Abstract
Administration of anesthetic agents fundamentally shifts the responsibility for maintenance of homeostasis from the patient and their intrinsic physiological regulatory mechanisms to the anesthesiologist. Continuous delivery of oxygen and nutrients to the brain is necessary to prevent irreversible injury and arises from a complex series of regulatory mechanisms that ensure uninterrupted cerebral blood flow. Our understanding of these regulatory mechanisms and the effects of anesthetics on them has been driven by the tireless work of pioneers in the field. It is of paramount importance that the anesthesiologist shares this understanding. Herein, we will review the physiological determinants of cerebral blood flow and how delivery of anesthesia impacts these processes.
Keywords: Cerebral blood flow, anesthetics, cerebral metabolism, neuroprotection, autoregulation
Introduction
Utilizing 10-fold more oxygen than would be predicted based on its weight the human brain is one of the most energetically dense organs in the body.1 This feature is unique to humans and, in part, is a consequence of the complexity of tasks, such as language, tool use, and social relationships, that the organ performs.2,3 As the brain has relatively little capacity for energy storage, a continuous supply of oxygen and nutrients must be delivered by uninterrupted cerebral blood flow (CBF).4 Multifaceted physiological regulatory mechanisms of CBF exist to ensure that this process occurs throughout the life of the organism. Disruption of CBF by disease, trauma, or iatrogenic causes can give rise to profound irreversible injury in the form of stroke.
Surgery is a form of controlled trauma that occurs concurrently with the establishment of some type of anesthesia. Neurosurgical interventions directly traumatize vulnerable tissues. Anesthetic agents either directly impact the primary functions of the brain and CBF secondarily and/or indirectly alter the hemodynamic factors that contribute to CBF. It is paramount that the anesthesiologist be cognizant of these effects to facilitate surgical procedures and to avoid undue iatrogenic injury during the perioperative period.
In this review, we will discuss the physiological mechanisms responsible for maintaining CBF homeostasis and their interplay with anesthetic agents. Special attention will be given to the contributions of Dr. Richard J Traystman, former editor-in-chief of the Journal of Cerebral Blood Flow and Metabolism, to our understanding of this field.
Physiological determinants of CBF
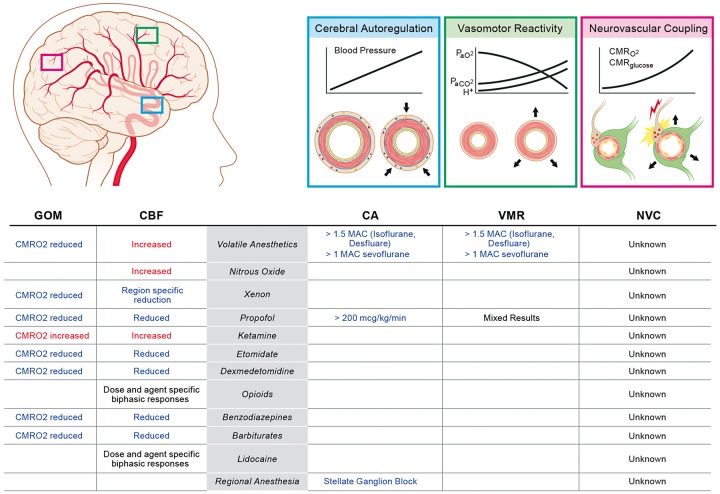
Prior to an examination of the effects of anesthetics on CBF, we will review the physiological mechanisms that define flow. Under conditions of normothermia and normoxia, CBF must remain at 50–60 ml/100 g/min to meet the metabolic demands of the functioning brain, with women having slightly higher flow rates.5,6 Reserve blood flow exists to a point, but ischemic injury generally occurs once CBF drops below 22 ml/100 g/min, although concurrent pathology such as traumatic brain injury (TBI) or hypothermia can change this threshold.7 Regulation of CBF is the result of a host of complex and incompletely described intra- and inter-cellular signaling events. The regulatory mechanisms that preserve CBF can broadly be categorized into cerebral autoregulation (CA), neurovascular coupling (NVC), and vasomotor reactivity (VMR).8 These mechanisms will be reviewed here in brief; for additional details the reader is directed to dedicated reviews of the subject.9–12 While categorization of these regulatory mechanisms as discrete entities provides a useful conceptual framework for this discussion, it should be recognized that significant overlap exists between these mechanisms.
Cerebral autoregulation
In response to any number of extrinsic and intrinsic stimuli, systemic blood pressure can rise or fall dramatically on a timeframe of seconds to minutes to hours, days, and years. Autoregulation of blood flow exists to ensure that perfusion of vital organs remains intact and stable across the dynamic range of pressures the vascular bed is exposed to. CA can broadly be defined as a proportional change in cerebral vascular resistance in response to changes in perfusion pressure to maintain a constant blood flow (Figure 1, blue inlay).13 Although the result of the processes that give rise to CA is measurable in clinical and laboratory settings, a full understanding of the processes themselves has remained elusive.
Figure 1.
Summary of the effects of anesthetic agents on global oxidative-metabolism (GOM) and cerebral blood flow (CBF) as well as the endogenous regulatory mechanisms such as cerebral autoregulation (CA), vasomotor reactivity (VMR) and neurovascular coupling (NVC). See text for additional details.
The range of mean arterial pressure (MAP) the CA mechanism can respond to was first described in humans by Lassen in 1959. In his seminal work, Lassen reviewed previous reports of CBF measured in 376 different individuals at 11 different MAP states which were established by normal physiology, administration of vasoactive agents, or systemic disease.14 From this work, the “autoregulation curve” was generated, which depicts a plateau region wherein CBF is stable across an MAP range of ∼50–160 mmHg (Figure 2(a)). Since its first inception, the nature of the autoregulation curve has been used to guide intra-individual clinical management of patients. This practice assumes that the static inter-individual continuum reported by Lassen can be applied to the physiological experience of singular patients.
Figure 2.
