Abstract
Background:
Egg allergy is phenotypically heterogeneous. A subset of egg allergic individuals can tolerate egg in an extensively heated form. Inclusion of baked-egg (BE) into their diet accelerates resolution of egg allergy. Conversely, BE reactivity is associated with persistent disease. The immune basis of this clinical heterogeneity is unknown.
Objectives:
To study egg-specific antibody, basophil, and T cell responses in children with reactivity or tolerance to BE.
Methods:
All participants underwent double-blind placebo-controlled challenges to BE, and those who tolerated BE were challenged to unheated egg white protein to confirm clinical egg reactivity. Laboratory studies included serum antibodies, basophil activation tests, and CD154-based detection of egg responsive T cells by flow cytometry.
Results:
Of the 129 children studied, BE reactive participants had significantly higher levels of egg, ovalbumin, and ovomucoid-specific IgE, lower ratios of egg-specific IgG4/IgE, and increased basophil activation in response to egg. Among all participants, CD154-based profiling revealed egg-responsive T cells producing IL-4 and IL-13, but little IL-10 or IFN-γ, as well as presence of egg-responsive Foxp3+CD25+CD127low Tregs. Egg-responsive T cells expressed CCR4, CCR6, and CXCR5, indicating capacity for homing to skin, mucosa, and B cell follicles. However, neither the frequency nor phenotype of egg-responsive T cells were different in those with tolerance or reactivity to BE.
Conclusions:
Egg-specific antibody and basophil responses, but not T cell responses, are higher in those with reactivity versus tolerance to BE. The egg-specific antibody and T cell responses were highly heterogeneous in this cohort. The clinical implications of this immune heterogeneity will need to be studied longitudinally.
Keywords: Egg allergy, food allergy, IgE, IgG4, basophil, CD4+ T lymphocyte, Treg, Th2
Capsule Summary
In a study of 129 children challenged to baked and unheated forms of egg, significantly lower egg-specific IgE and basophil activation, but no differences in egg-specific Th2 cells or Tregs, were associated with tolerance to BE.
Introduction
Over the past decade, it has become increasingly clear that a large subset of children with egg allergy are able to tolerate egg protein after it has been extensively heated, i.e. heat-denatured.1-4 Specific predictors of those patients most likely to tolerate baked egg (BE) have been identified, including prick skin testing, egg-specific IgE and ovomucoid-specific IgE.5, 6 Children with tolerance to heated egg are more likely to outgrow their egg allergy by 2 years of life 7. Longitudinal studies have also demonstrated that exposure to heated egg enhances eventual tolerance to less heated forms of egg. 8, 9 In addition to these clinical outcomes, studies have also helped to define the immunologic changes that occur with regular exposure to BE in egg-allergic patients.4, 8
Despite these advances, there is a lack of information about detailed immunologic characteristics that may differentiate egg allergic patients who do or do not tolerate extensively heated egg. In this study, we had the opportunity to conduct detailed studies profiling basophil and T cell responsiveness as well as serologic measures at baseline in a large cohort of children who underwent double blind placebo controlled challenges to baked or unheated egg.
Methods
Participants.
Baseline blood samples were obtained from children aged 3-16 years at the time of enrollment (prior to egg exposure through diet or immunotherapy) in a multi-center CoFAR intervention trial comparing the efficacy of BE diet or egg oral immunotherapy (OIT) in the treatment of egg allergy (CoFAR7, NCT01846208). These participants included children with an egg-specific IgE > 5 kUA/L who were avoiding all forms of egg in the diet. Participants underwent an initial double-blind placebo controlled food challenge (DBPCFC) to BE in the form of a muffin. Those who reacted during the BE challenge were categorized as BE reactive. Those who tolerated the BE challenge then underwent a DBPCFC to unheated egg white protein to confirm reactivity to unheated egg. This group was categorized as BE tolerant. After enrollment of the BE reactive group was complete (40 participants per the protocol), the baseline blood sample was drawn post-BE challenge from the remaining BE tolerant participants (to determine their qualification for the study before conducting immune profiling). The National Institute of Allergy and Infectious Diseases Allergy and Asthma Data and Safety Monitoring Board, and the Institutional Review Boards at Icahn School of Medicine at Mount Sinai, National Jewish Health, Johns Hopkins Medical School, University of Arkansas for Medical Sciences, and University of North Carolina, Chapel Hill approved study procedures. Written informed consent was obtained from the parent or guardian.
Blood processing and PBMC isolation.
Blood samples were obtained in 10 ml sodium-heparin Vacutainer tubes at the 5 clinical sites. An aliquot was retained for basophil activation tests on site, and the remaining whole blood was shipped overnight in temperature-controlled Greenbox™ shipping containers (ThermoSafe, Arlington Heights, IL) assembled according to standard operating procedures. Temperature loggers were included to ensure that temperatures were maintained between 20 and 30 °C. Samples from the Icahn School of Medicine at Mount Sinai clinical site were stored at room temperature and processed the next day to maintain consistency with the other sites. An aliquot of whole blood was used for phenotypic analysis by flow cytometry using antibodies shown in Table E1. Blood was centrifuged to obtain plasma prior to isolation of peripheral blood mononuclear cells (PBMCs) by Ficoll-Paque PLUS (GE Healthcare, Uppsala, Sweden). Cells were washed, counted, and cultured in AimV (ThermoFisher, Grand Island,NY) with 2.5% autologous plasma at a density of 4 × 106 cells in 1 ml in 24-well culture plates.
Basophil activation.
Basophil activation assays were performed at the clinical sites, within 2 hours of blood draw. Whole blood was stimulated for 30 min with media containing 2 ng/ml IL-3 plus egg white protein (Deb-El Food Products, Elizabeth, NJ, 1 to 0.001 μg/ml), anti-IgE or fMLP as positive controls, or media +/− IL-3 as negative controls. After stopping degranulation with EDTA, samples were stained for surface activation markers CD63 and CD203c (full antibody staining panel shown in Table E2).
PBMC stimulations.
4 × 106 PBMCs in 1 ml of AimV media were stimulated with 300 μg/ml of egg white (EW) protein (Deb-El Food Products, Elizabeth, NJ), or 5 μl anti-CD3/CD28 stimulation beads (Life Technologies AS, Norway) for a bead:cell ratio of 1:20. Duplicate cultures were used for unstimulated and egg stimulated conditions. EW had been cleaned of endotoxin using Detoxi-Gel columns (ThermoFisher). Residual endotoxin levels were approximately 0.3 EU/ml of working solution of egg stimulant (99.85% removal, and within the acceptable range for culture reagents). The dose of antigen was based on pilot experiments conducted to optimize detection of egg-responsive T cells by flow cytometry. Cells were cultured in standard tissue culture incubators. GolgiPlug (BD Biosciences, San Jose, CA) was added 4 hours prior to harvesting.
T cell phenotypic analysis.
