Historically, after introduction of an antibiotic for treatment of gonorrhea, strains of N. gonorrhoeae emerge that display clinical resistance due to spontaneous mutation or acquisition of resistance genes. Genetic exchange between members of the Neisseria genus occurring by transformation can cause significant changes in gonococci that impact the structure of an antibiotic target or expression of genes involved in resistance. The results presented here provide a framework for understanding how mosaic-like DNA sequences from commensal Neisseria that recombine within the gonococcal mtr efflux pump locus function to decrease bacterial susceptibility to antimicrobials, including antibiotics used in therapy of gonorrhea.
KEYWORDS: antibiotic resistance, efflux, gonorrhea, molecular genetics, transformation
ABSTRACT
Recent reports suggest that mosaic-like sequences within the mtr (multiple transferable resistance) efflux pump locus of Neisseria gonorrhoeae, likely originating from commensal Neisseria sp. by transformation, can increase the ability of gonococci to resist structurally diverse antimicrobials. Thus, acquisition of numerous nucleotide changes within the mtrR gene encoding the transcriptional repressor (MtrR) of the mtrCDE efflux pump-encoding operon or overlapping promoter region for both along with those that cause amino acid changes in the MtrD transporter protein were recently reported to decrease gonococcal susceptibility to numerous antimicrobials, including azithromycin (Azi) (C. B. Wadsworth, B. J. Arnold, M. R. A. Satar, and Y. H. Grad, mBio 9:e01419-18, 2018, https://doi.org/10.1128/mBio.01419-18). We performed detailed genetic and molecular studies to define the mechanistic basis for why such strains can exhibit decreased susceptibility to MtrCDE antimicrobial substrates, including Azi. We report that a strong cis-acting transcriptional impact of a single nucleotide change within the −35 hexamer of the mtrCDE promoter as well gain-of-function amino acid changes at the C-terminal region of MtrD can mechanistically account for the decreased antimicrobial susceptibility of gonococci with a mosaic-like mtr locus.
INTRODUCTION
Neisseria gonorrhoeae is the etiologic agent of the sexually transmitted infection (STI) gonorrhea. Gonorrhea is the second most reported condition in the United States (468,514 cases were reported in 2016) (1) and a major worldwide public health problem given its estimated yearly incidence of 78 million infections (2). Historically, the gonococcus has developed resistance to all drugs used for treatment since the introduction of sulfonamides in the late 1930s (3), and concern exists that without new effective antibiotics some gonorrheal infections in the future may be untreatable (4, 5). Currently, a dual antibiotic treatment regimen of ceftriaxone (Cro) (single intramuscular injection of 250 to 500 mg) and azithromycin (Azi) (single oral dose of 1 to 2 g) is used in many western countries (6, 7), but their continued efficacy for use in curing gonorrheal infections is threatened as strains resistant to either or both antibiotics have emerged in the past decade (8–11).
The gonococcus has adapted numerous strategies to survive attacks by antimicrobials, including the use of multidrug efflux pumps to export toxic compounds (3, 12, 13). Five gonococcal efflux pumps that export a wide range of substrates have been described (13). Of these, the best-studied efflux pump is MtrCDE, which belongs to the resistance-nodulation-division family possessed by many Gram-negative bacteria. MtrCDE captures and exports structurally diverse, but generally amphipathic, antimicrobial agents, including macrolides, beta-lactams, cationic antimicrobial peptides, dyes, and detergents (13). The contribution of the MtrCDE efflux pump in antimicrobial resistance expressed by gonococci can be enhanced by cis- or trans-acting mutations that result in overexpression of the mtrCDE efflux pump operon (13). Importantly, overproduction of the MtrCDE efflux pump due to relief of transcriptional repression of mtrCDE can contribute to clinically relevant levels of resistance to beta-lactams and macrolides (13).
Expression of mtrCDE in wild-type (WT) gonococci is subject to repression by MtrR (14, 15) and, in the presence of an inducer, activation by MtrA (16). Both MtrA and MtrR bind to regions within a 250-bp sequence that contains overlapping, divergent promoters for mtrR and mtrCDE transcription (17). Loss of MtrR repression of mtrCDE can result from point mutations in the MtrR-binding site (14), a single base pair deletion within a 13-bp inverted repeat sequence in the mtrR promoter (18), a point mutation that creates a new mtrCDE promoter (19), or missense/nonsense mutations in the mtrR gene (20–22). In addition to decreasing gonococcal susceptibility to antibiotics, these regulatory mutations can also enhance the fitness of gonococci during experimental infection of the lower genital tract of female mice (23), which supports the concept that the MtrCDE efflux pump is of importance during infection due to its ability to export host-derived antimicrobials such as cationic antimicrobial peptides and progesterone (24).
While single-site regulatory mutations impacting mtrCDE expression have been extensively studied (14, 19–22, 25), increasing evidence suggests that entry and recombination of donor DNA from commensal Neisseria spp. into the mtr locus can result in multiple nucleotide changes that can decrease gonococcal susceptibility to antimicrobials including Azi and Cro. Thus, the presence of mosaic-like sequences within the mtrR region likely resulting from transformation by DNA from N. lactamica or N. meningitidis has been reported in worldwide-isolated gonococcal strains (26–30). Recent work by Wadsworth et al. (30) showed that gonococci bearing diverse mosaic-like sequences within the mtrR/mtrCDE promoter region have elevated expression of mtr-associated genes and decreased susceptibility to Azi. Importantly, mosaic-like sequences within the mtrD inner membrane transporter protein-encoding gene showed strong linkage disequilibrium and epistatic effects that likely enhance the activity of the MtrCDE efflux pump (30). Taken together, the available information strongly suggests that mosaic-like sequences in the mtr locus can result in increased expression of the mtrCDE efflux pump operon as well as providing a gain-of-function property to MtrD that enhances its ability to export antimicrobials. In this study, we examined gonococcal clinical isolates that possess a mosaic-like mtr locus similar to other clinical isolates (30). We describe both a single nucleotide change in the overlapping mtrR/mtrCDE promoters and a likely mechanism for its impact on gene transcription, and amino acid changes in the C-terminal domain of MtrD that were linked to the decreased antimicrobial susceptibility phenotype expressed by gonococci with a mosaic-like mtr locus.
RESULTS
Importance of the MtrCDE efflux pump in reduced susceptibility to Azi and other antimicrobials in gonococcal clinical isolates bearing a mosaic-like mtr locus.
Public health laboratories associated with the Gonococcal Isolate Surveillance Project (GISP) alert the Centers for Disease Control and Prevention (CDC) to N. gonorrhoeae isolates if the Azi MIC is 2 µg/ml or greater. High-level MICs of Azi (≥ 256 µg/ml [3]) are typically due to mutations in the four 23S rRNA genes (31). However, in recent years it has become apparent that gonococci can display a so-called “less-Azi-susceptible” phenotype characterized by MIC values of 2 to 4 µg/ml (3, 25–30) that does not involve 23S rRNA mutations. This less-Azi-susceptible property may help gonococci escape the action of this macrolide during treatment, especially at extragenital sites of infection (e.g., the pharyngeal mucosa) where the pharmacokinetic properties of antibiotics are not optimal (32). It is therefore important to define how gonococci can develop decreased Azi susceptibility in the absence of 23S rRNA mutations as this could result in clinical failure of this macrolide during certain infections.
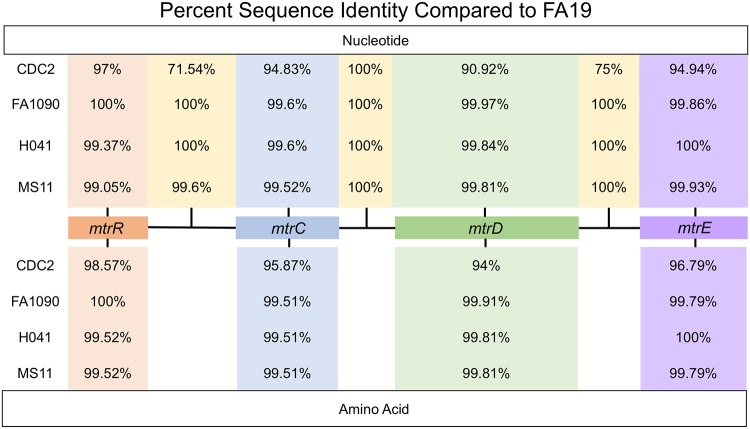
In order to study emergence of gonococcal clinical isolates with reduced susceptibility to Azi and to ascertain the contribution, if any, of the MtrCDE efflux pump system to this phenotype, we analyzed eight clinical strains collected in 2014 that expressed a less-Azi-susceptible property (MIC of 2 µg/ml); details of these strains are provided in Materials and Methods and Table S1 in the supplemental material. Whole-genome sequencing (WGS) performed on these eight strains and detailed bioinformatic analysis revealed that they lacked known 23S rRNA mutations associated with high levels of Azi resistance (data not presented). The sequences of the genes within the mtr locus of the 8 strains were identical, and they contained multiple nucleotide differences compared to antibiotic-sensitive reference strain FA19; the details of the WGS performed on these and other strains will be presented elsewhere (O. O. Soge and A. J. Abrams, unpublished data). Briefly, an alignment of the nucleotide sequence of the entire mtr locus in clinical strain LRRBGS0002 (here termed CDC2), displaying a less Azi-susceptible phenotype, with that of FA19 is shown in Fig. S1A. The nucleotide sequence of the mtr locus possessed by strain CDC2 was most dissimilar to FA19 (as well as three other gonococcal strains [MS11, FA1090, and H041]) in the mtrR/mtrCDE promoter region, the mtrD gene, and the noncoding region between mtrD and mtrE (Fig. 1). A BLAST search against Neisseria (NCBI:txid482) nucleotide sequences in NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi) determined that the entire mtr locus sequence possessed by CDC2 was most similar (95% identity) to that of N. polysaccharea M18661 (GenBank CP031325.1). Analysis of a phylogenetic tree based on the mtr loci of CDC2, FA19, and three clinical strains (GCGS0276, GCGS0402, and GCGS0834) with mosaic-like mtr loci studied by Wadsworth et al. (30) indicated that the mtr locus of CDC2 was most similar to that possessed by strain GCGS0402 (Fig. S1B). In fact, the nucleotide sequences of the mtr loci of CDC2 and GCGS0402 were identical. Importantly, however, CDC2 lacked the Correia element (CE) (33) that is positioned adjacent to the mtrR-mtrCDE promoter region found in mtr mosaic-like strain GCGS0276 (30) (Fig. S1A).