Evolving understanding of the relationship between MAP and CBF. (a) Inter-individual static cerebral autoregulatory curve (adapted from Lassen14). (b) Intra-individual static cerebral autoregulatory curve (adapted from Numan et al.15). (c) Dynamic cerebral autoregulatory curve (adapted from Tan17).
MAP: mean arterial pressure; CBF: cerebral blood flow.
In the last three decades with the advent of non-invasive methodologies, such as transcranial doppler (TCD) ultrasonography which allows for the measurement of CBF velocity in real time, the validity of this assumption has been called into question. This technology has made it possible to evaluate the intra-individual relationship between CBF and MAP. Utilizing technology which allows for real-time evaluation of the CBF in singular individuals across a range of systemic blood pressures an autoregulatory curve quite unlike Lassen's has been described. Analysis of individual static CA relationships from multiple studies indicate that increases in cerebral vascular resistance provide effective CA when systemic blood pressure is increased in most individuals, whereas the decreases in cerebral vascular resistance during decreases in MAP are usually insufficient to maintain CBF perfectly constant (Figure 2(b)).15
Evaluation of CBF in real time has also revealed that cerebrovasculature can respond rapidly to fluctuations in perfusion pressure and that these adaptations persist. This observation has led to the development of the concept of dynamic CA, i.e. changes in CBF occurring in response to MAP alteration over a timeframe of seconds to minutes. Dynamic CA is in contrast to static CA which arises over minutes to hours to days.16 When a dynamic CA paradigm is used to generate an intra-individual autoregulatory curve, a frequency-dependent narrow plateau region is observed (Figure 2(c)).17 The significance of this observation to the practice of anesthesiology is unknown, but with the availability of non-invasive technologies which allow for real-time monitoring of CBF surrogates, it is now possible to perform the clinical studies necessary to make this determination.
Application of CBF-surrogate monitoring in real time to evaluate CA may be of interest to anesthesiologists and intensivists during states of suspected neurological stress due to iatrogenesis or disease. During non-pulsatile cardiopulmonary bypass (CPB) wherein the time-domain fluctuation of MAP is reduced from seconds to minutes, it has been found that the intra-individual lower limit of the CA is incredibly variable and unpredictable.18 Further, hemodynamic management during CPB guided by CBF-surrogate monitoring may result in a reduction in injury.19 Additionally, application of the technology has revealed that CA is preserved during hypothermia.20
Acute regulation of vascular tone generally is mediated by the autonomic nervous system (ANS); however, the contribution of the ANS to CBF and CA is obscure. Cerebral vasculature is known to be highly innervated by the ANS, and postsynaptic adrenergic receptors are present on vascular smooth muscle.21 While the presence of these elements would suggest that the ANS regulates CBF, early work by Traystman and Rapela demonstrated that direct stimulation of the stellate ganglion produces minimal reduction of CBF.22 Similarly, denervation does not alter resting CBF and only modestly reduces the upper limit of the autoregulatory curve.23 Further, both vasoconstriction and vasodilation have been observed following direct application of catecholamines to isolated cerebral artery preparations.24–26 While controversial, an emerging consensus is that the contribution of the ANS to CA is minimal in comparison to the intrinsic mechanisms which establish cerebral vascular tone.27–31
The intrinsic mechanisms of the cerebral vasculature to set its myogenic tone have been intensely studied. The vascular smooth muscle, endothelium, and neighboring neurons and astrocytes, collectively referred to as the neurovascular unit, play direct and modulatory roles in establishing myogenic tone.32 Smooth muscle of the cerebral vasculature is able to respond to transmural pressure gradients to establish a resting tone matched to the luminal pressure. Mechano-sensitive transient receptor potential (TRP) channel family members present throughout the cerebral vasculature have recently been identified as a component of the pressure detection mechanism of the neurovascular unit.33 These channels respond to stretch and/or shear forces resulting in cation conductance ultimately leading to vasoconstriction.34,35 The remaining components of the neurovascular unit modulate the myogenic tone established by the vascular smooth muscle.
In addition to mediating vasoconstriction by vascular smooth muscle, TRP channel activity on vascular endothelium may lead to smooth muscle relaxation. In isolated cerebral vascular endothelium, activation of TRP channels results in the generation of nitric oxide.36 Nitric oxide has long been recognized as one of the endothelium-derived relaxation factors in the cerebral vasculature. Following up on work performed in small mammals, Traystman and his colleagues demonstrated that inhibition of nitric oxide synthesis results in increased cerebral vascular resistance and decreased CBF in piglets and non-human primates.37–39 Later work demonstrated similar activity in humans.40,41 The endothelium is also a source of vasodilatory arachidonic acid-derived prostanoids.42 Inhibition of prostanoid production reduces regional CBF and is synergistic with inhibition of nitric oxide production.43 These finding have led to the appreciation for the role of endothelium-derived nitric oxide and prostanoids in establishing the basal tone of the cerebral vasculature.
