIDE). Twenty-four-week CDP was defined as an increase in the EDSS score of ⩾1.0 point(s) from a baseline score of ⩾1.0 or ⩾1.5 points from a baseline score of 0 confirmed after 24 weeks.13 Twenty-four-week sustained worsening on the MSFCS, based in part on an analysis by Rudick et al.,8 was defined as ⩾20% worsening in T25FW score, ⩾20% worsening in 9HPT score (mean of both hands), or a decrease of ⩾4 points in SDMT score (clinically meaningful change)19 sustained for 24 weeks. Additional analyses were run using alternative methods for evaluating 9HPT. These included examining a 20% worsening in 9HPT score for the dominant hand only or for either the dominant hand or the non-dominant hand. Finally, in order to capture clinically meaningful changes due to MS from the patents’ perspective, worsening from baseline in MSIS-29 PHYS score was analyzed at week 96. An increase from baseline on the MSIS-29 of ⩾7.5 points has been shown to indicate clinically meaningful worsening in a large clinical study population.20
EDSS, T25FW, and 9HPT scores were assessed at baseline and every 12 weeks until week 144 (or end of study). SDMT (oral response format) and MSIS-29 (version 1) scores were assessed at baseline and every 24 weeks until week 144 or end of study.
Subgroups based on baseline demographics were age (⩽35, >35 years) and sex (female, male). Subgroups based on baseline disease characteristics were disability as defined by EDSS score (<3.5, ⩾3.5), relapses in previous 12 months (one or less, two or more), disease duration (<3, ⩾3 to <10, or ⩾10 years), gadolinium-enhancing (Gd+) lesions (absent, present), T2 hyperintense lesion volume (<, ⩾ median), disease activity (highly active, less active; highly active was defined as two or more relapses in the year before randomization and one or more Gd+ lesion(s) on baseline MRI, less active otherwise), prior DMT use (yes, no; excluding steroids but including any prior disease-modifying or immunomodulatory therapy for MS, such as alemtuzumab, azathioprine, cladribine, cyclophosphamide, fingolimod, fumaric acid, glatiramer acetate, immune globulin, IFN beta-1a, IFN beta-1b, laquinimod, methotrexate, mitoxantrone, mycophenolic acid, natalizumab, teriflunomide, or temsirolimus), and prior IFN beta use (yes, no; including IFN beta, IFN beta-1a, and IFN beta-1b).
Statistical analyses
All analyses were performed on the intention-to-treat population (randomized patients who received one or more dose(s) of study drug) with non-missing baseline assessments.13 p-values reported were not adjusted for multiple testing. Disability progression based on EDSS score was analyzed by a Cox proportional hazards model adjusted for baseline EDSS score (continuous variable), prior IFN beta use (yes, no), and baseline age (⩽35, >35 years), excluding covariates defining the subgroup. Among patients with one or more tentative progression event(s), a logistic model was used to estimate the probability of confirmation for patients with a missing EDSS assessment to confirm progression. The logistic model adjusted for treatment group, EDSS score at baseline (continuous variable), change in EDSS score from baseline to the time of tentative progression, and presence or absence of a relapse within the last 29 days of the tentative progression.13 For patients with multiple tentative progressions, the confirmed (if patient had a confirmed progression) or the last (if patient did not have any tentative progressions confirmed) tentative progression record was retained. In total, 50 imputed datasets were generated using the estimated probabilities from this logistic regression model. The Cox proportional hazards model was conducted on subgroups of each of the 50 datasets. Rubin’s rule21 was used to combine the HR, standard error of this estimate, and p-values.
MSFCS progression was analyzed by Cox proportional hazards model adjusted for prior IFN beta use (yes, no) and baseline age (⩽35, >35 years), excluding covariates defining the subgroup. Patients with a tentative progression at the end of treatment period visit and no confirmation assessment were censored at their last assessment. Data were re-censored at 2 years, that is, 96 weeks. Missing T25FW and 9HPT data were imputed using the method described in the supplemental material of Kappos et al.13 Missing SDMT values in post-baseline visits were imputed using last observation carried forward. For patients with missing SDMT values, the other endpoints were used to derive time to first sustained progression.