FIG 1.
Shown are the nucleotide and amino acid sequence percent identities of genes and intervening regions of the mtr locus possessed by gonococcal strains with respect to FA19 (CP012026.1). Accession numbers are provided in Table S1. Clustal Omega multiple sequence alignments were performed using N. gonorrhoeae strains FA19, CDC2, FA1090, H041, and MS11. Alignments were generated for each mtr gene using their nucleotide and amino acid sequences, and nucleotide sequences were aligned for the intergenic regions. Pairwise identity matrices were calculated, and the pairwise identity values for each alignment are shown.
(A) Shown is an alignment of the nucleotide sequences of the complete mtr locus possessed by gonococcal strains FA19, CDC2, and three clinical isolates studied by Wadsworth et al. (30). Nucleotides of note in CDC2 that differ from FA19 in either coding or noncoding regions are highlighted in red. The Correia element (CE) in strain GCGS0276 is highlighted in gray. (B) Shown is a phylogenetic tree for the mtr loci possessed by gonococcal strains FA19, CDC2, GCGS0276, GCGS0402, and GCGS0834. Download FIG S1, PDF file, 0.1 MB (124KB, pdf) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Gonococcal clinical strains and mtrD::kan mutants used in this study. Download Table S1, PDF file, 0.01 MB (8.6KB, pdf) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
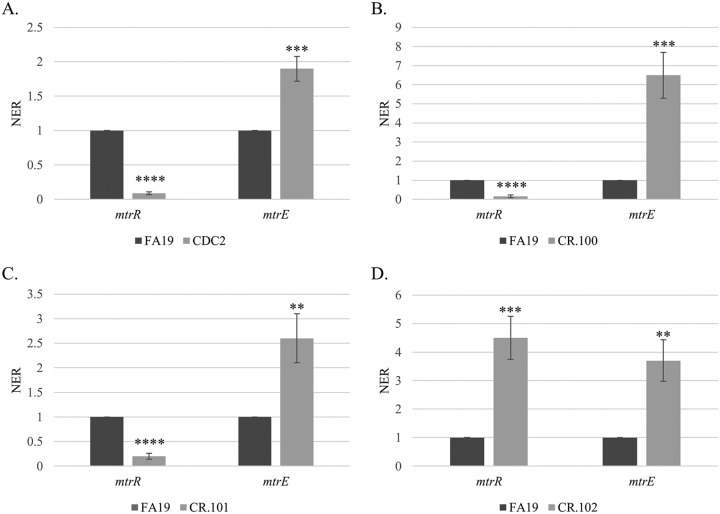
Since CDC2 contained numerous nucleotide sequence variations (with respect to FA19) in the mtrR coding region and the overlapping mtrR/mtrCDE promoter region, we hypothesized that it (as well as the seven other alert strains) might overproduce the MtrCDE pump, leading to decreased susceptibility to Azi and other antimicrobials. In order to confirm that this efflux pump is required for antimicrobial resistance in CDC2, as has been reported for non-mtr mosaic-like clinical isolates such as H041 (25), we created a mutant that lacked a functional MtrCDE efflux pump due to insertional inactivation (mtrD::kan) of the parental mtrD gene, which encodes the MtrD cytoplasmic membrane transporter. We also created other mutants that lacked functional MacAB or NorM efflux pumps to ascertain if their loss might also increase susceptibility of this strain to antimicrobials. Of these three efflux pumps, only loss of an active MtrCDE efflux pump rendered CDC2 and the other Azi alert isolates hypersusceptible to Azi (Table S1). Similarly, for CDC2 (Table 1) and the other Azi alert strains (data not presented), only the loss of the MtrCDE efflux pump (see CR.99 in Tables S1 and S2) resulted in hypersusceptibility to the tested antimicrobials.
TABLE 1.
Levels of antimicrobial susceptibility of gonococcal strains
| Strain | MIC (μg/ml)a
|
||||
|---|---|---|---|---|---|
| Azi | Ery | TX-100 | CV | EB | |
| FA19 | 0.25 | 0.25 | 100 | 1.25 | 1 |
| KH14 | 0.0625 | 0.0625 | 12.5 | 0.625 | 0.25 |
| JF1 | 0.5 | 1 | 200 | 1.25 | 2 |
| KH15 | 1 | 2 | >6,400 | 2.5 | 4 |
| CDC2 | 2 | 4 | >6,400 | 2.5 | 16 |
| CDC2mtrD::kan | <0.03 | 0.125 | ≤50 | 0.625 | 0.5 |
| CDC2norM::kan | 2 | 4 | >6,400 | 2.5 | 4 |
| CDC2macA::kan | 2 | 4 | >6,400 | 2.5 | 16 |
| CR.100 | 1 | 2 | >6,400 | 2.5 | 4 |
| CR.101 | 1 | 2 | >6,400 | 2.5 | 4 |
| CR.102 | 0.5 | 1 | 200 | 2.5 | 2 |
| CR.103 | 2 | 4 | >6,400 | 2.5 | 8 |
| CR.104 | 2 | 4 | >6,400 | 5 | 8 |
All MIC values are representative results from 3 to 5 independent determinations.
Genetic derivatives used in this study. Download Table S2, PDF file, 0.01 MB (11.7KB, pdf) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Since the MtrCDE efflux pump was essential for cross-resistance of CDC2 to antimicrobials, we focused on defining the impact of mosaic-like sequences in its mtr locus on MIC values of antimicrobials exported by this pump. For this purpose, we studied expression of the mtrR repressor gene and mtrE gene, which encodes the outer membrane protein (OMP) channel of the pump and is the last gene in the mtrCDE operon (Fig. 1) (3). Expression of these genes in FA19 and CDC2 was assessed at the levels of transcription and translation by qRT-PCR and Western immunoblotting, respectively. At the level of transcription, it was found that mtrR expression in FA19 was significantly higher than that of CDC2, while mtrE expression was higher in CDC2 (Fig. 2A). With respect to the MtrR repressor protein, its level in CDC2 and the other seven clinical Azi alert strains was substantially lower than that in strain FA19 (Fig. S2A). As expected from the low level of the MtrR repressor in CDC2, the level of the MtrE OMP in this strain was higher than that in FA19. In this respect, the level of MtrE in CDC2 was similar to that of strain JF1 (FA19ΔmtrR), but lower than that of KH15 (FA19 with single base pair deletion in the mtrR promoter, which is known to decrease mtrR expression and increase mtrCDE expression) (Fig. S2B).
FIG 2.
Shown are levels of expression of mtrR and mtrE genes in gonococcal strains FA19 and CDC2 (A), FA19 and CR.100 (B), FA19 and CR.101 (C), and FA19 and CR.102 (D). Gene transcript levels were quantified by qRT-PCR performed in triplicate with three biological replicates. Results are presented as average NER (normalized expression ratio) values (±SD) with P values. **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001.
Shown are levels of the MtrR (A) and MtrE (B) proteins in whole-cell lysates of gonococcal strains as determined by Western immunoblotting. The eight CDC alert strains are shown with a strain number. Included in these blots are lysates from WT strain FA19 and transformant strains JF1 and KH15 that lack MtrR due to deletion of the gene (JF1) or a single-base-pair deletion in the mtrR promoter that abrogates mtrR gene expression and elevates mtrCDE expression. An accompanying CBB-stained gel is shown in panel C. Download FIG S2, TIF file, 16.2 MB (16.6MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Increased expression of mtrCDE due to mosaic-like mtrR and mtrR-mtrCDE promoter regions can contribute to, but is not sufficient for, the decreased-Azi susceptibility phenotype of CDC2.