Astrocytes and neurons also modulate the tone of surrounding vasculature. Astrocytes are a source of arachidonic acid-derived epoxyeicosatrienoic acids which when released diffuse to vascular smooth muscle and mediate vasodilation through membrane hyperpolarization.44 Neurons also produce and release vasodilatory prostaglandins and nitric oxide.45 It should be recognized though that additional, as yet incompletely described, mechanisms must also contribute to CA. Systemic application of calcium channel antagonist which negates ion channel-dependent regulation of myogenic tone does not ablate compensation for orthostatic perturbations of blood pressure.46 Also, disruption of the endothelium secondary to chronic disease which would inhibit mechanical signal transduction does not impair pressure-dependent autoregulation.47
Neurovascular coupling
NVC describes the modulation of regional CBF to meet the metabolic demands of neural activity (Figure 1, pink inlay). This process occurs at the level of the cerebral microvasculature of plial arterioles outside the pia mater and penetrating parenchymal arterioles. The mechanism by which the tone of these vessels is matched to the needs of the surrounding neurons is complex and involves all members of the neurovascular unit. In response to excitatory glutamatergic inputs, active neurons release nitric oxide which diffuses to nearby vascular smooth muscle and promotes dilation.48 Adenosine produced from extracellular conversion of neuron- and astrocyte-derived ATP diffuses to vascular smooth muscle and induces changes in vascular tone.49–52 Neuron-derived prostaglandins also influence vascular tone.53,54 Prostaglandin E2 is produced by active pyramidal neurons and acts on EP2 and EP4 receptors present on cerebral vascular smooth muscle resulting in vasodilation.55 Additionally, GABAergic interneurons release numerous vasoactive mediators directly onto vascular smooth muscle.56 Metabolically active astrocytes which envelope the parenchymal arterioles also mediate the tone of the vascular smooth muscle.55 Astrocytes are a source of vasodilatory epoxyeicosatrienoic acids released during cortical activation.57,58 Astrocytes also modulate vascular tone through release of vasodilatory K+ and Ca2+ to the perivascular space.59,60 Finally, in response to neuronal activity, the endothelium itself propagates vasodilatory signals throughout the vasculature of active cortical regions.61 Collectively, these mechanisms allow for modulation of CBF that is temporally and spatially correlated with neuronal activity. Vessels surrounding active neurons to a distance of 0.5–1 mm respond within ∼ 1 s to the onset of neuronal activity to modulate CBF.62
Vasomotor reactivity
The cerebral vasculature tone is influenced by changes in arterial blood CO2 and, to a lesser extent, O2 tension through a process referred to here as VMR (Figure 1, green inlay).63 VMR to CO2 is stronger in the brain compared to other organs.64 Both plial arterioles and large caliber cerebral vessels respond.65 Alteration of cerebral vascular tone in response to changes in PaCO2 is an intrinsic property of the vasculature and occurs independent of adrenergic activity despite extensive innervation by the sympathetic nervous system.66 The mechanism responsible for cerebral vasodilation and vasoconstriction in response rising and falling PaCO2, respectively, is incompletely understood. Nitric oxide production is dispensable for VMR.67 Early studies indicated that alteration of extracellular pH due to diffusion of CO2 out of or into the vasculature is the dominant mechanism of VMR to CO2.68,69 However, more recent work suggests that transient changes in vascular smooth muscle intracellular pH also can modulate the response.70 This mechanism establishes a roughly linear relationship between CBF and acute PaCO2 changes with a 1–6% change in CBF for every 1 mmHg across a range of 20–80 mmHg.71 Adaptation to chronic alteration of PaCO2 occurs and CBF will tend to normalize over a 6–8-h period.72 Acute changes in CO2 tension impact the CA mechanism with loss of eucapnia, leading to a narrowing of the CA plateau.73 While impaired VMR to changes in PaCO2 is not predictive of stroke risk, it does correlate with increased mortality.74,75 This may indicate that impaired VMR is a symptom of systemic vascular disease which itself is the most detrimental pathology.
To guard against injury due to hypoxia or hyperoxia CBF is modulated such that oxygen delivery expressed by the product of CBF and CaO2 is roughly constant over PaO2 range of 30–430 mmHg.76 Vasodilation occurs independent of peripheral chemoreceptors and may be accompanied by a small increase in CMRO2, although not all studies detect this increase.76,77 Under hypoxic conditions, the hypocapnic cerebral vasoconstriction activity is attenuated.78 This prevents ischemic injury from arising due to hyperventilation-induced hypocapnia associated with hypoxic ventilatory drive.
Anesthetic agents and CBF
Anesthetic agents affect the dynamics of the cerebral vasculature through direct effects on the vessels as well as modulation of the endogenous regulatory mechanisms (Figure 1). In addition to impacting the delivery of oxygen and nutrients to metabolically active neurons, alteration of cerebral vessel dynamics by anesthetic agents can influence the tissue composition encountered during neurosurgical intervention. For these reasons, the anesthesiologist must have a full understanding of the effect of their interventions on the cerebral vasculature. Additionally, intrinsic neuroprotective properties have been attributed to anesthetic agents. The concept of neuroprotective intervention encompasses therapies which favorably shift the balance of cerebral oxygen supply and utilization as well as those that prolong survival in ischemic states. Where directly relevant neuroprotection will be discussed, in addition, the interested reader is directed to other recent reviews of this subject.79–82 Herein, emphasis is placed on the inclusion of studies conducted using human subjects as much as is made possible by the availability of data.
Volatile anesthetics
Modern halogenated volatile anesthetics, such as isoflurane, sevoflurane and desflurane, are thought to uncouple flow-metabolism matching. Suppression of CMRO2 is consistently observed in human and animal models exposed to volatile anesthetics using a host of assessment techniques. Assessment of CBF in humans exposed to volatile anesthetics using TCD and gas diffusion techniques generally report dose-dependent increases.83–86 However, magnetic resonance imaging (MRI)-based assessments reveal minimal changes in global CBF in humans, whereas non-human primates display dose-dependent increases.87–89 Further, conflicting data have been generated regarding sevoflurane with no change, increases and decreases in CBF being observed.88,90–96 The mismatch between CBF and oxidative metabolism likely arises from the direct effects of volatile anesthetics on cerebral vascular resistance.
Vasodilation mediated by volatile anesthetics occurs independent of the anesthetic depth and is the result of a direct effect on the cerebral vasculature.97 Compared to isoflurane, sevoflurane is a less potent cerebrovascular vasodilator when administered at MAC equivalent doses.98 A full understanding of the mechanism by which vasodilation occurs is being derived. ATP-sensitive K+ channels as well as increased production of endothelium-derived nitric oxide and prostanoids have been identified as being potentially modulated by volatile anesthetics.43,99,100
CA remains intact during isoflurane and desflurane administration at 1 MAC, but above 1.5 MAC CBF is pressure-passive with respect to MAP.101,102 However, concurrent hypercapnia does induce blockade of CA during administration of therapeutic doses of volatile anesthetic.103 Conversely, hypocapnia restores CA during supratherapeutic isoflurane administration.104 Sevoflurane at therapeutic doses has been reported to narrow the plateau region of the autoregulatory curve.105 VMR is preserved during isoflurane administration unless supratherapeutic doses are administered.106 Conversely, vasodilation due to hypercapnia as well as vasoconstriction due to hypocapnia are blunted by sevoflurane.107
Volatile anesthetics have long been postulated to act as neuroprotective agents in the setting of cerebral hypoperfusion. Investigations in small animals and non-human primates suggest that use of volatile anesthetics could limit injury following experimentally induced ischemia.79 Retrospective clinical studies have produced data to suggest the same. Isoflurane use during carotid endarterectomy appears to decrease the critical CBF below which electroencephalogram (EEG) evidence of injury arises.108 Similarly, desflurane administration preserves cerebral oxygenation during temporary cerebral artery clipping.109 However, in specific circumstances such as the neonatal period, toxicity attributable to volatile anesthetics has been described in preclinical trials.110,111 In the absence of results from prospective randomized controlled trials, it is premature to conclude that sufficient evidence exists to support the notion that volatile anesthetics are neuroprotective or neurotoxic agents in humans.