Harvested PBMCs were stained for viability (Live/Dead Fixable stain, Invitrogen), washed and stained for surface markers, and washed for fixation and permeabilization. To detect intracellular CD154 and cytokines, cells were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) and treated with permeabilization buffer (eBioscience, San Diego, CA) before staining with labeled antibodies. For FoxP3 staining, cells were processed using the FoxP3/Transcription Factor Staining Buffer Set (eBioscience) before staining with antibodies. Antibody panels used for surface and cytoplasmic staining are shown in Tables E3-4. Stained cells were subsequently analyzed on a LSR II Cytometer (BD Biosciences).
Skin Prick Test.
A skin prick test was performed while the participant was off antihistamines for an appropriate length of time (5 half-lives of the antihistamine being used). A skin test probe was pressed through a commercial extract of egg into the epidermis. Positive (histamine) and negative (saline-glycerin) controls were also used. The egg skin prick test score was calculated by subtracting the diameter of the saline wheal from the diameter of the egg wheal.
Immunoglobulin Measurements.
Frozen plasma samples were thawed and IgE, IgG, and IgG4 antibodies specific for egg, ovalbumin, and ovomucoid were measured by ImmunoCAP (Thermo Fisher Scientific). The range of detection for the assays were 0.01 to 100 kUA/L for IgE, 2 to 200 mgA/L for IgG, 0.07 to 30 mgA/L for IgG4, 5000 kU/L upper limit for total IgE. Values obtained outside of this range of detection were truncated for analysis as follows (lower, upper): IgE (0.05, 101 kUA/L), IgG (1, 201 mgA/L), IgG4 (0.035, 31 mgA/L), total IgE (NA, 5001 kU/L).
Statistical Analysis.
The primary analysis sought to identify differences in immunological markers between the BE reactive and BE tolerant groups. Continuous variables were summarized by medians and ranges and compared between groups using a Wilcoxon Rank Sum test. Categorical variables were summarized by counts and percents and compared between groups using a Fisher’s Exact test. Spearman correlations were used to assess associations between continuous variables. A secondary analysis looked at the same variables to determine if there was a difference for participants with a sample drawn pre- or post-BE oral food challenge. A p-value of <0.01 was considered significant to control for multiple comparisons.
Results
Study Population
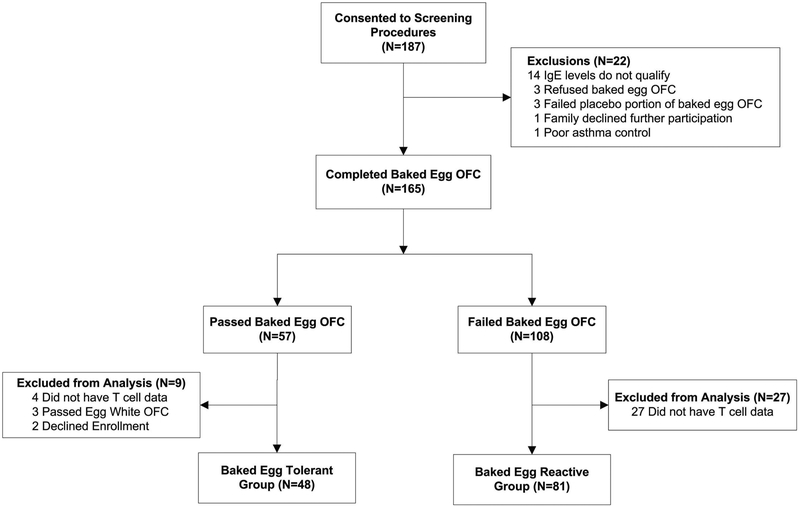
The CONSORT diagram is shown in Figure 1, and participant characteristics in Table 1. 187 participants consented to screening procedures, and 165 underwent a BE OFC. 57 participants successfully tolerated the BE oral food challenge (OFC) and were therefore categorized as BE tolerant. 48 were included in analysis (BE tolerant group). Nine participants were excluded from the analysis due to passing the egg white OFC (not egg allergic), declining to enroll, or not having T cell data. 108 participants reacted to the BE OFC and were therefore categorized as BE reactive. 81 participants were included in analysis, the remainder were excluded due to lack of T cell data primarily due to completion of enrollment in the BE reactive group and change in timing of specimen collection. Enrollment occurred between July 2013 and August 2015, and did not reach the target enrollment (96) in the BE tolerant group.
Figure 1: CONSORT diagram showing participant disposition.
Table 1:
Participant Characteristics
| Baked Egg Reactive | Baked Egg Tolerant |
p-value | |
|---|---|---|---|
| N | 81 | 48 | |
| Gender (M/F) | 42/39 | 31/17 | 0.20 |
| Age, years (range) | 8.0 (3.7,16.0) | 7.4 (3.7,16.8) | 0.49 |
| Asthma, N (%) | 49 (60.5) | 31 (64.6) | 0.71 |
| Allergic Rhinitis, N (%) | 66 (81.5) | 40 (83.3) | 1.00 |
| Atopic Dermatitis, N (%) | 51 (63.0) | 30 (62.5) | 1.00 |
| Other Food Allergy, N (%) | 72 (88.9) | 44 (91.7) | 0.77 |
| Successfully Consumed Dose (mg protein), Baked Egg OFC (range) | 300 (0,1500) | 2000 (2000,2000) | |
| Successfully Consumed Dose (mg protein), Egg White OFC (range) | ND | 144 (1,444) | |
| Egg Skin Prick Test (mm) | 12 (7,16) | 10 (6,13) | 0.05 |
| Egg IgE (kUA/L) | 25.4 (12.4,70.0) | 11.6 (6.7,26.7) | 0.001 |
| Ovalbumin IgE (kUA/L) | 15.7 (5.9,31.4) | 5.7 (3.3,16.6) | 0.0004 |
| Ovomucoid IgE (kUA/L) | 15.1 (6.2,40.3) | 7.0 (3.0,13.3) | 0.001 |
| Egg IgG (mgA/L) | 5.2 (2.9,8.5) | 7.4 (3.9,17.9) | 0.02 |
| Egg IgG4 (mgA/L) | 0.6 (0.2,1.1) | 0.7 (0.2,2.5) | 0.15 |
| Ovalbumin IgG4 (mgA/L) | 0.34 (0.14,1.04) | 0.48 (0.11,1.93) | 0.32 |
| Ovomucoid IgG4 (mgA/L) | 0.11 (0.04,0.33) | 0.15 (0.04,0.86) | 0.19 |
| Egg IgG4/IgE ratio | 6.4 (3.4,14.9) | 23.2 (6.8,80.8) | 0.0003 |
Median with interquartile range is shown unless otherwise stated.
All participants were avoiding egg in the diet prior to enrollment. BE reactive participants had a median successfully consumed dose of 300 mg of baked egg protein given in the form of a muffin during the BE OFC. BE tolerant participants, by definition, tolerated the full challenge dose equivalent to 2000 mg of baked egg protein during the BE OFC. When re-challenged with unheated egg white powder to confirm egg allergy, the median successfully consumed dose was 144 mg of egg white protein.
Immune Cell Subsets
To determine if there were any global differences in immune cell populations between BE reactive and tolerant groups, we compared the frequencies of immune cell subsets in whole blood by flow cytometry. We identified CD14+ and CD16+ monocytes, pDCs, cDCs, neutrophils, basophils, eosinophils, NK cells, and B and T cells (Table 2). There was no significant difference in the representation of these populations between the BE reactive and tolerant groups.