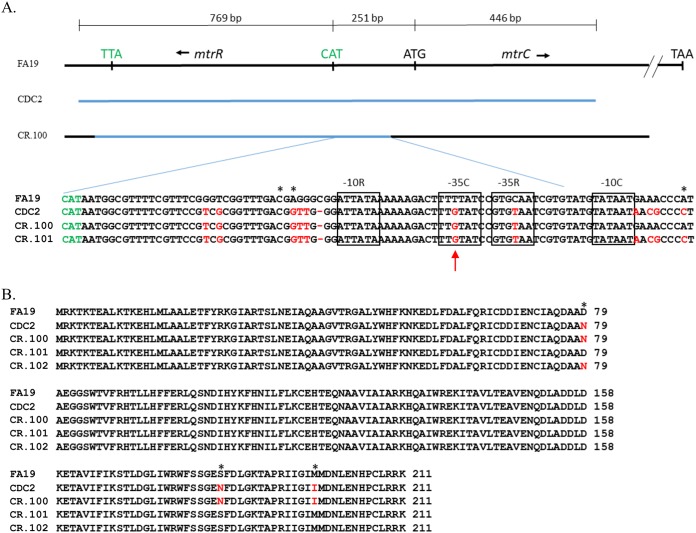
We hypothesized that the less-Azi-susceptible phenotype of CDC2 was due, in part, to enhanced levels of the MtrCDE efflux pump resulting from cis- and/or trans-acting mutations generated by the mosaic-like sequence that influence mtrR and mtrCDE expression. To test this hypothesis, we replaced the FA19 mtrR coding and mtrR-mtrCDE promoter sequences with that possessed by CDC2 and tested if this would influence expression of these genes as well as increasing MICs of Azi and other antimicrobials recognized by the MtrCDE efflux pump. For this purpose, a PCR-generated DNA fragment containing the mtrR coding region, the mtrR-mtrCDE intervening region, and the 5′ end of mtrC present in CDC2 (summarized in Fig. 3A) was used to transform strain FA19 for increased resistance to a known MtrCDE substrate, Triton X-100 (TX-100). A resulting transformant termed CR.100 (Tables 1 and 2) was selected for more detailed studies. DNA sequencing revealed that CR.100 had mtrR coding and mtrR-mtrCDE promoter sequences that contained most, but not all, of the CDC2 donor mosaic-like DNA in this region (Fig. 3A). With respect to the nucleotide changes in the noncoding sequence, CR.100 had the CDC2 mosaic-like sequence that included one nucleotide change within the −35 hexamer of the mtrR promoter (C to T) and one change within the −35 hexamer of the mtrCDE promoter (T to G) (Fig. 3A and Table 2) that have been observed in other strains (27, 30). In this respect, Wadsworth et al. found that the −35 mtrR promoter mutation was present in GCGS0402, while the −35 mtrCDE promoter mutation was present in all three of the mtr mosaic-like strains used in their study (30). Although a nucleotide difference was also noted in the previously identified transcriptional start site (TSS) for mtrCDE transcription (18), primer extension analysis revealed that transcription of mtrCDE in strains CDC2 and FA19 was similarly initiated (Fig. S3). CR.100 also contained the CDC2-derived mosaic-like sequence in mtrR, which was characterized by missense mutations in codons 79 (D79N), 183 (S183N), and 197 (M197I) (Fig. 3B).
FIG 3.
(A) Shown is the region of the mtrR-mtrC that was PCR amplified from chromosomal DNA of strain CDC2 (blue line) used to transform strain FA19. The region of recombination in transformant strain CR.100 is shown by a blue line. The nucleotide sequences of the mtrR/mtrC promoter region (mtrCDE coding strand) from strains FA19, CDC2, CR.100, and CR.101 are shown below. The translation start codon for mtrR is shown in green. The −10 and −35 hexamers of the mtrR and mtrCDE promoters are boxed. The TSS sites for both promoters are shown by asterisks. The red arrow shows the point mutation in the −35 hexamer of the mtrCDE sigma-70 promoter. Differences in nucleotide sequence or deletions are highlighted in red. (B) The predicted amino acid sequences of MtrR produced by strains FA19, CDC2, CR.100, CR.101, and CR.102 are shown. Differences at sites 79, 183, and 197 are highlighted in red and with asterisks.
TABLE 2.
Summary of important mtr genetic changes and Azi and TX-100 MIC values
| Strain | MtrR | mtrCDE promoter/mtrR promoter | MtrD | MIC (μg/ml) |
|
|---|---|---|---|---|---|
| Azi | TX-100 | ||||
| CDC2 | D79N, S183N, M197I | −35 (T to G)/−35 (C to T) | Mosaic-like | 2 | >6,400 |
| FA19 | WT | WT/WT | WT | 0.25 | 100 |
| CR.100a | D79N, S183N, M197I | −35 (T to G)/−35 (C to T) | WT | 1 | >6,400 |
| CR.101 | WT | −35 (T to G)/−35 (C to T) | WT | 1 | >6,400 |
| CR.102 | D79N | WT/WT | WT | 0.5 | 200 |
| CR.103 | D79N, S183N, M197I | −35 (T to G)/−35 (C to T) | 3′-end mosaic-like | 2 | >6,400 |
| CR.104 | D79N, S183N, M197I | −35 (T to G)/−35 (C to T) | S821A, K823E | 2 | >6,400 |
Strains CR.100 to CR.104 are all in the FA19 genetic background.
(A). Shown are results from a primer extension experiment that identified the mtrC TSS in gonococcal strains FA19 and CDC2. The nucleotide sequence from the noncoding strand is shown adjacent to the autoradiogram with the start sites highlighted in red. (B) Shown are the nucleotide sequences of the mtrCDE promoter region from strains FA19 and CDC2 with the G nucleotide change (CDC2) in the −35 hexamer shown in green and the TSS sites highlighted by red asterisks. Download FIG S3, TIF file, 13.0 MB (13.4MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
As is shown in Table 1, transformant strain CR.100 was more resistant than parent strain FA19 to a panel of MtrCDE substrate antimicrobials but was 2-fold less resistant than donor strain CDC2 to macrolides Azi and erythromycin (Ery) and 4-fold less resistant to ethidium bromide (EB). An examination of transcript levels of mtrR and mtrE in FA19 versus CR.100 showed that mtrR expression was decreased in CR.100 while mtrE expression was increased (Fig. 2B). Although the MtrR repressor protein was readily detected in whole-cell lysates of FA19, it was much lower in transformant strain CR.100 (Fig. 4). This result indicated that acquisition of the mosaic-like sequence encompassing the mtrR coding and mtrR-mtrCDE promoter sequences resulted in transcriptional repression of mtrR and derepression of mtrCDE. However, it was unclear as to whether these gene expression differences and increased MICs of antimicrobials were due to the mtrR coding or promoter mutations present in CR.100.
FIG 4.
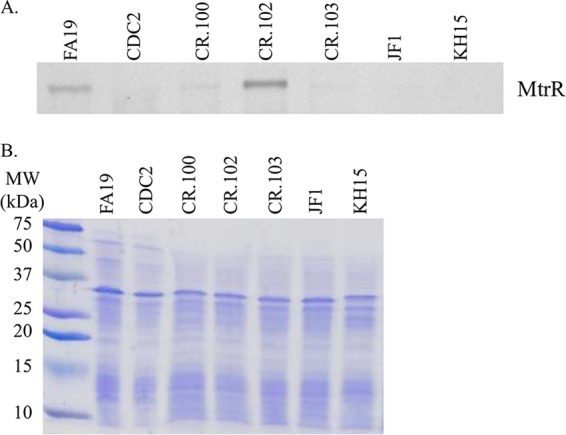
(A) Shown are levels of MtrR repressor protein in whole-cell lysates of gonococcal strains as determined by Western immunoblotting. (B) The SDS-PAGE gel stained with CBB showing near-equivalent levels of protein (15 μg) loaded in each well is shown. Gonococcal strains are identified at the top of each well.
In order to separate potential influences of cis- or trans-acting mutations in the overlapping mtrR/mtrCDE promoter regions from that of the mtrR coding region, respectively, on levels of antimicrobial resistance and gene expression in CR.100, we generated a pair of PCR products that singularly covered these regions in CDC2. We found that both regions could transform WT strain FA19 for decreased susceptibility to TX-100 or Azi. Recovered transformants were termed CR.101 and CR.102. DNA sequencing of PCR products from CR.101 and CR.102 was performed to determine the extent of recombination of the donor mosaic-like sequence in the mtrR coding or upstream noncoding region. With the promoter region-bearing PCR product, we found that complete replacement of the wild-type mtrR-mtrCDE intervening region by the donor sequence from CR.100 had occurred in CR.101 (Table 2 and Fig. 3A). In contrast, with the mtrR coding PCR product, only the MtrR D79N mutation was present in CR.102; the MtrR amino acid alignment information is provided in Fig. 3B and summarized in Table 2. Antimicrobial susceptibility testing results (Table 1) showed that the MICs of macrolides against CR.101 (mosaic-like mtrR-mtrCDE intervening region) were 2-fold higher than those of CR.102 (MtrR D79N). Interestingly, TX-100 resistance was >32-fold higher in CR.101 than CR.102 (MtrR D79N); the MIC against CR.101 resembled that seen with KH15 (Table 1), which has a single-base-pair deletion in the mtrR promoter that abrogates mtrR gene expression and shifts RNA polymerase recognition to the mtrCDE promoter (18). In contrast, the TX-100 MIC versus CR.102 was similar to that of JF1 (Table 1), which has a deletion of mtrR but retains a wild-type mtrR promoter (34).