Nitrous oxide
Nitrous oxide is the oldest anesthetic gas still in regular use. When inhaled, it produces dose-dependent analgesia, dissociative amnesia, anxiolysis, and somnolence. Due to its low potency at atmospheric pressures, a state general anesthesia cannot be induced by nitrous oxide alone. Under hyperbaric conditions, general anesthesia can be achieved by nitrous oxide alone; however, this is associated with significant hemodynamic derangement.112 Numerous biological targets for nitrous oxide have been described with antagonism of the NMDA receptor thought to be responsible for its anesthetic properties.113 The efficacy of nitrous oxide as part of the anesthesia provided to neurologically vulnerable patients has been contested.
Rapid agent elimination following surgical intervention is desirable to allow for early clinical assessment in the postoperative period, but this feature of nitrous oxide is not widely accepted to outweigh the potential risks associated with its use.114 Classical teaching is that nitrous oxide increases CBF, CMRO2, intracranial pressure (ICP), and moves into the potential space created by surgically induced pneumocephalus or could worsen venous air embolism and should therefore be avoided. Data supporting this teaching come from studies to model the use of nitrous oxide as a MAC sparing agent in general anesthetic states established by other agents. The varying nature of these models may give rise to misunderstanding of the intrinsic activity of nitrous oxide. When administered as a single agent, nitrous oxide results in increased CBF and no change or decreased CMRO2.115–119 However, when used as an adjunct to other anesthetics, the addition of nitrous oxide has been reported to enhance, blunt, or have no effect on CBF and CMRO2.120–127 Further complicating this picture is the observation that the temporal relationship between agent exposure and outcome is inconsistent.128 VMR remains intact when nitrous oxide is administered alone or in combination with other anesthetic agents.129
Data on outcomes associated with administration of nitrous oxide to neurologically vulnerable patients come from retrospective analysis of the IHAST trial.130 When nitrous oxide was used as part of general anesthesia provided to patients undergoing surgical repair of ruptured subarachnoid hemorrhage, there were no long-term neurological deficits attributable to the drug.131 Subgroup analysis of patients who underwent temporary occlusion of major cerebral vessels as part of the surgical intervention did reveal an increase in the occurrence of neurological deficits in the immediate perioperative period associated with nitrous oxide exposure; however, this association was no longer present three months following surgery.132 These results have led to a renewed interest in the use of nitrous oxide as part of the anesthesia provided during neurosurgical interventions.129
Xenon
A noble gas naturally occurring at low concentration in Earth's atmosphere, 0.0875 ppm, xenon been recognized as an agent able to achieve general anesthesia since the 1940s.133,134 The mechanism by which xenon induces a general anesthetic state is believed to arise from inhibition of NMDA receptors by tight association with the glycine-binding site.135,136 Xenon has features of an ideal anesthetic including rapid induction of and emergence from a general anesthetic state following initiation and discontinuation of administration, hemodynamic neutrality, and neuroprotective properties.137,138 Scarcity, cost of production, and competition with use in industrial applications all limit clinical use of xenon.
Studies conducted to examine the effect of xenon on CBF and CMRO2 using rodent, porcine, and non-human primate systems have produced conflicting results. In an elegant study by Laitio et al., regional CBF was measured in humans under general anesthesia induced and maintained by 1 MAC of xenon alone. In this study, adjunct remifentanil was used at the time of intubation in response to signs of responsiveness to laryngoscopy; however, a period of 10 remifentanil half-lives was allowed to pass prior to measurement of CBF by 15O-H2O positron emission tomography (PET) Laitio et al. found that areas of dense concentrations of soma, the cortex, cerebellum, and thalamus experience an overall reduction in blood flow, whereas structures arising from axon projections, white matter tracts, had an increase in blood flow during xenon administration.139 In a follow-up study, this group was able to confirm that reduction of CBF correlated with reduction of CMRO2.140 These findings are largely in concordance with those reported by Rex et al.141,142 The matched reduction in CBF and CMRO2 induced by xenon is in stark contrast to other NMDA receptor antagonists, such as nitrous oxide and ketamine which, as discussed above and below, increase CBF.
Propofol
Propofol is an intravenous anesthetic that achieves dose-dependent sedation or general anesthesia. The mechanism by which reduced consciousness occurs is by a direct interaction between propofol and the GABAA receptor leading to potentiation of the receptor activity.143,144 Compared to volatile anesthetics delivered at Bispectral Index (BIS)-equivalent equipotent doses, propofol causes significant reduction of CBF and similar decreases in CMRO2.122,145 The mechanism by which CBF reduction occurs is thought to arise from intact flow-oxidative metabolism coupling as vasodilation is observed when propofol is applied directly to vessels in in vitro preparations.146 CA remains intact during propofol administration up to doses of 200 µg/kg/min.102 An early study using equipotent doses of propofol and sevoflurane titrated to BIS suggested that propofol blunts reactivity VMR.147 However, recent studies using NIRS spectroscopy have demonstrated that reactivity to hypocapnia secondary to hyperventilation is similar when either propofol or sevoflurane is used to maintain general anesthesia.148–150
Evidence for neuroprotection that may be attributable to propofol comes from the prospective SIESTA and Goliath trials which suggest an association between general anesthesia maintained with propofol and improved neurological outcome following endovascular clot retrieval for stroke.151,152 However, neither trial was structured to investigate an agent-specific effect, rather a state-specific effect, sedation versus general anesthesia, was the primary comparison of interest for these studies. A single agent-specific study exists wherein propofol administration was titrated to burst suppression on EEG during cardiac valve surgery; this study failed to demonstrate improved neurological outcomes following propofol administration.153 Interpretation of the significance of this study is difficult, as burst suppression is itself thought to give rise to adverse neurological outcomes.154 Additional prospective studies examining the administration of therapeutic doses of propofol are likely indicated at this time.