Analyses of patients with a clinically meaningful worsening in MSIS-29 PHYS score were based on logistic regression models, adjusted for baseline MSIS-29 PHYS score, baseline Beck Depression Inventory-II score, prior IFN beta use, and baseline age (⩽35, >35 years), but excluded covariates defining the subgroup. If a patient was missing data for <10 of the 20 items that make up the PHYS score, then the mean of the non-missing items was used for the missing items. If the patient was missing ⩾10 of the 20 items that make up the PHYS score, or missing the questionnaire entirely, or if the questionnaire was completed after the patient switched to alternative MS medication, a random effects model was used to estimate MSIS-29 PHYS score.
Results
The intention-to-treat population of DECIDE included 1841 patients; 922 were randomized to IM IFN beta-1a and 919 were randomized to daclizumab beta.13 Details of the demographics and baseline characteristics of the DECIDE study population are published.13 Relevant demographics and baseline characteristics are shown in Table 1.
Table 1.
Patient demographics and baseline disease characteristics in DECIDE.
| Characteristic | IM IFN beta-1a (n = 922) | Daclizumab beta (n = 919) |
|---|---|---|
| Age, years, mean (SD) | 36.2 (9.3) | 36.4 (9.4) |
| Female, n (%) | 627 (68) | 625 (68) |
| White, n (%) | 828 (90) | 823 (90) |
| Time since MS diagnosis, years, mean (median) | 4.1 (2.0) | 4.2 (2.0) |
| Number of relapses in previous year, mean (SD) | 1.6 (0.8) | 1.5 (0.7) |
| Number of relapses in previous 3 years,a mean (SD) | 2.7 (1.3) | 2.7 (1.2) |
| Baseline EDSS score | ||
| Mean (SD) | 2.5 (1.3) | 2.5 (1.2) |
| Median (range) | 2.3 (0−6.0) | 2.0 (0−5.5) |
| SDMT score,b mean (SD) | 47.7 (16.1) | 48.5 (15.9) |
| MSIS-29 PHYS score,c mean (SD) | 21.9 (19.2) | 21.5 (19.8) |
| MSIS-29 PSYCH score,d mean (SD) | 28.6 (21.1) | 28.8 (21.8) |
| MSFC score,e median (25th, 75th percentiles) | 0.118 (−0.377, 0.482) | 0.139 (−0.335, 0.491) |
| T25FW z-score | 0.223 (−0.042, 0.372) | 0.223 (−0.034, 0.372) |
| 9HPT z-score | 0.035 (−0.622, 0.633) | 0.065 (−0.597, 0.661) |
| PASAT-3 z-scorec | 0.264 (−0.619, 0.794) | 0.352 (−0.531, 0.794) |
IM: intramuscular; IFN: interferon; SD: standard deviation; MS: multiple sclerosis; EDSS: Expanded Disability Status Scale; SDMT: Symbol Digit Modalities Test; MSIS-29: 29-Item Multiple Sclerosis Impact Scale; PHYS: physical impact subscale; PSYCH: psychological impact subscale; MSFC: multiple sclerosis functional composite; T25FW: Timed 25-Foot Walk; 9HPT: 9-Hole Peg Test; PASAT-3: 3-Second Paced Auditory Serial Addition Test.
Daclizumab beta, n = 918.
IM IFN beta-1a, n = 880; daclizumab beta, n = 884.
IM IFN beta-1a, n = 912; daclizumab beta, n = 906.
IM IFN beta-1a, n = 912; daclizumab beta, n = 904.
IM IFN beta-1a, n = 920; daclizumab beta, n = 916.