The results from antimicrobial susceptibility testing suggested that mtrR coding and noncoding mosaic-like sequences have different impacts on expression of mtr-associated genes. Indeed, we found that while the MtrR D79N mutation in CR.102, which is also possessed by GCGS0402 and GCGS0834 (30), resulted in increased levels of mtrE expression compared to parental strain FA19, it also, unlike CDC2 and CR.100, had increased levels of the mtrR transcript (Fig. 2D) and the MtrR repressor protein (Fig. 4). Although position 79 of MtrR is outside the DNA-binding domain of this repressor (14, 15), we suggest that this amino acid change causes a decrease in MtrR function, resulting in derepression of mtrCDE expression. Nevertheless, the consequence of this mutation did not endow gonococci with either the antimicrobial resistance profile or the mtrR gene expression profile observed for CDC2 or CR.100, which also have mtrR/mtrCDE promoter mutations (Table 1 and Fig. 2).
Based on the findings with CR.102, we next tested whether potentially cis-acting mutations in the mtrR-mtrCDE promoter region influence expression of mtrR or mtrCDE. As was observed with clinical isolate CDC2 and transformant strain CR.100, the presence of the mosaic-like mtrR-mtrCDE promoter region in CR.101 resulted in a decreased level of mtrR expression but increased amounts of the mtrE transcript compared to parental strain FA19 (Fig. 2C). We hypothesize that mutations upstream of mtrR, especially the single nucleotide changes in the adjacent −35 hexamers of the mtrR and mtrCDE promoters (Fig. 3A), negatively impact expression of mtrR. Such repression of mtrR would not occur in CR.102 (MtrR D79N) where the wild-type FA19 promoter sequence is present (Fig. 3A) and MtrR levels are elevated (Fig. 4). Coupled with the antimicrobial susceptibility data (Tables 1 and 2), this result indicated that the nucleotide changes in the promoter region were responsible for modulating expression of mtrR and mtrCDE in clinical isolate CDC2 and transformant strain CR.100.
A single nucleotide change in the mosaic-like mtrR/mtrCDE promoter region can impact gene expression.
We hypothesized that the T-to-G change in the −35 hexamer region of the mtrCDE sigma-70 promoter in clinical isolate CDC2 and FA19 transformant strains CR.100 and CR.101 (red arrow, Fig. 3A) directly raised mtrCDE expression (Fig. 2A) and the MICs of MtrCDE antimicrobial substrates (Table 1); a similar nucleotide change was observed by others in mtr mosaic-like strains (27, 30). This nucleotide change would result in an improved −35 hexamer (5′-TTTTAT-3′ to 5′-TTGTAT-3′) of the mtrCDE promoter. To test the importance of this T-to-G change, two pLES94 mtrCpromoter-lacZ fusion (PmtrC-lacZ) constructs containing either the CDC2 mtrCDE promoter (CR.102pLES2.2) or an identical sequence but with the T nucleotide (CR.102pLES4.1) were introduced into CR.102 (FA19 MtrR D79N) by transformation; the lacZ fusions were integrated in the proAB region of the gonococcal chromosome (35). After verification of selected transformants by DNA sequencing of PCR products, levels of beta-galactosidase (β-Gal) were measured. As shown in Fig. 5, the β-Gal expression level was 3.5-fold higher in gonococci with the PmtrC-lacZ fusion that contained the G nucleotide in the −35 sequence of mtrCDE promoter compared to the variant that had the T nucleotide. This result suggests that the T-to-G change observed in the −35 hexamer of the mtrCDE promoter possessed by CDC2 as well as other isolates (30) results in a more effective mtrCDE promoter than that possessed by nonmosaic strain FA19. However, since MtrR can activate certain gonococcal genes (34), it was necessary to eliminate the (remote) possibility that the T-to-G nucleotide change facilitated binding of MtrR D79N to the mtrCDE promoter in an activating capacity. Accordingly, we introduced the PmtrC-lacZ fusion in pLES2.2 into strain JF1 (FA19 ΔmtrR). The results showed that compared to CR.102, expression of PmtrC-lacZ from the pLES2.2 fusion in JF1 was slightly elevated (data not presented), which is consistent with the MtrR D79N protein in CR.102 retaining a degree of transcriptional repressive activity as opposed to becoming an activator of mtrCDE.
FIG 5.
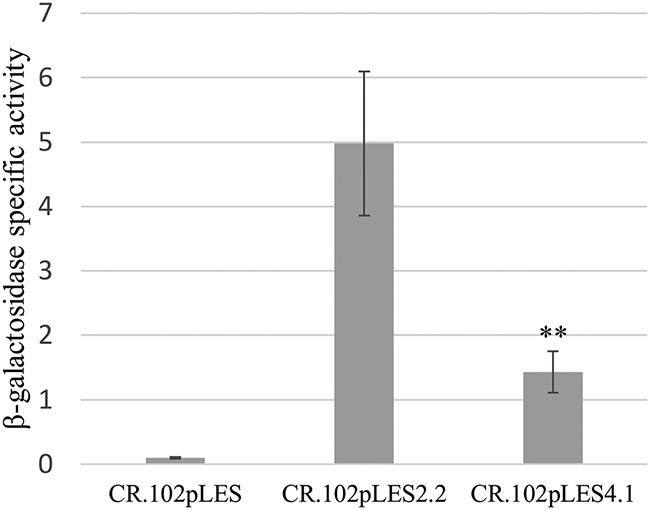
Shown are specific activities of β-Gal produced by gonococcal strain CR.102 (MtrR D79N) from pLES94 constructs without PmtrC-lacZ (CR.102pLES; control), or with PmtrC-lacZ with the CDC2 promoter (CR.102pLES2.2) or the same but with the WT −35 hexamer possessed by FA19 (CR.102pLES4.1). The results are shown as average values (±SD) with P values from three biologic replicates with each performed in triplicate. **, P ≤ 0.01.
Mutations in mtrD due to acquisition of a mosaic-like sequence can increase antimicrobial resistance.
The results from the gene expression studies described above implicated the T-to-G change in the −35 mtrCDE promoter hexamer as having a strong cis-acting influence that derepresses mtrCDE expression, leading to decreased susceptibility of gonococci to antimicrobials, including Azi. Collectively, these findings likely explain the levels of mtrR and mtrCDE gene expression in mtr mosaic-like strain CDC2 and other clinical isolates (27, 30) that possess this mutation. However, these transcriptional changes could not fully explain the higher MIC values of certain antimicrobials against CDC2. Thus, although transformant strain CR.100 had decreased susceptibility to all tested antimicrobials, only the MIC values of crystal violet (CV) and TX-100 matched that of CDC2 (Table 1). We hypothesized that amino acid differences in the MtrD efflux pump transporter protein possessed by CDC2 compared to FA19 and CR.100 might account for the higher Azi MIC seen with strain CDC2. Based on the structure of MtrD (PDB 4MTI) (36) and known similarity to AcrB (37), the following amino acid changes in MtrD possessed by strain CDC2 are predicted (E. Yu, personal communication) to impact its binding of antimicrobials: T42N, H46R, I48T, N101D, V662I, and K823E. To test whether these or other amino acid changes contribute to antimicrobial resistance in CDC2, three overlapping PCR products (summarized in Fig. S4) encompassing its entire mtrD gene were used together to transform strain CR.100 for resistance to 1 µg/ml of Azi. A resulting transformant (CR.103) was examined in antimicrobial susceptibility assays and was found to have identical levels of resistance as CDC2 to Azi (MIC of 2 µg/ml) and Ery (MIC of 4 µg/ml) but was 2-fold less resistant than CDC2 to the dye EB (Table 1). DNA sequencing of mtrD PCR products from CDC2, CR.100, and CR.103 showed that the nucleotide sequence of mtrD in these strains was identical to that of FA19 until codon 738, after which the CDC2 sequence had recombined in CR.103 until codon 1020. This recombination event generated twenty-three amino acid replacements in the C-terminal end of MtrD (Fig. 6). The amino acid changes in this C-terminal region of MtrD are located in the DC subdomain of the docking domain as well as in the PC2 subdomain of the pore region and transmembrane domains TM8 and TM9 (36). As assessed by qRT-PCR, CR.100 and CR.103 did not differ in levels of the mtrD transcript (data not presented), indicating that differences in levels of antimicrobial resistance between these strains were linked to structural alterations of MtrD located at the C-terminal end.
FIG 6.
Shown are the sequences of the MtrD protein produced by gonococcal strains as deduced by DNA sequencing. Amino acid differences of MtrD from strains CDC2, CR.100, CR.103, and CR.104 compared to FA19 are shown in green, with amino acids predicted to be sites for binding antimicrobials shown by red asterisks.