Ketamine
Ketamine is a phenylcyclidine derivative that when administered intravenously or intramuscular induces a state of general anesthesia with preservation of respiratory drive. In the catecholamine-replete patient, ketamine induces a weak and transient release of endogenous stores which counteracts the direct myocardial depressant effect of the drug. While many molecular targets for ketamine have been described, inhibition of the NMDA receptor is thought to give rise to its anesthetic properties. Enthusiasm for use of ketamine in the neurologically compromised patient was tempered by early preclinical reports of increased CMRO2 and small case series documenting elevated ICP associated with its use.155–159 However, recent studies conducted in human reveal that when used in conjunction with usual modern anesthetic practices, ketamine administration does not result in increased ICP.160 Alteration of CMRO2 in humans is minimal and is likely a consequence of increased metabolite supply rather than simply increased utilization.161,162 In addition, a recent meta-analysis of available human studies has demonstrated that ketamine administration increases CBF.163 Ketamine-mediated increase of CBF appears to arise from direct vasodilation of medium cerebral vessels.164 There is renewed interest in ketamine use due to the neuroprotective properties attributed to the drug. A full discussion of these properties is beyond the scope of this review; for additional information the reader is directed to an excellent recent review article on the subject.165
Etomidate
Etomidate is an imidazole derivative synthesized in the early 1970s and originally intended for use as an antifungal agent prior to appreciation of its sedative-hypnotic effects. When administered as a single bolus or continuous infusion, etomidate achieves sedation through potentiation and direct activation of synaptic and extrasynaptic GABA receptors.166 Unique among anesthetic agents, administration of etomidate is not associated with hemodynamic depression. Using animal models of focal and global ischemia, reports have described etomidate as having neuroprotective properties.167,168 Etomidate administration to humans has been shown to result in reductions of ICP, CBF, and CMRO2.169–172 Reduction of CBF is speculated to arise secondarily from reduction of CMRO2 as well as direct vasoconstrictive properties of the drug itself possibly through inhibition of nitric oxide signaling.170,173,174
Due to its unique hemodynamic neutrality, an argument has been advanced for use of etomidate in the context of vulnerable cerebral perfusion in place of barbiturates.175 However, administration during cerebral aneurysm clipping procedures and temporary vessel occlusion has revealed that tissue hypoxia attributable to etomidate occurs.109,176 Further, retrospective analysis of the data from the IHAST trial did not demonstrate an association between etomidate administration and neurological outcome following temporary vessel clipping.177 Finally, reduced cerebral oxygenation has been observed following administration of induction doses of etomidate administered to patients with intact cerebral vasculature.178 These results suggest that cerebral vasoconstriction and CBF reduction mediated by etomidate are not matched by reduction of CMRO2 and the presumption that neuroprotection arises in ischemic states is questionable.
Dexmedetomidine
Dexmedetomidine (Dex) is a centrally acting α2-adrenergic agonist that achieves sedation, anxiolysis, and analgesia without concurrent respiratory depression.179,180 Dex administered by continuous infusion is used as a primary sedative in the ICU setting or for procedural sedation and has been found to have opioid sparing effects when used as an adjunct for intracranial procedures.181–184 Dex reduces CBF possibly through direct activation of postsynaptic α2-adrenergic receptors located on cortical vasculature resulting in vasoconstriction.185–187 Clonidine, another α2-adrenergic agonist, also reduces CBF.188 In addition, Dex administration results in a reduction in CMRO2.189 In humans, the reduction in CBF induced by Dex occurs without producing significant alteration of cerebral oxygenation.186,189–191 However, concurrent hypoxia or systemic hypotension may result in impaired cerebral oxygenation during Dex administration.185,192 These results raise cause for caution with the use of Dex as part of an anesthetic for the patient with hemodynamic instability.
Opioids
As a class, opioids have generally been considered to be neutral in regards to alteration of CBF and CMRO2. Studies supporting this belief were generated using low-resolution detection techniques, such as TCD wherein flow velocity through the MCA is used as a surrogate for global CBF without consideration for regional differences. High-resolution studies using MRI and PET-based detection techniques have revealed that regional CBF is affected by opioids and use of class-wide generalities may incorrect. Fentanyl administration to opioid-naive humans has been found to increase regional CBF in areas of the prefrontal cortex and caudate.193,194 Morphine and hydromorphone have been found to have similar effects.195,196 Conversely, sufentanil and remifentanil administration have a biphasic effect on regional CBF with initial increases at low to moderate doses (<0.15 µg/kg/min remifentanil) followed by dose-dependent decreases in CBF and CMRO2 at supratherapeutic doses (>2 µg/kg/min remifentanil) under normocapnic conditions in brain regions known to be involved in pain processing.197–200 VMR appears to be intact during remifentanil infusion.201,202 Alteration of regional CBF has been speculated to be reflective of the mechanisms giving rise to the analgesic and euphoric properties of the drugs.195,203
Outcome studies comparing the use of different opioids in conjunction with propofol-based anesthetics have not revealed a difference in dreaded perioperative complications attributable to opioid choice. However, these studies do suggest a more rapid recovery following discontinuation of anesthesia when remifentanil is used, although this effect is likely attributable to remifentanil's unique pharmacokinetic profile rather than arising from differences in intraoperative cerebrovasculature dynamics.204–206 The lack of dreaded complications observed suggests that the clinical significance of the alteration of regional CBF induced by different opioids is small or non-existent. Additionally, neuroprotective properties have been attributed to opioid receptor subtype-specific agnonists.207,208 It is possible that this activity arises from preservation of CA and VMR in the face of ischemic injury.209,210 In addition, preclinical data suggest that endogenous opioids may play a protective role following TBI.211 However, at present, no opioid receptor-specific agonist agent has been evaluated in clinical trials for therapeutic efficacy in ischemic states or TBI.