Table 2:
Leukocyte Subset Frequency (% of CD45)
| Baked Egg Reactive | Baked Egg Tolerant | |||||
|---|---|---|---|---|---|---|
| Median | Max | Min | Median | Max | Min | |
| Neutrophils | 28.38 | 64.70 | 8.68 | 28.26 | 89.05 | 12.07 |
| Eosinophils | 7.25 | 20.43 | 1.07 | 6.90 | 64.70 | 1.66 |
| Basophils | 0.75 | 1.72 | 0.08 | 0.64 | 5.33 | 0.07 |
| pDCs | 0.20 | 0.74 | 0.01 | 0.17 | 0.88 | 0.01 |
| mDCs | 0.29 | 3.64 | 0.02 | 0.27 | 1.34 | 0.03 |
| NK T cell | 0.56 | 4.08 | 0.03 | 0.70 | 7.01 | 0.13 |
| CD4+ T cell | 18.41 | 33.33 | 0.59 | 18.99 | 73.10 | 3.74 |
| CD8+ T cell | 9.60 | 22.33 | 1.57 | 10.04 | 25.86 | 2.74 |
| B cells | 6.89 | 16.07 | 1.44 | 6.88 | 31.13 | 3.30 |
| NK CD56Bright | 0.18 | 0.61 | 0.05 | 0.19 | 0.84 | 0.04 |
| NK CD56dim | 2.60 | 9.18 | 0.71 | 2.96 | 11.08 | 0.34 |
| CD14+ Monocyte | 3.66 | 7.85 | 0.11 | 3.62 | 21.54 | 0.02 |
| CD16+ Monocyte | 0.29 | 2.91 | 0.06 | 0.41 | 2.24 | 0.04 |
Specific Immunoglobulins
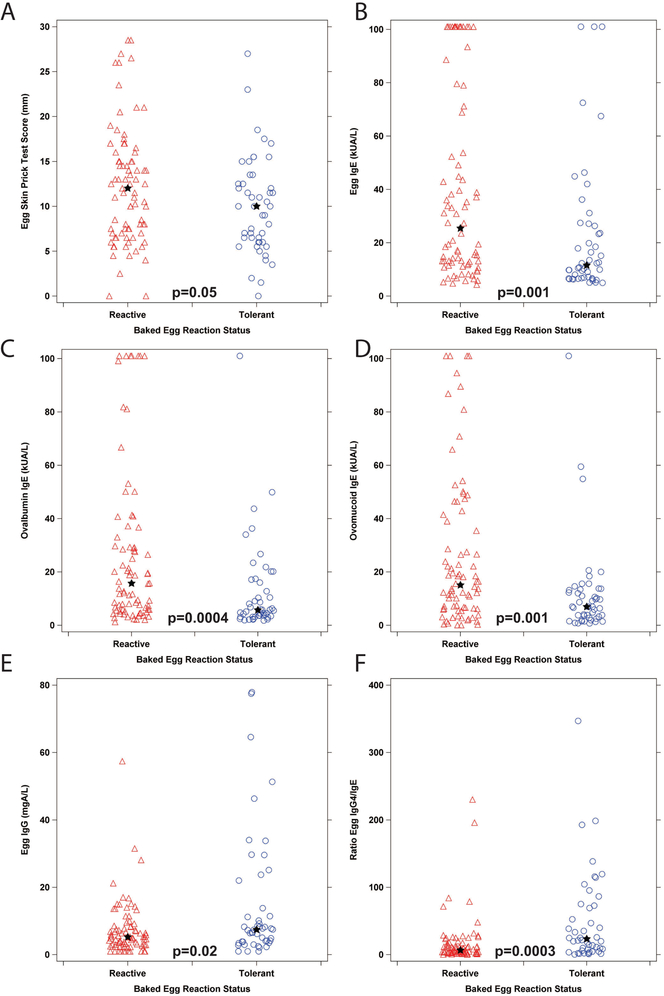
We compared skin prick tests (SPT) and immunoglobulin levels in the two groups of egg allergic participants, as shown in Figure 2, Table 1. Egg SPT scores were higher but did not reach the level of statistical significance in the BE reactive versus the BE tolerant group (Fig 2A). Egg-specific IgE levels were significantly (p=0.001) elevated in the BE reactive group versus tolerant group (Fig 2B). IgE binding to the major egg white allergens, ovomucoid and ovalbumin, was also significantly higher in the BE reactive group versus the BE tolerant group (Fig 2C, 2D, p=0.001, 0.0004 respectively). Total IgE levels were similar between groups (not shown). Egg-specific IgG (Fig 2E) and egg-, ovalbumin-, and ovomucoid-specific IgG4 levels (data not shown) were higher but did not reach the level of statistical significance in the BE tolerant group compared to the BE reactive group. Egg IgG4/IgE ratios were significantly higher in BE tolerant versus BE reactive participants (Fig 2F, p=0.0003), while the percent egg IgE (ratio of egg-specific to total IgE) was not significantly different between groups (data not shown). Thus, the dominant difference between groups when comparing egg-specific immunoglobulins was the magnitude of the egg and egg component-specific IgE responses.
Figure 2: Egg-specific Immunoglobulins.
A. Egg skin prick test. B. Egg-specific IgE. C. Ovalbumin-specific IgE. D. Ovomucoid-specific IgE. E. Egg-specific IgG. F. Egg IgG4/IgE ratio. The star indicates the group median, p-values from a Wilcoxon Rank-Sum test comparing the groups are indicated on each graph.
Basophil Activation Tests
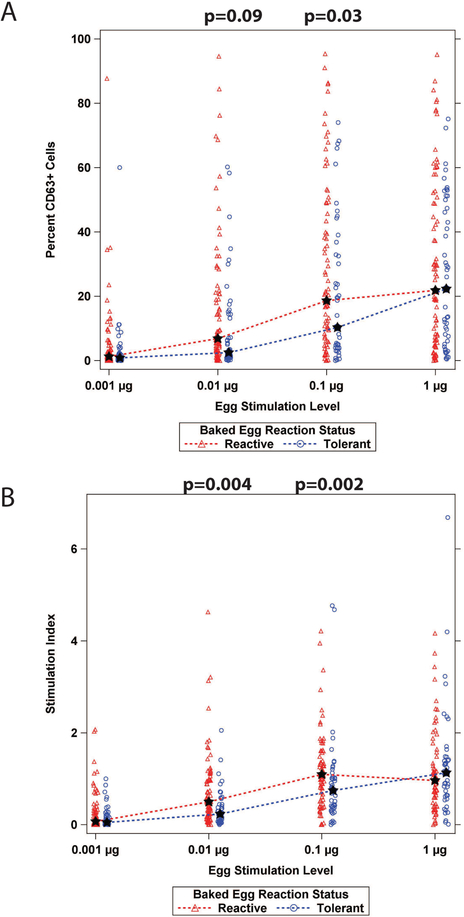
We performed basophil activation tests in response to in vitro stimulation of whole blood with egg white protein. Expression of the degranulation marker CD63 was used to quantify the magnitude of activation. As shown in Fig 3A, BE reactive participants had higher basophil activation compared to BE tolerant participants at intermediate stimulation levels of egg white (0.01 and 0.1 μg), while the two groups reached similar levels of basophil activation at the lowest and highest levels of 0.001 and 1 μg of egg white protein. When expressed as a stimulation index that normalized the egg-stimulated values to that of the positive control (anti-IgE stimulation), the differences in basophil activation between the BE reactive and BE tolerant participants at 0.01 and 0.1μg doses were significantly different (p=0.002, p=0.004) (Fig 3B). Again, maximal reactivity at 1 μg egg white protein was similar between the groups. These data suggest a lower threshold of basophil activation at intermediate antigen doses in the BE reactive group.