Shown is the strategy used to construct CR.103. Three regions of mtrD from CDC2 were amplified by PCR. The oligonucleotide primers and the length of the products are shown. These PCR products were used to transform strain CR.100 for resistance to 1 µg/ml of Azi. The region of recombination in strain CR.103 is shown by the blue rectangle. Download FIG S4, TIF file, 16.8 MB (17.2MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
In order to verify that amino acid changes in the C-terminal region of MtrD from CR.103 could increase MIC values of Azi, we prepared an mtrD 3′-end PCR product described above from CR.103 and used it to transform CR.100 with selection for resistance to 1 µg/ml of Azi. Six individual transformants were assessed in antimicrobial susceptibility testing assays using Azi and Ery, and all showed an elevated level of resistance to these macrolides comparable to that observed with donor strain CR.103 (data not presented). Differences were detected in the nucleotide sequence of the donated 3′ end of mtrD in the transformants, indicating that unique sites of recombination had occurred. Translation of the nucleotide sequence to amino acid sequence showed similar as well as unique amino acid changes in each transformant strain (data not presented). Importantly, however, only two amino acid changes, positioned at 821 (Ser to Ala) and 823 (Lys to Glu), were common in all six transformants. In fact, one transformant (CR.104) had only these two amino acid changes (Fig. 6 and Table 2) compared to recipient strain CR.100. We also sequenced the entire mtr locus (6,793 bp) possessed by strains FA19 and CR.104 and found that except for the nucleotide differences in the mtrR coding region, mtrR-mtrCDE intervening region, and the mtrD allele of CR.104, the mtr loci in these strains were otherwise identical (data not presented). The MIC values of antimicrobials against CR.104 were identical to that of donor strain CR.103 (Table 1); the sole exception was with CV, to which CR.104 was 2-fold more resistant.
DISCUSSION
Recombination of commensal neisserial DNA sequences into the chromosome of N. gonorrhoeae and N. meningitidis has been well established (27, 30, 38–40). Such horizontal transmission of DNA can endow gonococci or meningococci with decreased susceptibility to antibiotics that target the respective gene product. The best example of the consequence of this genetic exchange event is that of mosaic sequences in the pbp2 gene (also known as penA), which encodes the beta-lactam-sensitive target penicillin-binding protein 2 (PBP2) (41). The extensive remodeling of PBP2 that occurs due to the multiple (up to 60) amino acid changes due to a mosaic pbp2 decreases the acylation rate of penicillin and third-generation cephalosporins (cefixime and ceftriaxone) (42). pbp2 mosaic gonococcal strains also frequently contain cis-acting regulatory mutations that increase expression of the mtrCDE efflux pump operon (reviewed in reference 3). Loss of the MtrCDE efflux pump in gonococci with chromosomally mediated penicillin resistance can result in a return to a penicillin-sensitive phenotype (25, 43), while a similar loss can result in a 2- to 4-fold increase in susceptibility to Cro and Azi (25). Thus, mutations that derepress mtrCDE expression can work with mosaic pbp2 to decrease gonococcal susceptibility to beta-lactams.
The mosaic-like mtrR/mtrCDE promoter region described here and that reported by Wadsworth et al. (30) emphasize that in gonococci nucleotide changes due to acquisition of mosaic-like mtr sequences can elevate mtrCDE expression and MICs of antibiotics; a graphic model describing the influence of these nucleotide changes was recently presented in a commentary (44) by the corresponding author (W. M. Shafer) of the work by Wadsworth et al. (30). We propose that the single nucleotide change (T to G) within the −35 hexamer of the mtrCDE promoter provides increased expression of mtrCDE, as this change results in a consensus −35 hexamer sequence for sigma-70 promoters. Further, since this nucleotide change is also within the 13-bp inverted repeat positioned between the −10 and −35 regions of the mtrR promoter, it could alter the ability of this sequence to form a DNA secondary structure with regulatory activity. Thus, the position of this nucleotide change would maintain the spacing between the −10 and −35 hexamers of the mtrR promoter but would reduce the T:A bp repeat from 6 to 4. This reduction in the T:A bp repeat may impact promoter recognition by RNA polymerase, which preferentially shifts its recognition from the mtrR promoter to the mtrCDE promoter that has an improved −35 hexamer sequence. It should also be noted that mtr mosaic-like CDC2 exhibited a low level of mtrR expression, possibly due to the nucleotide change in the −35 hexamer of the mtrR promoter (Fig. 2 and 3). We hypothesize that this may also contribute to the low levels of MtrR in this and other clinical isolates. Interestingly, when the MtrR D79N mutation from CDC2 was placed into strain FA19, which has a WT mtrR promoter, levels of MtrR increased significantly (Fig. 4), indicating that this mutation can cause dysregulation of autoregulation of mtrR expression.
While the −35 mtrCDE promoter mutation can endow gonococci with increased expression of mtrCDE (Fig. 2) and elevate gonococcal resistance to antimicrobials, it did not account for the Azi MIC observed with clinical isolate CDC2 (Tables 1 and 2). In this respect, the results presented here and those of Wadsworth et al. (30) show that amino acid changes in mtrD, which result from importation of commensal neisserial DNA, are also necessary for the less-Azi-susceptible phenotype of mtr mosaic-like strains such as CDC2 and GCGS0402. Based on the published MtrD structure (36), many of the amino acid positions in MtrD that are changed in CDC2 versus antibiotic-sensitive strain FA19 could influence drug binding, efflux activity, or protein stability. Results from our transformation experiments indicated, however, that amino acid changes in the C-terminal end of MtrD, especially at positions 821 (S821A) and 823 (K823E), are sites for gain-of-function mutations that can contribute to antimicrobial resistance seen in mtr mosaic-like strains. These two amino acids are within the PC2 region of MtrD that is part of the pore domain of this transporter and is located at the outermost region that faces the periplasm. Based on the structural comparison of MtrD with the orthologous AcrB protein (37), position 823 in MtrD is predicted to be a site for binding antimicrobials (E. Yu, personal communication). Amino acid changes located elsewhere in MtrD could also have a similar impact, as indicated by the higher EB MIC value against CDC2 versus transformant strains CR.103 and CR.104. We note that the S821A and K823E changes are also present in the MtrD protein of the GCGS0402 and GCGS0834 strains studied by Wadsworth et al. (30); interestingly, GCGS0276, which also possesses a mosaic-like mtrD, has a K823D change that may have a similar functional impact on MtrD activity as the K823E mutation (30).
Active international and national surveillance systems for determining trends in gonococcal resistance to antibiotics coupled with whole-genome sequencing and bioinformatic analyses have been instrumental in detecting gonococci with resistance determinants, including those generated by mosaic-like mtr sequences (45–49). The detailed molecular, genetic, and phenotypic analyses made possible by these efforts have provided new insights regarding the impact of the gonococcal MtrCDE efflux pump in determining levels of bacterial resistance to clinically important antibiotics. The worldwide distribution of strains with mosaic-like mtr loci indicates that future diagnostic tests for resistance determinants should include functionally important nucleotide changes that were not described in earlier studies (summarized in reference 13). Further, the presence of such gonococcal strains reemphasizes that genetic exchange between gonococci and commensal Neisseria, which is likely to occur in the pharyngeal cavity, can be a major mechanism by which N. gonorrhoeae develops resistance to antibiotics. Thus, in order to enhance monitoring of antibiotic resistance trends, it would be prudent to routinely sample both genital and extragenital (especially the oral mucosae) sites for the presence of gonococci in patients suspected of having gonorrhea so as to better detect emergence of antibiotic-resistant strains in the community.
MATERIALS AND METHODS
Gonococcal strains, growth conditions, oligonucleotide primers, and determination of susceptibility to antimicrobial agents.
The gonococcal strains used in this study are presented in Tables S1 and S2 in the supplemental material. The clinical strains used in this investigation were kindly provided by Edward Bannister (Dallas, TX) and the Dallas, TX, GISP sentinel site. These strains were provided to CDC without patient identifiers, and as such, there was no involvement of human subjects in the research reported here. For most genetic studies we used antimicrobial-sensitive strain FA19 with an introduced rpsL mutation (FA19 Strr) that confers high-level resistance to streptomycin, but for brevity it is referred to as FA19 throughout the text (Table S2). Eight gonococcal clinical strains displaying decreased Azi susceptibility between 2014 and 2015 were also included in this study (Table S1). The details of their WGSs and bioinformatic analysis will be presented elsewhere (Soge and Abrams, unpublished). Briefly, the eight strains were selected based on their representation within one of four different clades that were identified by analysis of a generated phylogenetic tree from thirty-seven strains. Of these, strain LRRBGS0002 (CDC2; Table S2) was the most extensively studied. Gonococcal strains were grown overnight at 37°C under 5% (vol/vol) CO2 on GCB agar containing defined supplements I and II (21). The sequences of oligonucleotide primers used in this study are shown in Table S2. The MICs of antimicrobials were determined by the agar dilution method (50) using the GISP protocol (51). Antimicrobials were purchased from Sigma Chemical Co. (St. Louis, MO).
Bioinformatic analyses of WGS information.