An additional point of note is the finding that administration of the opioid receptor antagonist, naloxone, has been found to result in decreased CBF.212 Naloxone and related opioid receptor antagonists have been evaluated in the context of stroke injury, but efficacy has not been demonstrated.213–215 Neuroprotective properties have been attributed to naloxone in the context of incomplete spinal cord injury.216 This effect may arise from preservation of blood flow to the spinal cord through alteration of the expression of mediators of vasogenic tone.217,218 It is possible that the CBF alteration and/or induction of a neuro-protected state that occurs following administration of exogenous opioids or opioid receptor antagonists arises from competition with endogenous opioids or alteration of opioid receptor signaling in a subtype and location-specific fashion.
Benzodiazepines
Benzodiazepines are commonly used to achieve anxiolysis, amnesia, and sedation during conscious sedation and, when administered at high doses, induce general anesthesia through potentiation of GABA receptors. Acute administration of benzodiazepines results in dose-dependent matched reductions in CBF and CMRO2.219–222 Enhancement of GABAergic activity seems to generally cause a reduction in CBF as, like benzodiazepine, the non-benzodiazepine GABA receptor modulator zolpidem has similar effects.223 Studies assessing the cerebrovascular response to benzodiazepine administration have generally been performed in healthy volunteers naive to the drug following acute administration. A single study has investigated the relationship between chronic benzodiazepine use and CBF and revealed development of tolerance to the CBF reduction effects.224 Regional CBF alteration has been observed with the largest declines in areas of the brain involved in memory formation, attention, and arousal; while the location of these changes correlates with the areas of the brains associated with the clinical effects observed following administration of benzodiazepines, a causal relationship has not yet been established.225,226 Interestingly, unlike the sedative effects, the reduction of CBF following midazolam administration is not reversed by doses of flumazenil, an observation that may be explained by direct vasoconstriction mediated by flumazenil.227,228 This effect though is controversial as some reports describe a recovery of CBF following administration of flumazenil following midazolam.222,229
Barbiturates
While short-acting barbiturates (e.g. thiopental) are no longer available for clinical use in the United States, a discussion on barbiturates is included here for completeness sake. With the exception of methohexital use in electroconvulsive therapy, thiopental was the only barbiturate used to induce and maintain general anesthesia in modern anesthetic practice. Unconsciousness occurs secondary to direct activation and potentiation of the GABAA receptor by barbiturate binding.230 Barbiturate administration results in reduced CBF and CMRO2 with preservation of CA and blunting of VMR.231–234 Many studies using small and large animal models of cerebral ischemia have demonstrated neuroprotection attributable to barbiturate administration.235 Thiopental has been subject to many clinical trials aimed at determining if its neuroprotective properties are clinically accessible. The results of these studies have been mixed and if beneficial affects are present, the timing of thiopental administration surrounding the onset of ischemia may be crucial.236 Additionally, retrospective analysis of data generated in the IHAST study demonstrated no association between thiopental administration and neurological outcome.177
Lidocaine
While not a general anesthetic agent itself, lidocaine is often administered during induction of general anesthesia at doses known to effect CBF. Data supporting this practice come from the observations that lidocaine administration blunts the pain associated with propofol injection and limits hemodynamic alteration during laryngoscopy.237,238 In humans, a moderate bolus (0.5 mg/kg) of lidocaine increases regional CBF whereas a large dose (5 mg/mg) results in modest reduction of CBF and CMRO2.239–241 In addition, bolus administration is associated with reduced ICP.242 No studies exist which have examined the effects of lidocaine on CBF when administered with other agents during induction of anesthesia. Studies conducted in small mammals suggest that lidocaine may have neuroprotective properties during threatened CBF.243–245 Lidocaine has been examined in humans in the context of post-operative cognitive dysfunction occurrence; these studies have produced conflicting results, and at this time, no uniform body of evidence exists to define the clinical utility of the drug in this context.246–249
Regional anesthesia
As certain blocks alter intracranial sympathetic inputs, the possibility exists for disruption of CBF secondary to alteration of incoming supply. While contribution of the ANS to CBF is controversial, it has been found that direct stellate ganglion blockade results in a modest increase in CBF prominent on the side ipsilateral to the block.28 Interscalene block has been found not to result in significant change to CBF.250 Interestingly, afferent inputs during spontaneous movement increase regional CBF and peripheral regional blocks blunt this response.251–253
Conclusions
Regulation of CBF to ensure uninterrupted delivery of oxygen and nutrients is necessary for the prevention of irreversible ischemic injury to the brain. Through the work of pioneers in the field like Dr. Richard J Traystman, we are beginning to understand not just how the CBF regulatory mechanisms function but also how therapeutics interact with these processes. Clinical studies informed by this understanding are ongoing. While no single agent has yet been identified as being the “silver bullet” for neuroprotection, there is cause for hope. Reason for this hope was best expressed by Dr. Traystman himself when he wrote,
(a)nesthetics have shown neuroprotective potential, but thus far, no single leader has come forward despite much research in this area. While it would be easy to give up looking for an anesthetic neuroprotective agent considering this poor track record, the lure of the benefit to patients of actually finding a neuroprotective agent is great. I say, we keep looking and be optimistic. It is just a matter of time!235