Twenty-four-week CDP
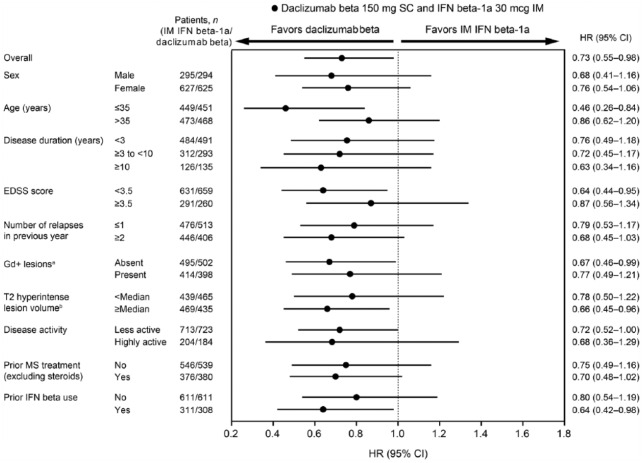
Across all subgroups, point estimates of the risk of 24-week CDP showed consistent trends favoring daclizumab beta over IM IFN beta-1a (Figure 1) and supported the results observed for 24-week CDP in the overall study population.13 For this outcome measure, minor variations in treatment effect estimates were observed and there was no convincing evidence of effect modification. HRs ranged from 0.46 to 0.87, where the greatest risk reduction was observed in patients ⩽35 years of age.
Figure 1.
Forest plot for 24-week confirmed disability progression for daclizumab beta versus IM IFN beta-1a by baseline demographics and disease characteristics.
CI: confidence interval; EDSS: Expanded Disability Status Scale; Gd+: gadolinium-enhancing; IFN: interferon; IM: intramuscular; MS: multiple sclerosis; SC: subcutaneous.
aMissing baseline Gd+ lesions data: IM IFN beta-1a, n = 13; daclizumab beta, n = 19.
bMissing baseline T2 hyperintense lesion volume data: IM IFN beta-1a, n = 14; daclizumab beta, n = 19.
cMissing baseline disease activity data: IM IFN beta-1a, n = 5; daclizumab beta, n = 12.
Twenty-four-week sustained MSFCS progression
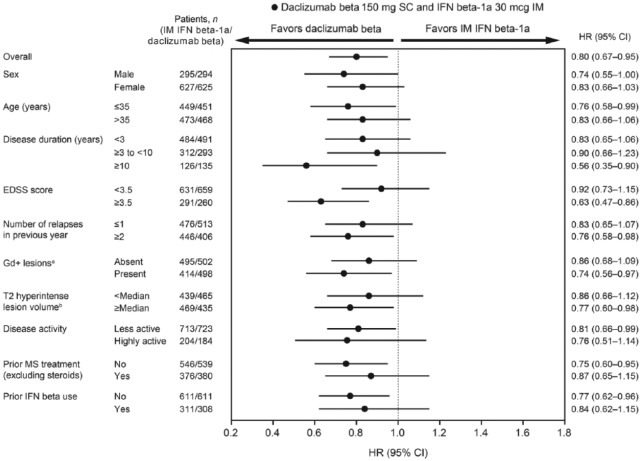
Fewer daclizumab beta (24% (224/919)) versus IM IFN beta-1a patients (28% (259/922)) met the criteria for 24-week sustained MSFCS progression at week 96. Of patients who progressed, MSFCS progression was most commonly driven by SDMT (IM IFN beta-1a, 56% (146/259); daclizumab beta, 55% (124/224)), followed by T25FW (IM IFN beta-1a, 34% (89/259); daclizumab beta, 33% (75/224)) and 9HPT scores (IM IFN beta-1a, 6% (16/259); daclizumab beta, 8% (17/224)). The rest of the patients progressed on two or more components at the same time. In the overall study population, treatment with daclizumab beta resulted in a 20% reduction (HR, 0.80; 95% CI, 0.67–0.95; p = 0.0132) in risk of 24-week sustained MSFCS progression compared with IM IFN beta-1a. Point estimates of risk of 24-week sustained progression of the MSFCS show consistent trends favoring daclizumab beta over IM IFN beta-1a across all subgroups. HRs ranged from 0.56 to 0.92. While there were minor variations in treatment effect estimates, there was no convincing evidence of effect modification (Figure 2). Nominal statistical significance of risk reduction was noted for age ⩽ 35 years, baseline EDSS score ⩾3.5, two or more relapses in the previous year, presence of baseline Gd+ lesions, T2 hyperintense lesion volume ⩾median, less active disease activity at baseline, no prior DMT use, no prior IFN beta use, and time since diagnosis ⩾10 years. Similar results were observed when alternative methods were used for assessing 9HPT score (both dominant and non-dominant hands included, dominant hand only; Figure S1).