Figure 3: Basophil Activation Test.
A. Percent CD63+ cells by egg stimulation level. B. Stimulation index was calculated as the percent of cells positive for CD63 after egg stimulation divided by the percent of cells positive for CD63 after positive control anti-IgE stimulation. The star indicates the group median; p-values from a Wilcoxon Rank Sum test at each stimulation level comparing the groups are shown.
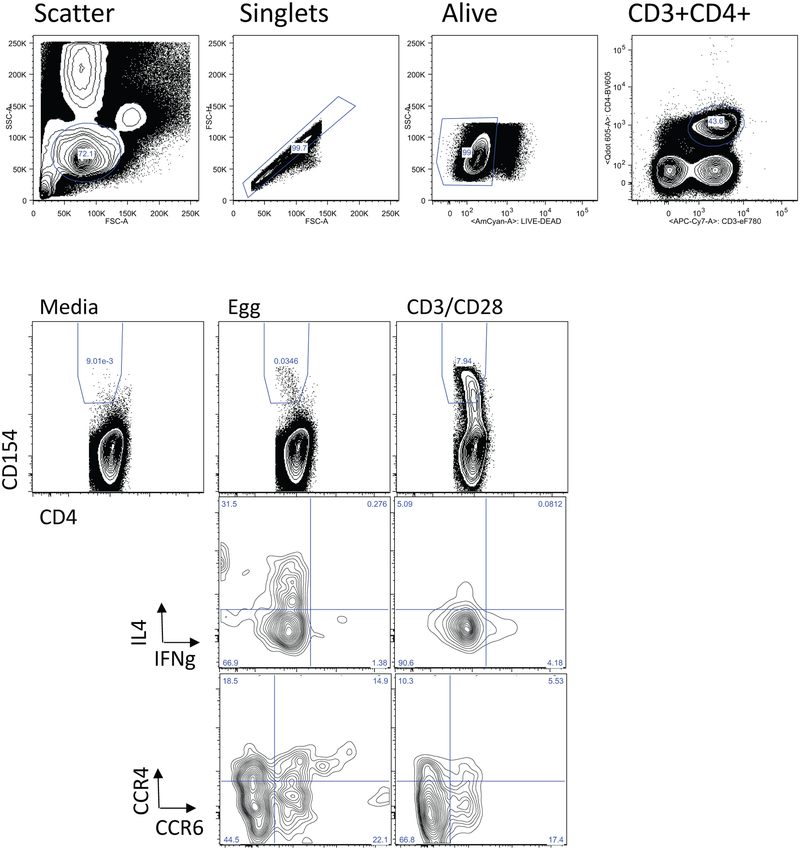
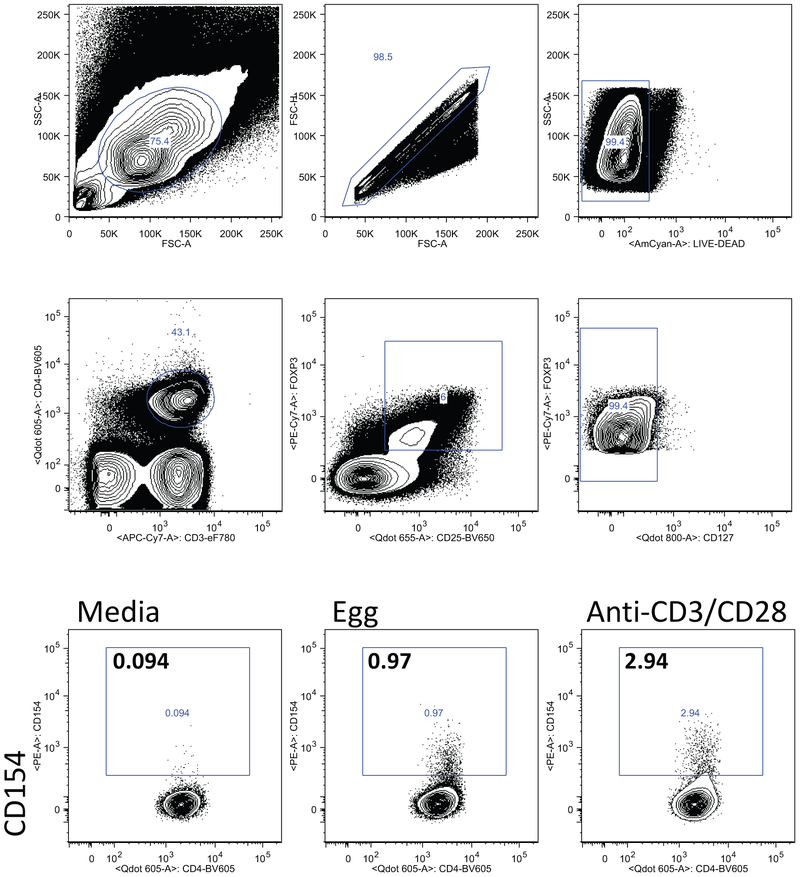
Egg-responsive T cells
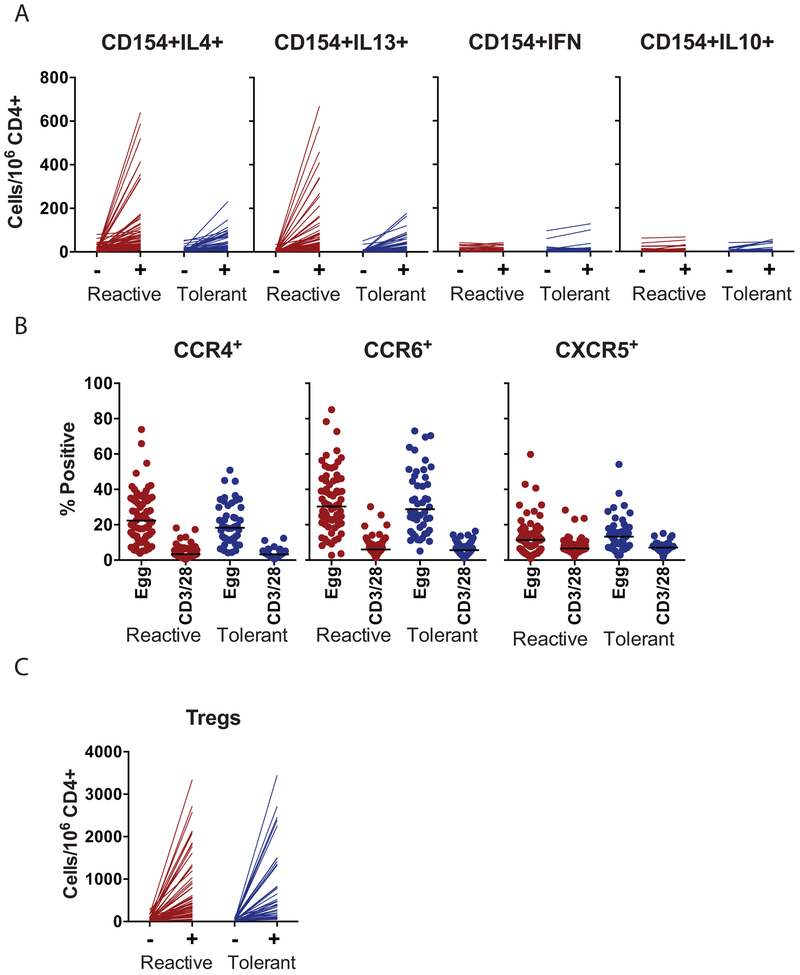
We stimulated fresh PBMCs with egg white protein and identified egg-responsive cells by upregulation of CD154 after 6 or 18h of stimulation. Based on previous piloting of the kinetics of CD154 upregulation of different T cell subsets, we examined CD154 expression on effector CD4+ T cells after 6h of stimulation, and on Foxp3+ CD25+ CD127− Tregs after 18h of stimulation. Gating and representative flow cytometry plots for the two populations are shown in Figs E1 and E2. As shown in Fig 4A, there was an induction of CD154+ cells co-expressing IL-4 and IL-13 after 6h of stimulation with egg white antigen in both the BE reactive and tolerant groups. Frequency of CD154+IL-10+ and CD154+IFNg+ co-expressing cells remained low after egg stimulation in both groups. Although the average frequencies of CD154+IL-4+ and CD154+IL-13+ cells in the BE reactive and tolerant groups were not statistically significantly different, there was a subset of individuals with high Th2 responses in the BE reactive group that was not observed in the BE tolerant group. In both groups, egg-responsive Th2 cells (defined as CD154+IL4+ or CD154+IL13+) significantly correlated with egg-specific IgE (data not shown). Because some of the BE tolerant group had T cell assays performed after the BE OFC, the frequency of egg-responsive Th2 cells was compared in those analyzed prior to BE OFC (n=31) vs those analyzed after BE OFC (n=17). There was no significant difference in frequency of CD154+Th2 cells when assayed before or after BE OFC in the BE tolerant group.