To compare the sequences of the mtr loci among the strains, sequences for strains FA19, FA1090, and MS11 were retrieved from the NCBI genome sequence database (https://www.ncbi.nlm.nih.gov/nuccore/) or the NCBI Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra); the H041 WGS sequence was kindly provided by R. Nicholas (University of North Carolina, Chapel Hill, NC). Sequence read files for LRRB strains used in this study (Table S1) were downloaded from the NCBI Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra) into CLC Genomics Workbench (Qiagen), where we performed trimming, de novo assembly, and a BLAST search of the FA19 mtr locus sequence (GenBank accession number CP012026.1, nucleotides 1104741 to 1111533) against the newly assembled sequences in order to recover their mtr locus sequences for alignment. Raw sequence files for GCGS0276, GCGS0402, and GCGS0834 (30) were downloaded from NCBI SRA and trimmed and assembled according to published methods with Cutadapt (52) and SPAdes (53). The NCBI-blast toolkit (BLASTN [https://blast.ncbi.nlm.nih.gov/Blast.cgi]) retrieved the sequences for the mtr operon based on FA19 mtr locus sequence. Alignments were performed using the Clustal Omega multiple sequence alignment tool for nucleotide and amino acid sequences (54, 55), and percent identity values were obtained from the resulting pairwise identity matrix. The Newick file derived from the alignment was visualized using Interactive Tree of Life (iTOL) (56).
Strain constructions.
To construct mutants of the eight less-Azi-susceptible clinical isolates, genomic DNA from strains KH14 (FA19mtrD::kan), FA19norM::kan, and FA19macA::kan was used in transformation experiments as described previously (21) and verified by PCR using oligonucleotide primer pairs for each gene (Table S3). Strains KH14 and FA19norM::kan have been previously described (21, 57). FA19macA::kan was constructed in this study. Briefly, primers macAF and macAR (Table S2) were used to PCR amplify macA from FA19 genomic DNA. The resulting PCR product was cloned into the pBAD-TOPO vector as described by the manufacturer (Invitrogen) to create pBADmacA, which was then digested with SmaI. The nonpolar kanamycin (Kan) resistance cassette aphA3 (58) was cloned into the SmaI site, and the resulting plasmid (pBADmacA::kan) was transformed into FA19. Transformants were selected on GCB agar containing 50 µg/ml of Kan and were verified by PCR. PCR products were generated using CDC2 genomic DNA and primers CEL1 and KH9#12B (to construct CR.100), primers CEL4 and mtrCpromR (to construct CR.101), or primers CEL1 and KH9#10B (to construct CR.102). The resulting PCR products were transformed into FA19 as previously described (18). CR.100 clones were selected on GC plates supplemented with 3,600 μg/ml of TX-100. CR.101 was selected on GC plates supplemented with 0.25 μg/ml of Azi. CR.102 was selected on GC plates supplemented with 400 μg/ml of TX-100. The presence of mutations in the clones was verified by sequencing. For construction of CR.103, PCR products were generated with primers mtrD11Rev and mtrD3, mtrD3Rev and mtrD1, and mtrE12 and mtrD10 using CDC2 genomic DNA as the template. The 3 PCR products were used together to transform strain CR.100 using a selection of 1 µg/ml of Azi; a resulting transformant was termed CR.103. For construction of CR.104, a PCR product was generated with primers mtrD10 and mtrE12 on genomic DNA from CR.103. The resulting PCR was transformed into CR.100, and clones were selected on GC agar plates supplemented with 1 μg/ml of Azi.
Sequences of oligonucleotide primers. Download Table S3, PDF file, 0.02 MB (16KB, pdf) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Quantitative reverse transcriptase PCR (qRT-PCR).
RNA was extracted from strains FA19, CDC2, CR.100, CR.101, CR.102, and CR.104 at mid-logarithmic phase of growth in GC broth plus supplements by the TRIzol method as directed by the manufacturer (Thermo Fisher Scientific, Waltham, MA) and was performed as described previously (59). Briefly, genomic DNA (gDNA) was removed by RNase-free DNase treatment and gDNA Wipeout (Qiagen, Germantown, MD). The resulting RNA was then reverse transcribed to cDNA using the QuantiTect reverse transcriptase kit (Qiagen) as described previously (57). Primers 16Smai_qRTF and 16Smai_qRTR were used for 16S rRNA. Primers mtrEqPCR-F and mtrEqPCR-R were used for the mtrE gene, and primers mtrD8 and mtrD13 were used for the mtrD gene. Primers mtrR_qRT_F and mtrR_qRT_R were used for the mtrR gene. Sequences of primers are shown in Table S2. Results were calculated as normalized expression ratios (NERs) using 16S rRNA expression levels. Statistical significance was calculated by Student’s t test.
Western immunoblot analysis.
Whole-cell lysates were prepared from gonococcal strains grown overnight on GC agar plates with supplements as described previously and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (60). Coomassie brilliant blue (CBB) staining of duplicate SDS-PAGE gels was performed to calibrate and verify consistent loading of proteins (15 µg) loaded into each well. The concentration of protein in whole-cell lysates was estimated by using a NanoDrop spectrometer at 280 nM. Western immunoblotting using a mouse anti-MtrE serum (kindly provided by A. Jerse) and a rabbit anti-MtrR serum was performed as described previously (23, 34).
β-Gal assays.
lacZ fusions were constructed using the pLES94 system as previously described (35). Briefly, PCRs were performed on genomic DNA from strain CDC2 using primers C2 and C3PmtrC for pLES2.2 and C4 and C3PmtrC for pLES4.1. The resulting PCR products were cloned into the BamHI site of pLES94. pLES94, pLES2.2, and pLES4.1 and were introduced into strain CR.102 or JF1 by transformation with selection on GC agar plates supplemented with 1 µg/ml of chloramphenicol. β-Gal assays were performed in triplicate as described by Folster et al. (34) from lysates of gonococcal strains after growth overnight on GC agar plates supplemented with chloramphenicol. The β-Gal specific activity was calculated using the formula A420 × 1,000/A280 (mg/ml) × time (min) × volume (ml).
Primer extension analysis.
Total RNA from strains FA19 and CDC2 was prepared from mid- and late-logarithmic phase GCB broth cultures by the TRIzol method as directed by the manufacturer (Thermo Fisher Scientific, Waltham, MA). Primer extension experiments were performed as described previously (18) on 6 µg of total RNA with primer PEmtrC181. The primer extension transcription start site of the mtrC gene was determined by electrophoresis of the extension products on a 6% (wt/vol) DNA sequencing acrylamide gel adjacent to reference sequencing reactions.
WGS of CDC strains used in this study.
The whole genome sequences of the CDC strains 1, 2, 5, 7, 13, 17, 27, and 37 (Table S1) are provided at NCBI under Sequence Read Archive accession numbers SRR4416029 (CDC1), SRR4416030 (CDC2), SRR4416060 (CDC5), SRR4416062 (CDC7), SRR4416033 (CDC13), SRR4416037 (CDC17), SRR4416048 (CDC27), and SRR4416059 (CDC37).
ACKNOWLEDGMENTS
We thank E. Yu (Case Western University) for his insightful comments regarding MtrD structure and impact of mutations with respect to binding antimicrobials. We also thank the Gonococcal Isolate Surveillance Project (GISP) for the use of isolates and corresponding data for this analysis. Edward Bannister (Dallas, TX) and the Dallas, TX, GISP sentinel site collected, isolated, and provided epidemiological data for the isolates used in this analysis. The University of Washington GISP Regional Laboratory (Seattle, WA) determined and provided antimicrobial susceptibility result data for the isolates used in this analysis. We also thank Steven Johnson, Hsi Liu, Matthew Schmerer, Sandra Seby, Jesse Thomas, and Eshaw Vidyaprakash for thoughtful and informative discussions.
The contents of this article are solely the responsibility of the authors and do not necessarily reflect the official views of the National Institutes of Health, the Centers for Disease Control and Prevention, the U.S. Department of Veterans Affairs, or the United States government.
We have no competing interest to declare.
This work was supported by NIH grant R37AI21150-33 (W.M.S.) and funds from an Intergovernmental Personnel Act from the CDC to C.E.R.-L., J.L.R., and W.M.S. W.M.S. is the recipient of a Senior Research Career Scientist Award from the Biomedical Laboratory Research and Development Service of the U.S. Department of Veterans Affairs. CDC-based coauthors were funded by CDC. Their work was in part made possible through support from CDC’s Advanced Molecular Detection (AMD-18) and Combating Antibiotic Resistant Bacteria (CARB) programs.
Footnotes
Citation Rouquette-Loughlin CE, Reimche JL, Balthazar JT, Dhulipala V, Gernert KM, Kersh EN, Pham CD, Pettus K, Abrams AJ, Trees DL, St Cyr S, Shafer WM. 2018. Mechanistic basis for decreased antimicrobial susceptibility in a clinical isolate of Neisseria gonorrhoeae possessing a mosaic-like mtr efflux pump locus. mBio 9:e02281-18. https://doi.org/10.1128/mBio.02281-18.
Contributor Information
Michael S. Gilmore, Harvard Medical School.
Michael Apicella, University of Iowa.
Melissa Brown, Flinders University.