Figure 2.
Forest plot for 24-week sustained modified Multiple Sclerosis Functional Composite progression for daclizumab beta versus IM IFN beta-1a by baseline demographics and disease characteristics.
CI: confidence interval; EDSS: Expanded Disability Status Scale; Gd+: gadolinium-enhancing; IFN: interferon; IM: intramuscular; MS: multiple sclerosis; SC: subcutaneous.
aMissing baseline Gd+ lesions data: IM IFN beta-1a, n = 13; daclizumab beta, n = 19.
bMissing baseline T2 hyperintense lesion volume data: IM IFN beta-1a, n = 14; daclizumab beta, n = 19.
cMissing baseline disease activity data: IM IFN beta-1a, n = 5; daclizumab beta, n = 12.
Clinically meaningful worsening in MSIS-29 PHYS score
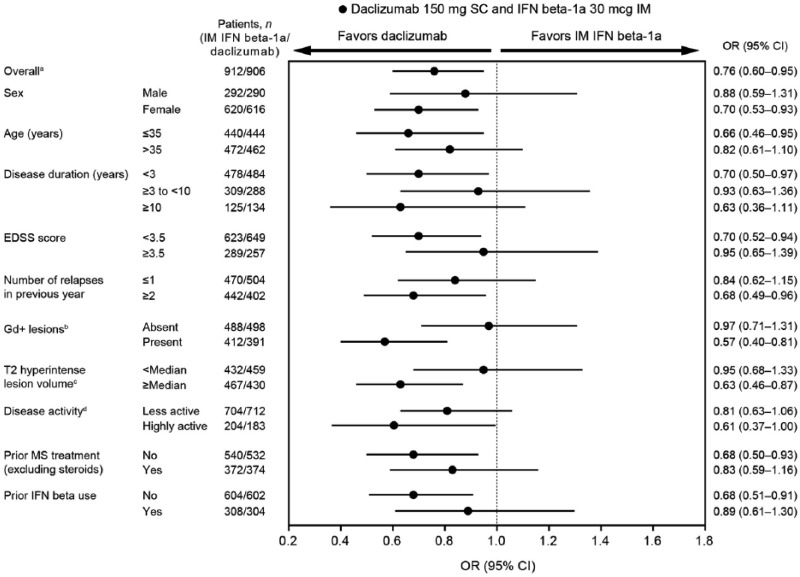
Treatment with daclizumab beta resulted in a 24% reduction in the odds of a clinically meaningful worsening in MSIS-29 PHYS score at week 96 versus IM IFN beta-1a (OR, 0.76; 95% CI, 0.60–0.95; p = 0.0176).13 ORs of the risk of clinically meaningful worsening in MSIS-29 PHYS score at week 96 show trends favoring daclizumab beta over IM IFN beta-1a across all subgroups (Figure 3). ORs ranged from 0.57 to 0.97. Nominal statistical significance of risk reduction was noted for female sex, age ⩽ 35 years, disease duration <3 years, baseline EDSS score <3.5, two or more relapses in previous year, presence of baseline Gd+ lesions, T2 hyperintense lesion volume ⩾median, no prior DMT use, and no prior IFN beta use.