Figure 4: Egg-responsive T cells.
A. Frequency of cells co-expressing CD154 and the cytokines IL-4, IL-13, IFN-γ, or IL-10 after 6h of stimulation with egg antigen (+) or media alone. Reactive and tolerant refers to responsiveness to baked egg. B. Percent of CD154+ cells after stimulation with egg or anti-CD3/CD28 that co-express CCR4, CCR6, or CXCR5. Bars indicate medians. C. Frequency of Foxp3+CD25+CD127− cells that express CD154 after 18h of stimulation with egg allergen.
We examined the expression of the chemokine receptors CCR4, CCR6, and CXCR5 on CD154+ cells from the BE tolerant and reactive participants after stimulation with egg or anti-CD3/CD28 antibody stimulation for 6h. All three chemokine receptors were enriched on egg-activated T cells as compared to polyclonally activated T cells and no significant differences were observed between the BE tolerant and reactive participants (Figure 4B). These data indicate that egg-specific CD4+ T cell populations include cells with the capacity to home to the skin (CCR4+), mucosa (CCR6+) and B cell follicles (CXCR5+). We also quantified regulatory T cells (Foxp3+CD25+CD127low) upregulating CD154 after 18h of stimulation with egg antigen. We observed a large induction of CD154+ on cells expressing Treg markers. This activation of Tregs was not significantly different when comparing BE reactive and tolerant participants.
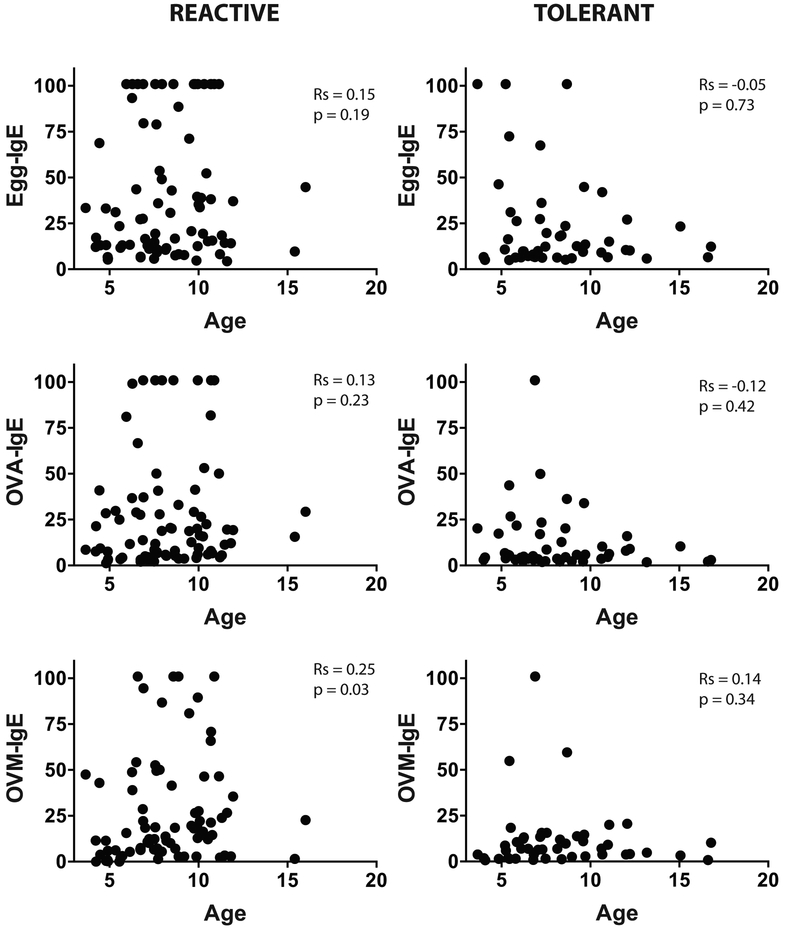
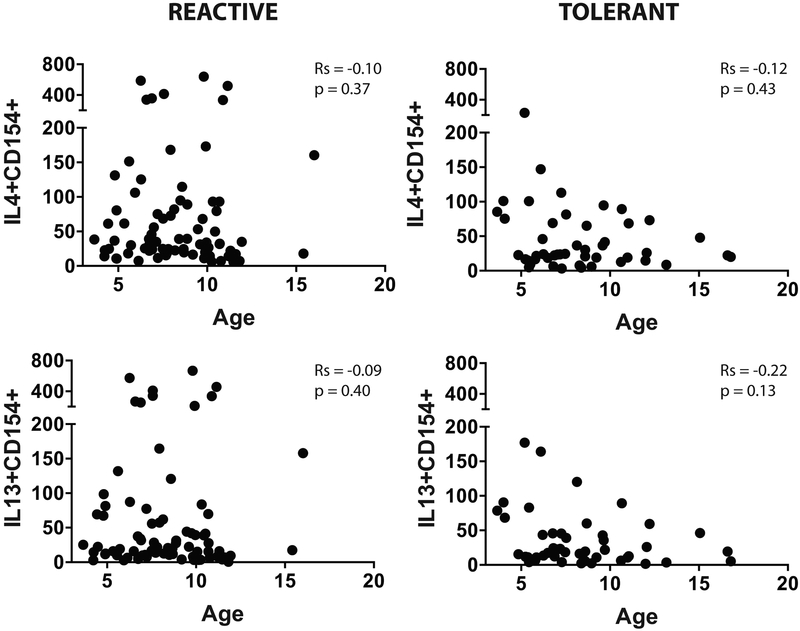
Age and egg-specific immune response
We examined the association between age and specific IgE to egg, ovalbumin, and ovomucoid (Fig E3), and between age and egg-responsive CD154+IL4+ or CD154+IL13+ cells (Fig E4) in the BE reactive and tolerant groups. We found no significant association between age and any of these parameters.