REFERENCES
- 1.CDC. 2017. Sexually transmitted disease surveillance 2016. CDC, Atlanta, GA. https://www.cdc.gov/std/stats. Accessed 31 July 2018.
- 2.Newman L, Rowley J, Vander Hoorn S, Saman Wijesooriya N, Unemo M, Low N, Stevens G, Gottlieb S, Kiarie J, Temmerman M. 2015. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 10:e0143304. doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unemo M, Shafer WM. 2014. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century—past, evolution, and future. Clin Microbiol Rev 27:587–613. doi: 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolan GA, Sparling PF, Wasserheit JN. 2012. The emerging threat of untreatable gonococcal infection. N Engl J Med 366:485–487. doi: 10.1056/NEJMp1112456. [DOI] [PubMed] [Google Scholar]
- 5.Unemo M, del Rio C, Shafer WM. 2016. Antimicrobial resistance expressed by Neisseria gonorrhoeae: a major global public health problem in the 21st century. Microbiol Spectr 4 doi: 10.1128/microbiolspec.EI10-0009-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Workowski KA, Bolan GA. 2015. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 7.Bignell C, Unemo M. 2013. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS 24:85–92. doi: 10.1177/0956462412472837. [DOI] [PubMed] [Google Scholar]
- 8.Fifer H, Natarajan U, Alexander S, Hughes G, Golparian D, Unemo M. 2016. Failure of dual antimicrobial therapy in treatment of gonorrhea. N Engl J Med 374:2504–2506. doi: 10.1056/NEJMc1512757. [DOI] [PubMed] [Google Scholar]
- 9.Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, Nakayama S, Kitawaki J, Unemo M. 2011. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother 55:3538–3545. doi: 10.1128/AAC.00325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. 2012. High-level cefixime- and ceftriaxone-resistant N. gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother 56:1273–1280. doi: 10.1128/AAC.05760-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Public Health England. 2018. UK case of Neisseria gonorrhoeae with high-level resistance to azithromycin and resistance to ceftriaxone acquired abroad. Health protection report advanced access report. Public Health; England, London, United Kingdom. [Google Scholar]
- 12.Rice PA, Shafer WM, Ram S, Jerse AE. 2017. Neisseria gonorrhoeae: drug resistance, mouse models and vaccine development. Annu Rev Microbiol 71:665–686. doi: 10.1146/annurev-micro-090816-093530. [DOI] [PubMed] [Google Scholar]
- 13.Shafer WM, Yu EW, Rouquette-Loughlin C, Golparian D, Jerse AE, Unemo M. 2016. Efflux pumps in Neisseria gonorrhoeae: contributions to antibiotic resistance and virulence, p 439–469. In Li X-Z, Elkins CA, Zgurskaya HI (ed), Efflux-mediated antimicrobial resistance in bacteria: mechanisms, regulation and clinical implications. Adis, Cham, Switzerland. [Google Scholar]
- 14.Lucas CE, Balthazar JT, Hagman KE, Shafer WM. 1997. The MtrR repressor binds the DNA sequence between the mtrR and mtrC genes of Neisseria gonorrhoeae. J Bacteriol 179:4123–4128. doi: 10.1128/jb.179.13.4123-4128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann KM, Williams D, Shafer WM, Brennan RG. 2005. Characterization of the multiple transferrable repressor, MtrR, from Neisseria gonorrhoeae. J Bacteriol 187:5008–5012. doi: 10.1128/JB.187.14.5008-5012.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rouquette C, Harmon JB, Shafer WM. 1999. Induction of the mtrCDE-encoded efflux pump system of Neisseria gonorrhoeae requires MtrA, an AraC-like protein. Mol Microbiol 33:651–658. doi: 10.1046/j.1365-2958.1999.01517.x. [DOI] [PubMed] [Google Scholar]
- 17.Zalucki YM, Dhulipala V, Shafer WM. 2012. Dueling regulatory properties of a transcriptional activator (MtrA) and repressor (MtrR) that control efflux pump gene expression in Neisseria gonorrhoeae. mBio 3:e00446-12. doi: 10.1128/mBio.00446-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagman KE, Shafer WM. 1995. Transcriptional control of the mtr efflux system in Neisseria gonorrhoeae. J Bacteriol 177:4162–4165. doi: 10.1128/jb.177.14.4162-4165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohneck EA, Zalucki YM, Johnson PJ, Dhulipala V, Golparian D, Unemo M, Jerse AE, Shafer WM. 2011. A novel mechanism of high-level, broad-spectrum antibiotic resistance caused by a single base pair change in Neisseria gonorrhoeae. mBio 2:e00187-11. doi: 10.1128/mBio.00187-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan W, Spratt BG. 1994. Regulation of the permeability of the gonococcal cell envelope by the mtr system. Mol Microbiol 11:769–775. doi: 10.1111/j.1365-2958.1994.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 21.Hagman KE, Pan W, Spratt BG, Balthazar JT, Judd RC, Shafer WM. 1995. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 141:611–622. doi: 10.1099/13500872-141-3-611. [DOI] [PubMed] [Google Scholar]
- 22.Shafer WM, Balthazar JT, Hagman KE, Morse SA. 1995. Missense mutations that alter the DNA-binding domain of the MtrR protein occur frequently in rectal isolates of Neisseria gonorrhoeae that are resistant to faecal lipids. Microbiology 141:907. doi: 10.1099/13500872-141-4-907. [DOI] [PubMed] [Google Scholar]
- 23.Warner DM, Shafer WM, Jerse AE. 2008. Clinically relevant mutations that cause derepression of the Neisseria gonorrhoeae MtrC-MtrD-MtrE efflux pump system confer different levels of antimicrobial resistance and in vivo fitness. Mol Microbiol 70:462–478. doi: 10.1111/j.1365-2958.2008.06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jerse AE, Sharma ND, Simms AN, Crow ET, Snyder LA, Shafer WM. 2003. A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect Immun 71:5576–5582. doi: 10.1128/IAI.71.10.5576-5582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golparian D, Shafer WM, Ohnishi M, Unemo M. 2014. Importance of multidrug efflux pumps in the antimicrobial resistance property of clinical multidrug-resistant isolates of Neisseria gonorrhoeae: rationale for targeting efflux systems for drug development. Antimicrob Agents Chemother 58:3556–3559. doi: 10.1128/AAC.00038-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grad YH, Harris SR, Kirkcaldy RD, Green AG, Marks DS, Bentley SD, Trees D, Lipsitch M. 2016. Genomic epidemiology of gonococcal resistance to extended-spectrum cephalosporins, macrolides, and fluoroquinolones in the United States, 2001-2013. J Infect Dis 214:1579–1587. doi: 10.1093/infdis/jiw420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trembizki E, Doyle C, Jennison A, Smith H, Bates J, Lahra M, Whiley D. 2014. A Neisseria gonorrhoeae strain with a meningococcal mtrR sequence. J Med Microbiol 63:1113–1115. doi: 10.1099/jmm.0.074286-0. [DOI] [PubMed] [Google Scholar]
- 28.Demczuk W, Martin I, Peterson S, Bharat A, Van Domselaar G, Graham M, Lefebvre B, Allen V, Hoang L, Tyrrell G, Horsman G, Wylie J, Haldane D, Archibald C, Wong T, Unemo M, Mulvey MR. 2016. Genomic epidemiology and molecular resistance mechanisms of azithromycin-resistant Neisseria gonorrhoeae in Canada from 1997 to 2014. J Clin Microbiol 54:1304–1313. doi: 10.1128/JCM.03195-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whiley DM, Kundu RI, Jennison AV, Buckley C, Limnios A, Hogan T, Enriquez R, El Nasser J, George CR, Lahra MM. 2018. Azithromycin-resistant Neisseria gonorrhoeae spreading amongst men who have sex with men (MSM) and heterosexuals in New South Wales, Australia, 2017. J Antimicrob Chemother 73:1242–1246. doi: 10.1093/jac/dky017. [DOI] [PubMed] [Google Scholar]
- 30.Wadsworth CB, Arnold BJ, Sater MRA, Grad YH. 2018. Azithromycin resistance through interspecific acquisition of an epistasis-dependent efflux pump component and transcriptional regulator in Neisseria gonorrhoeae. mBio 9:e01419-18. doi: 10.1128/mBio.01419-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galarza PG, Abad R, Canigia LF, Buscemi L, Pagano I, Oviedo C, Vázquez JA. 2010. New mutation in 23S rRNA gene associated with high level of azithromycin resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother 54:1652–1653. doi: 10.1128/AAC.01506-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barza M. 1994. Challenge to antibiotic activity in tissues. Clin Infect Dis 19:910–915. doi: 10.1093/clinids/19.5.910. [DOI] [PubMed] [Google Scholar]
- 33.Correia FF, Inouye S, Inouye M. 1988. A family of small repeated elements with some transposon-like properties in the genome of Neisseria gonorrhoeae. J Biol Chem 263:12194–12198. [PubMed] [Google Scholar]
- 34.Folster JP, Johnson PJ, Jackson L, Dhulipali V, Dyer DW, Shafer WM. 2009. MtrR modulates rpoH expression and levels of antimicrobial resistance in Neisseria gonorrhoeae. J Bacteriol 191:287–297. doi: 10.1128/JB.01165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silver LE, Clark VL. 1995. Construction of a translational lacZ fusion system to study gene regulation in Neisseria gonorrhoeae. Gene 166:101–104. doi: 10.1016/0378-1119(95)00605-6. [DOI] [PubMed] [Google Scholar]