Figure 3.
Forest plot for proportion of patients with clinically meaningful worsening in 29-Item Multiple Sclerosis Impact Scale physical impact subscale score at week 96 for daclizumab beta versus IM IFN beta-1a by baseline demographics and disease characteristics.
aOnly patients with available baseline assessment were included in the overall MSIS-29 PHYS analysis.
bPatients included in the overall MSIS-29 PHYS analysis with missing baseline Gd+ lesions data: IM IFN beta-1a, n = 12; daclizumab beta, n = 17.
cPatients included in the overall MSIS-29 PHYS analysis with missing baseline T2 hyperintense lesion volume data: IM IFN beta-1a, n = 13; daclizumab beta, n = 17.
dPatients included in the overall MSIS-29 PHYS analysis missing baseline disease activity data: IM IFN beta-1a, n = 4; daclizumab beta, n = 11.
Discussion
In the overall study population of DECIDE, treatment with daclizumab beta resulted in significant reductions in risk of 24-week CDP as measured using the EDSS, and risk of 24-week sustained progression on the MSFCS, a version of the MSFC replacing the PASAT-3 with the SDMT. Additionally, patients receiving daclizumab beta had reduced risk of experiencing a clinically meaningful worsening in MSIS-29 PHYS score compared with IM IFN beta-1a. Daclizumab beta treatment also showed consistent benefit versus IM IFN beta-1a across multiple patient subgroups, thus supporting the treatment effect seen in the overall population for each of the outcome measures examined independent of baseline characteristics. Treatment effect did not reach statistical significance for all subgroups for any of the three outcome measures, however, age ⩽ 35 years and baseline T2 hyperintense lesion volume ⩾median reached nominal significance for all three measures.
In this study, daclizumab beta demonstrated greater efficacy versus IM IFN beta-1a on two distinct measures of disability progression, 24-week CDP as measured by EDSS and the MSFCS. Despite its wide use, the EDSS has been criticized for a lack of sensitivity to change and inadequate assessment of cognition.5,22 The MSFC was developed to address these limitations and provide information supplemental to that provided by the EDSS.7 Both a 15% and a 20% worsening from baseline in at least one MSFC component (sustained for 3 months) were found to be sensitive measures of disability progression.8
This study examined MSFCS progression sustained for 6 months, which is considered more robust than the 3-month interval and is recommended by the European Medicines Agency when examining CDP.2,23 This study also explored three methodologies for the 9HPT component of the MSFCS: mean of both hands, dominant hand only, and either the dominant or non-dominant hand. The results of this MSFCS analysis did not appear to be impacted by choice of methodology.
Rudick et al. reported that, of patients who progressed on the MSFCS using a 20% worsening, the majority of patients progressed first on the T25FW (51% of placebo and 54% of natalizumab), while few patients progressed first on the PASAT-3 (5% of placebo and 6% of natalizumab).8 In contrast, the present analysis found that the majority of the patients with MSFCS progression worsened first on the SDMT, suggesting that the SDMT has potentially greater sensitivity compared with the PASAT-3 in detecting cognitive decline.
In contrast to the EDSS and MSFC, which are clinical assessment measures administered by physicians or trained professionals, the patient-reported MSIS-29 was developed as a disease-specific tool meant to capture the impact of MS from the perspective of the patient.24 Point estimates from the subgroup analyses of the proportion of patients with clinically meaningful worsening in the MSIS-29 PHYS consistently favored daclizumab beta versus IM IFN beta-1a.
These analyses should be interpreted as exploratory and hypothesis generating for future studies. Some subgroups had small sample sizes, which resulted in wider CIs for these subgroups.15 Additionally, no adjustments were made for multiple testing. Inherent differences in the properties of the tools (e.g. clinician-administered vs patient-reported) and the different functions assessed by each also may contribute to the differences observed across them.25
Overall, the results of these post hoc subgroup analyses of outcome measures assessing disability progression, as well as patient-reported function, indicate that the efficacy of daclizumab beta treatment compared with IM IFN beta-1a was superior and consistent across a range of baseline demographic and disease characteristics in patients with RRMS in DECIDE.