Discussion
We investigated the immune basis underlying reactivity or tolerance to BE among 129 pediatric egg allergic participants who underwent oral egg challenges during baseline evaluations for a clinical trial comparing the efficacy of BE diet to egg oral immunotherapy. A large subset of children with egg allergy has been reported to tolerate extensively heated or baked forms of egg 2-4. The proportion of BE reactive and tolerant individuals screened and enrolled in this study does not reflect those findings. Most individuals screened were BE reactive, and we were not able to reach target enrollment in the BE tolerant group, most likely due to the fact that many BE tolerant children are now identified in their local clinical centers and were no longer eligible for this study.
We found significant differences between BE reactive and tolerant groups at the level of egg-specific immunoglobulins and egg-induced basophil activation, but not in egg-specific T cell responses. Heating alters the conformational structure and gastrointestinal absorption of heat-sensitive allergens in egg 10. Of the two major egg allergens, ovomucoid is more resistant to heating and digestion than ovalbumin, and therefore a difference in specificity of the IgE response could explain reactivity versus tolerance to BE. We found that BE reactivity was associated with significantly higher levels of IgE specific for ovomucoid, as well as ovalbumin and total egg white. These results are consistent with previous single-center studies 4, 11, and indicate that the magnitude rather than the specificity of the IgE response to whole or component proteins is the most relevant difference between BE reactive and tolerant participants. Previous studies comparing immunoglobulin responses in patients with reactivity or tolerance to heated milk identified the affinity of IgE binding as an important factor discriminating the two groups 12. Furthermore, there may be important differences at the level of binding to clinically-relevant epitopes (i.e. those that survive heating and digestion and are absorbed) that are obscured by measurement of IgE binding to the entire allergen. In milk allergy, IgE from sera of baked milk reactive individuals binds to a greater number of epitopes compared to baked milk tolerant individuals 12. Egg-specific IgG and IgG4, which could function to suppress reactivity to egg, were not significantly different between BE reactive or tolerant participants, likely reflecting the lack of exposure to egg in both groups. Basophil activation as a functional assay reflected this lower level of egg-specific IgE, and higher threshold of reactivity, in BE tolerant participants. It should be noted that despite the significant difference between groups, there was a great deal of overlap in IgE binding and basophil activation, indicating that other factors must contribute to the difference in clinical reactivity of these participants to BE. In addition to immune parameters, these differences may also include factors upstream of immune activation, such as differences in digestion or absorption of BE.
We examined the immunoglobulin, basophil, and T cell response to unheated egg protein or components. This was chosen over using baked egg for several reasons. First, BE reactive and tolerant individuals have a different prognosis in their resolution of allergy to unheated egg, with BE tolerant individuals more likely to outgrow their allergy to unheated egg. The primary outcome of the clinical trial in which these participants were enrolled is testing the impact of intervention (immunotherapy versus baked egg diet) on the development of sustained unresponsiveness to unheated egg. Furthermore, tolerance to baked egg is a phenotype associated with increased rate of resolution of allergy to unheated egg. Thus, there is evidence for a clinical difference between the two groups in the development of tolerance to unheated egg, and our goal was to examine whether there was an immune basis for that difference.
There is also a question of the immune basis underlying the difference in clinical reactivity to baked egg. This is a question that we have not directly addressed here through assessment of immune reactivity to baked egg in vitro. We and others have previously examined how heating of egg influences clinical reactivity using in vitro model systems and in vivo mouse models 1, 10, 13-15. These previous findings are summarized in Fig 5A. Heating destroys conformational epitopes, makes antigens more susceptible to degradation by digestive enzymes, and suppresses uptake across intestinal epithelium, leading to a suppression of immune activation. Mechanisms that contribute to maintained reactivity to heated egg in BE reactive subjects include IgE recognition of linear epitopes that are maintained after heat denaturation, and recognition of epitopes that are not destroyed by digestion. Furthermore, as we show here, BE reactive individuals had increased egg- and component-specific IgE and a lower threshold of reactivity of basophils to egg, indicating that they are more likely to respond despite the lower amount of antigen that can survive digestion and be absorbed intact across the epithelial barrier (Fig 5A).
Figure 5:
(A) Mechanisms contributing to clinical reactions to baked egg. Native egg antigens that contain IgE-binding epitopes, survive digestion, and are absorbed across the epithelial barrier can trigger effector cells in egg allergic individuals. Heating destroys conformational epitopes, increases susceptibility to digestion, and reduces uptake across epithelium, resulting in no effector cell activation in BE tolerant subjects. BE reactive subjects recognize sequential epitopes in digestion-resistant regions of egg antigens. Furthermore, increased level of egg-specific IgE lowers reaction threshold, resulting in effector cell activation. (B) Hypothesized relationship between egg-specific Th2 cell frequency and resolution of egg allergy. Left: If resolution is dependent on a loss of egg-specific Th2 cells, and the rate of Th2 cell loss is similar between BE reactive and tolerant subjects, a higher starting value in the BE reactive group would be associated with delayed resolution. Right: A high Th2 frequency may be associated with persistence of Th2 cells and egg allergy. The finding of high Th2 frequency only in BE reactive individuals would confer an increased persistence of egg allergy in this group.
We observed no significant differences in the proportions of peripheral blood immune subsets between those who were reactive or tolerant to BE. In a previous study using a subset of this cohort, we had identified differences between groups in the transcriptional response to egg stimulation 16. We found that genes associated with virally-infected DCs, type I interferons, and IFN-γ were differentially expressed between BE tolerant and reactive participants. Here, we did not find any numerical difference in DCs or other innate cells, but there may be functional differences within the innate populations that differ between egg allergy phenotypes.