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Quastel JH, Wheatley AH. Oxidations by the brain. Biochem J 1932; 26: 725–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoenemann PT. Evolution of the size and functional areas of the human brain. Annu Rev Anthropol 2006; 35: 379–406. [Google Scholar]
- 3.Seymour RS, Bosiocic V, Snelling EP. Fossil skulls reveal that blood flow rate to the brain increased faster than brain volume during human evolution. Royal Soc Open Sci 2016; 3: 160305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 2011; 14: 724–738. [DOI] [PubMed] [Google Scholar]
- 5.Rostrup E, Knudsen GM, Law I, et al. The relationship between cerebral blood flow and volume in humans. Neuroimage 2005; 24: 1–11. [DOI] [PubMed] [Google Scholar]
- 6.Satterthwaite TD, Shinohara RT, Wolf DH, et al. Impact of puberty on the evolution of cerebral perfusion during adolescence. Proc Natl Acad Sci USA 2014; 111: 8643–8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velly L, Bruder N. Cerebral metabolism and function. In: Ichai C, Quintard H, Orban J-C. (eds). Metabolic disorders and critically ill patients: from pathophysiology to treatment, Cham: Springer International Publishing, 2018, pp. 285–300. [Google Scholar]
- 8.Tzeng YC, Panerai RB. CrossTalk proposal: dynamic cerebral autoregulation should be quantified using spontaneous blood pressure fluctuations. J Physiol 2018; 596: 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willie CK, Tzeng YC, Fisher JA, et al. Integrative regulation of human brain blood flow. J Physiol 2014; 592: 841–859. [DOI] [PMC free article] [PubMed]
- 10.Armstead WM. Cerebral blood flow autoregulation and dysautoregulation. Anesthesiol Clin 2016; 34: 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips AA, Chan FH, Zheng MM, et al. Neurovascular coupling in humans: physiology, methodological advances and clinical implications. J Cereb Blood Flow Metab 2016; 36: 647–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McBryde FD, Malpas SC, Paton JF. Intracranial mechanisms for preserving brain blood flow in health and disease. Acta Physiol (Oxf) 2017; 219: 274–287. [DOI] [PubMed] [Google Scholar]
- 13.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev 1990; 2: 161–192. [PubMed] [Google Scholar]
- 14.Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev 1959; 39: 183–238. [DOI] [PubMed] [Google Scholar]
- 15.Numan T, Bain AR, Hoiland RL, et al. Static autoregulation in humans: a review and reanalysis. Med Eng Phys 2014; 36: 1487–1495. [DOI] [PubMed] [Google Scholar]
- 16.Tzeng Y-C, Ainslie PN. Blood pressure regulation IX: cerebral autoregulation under blood pressure challenges. Eur J Appl Physiol 2014; 114: 545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan CO. Defining the characteristic relationship between arterial pressure and cerebral flow. J Appl Physiol (1985) 2012; 113: 1194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi B, Ono M, Brown C, et al. Predicting the limits of cerebral autoregulation during cardiopulmonary bypass. Anesth Analg 2012; 114: 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hori D, Nomura Y, Ono M, et al. Optimal blood pressure during cardiopulmonary bypass defined by cerebral autoregulation monitoring. J Thorac Cardiovasc Surg 2017; 154: 1590–1598 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goswami D, McLeod K, Leonard S, et al. Static cerebrovascular pressure autoregulation remains intact during deep hypothermia. Paediatr Anaesth 2017; 27: 911–917. [DOI] [PubMed] [Google Scholar]
- 21.Edvinsson L. Sympathetic control of cerebral circulation. Trends Neurosci 1982; 5: 425–429. [Google Scholar]
- 22.Traystman RJ, Rapela CE. Effect of sympathetic nerve stimulation on cerebral and cephalic blood flow in dogs. Circ Res 1975; 36: 620–630. [DOI] [PubMed] [Google Scholar]
- 23.Sadoshima S, Fujii K, Yao H, et al. Regional cerebral blood flow autoregulation in normotensive and spontaneously hypertensive rats – effects of sympathetic denervation. Stroke 1986; 17: 981–984. [DOI] [PubMed] [Google Scholar]
- 24.Edvinsson L, Owman C. Pharmacological characterization of adrenergic alpha and beta receptors mediating the vasomotor responses of cerebral arteries in vitro. Circ Res 1974; 35: 835–849. [DOI] [PubMed] [Google Scholar]
- 25.Edvinsson L, Aubineau P, Owman C, et al. Sympathetic innervation of cerebral arteries: prejunctional supersensitivity to norepinephrine after sympathectomy or cocaine treatment. Stroke 1975; 6: 525–530. [DOI] [PubMed] [Google Scholar]
- 26.Winquist RJ, Bohr DF. Characterization of the rat basilar artery in vitro. Experientia 1982; 38: 1187–1188. [DOI] [PubMed] [Google Scholar]
- 27.Ainslie PN, Brassard P. Why is the neural control of cerebral autoregulation so controversial? F1000Prime Rep 2014; 6: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ter Laan M, van Dijk JMC, Elting JWJ, et al. Sympathetic regulation of cerebral blood flow in humans: a review. Br J Anaesth 2013; 111: 361–367. [DOI] [PubMed] [Google Scholar]
- 29.Heistad DD, Marcus ML. Evidence that neural mechanisms do not have important effects on cerebral blood flow. Circ Res 1978; 42: 295–302. [DOI] [PubMed] [Google Scholar]
- 30.van Lieshout JJ, Secher NH. Point:Counterpoint: sympathetic activity does/does not influence cerebral blood flow. Point: sympathetic activity does influence cerebral blood flow. J Appl Physiol (1985) 2008; 105: 1364–1366. [DOI] [PubMed] [Google Scholar]
- 31.Strandgaard S, Sigurdsson ST. Point:Counterpoint: sympathetic activity does/does not influence cerebral blood flow. Counterpoint: sympathetic nerve activity does not influence cerebral blood flow. J Appl Physiol (1985) 2008; 105: 1366–1367; discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 32.Lok J, Gupta P, Guo S, et al. Cell-cell signaling in the neurovascular unit. Neurochem Res 2007; 32: 2032–2045. [DOI] [PubMed] [Google Scholar]
- 33.Brayden JE, Earley S, Nelson MT, et al. Transient receptor potential (TRP) channels, vascular tone and autoregulation of cerebral blood flow. Clin Exp Pharmacol Physiol 2008; 35: 1116–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zechariah A and Welsh DG. Chapter 15 – connecting TRP channels and cerebrovascular diseases A2. In: Szallasi A. (Ed), TRP channels as therapeutic targets. Boston: Academic Press, 2015, pp.263–277.