Supplementary Material
Acknowledgments
Rebecca Jarvis, PhD, from Excel Scientific Solutions (Southport, CT, USA) wrote the first draft of the manuscript based on input from authors, and Kristen DeYoung from Excel Scientific Solutions (Southport, CT, USA) copyedited and styled the manuscript per journal requirements. Biogen and AbbVie Inc. reviewed and provided feedback on the paper to the authors. The authors had full editorial control of the paper and provided their final approval of all content. ClinicalTrials.gov identifier: NCT01064401 (Efficacy and Safety of BIIB019 (Daclizumab High-Yield Process) Versus Interferon β-1a in Participants With Relapsing-Remitting Multiple Sclerosis ((DECIDE)); https://clinicaltrials.gov/ct2/show/NCT01064401).
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Cohan has received consulting fees from Biogen, Genzyme, Mallinckrodt, and Novartis; participated in speaker bureaus for Acorda, Biogen, Genentech, Genzyme, and Novartis; and received research support from Biogen, Genentech, Genzyme, Mallinckrodt, Novartis, Opexa, and Roche. Ludwig Kappos’ institution (University Hospital Basel) received steering committee/consulting fees from Actelion, Addex, Bayer HealthCare, Biogen, Biotica, Genzyme, Lilly, Merck, Mitsubishi, Novartis, Ono, Pfizer, Receptos, Sanofi-Aventis, Santhera, Siemens, Teva, UCB, and Xenoport in the last 3 years, which were used exclusively for research support. Professor Kappos has received speaker fees from Bayer HealthCare, Biogen, Merck, Novartis, Sanofi-Aventis, and Teva; support of educational activities from Bayer HealthCare, Biogen, CSL Behring, Genzyme, Merck, Novartis, Sanofi-Aventis, and Teva; license fees for Neurostatus products; grants from Bayer HealthCare, Biogen, the European Union, Merck, Novartis, Roche, Roche Research Foundations, the Swiss Multiple Sclerosis Society, and the Swiss National Research Foundation. The respective payments were payed to University Hospital Basel and used exclusively for research support. Professor Giovannoni has served on advisory boards for AbbVie Biotherapeutics Inc., Biogen, Canbex, Ironwood, Merck, Merck Serono, Novartis, Roche, Sanofi-Genzyme, Synthon, Teva and Vertex; received speaker fees from AbbVie Biotherapeutics Inc., Bayer HealthCare, Biogen, Genzyme, Merck Serono, Sanofi-Aventis, and Teva; is co-editor in chief of Multiple Sclerosis and Related Disorders; and has received research support unrelated to study from Biogen, Genzyme, Ironwood, Merck Serono and Novartis. Dr Wiendl has received consulting fees/honoraria from Bayer HealthCare, Biogen, Fresenius Medical Care, GlaxoSmithKline, GW Pharmaceuticals, Merck Serono, Novartis, Sanofi-Genzyme and Teva; received grants from and contracts with Bayer HealthCare, Biogen, Deutsche Forschungsgesellschaft, the Else Kröner-Fresenius Foundation, the German Ministry for Education and Research, the Hertie Foundation, the Interdisciplinary Center for Clinical Studies in Münster, Germany, Merck Serono, Novartis, the NRW Ministry of Education and Research, the RE Children’s Foundation, Sanofi-Genzyme and Teva. Professor Selmaj has received consulting fees from Genzyme, Novartis, Ono, Roche, Synthon and Teva; and speaker fees from Biogen. Dr Havrdová has received honoraria/research support from Actelion, Biogen, Merck Serono, Novartis, Celgene, Sanofi-Genzyme and Teva; and has served on advisory boards for Biogen, Celgene, Merck Serono, Novartis, Sanofi-Genzyme, Roche, and Teva. She has been supported by the Ministry of Education of Czech Republic, program PRVOUK-P26/LF1/4. Dr Rose has received research support from AbbVie Biotherapeutics Inc., Biogen, Cumming Foundation, the National Institutes of Health, the National Multiple Sclerosis Society, Teva and the U.S. Department of Veterans Affairs. Dr Greenberg was an employee of AbbVie at the time of this analysis and the writing of this manuscript; holds stock/stock options in AbbVie. Drs. Phillips, Lima and Sabatella are full-time employees of and hold stock/stock options in Biogen. Dr Wang and Dr Ma were employees of Biogen at the time of this analysis and the writing of this manuscript; both hold stock in Biogen. Dr Wang’s current affiliation is with Shire, Lexington, MA, USA. Dr Ma’s current affiliation is with Institute of Statistics and Big Data, Renmin University of China.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Biogen (Cambridge, MA, USA) and AbbVie (Redwood City, CA, USA).