This is the first phenotypic analysis of the T cell response to egg using CD154-based flow cytometric profiling, an approach that minimizes time in culture while identifying functional parameters such as cytokine production and homing receptor expression. Egg-responsive T cells were Th2 skewed, with an absence of detectable Th1 cells and only low frequencies of IL-10+ cells. This is consistent with Th2 skewing identified by proliferation-based approaches 17. Although we do not have egg-tolerant controls in this study, we have previously shown that Th2-related and pro-inflammatory cytokine secretion (IL-5, IL-9, TNFα) is significantly elevated in egg allergy compared to atopic controls that were egg tolerant 16. In addition to their Th2 profile, egg-responsive T cells were enriched for homing receptors which would support trafficking to the skin or intestinal mucosa or to the B cell follicle. The phenotype of egg-responsive T cells was similar to that described for peanut using a similar profiling approach (Chiang et al, JACI, in press), with the exception of CD25 which was more highly expressed on egg-responsive T cells. We also identified a population of Foxp3+CD25+CD127low Tregs expressing CD154 after 18h of egg allergen exposure. We hypothesized that there would be differences in the egg-responsive Th2 and Treg cell profiles when comparing BE reactive and BE tolerant participants because BE tolerance has been associated with an increased frequency of egg allergy resolution 7. Tolerance to extensively heated forms of milk, which also is associated with increased rate of allergy resolution, was associated with an increased frequency of milk-responsive Tregs 18. However, Th2 and Treg responses were not statistically different when comparing those with reactivity or tolerance to BE. The similarity in the distribution of egg-reactive Tregs in the BE reactive and tolerant participants suggests that a deficit in Treg numbers is unlikely to explain the increased IgE response in the BE reactive participants, though the possibility of a difference in Treg function cannot be excluded. Interestingly, there was a subset of individuals with a high frequency of egg-responsive Th2 cells that was observed only in the BE reactive group. We speculate that these high Th2 responders may be more resistant to resolution of their egg allergy. In the summary Figure 5B, we speculate on the relationship of Th2 responses to rate of egg allergy resolution in the BE reactive and tolerant groups. In follow-up studies of this cohort, it will be of interest to determine how the “Th2 high” participants respond to egg immunotherapy.
In summary, an examination of antibody and T cell responses to egg demonstrated that BE reactive participants have significantly higher egg-specific IgE and basophil responses, but Th2 and Treg responses to egg were not different when comparing these two clinical phenotypes. A limitation of the study is the reductionist approach of in vitro activation that does not incorporate important factors such as gastrointestinal digestion or absorption of antigens known to be influenced by heating of egg. We examine the immune basis for differences in reactivity to unheated egg which is the basis of the clinical trial, but not the immune mechanisms responsible for differences in reactivity to baked egg. Furthermore, greater resolution at the level of epitope-specific immune responses may be needed to discriminate between these phenotypes of egg allergy. The data emphasizes the considerable heterogeneity of egg-specific antibody and T cell responses in this cohort; it will be of significant interest to determine if the immune heterogeneity observed at baseline has clinical implications in follow-up studies.
Extended Data
Figure E1:
Gating strategy (top row), and representative flow cytometry results showing CD154 expression of stimulation with egg, media (negative control), or anti-CD3/CD28 (positive control). Bottom panels show expression of IL-4 and IFN-γ, or CCR4 and CCR6 in CD154+ cells after stimulation with egg or anti-CD3/CD28.
Figure E2:
Gating strategy for identification of Tregs (CD3+CD4+Foxp3+CD25+CD127low), and representative flow cytometry results showing CD154 expression in gated Tregs after stimulation with egg, media (negative control), or anti-CD3/CD28 (positive control).
Figure E3:
Egg-specific IgE, ovalbumin (OVA) specific IgE, and ovomucoid (OVM) specific IgE versus age of participant in the BE reactive and tolerant groups. Spearman correlation coefficient (r) and p value are provided on each graph.
Figure E4:
Frequency of CD154+IL4+ and CD154+IL13+ cells after egg stimulation versus age of participant in the BE reactive and tolerant groups. Spearman correlation coefficient (r) and p value are provided on each graph.
Table E1:
Whole blood phenotyping panel
| Target | Clone | Fluorophore | Company |
|---|---|---|---|
| Target | Clone | Fluorophore | Company |
| CD45 | H130 | Pacific Orange |
Invitrogen |
| CD3 | UCHT1 | AF700 | BD Biosciences |
| CD4 | rpa-t4 | PE-CF594 | BD Biosciences |
| CD8 | SK1 | APC | Biolegend |
| CD19 | HIB19 | PE | Biolegend |
| CD56 | HCD56 | BV605 | Biolegend |
| CD14 | HCD14 | PE-Cy7 | Biolegend |
| CD16 | 3G8 | APC-H7 | BD Biosciences |
| HLADR | LN3 | AF488 | Biolegend |
| CD123 | 6H6 | BV650 | Biolegend |
| CD11c | 3.9 | PacBlue | Biolegend |
Table E2:
Basophil activation staining panel
| Marker | Clone | Fluorophore | Company |
|---|---|---|---|
| CD3 | UCHT1 | APC | BD Biosciences |
| CD14 | M5E2 | APC | BD Biosciences |
| CD19 | HIB19 | APC | BD Biosciences |
| CD41a | HIP8 | APC | BD Biosciences |
| HLA-DR | L243 | PE-Cy7 | BD Biosciences |
| CD123 | 9F5 | PE-Cy5 | BD Biosciences |
| CD63 | 557288 | FITC | BD Biosciences |
| CD203c | 97A6 | PE | Beckman Coulter Life Sciences |
Table E3:
Effector T cell staining panel (6h)
| Marker | Clone | Fluorophore | Company |
|---|---|---|---|
| Live/Dead Aqua | N/A | AmCyan | Thermo Fisher Scientific |
| CD3 | SK7 | APC-Cy7 | eBioscience |
| CD4 | OKT4 | Brilliant Violet 605 | BioLegend |
| CD25 | BC96 | Brilliant Violet 650 | BioLegend |
| CD127 | A019D5 | Brilliant Violet 785 | BioLegend |
| CXCR5 | RF8B2 | Alexa Fluor 488 | BD Biosciences |
| CCR4 | 1G1 | PerCP-Cy5.5 | BD Biosciences |
| CCR6 | 11A9 | PE-Cy7 | BD Biosciences |
| CD154 | 24-31 | PE | eBioscience |
| IFNg | B27 | Alexa Fluor 700 | BD Biosciences |
| IL-4 | MP4-25D2 | Alexa Fluor 647 | BioLegend |
| IL-10 | JES3-19F1 | PE-CF594 | BD Biosciences |
| IL-13 | JES10-5A2 | BD Horizon V450 | BD Biosciences |
Table E4:
Regulatory T cell staining panel (18h)
| Marker | Clone | Fluorophore | Company |
|---|---|---|---|
| Live/Dead Aqua | N/A | AmCyan | Thermo Fisher Scientific |
| CD3 | SK7 | APC-Cy7 | eBioscience |
| CD4 | OKT4 | Briliant Violet 605 | BioLegend |
| CD25 | BC96 | Briliant Violet 650 | BioLegend |
| CD127 | A019D5 | Briliant Violet 785 | BioLegend |
| CCR4 | 1G1 | PerCP-Cy5.5 | BD Biosciences |
| CCR6 | 11A9 | PE-Cy7 | BD Biosciences |
| CCR9 | 112509 | Alexa Fluor 488 | BD Biosciences |
| CD154 | 24-31 | PE | eBioscience |
| IFNg | B27 | Alexa Fluor 700 | BD Biosciences |
| IL-10 | JES3-19F1 | PE-CF594 | BD Biosciences |
| FoxP3 | 206D | Alexa Fluor 647 | BioLegend |
Key Messages.