- 36.Bolla JR, Su C-C, Do SV, Radhakrishnan A, Kumar N, Long F, Chou T-H, Delmar JA, Lei H-T, Rajashankar KR, Shafer WM, Yu EW. 2014. Crystal structure of the Neisseria gonorrhoeae MtrD inner membrane multidrug efflux pump. PLoS One 9:e97903. doi: 10.1371/journal.pone.0097903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murakami S, Nakashima R, Yamashita E, Yamaguchi A. 2002. Crystal structure of bacterial multidrug efflux transporter AcrB. Nature 419:587–593. doi: 10.1038/nature01050. [DOI] [PubMed] [Google Scholar]
- 38.Spratt BG, Bowler LD, Zhang QY, Zhou J, Smith JM. 1992. Role of interspecies transfer of chromosomal genes in the evolution of penicillin resistance in pathogenic and commensal Neisseria species. J Mol Evol 34:115–125. [DOI] [PubMed] [Google Scholar]
- 39.Bowler LD, Zhang QY, Riou JY, Spratt BG. 1994. Interspecies recombination between the penA genes of Neisseria meningitidis and commensal Neisseria species during the emergence of penicillin resistance in Neisseria meningitidis: natural events and laboratory stimulation. J Bacteriol 176:333–337. doi: 10.1128/jb.176.2.333-337.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimuta K, Unemo M, Nakayama S, Morita-Ishihara T, Dorin M, Kawahata T, Ohnishi M. 2013. Antimicrobial resistance and molecular typing of Neisseria gonorrhoeae isolates in Kyoto and Osaka, Japan, 2010 to 2012: intensified surveillance after identification of the first strain (H041) with high-level ceftriaxone resistance. Antimicrob Agents Chemother 57:5225–5232. doi: 10.1128/AAC.01295-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spratt BG. 1988. Hybrid penicillin-binding proteins in penicillin-resistant strains of Neisseria gonorrhoeae. Nature 332:173–176. doi: 10.1038/332173a0. [DOI] [PubMed] [Google Scholar]
- 42.Tomberg J, Unemo M, Davies C, Nicholas RA. 2010. Molecular and structural analysis of mosaic variants of penicillin-binding protein 2 conferring decreased susceptibility to expanded-spectrum cephalosporins in Neisseria gonorrhoeae: role of epistatic mutations. Biochemistry 49:8062–8070. doi: 10.1021/bi101167x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veal WL, Nicholas RA, Shafer WM. 2002. Overexpression of the MtrC-MtrD-MtrE efflux pump due to an mtrR mutation is required for chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. J Bacteriol 184:5619–5624. doi: 10.1128/JB.184.20.5619-5624.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shafer WM. 2018. Mosaic drug efflux gene sequences from commensal Neisseria can lead to low-level azithromycin resistance expressed by Neisseria gonorrhoeae clinical isolates. mBio 9:e01747-18. doi: 10.1128/mBio.01747-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dillon JAR, Trecker MA, Thakur SD. 2013. Two decades of the gonococcal antimicrobial surveillance program in South America and the Caribbean: challenges and opportunities. Sex Transm Infect 89:iv36–iv41. doi: 10.1136/sextrans-2012-050905. [DOI] [PubMed] [Google Scholar]
- 46.Kirkcaldy RD, Kidd S, Weinstock HS, Papp JR, Bolan GA. 2013. Trends in antimicrobial resistance in Neisseria gonorrhoeae in the USA: the Gonococcal Isolate Surveillance Project (GISP), January 2006-June 2012. Sex Transm Infect 89(Suppl 4):iv5–iv10. doi: 10.1136/sextrans-2013-051162. [DOI] [PubMed] [Google Scholar]
- 47.Kubanova A, Frigo N, Kubanov A, Sidorenko S, Priputnevich T, Vachnina T, Al-Khafaji N, Polevshikova S, Solomka V, Domeika M, Unemo M. 2008. National surveillance of antimicrobial susceptibility in Neisseria gonorrhoeae in 2005-2006 and recommendations of first-line antimicrobial drugs for gonorrhoea treatment in Russia. Sex Transm Infect 84:285–289. doi: 10.1136/sti.2007.029033. [DOI] [PubMed] [Google Scholar]
- 48.Lahra MM, Lo YR, Whiley DM. 2013. Gonococcal antimicrobial resistance in the Western Pacific Region. Sex Transm Infect 89:iv19–iv23. doi: 10.1136/sextrans-2012-050906. [DOI] [PubMed] [Google Scholar]
- 49.Johnson SR, Sandul AL, Parekh M, Wang SA, Knapp JS, Trees DL. 2003. Mutations causing in vitro resistance to azithromycin in Neisseria gonorrhoeae. Int J Antimicrob Agents 21:414–419. doi: 10.1016/S0924-8579(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 50.Sarubbi FA Jr, Blackman E, Sparling PF. 1974. Genetic mapping of linked antibiotic resistance loci in Neisseria gonorrhoeae. J Bacteriol 120:1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention. 2016. Division of Sexually Transmitted Diseases Prevention. Gonococcal Isolate Surveillance Project (GISP). Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/std/gisp/. [Google Scholar]
- 52.Martin M. 2011. CutAdapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17:10. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 53.Bankevich A, Nurk S, Antipov D, Gurevich A, Dvorkin M, Kulikov AS, Lesin V, Nikolendo S, Pham S, Prjibelski A, Pyshkin A, Sirotkin A, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sievers F, Wilm A, Dineen DG, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins D. 2014. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goujon M, McWilliam H, Li W, Valentin F, Squizzato S, Paern J, Lopez R. 2010. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res 38:W695–W699. doi: 10.1093/nar/gkq313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Letunic I, Bork P. 2016. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 44:W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rouquette-Loughlin C, Dunham SA, Kuhn M, Balthazar J, Shafer WM. 2003. The NorM efflux pump of Neisseria gonorrhoeae and Neisseria meningitidis recognizes antimicrobial cationic compounds. J Bacteriol 185:1101–1106. doi: 10.1128/JB.185.3.1101-1106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ménard R, Sansonetti PJ, Parsot C. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol 175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rouquette-Loughlin CE, Zalucki YM, Dhulipala VL, Balthazar JT, Doyle RG, Nicholas RA, Begum AA, Raterman EL, Jerse AE, Shafer WM. 2017. Control of gdhR expression in Neisseria gonorrhoeae via autoregulation and a master repressor (MtrR) of a drug efflux pump operon. mBio 8:e00449-17. doi: 10.1128/mBio.00449-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Shown is an alignment of the nucleotide sequences of the complete mtr locus possessed by gonococcal strains FA19, CDC2, and three clinical isolates studied by Wadsworth et al. (30). Nucleotides of note in CDC2 that differ from FA19 in either coding or noncoding regions are highlighted in red. The Correia element (CE) in strain GCGS0276 is highlighted in gray. (B) Shown is a phylogenetic tree for the mtr loci possessed by gonococcal strains FA19, CDC2, GCGS0276, GCGS0402, and GCGS0834. Download FIG S1, PDF file, 0.1 MB (124KB, pdf) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Gonococcal clinical strains and mtrD::kan mutants used in this study. Download Table S1, PDF file, 0.01 MB (8.6KB, pdf) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Genetic derivatives used in this study. Download Table S2, PDF file, 0.01 MB (11.7KB, pdf) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Shown are levels of the MtrR (A) and MtrE (B) proteins in whole-cell lysates of gonococcal strains as determined by Western immunoblotting. The eight CDC alert strains are shown with a strain number. Included in these blots are lysates from WT strain FA19 and transformant strains JF1 and KH15 that lack MtrR due to deletion of the gene (JF1) or a single-base-pair deletion in the mtrR promoter that abrogates mtrR gene expression and elevates mtrCDE expression. An accompanying CBB-stained gel is shown in panel C. Download FIG S2, TIF file, 16.2 MB (16.6MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
(A). Shown are results from a primer extension experiment that identified the mtrC TSS in gonococcal strains FA19 and CDC2. The nucleotide sequence from the noncoding strand is shown adjacent to the autoradiogram with the start sites highlighted in red. (B) Shown are the nucleotide sequences of the mtrCDE promoter region from strains FA19 and CDC2 with the G nucleotide change (CDC2) in the −35 hexamer shown in green and the TSS sites highlighted by red asterisks. Download FIG S3, TIF file, 13.0 MB (13.4MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Shown is the strategy used to construct CR.103. Three regions of mtrD from CDC2 were amplified by PCR. The oligonucleotide primers and the length of the products are shown. These PCR products were used to transform strain CR.100 for resistance to 1 µg/ml of Azi. The region of recombination in strain CR.103 is shown by the blue rectangle. Download FIG S4, TIF file, 16.8 MB (17.2MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Sequences of oligonucleotide primers. Download Table S3, PDF file, 0.02 MB (16KB, pdf) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.