Contributor Information
Stanley Cohan, Providence Multiple Sclerosis Center, Providence Brain and Spine Institute, Providence St. Joseph Health, Portland, OR, USA.
Ludwig Kappos, Neurologic Clinic and Policlinic, Departments of Medicine, Clinical Research and Biomedical Engineering, University Hospital and University of Basel, Basel, Switzerland.
Gavin Giovannoni, Blizard Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University London, London, UK.
Heinz Wiendl, Department of Neurology, University of Münster, Münster, Germany.
Krzysztof Selmaj, Department of Neurology, Medical University of Lodz, Lodz, Poland.
Eva Kubala Havrdová, Department of Neurology and Center for Clinical Neuroscience, First Faculty of Medicine, Charles University, Prague, Czech Republic.
John Rose, Department of Neurology, University of Utah and Neurovirology Research Laboratory VASLCHCS, Imaging and Neuroscience Center, Salt Lake City, UT, USA.
Steven Greenberg, AbbVie Inc., North Chicago, IL, USA.
Glenn Phillips, Biogen, Cambridge, MA, USA.
Wei Ma, Biogen, Cambridge, MA, USA.
Ping Wang, Biogen, Cambridge, MA, USA.
Gabriel Lima, Biogen, Cambridge, MA, USA.
Guido Sabatella, Biogen, Cambridge, MA, USA.
References
- 1. Ebers GC, Heigenhauser L, Daumer M, et al. Disability as an outcome in MS clinical trials. Neurology 2008; 71: 624–631. [DOI] [PubMed] [Google Scholar]
- 2. Wiendl H, Meuth SG. Pharmacological approaches to delaying disability progression in patients with multiple sclerosis. Drugs 2015; 75: 947–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 4. Lavery AM, Verhey LH, Waldman AT. Outcome measures in relapsing-remitting multiple sclerosis: Capturing disability and disease progression in clinical trials. Mult Scler Int 2014; 2014: 262350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balcer LJ. Clinical outcome measures for research in multiple sclerosis. J Neuroophthalmol 2001; 21: 296–301. [DOI] [PubMed] [Google Scholar]
- 6. Cohen JA, Reingold SC, Polman CH, et al. Disability outcome measures in multiple sclerosis clinical trials: Current status and future prospects. Lancet Neurol 2012; 11: 467–476. [DOI] [PubMed] [Google Scholar]
- 7. Polman CH, Rudick RA. The multiple sclerosis functional composite: A clinically meaningful measure of disability. Neurology 2010; 74(Suppl. 3): S8–S15. [DOI] [PubMed] [Google Scholar]
- 8. Rudick RA, Polman CH, Cohen JA, et al. Assessing disability progression with the Multiple Sclerosis Functional Composite. Mult Scler 2009; 15: 984–997. [DOI] [PubMed] [Google Scholar]
- 9. Drake AS, Weinstock-Guttman B, Morrow SA, et al. Psychometrics and normative data for the Multiple Sclerosis Functional Composite: Replacing the PASAT with the Symbol Digit Modalities Test. Mult Scler 2010; 16: 228–237. [DOI] [PubMed] [Google Scholar]
- 10. Lopez-Gongora M, Querol L, Escartin A. A one-year follow-up study of the Symbol Digit Modalities Test (SDMT) and the Paced Auditory Serial Addition Test (PASAT) in relapsing-remitting multiple sclerosis: An appraisal of comparative longitudinal sensitivity. BMC Neurol 2015; 15: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Strober LB, Rao SM, Lee JC, et al. Cognitive impairment in multiple sclerosis: An 18 year follow-up study. Mult Scler Relat Disord 2014; 3: 473–481. [DOI] [PubMed] [Google Scholar]