BE reactivity is associated with increased egg and egg component-specific IgE antibodies as well as basophil activation compared to BE tolerance.
Egg-responsive T cells in the peripheral blood of egg allergic individuals are dominated by IL-4 and IL-13 expression, and express skin and mucosal homing receptors CCR4 and CCR6.
Frequency of egg-responsive Th2 cells and Tregs did not differ between BE reactive and tolerant individuals, but a subset of individuals with elevated frequency of egg-responsive Th2 cells was observed within the BE reactive group.
Acknowledgements
Funding: Supported by National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) grants U19AI066738 and U01AI066560. The project was also supported by grant numbers UL1 TR000154 (National Jewish), UL1 TR000067 (Mount Sinai), UL1 TR000039 (Arkansas), UL1 TR000083 (North Carolina), and UL1 TR000424 (Johns Hopkins) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH).
The following persons provided study coordination and support: C. Bronchick, K. Brown-Engelhardt, S. Carlisle, L. Christie, J. Ross, M. Groetch, A. Hiegel, S. House, J. Kamilaris, S. Leung, K. Morgan, K. Mudd, S. Noone, M. Paterakis, J. Payne, D. Pearson, K. Peyton, R. Reames, J. Sikes, G. Spears, P. Steele, J. Stone, and M. Taylor. We thank G. Grishina, M. Kamalakannan and M. Mishoe for their technical expertise. We thank the staff of the clinical research unit at each institution and the Statistical and Clinical Coordinating Center; without their participation, the study could not have been done. Greer (Lenoir, NC) and Phadia (Uppsala, Sweden) generously provided reagents. Finally, we thank the families who kindly participated.
Funding: National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) grants U19AI066738 and U01AI066560.
Abbreviations
- BE:
baked egg
- DBPCFC:
double-blind placebo-controlled food challenge
- cDC:
classical DC
- OFC:
oral food challenge
- PBMC:
peripheral blood mononuclear cells
- pDC:
plasmacytoid DC
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Cooke SK, Sampson HA. Allergenic properties of ovomucoid in man. J Immunol 1997; 159:2026–32. [PubMed] [Google Scholar]
- 2.Urisu A, Ando H, Morita Y, Wada E, Yasaki T, Yamada K, et al. Allergenic activity of heated and ovomucoid-depleted egg white. J Allergy Clin Immunol 1997; 100:171–6. [DOI] [PubMed] [Google Scholar]
- 3.Des Roches A, Nguyen M, Paradis L, Primeau MN, Singer S. Tolerance to cooked egg in an egg allergic population. Allergy 2006; 61:900–1. [DOI] [PubMed] [Google Scholar]
- 4.Lemon-Mule H, Sampson HA, Sicherer SH, Shreffler WG, Noone S, Nowak-Wegrzyn A. Immunologic changes in children with egg allergy ingesting extensively heated egg. J Allergy Clin Immunol 2008; 122:977–83 e1. [DOI] [PubMed] [Google Scholar]
- 5.Bartnikas LM, Sheehan WJ, Tuttle KL, Petty CR, Schneider LC, Phipatanakul W. Ovomucoid specific immunoglobulin E as a predictor of tolerance to cooked egg. Allergy Rhinol (Providence) 2015; 6:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortot CF, Sheehan WJ, Permaul P, Friedlander JL, Baxi SN, Gaffin JM, et al. Role of specific IgE and skin-prick testing in predicting food challenge results to baked egg. Allergy Asthma Proc 2012; 33:275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters RL, Dharmage SC, Gurrin LC, Koplin JJ, Ponsonby AL, Lowe AJ, et al. The natural history and clinical predictors of egg allergy in the first 2 years of life: a prospective, population-based cohort study. J Allergy Clin Immunol 2014; 133:485–91. [DOI] [PubMed] [Google Scholar]
- 8.Leonard SA, Sampson HA, Sicherer SH, Noone S, Moshier EL, Godbold J, et al. Dietary baked egg accelerates resolution of egg allergy in children. J Allergy Clin Immunol 2012; 130:473–80 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leonard SA, Nowak-Wegrzyn AH. Baked Milk and Egg Diets for Milk and Egg Allergy Management. Immunol Allergy Clin North Am 2016; 36:147–59. [DOI] [PubMed] [Google Scholar]
- 10.Martos G, Lopez-Exposito I, Bencharitiwong R, Berin MC, Nowak-Wegrzyn A. Mechanisms underlying differential food allergy response to heated egg. J Allergy Clin Immunol 2011; 127:990–7 e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartnikas LM, Sheehan WJ, Larabee KS, Petty C, Schneider LC, Phipatanakul W. Ovomucoid is not superior to egg white testing in predicting tolerance to baked egg. J Allergy Clin Immunol Pract 2013; 1:354–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Lin J, Bardina L, Goldis M, Nowak-Wegrzyn A, Shreffler WG, et al. Correlation of IgE/IgG4 milk epitopes and affinity of milk-specific IgE antibodies with different phenotypes of clinical milk allergy. J Allergy Clin Immunol 2010; 125:695–702, e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarvinen KM, Beyer K, Vila L, Bardina L, Mishoe M, Sampson HA. Specificity of IgE antibodies to sequential epitopes of hen's egg ovomucoid as a marker for persistence of egg allergy. Allergy 2007; 62:758–65. [DOI] [PubMed] [Google Scholar]
- 14.Bloom KA, Huang FR, Bencharitiwong R, Bardina L, Ross A, Sampson HA, et al. Effect of heat treatment on milk and egg proteins allergenicity. Pediatr Allergy Immunol 2014; 25:740–6. [DOI] [PubMed] [Google Scholar]
- 15.Golias J, Schwarzer M, Wallner M, Kverka M, Kozakova H, Srutkova D, et al. Heat-induced structural changes affect OVA-antigen processing and reduce allergic response in mouse model of food allergy. PLoS One 2012; 7:e37156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosoy R, Agashe C, Grishin A, Leung DY, Wood RA, Sicherer SH, et al. Transcriptional Profiling of Egg Allergy and Relationship to Disease Phenotype. PLoS One 2016; 11:e0163831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qamar N, Fishbein AB, Erickson KA, Cai M, Szychlinski C, Bryce PJ, et al. Naturally occurring tolerance acquisition to foods in previously allergic children is characterized by antigen specificity and associated with increased subsets of regulatory T cells. Clin Exp Allergy 2015; 45:1663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shreffler WG, Wanich N, Moloney M, Nowak-Wegrzyn A, Sampson HA. Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. J Allergy Clin Immunol 2009; 123:43–52 e7. [DOI] [PubMed] [Google Scholar]