- 12. Cohen JA, Cutter GR, Fischer JS, et al. Use of the Multiple Sclerosis Functional Composite as an outcome measure in a phase 3 clinical trial. Arch Neurol 2001; 58: 961–967. [DOI] [PubMed] [Google Scholar]
- 13. Kappos L, Wiendl H, Selmaj K, et al. Daclizumab HYP versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N Engl J Med 2015; 373: 1418–1428. [DOI] [PubMed] [Google Scholar]
- 14. Liu Y, Vollmer T, Havrdova E, et al. Impact of daclizumab versus interferon beta-1a on patient-reported outcomes in relapsing-remitting multiple sclerosis. Mult Scler Relat Disord 2017; 11: 18–24. [DOI] [PubMed] [Google Scholar]
- 15. Rudick RA. MS clinical trials: What can subgroup analyses teach us? Lancet Neurol 2012; 11: 386–388. [DOI] [PubMed] [Google Scholar]
- 16. Lucchinetti C, Bruck W, Parisi J, et al. Heterogeneity of Multiple Sclerosis Lesions: Implications for the Pathogenesis of Demyelination. Ann Neurol 2000; 47: 707–717. [DOI] [PubMed] [Google Scholar]
- 17. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline: Guideline for good clinical practice, http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf (1996, accessed 2 February 2015).
- 18. Kappos L. Definitions for a standardised, quantified neurological examination and assessment of Kurtzke’s Functional Systems and Expanded Disability Status Scale in Multiple Sclerosis, https://www.neurostatus.net/media/specimen/Definitions_0309_specimen.pdf (2009, accessed 4 May 2017).
- 19. Benedict RH, Morrow S, Rodgers J, et al. Characterizing cognitive function during relapse in multiple sclerosis. Mult Scler 2014; 20: 1745–1752. [DOI] [PubMed] [Google Scholar]
- 20. Phillips GA, Wyrwich KW, Guo S, et al. Responder definition of the Multiple Sclerosis Impact Scale physical impact subscale for patients with physical worsening. Mult Scler 2014; 20: 1753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rubin DB. Multiple imputation for nonresponse in surveys. New York: John Wiley & Sons, 1987. [Google Scholar]
- 22. Karabudak R, Dahdaleh M, Aljumah M, et al. Functional clinical outcomes in multiple sclerosis: Current status and future prospects. Mult Scler Relat Disord 2015; 4: 192–201. [DOI] [PubMed] [Google Scholar]
- 23. European Medicines Agency. Guideline on clinical investigation of medicinal products for the treatment of Multiple Sclerosis, http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/03/WC500185161.pdf (2015, accessed 16 September 2016).
- 24. Hobart J, Lamping D, Fitzpatrick R, et al. The Multiple Sclerosis Impact Scale (MSIS-29): A new patient-based outcome measure. Brain 2001; 124: 962–973. [DOI] [PubMed] [Google Scholar]
- 25. Costelloe L, O’Rourke K, McGuigan C, et al. The longitudinal relationship between the patient-reported Multiple Sclerosis Impact Scale and the clinician-assessed Multiple Sclerosis Functional Composite. Mult Scler 2008; 14: 255–258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.