- 35.Longden TA, Hill-Eubanks DC, Nelson MT. Ion channel networks in the control of cerebral blood flow. J Cereb Blood Flow Metab 2016; 36: 492–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dragovich MA, Chester D, Fu BM, et al. Mechanotransduction of the endothelial glycocalyx mediates nitric oxide production through activation of TRP channels. Am J Physiol Cell Physiol 2016; 311: C846–C853. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka K, Gotoh F, Gomi S, et al. Inhibition of nitric oxide synthesis induces a significant reduction in local cerebral blood flow in the rat. Neuroscience Lett 1991; 127: 129–32. [DOI] [PubMed] [Google Scholar]
- 38.Greenberg RS, Helfaer MA, Kirsch JR, et al. Nitric oxide synthase inhibition with NG-mono-methyl-L-arginine reversibly decreases cerebral blood flow in piglets. Crit Care Med 1994; 22: 384–392. [DOI] [PubMed] [Google Scholar]
- 39.McPherson RW, Kirsch JR, Tobin JR, et al. Cerebral blood flow in primates is increased by isoflurane over time and is decreased by nitric oxide synthase inhibition. Anesthesiology 1994; 80: 1320–1327. [DOI] [PubMed] [Google Scholar]
- 40.Joshi S, Young WL, Duong DH, et al. Intracarotid Infusion of the nitric oxide synthase inhibitor, l-NMMA, modestly decreases cerebral blood flow in human subjects. Anesthesiology 2000; 93: 699–707. [DOI] [PubMed] [Google Scholar]
- 41.White RP, Deane C, Vallance P, Markus HS. Nitric oxide synthase inhibition in humans reduces cerebral blood flow but not the hyperemic response to hypercapnia. Stroke 1998; 29: 467–472. [DOI] [PubMed] [Google Scholar]
- 42.Moncada S, Gryglewski R, Bunting S, et al. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature 1976; 263: 663–665. [DOI] [PubMed] [Google Scholar]
- 43.Moore LE, Kirsch JR, Helfaer MA, et al. Nitric oxide and prostanoids contribute to isoflurane-induced cerebral hyperemia in pigs. Anesthesiology 1994; 80: 1328–1337. [DOI] [PubMed] [Google Scholar]
- 44.Koehler RC, Gebremedhin D, Harder DR. Role of astrocytes in cerebrovascular regulation. J Appl Physiol (1985) 2006; 100: 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H, Hitron IM, Iadecola C, et al. Synaptic and vascular associations of neurons containing cyclooxygenase-2 and nitric oxide synthase in rat somatosensory cortex. Cereb Cortex 2005; 15: 1250–1260. [DOI] [PubMed] [Google Scholar]
- 46.Tzeng YC, Chan GS, Willie CK, et al. Determinants of human cerebral pressure-flow velocity relationships: new insights from vascular modelling and Ca(2)(+) channel blockade. J Physiol 2011; 589: 3263–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lavi S, Gaitini D, Milloul V, et al. Impaired cerebral CO2 vasoreactivity: association with endothelial dysfunction. Am J Physiol Heart Circ Physiol 2006; 291: H1856–H1861. [DOI] [PubMed] [Google Scholar]
- 48.Faraci FM, Breese KR. Nitric oxide mediates vasodilatation in response to activation of N-methyl-D-aspartate receptors in brain. Circ Res 1993; 72: 476–480. [DOI] [PubMed] [Google Scholar]
- 49.Iliff JJ, D'Ambrosio R, Ngai AC, et al. Adenosine receptors mediate glutamate-evoked arteriolar dilation in the rat cerebral cortex. Am J Physiol Heart Circ Physiol 2003; 284: H1631–H1637. [DOI] [PubMed] [Google Scholar]
- 50.Dirnagl U, Niwa K, Lindauer U, et al. Coupling of cerebral blood flow to neuronal activation: role of adenosine and nitric oxide. Am J Physiol 1994; 267: H296–H301. [DOI] [PubMed] [Google Scholar]
- 51.Araque A, Carmignoto G, Haydon PG, et al. Gliotransmitters travel in time and space. Neuron 2014; 81: 728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dahl G. ATP release through pannexon channels. Philos Trans R Soc Lond B Biol Sci 2015; 370: 20140191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lecrux C, Toussay X, Kocharyan A, et al. Pyramidal neurons are “neurogenic hubs” in the neurovascular coupling response to whisker stimulation. J Neurosci 2011; 31: 9836–9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Niwa K, Araki E, Morham SG, et al. Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. J Neurosci 2000; 20: 763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lacroix A, Toussay X, Anenberg E, et al. COX-2-derived prostaglandin E2 produced by pyramidal neurons contributes to neurovascular coupling in the rodent cerebral cortex. J Neurosci 2015; 35: 11791–11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cauli B, Tong XK, Rancillac A, et al. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci 2004; 24: 8940–8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peng X, Carhuapoma JR, Bhardwaj A, et al. Suppression of cortical functional hyperemia to vibrissal stimulation in the rat by epoxygenase inhibitors. Am J Physiol Heart Circ Physiol 2002; 283: H2029–H2037. [DOI] [PubMed] [Google Scholar]
- 58.Higashimori H, Blanco VM, Tuniki VR, et al. Role of epoxyeicosatrienoic acids as autocrine metabolites in glutamate-mediated K+ signaling in perivascular astrocytes. Am J Physiol Cell Physiol 2010; 299: C1068–C1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Filosa JA, Bonev AD, Straub SV, et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci 2006; 9: 1397–1403. [DOI] [PubMed] [Google Scholar]
- 60.Dunn KM, Hill-Eubanks DC, Liedtke WB, et al. TRPV4 channels stimulate Ca2+-induced Ca2+ release in astrocytic endfeet and amplify neurovascular coupling responses. Proc Natl Acad Sci USA 2013; 110: 6157–6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen BR, Kozberg MG, Bouchard MB, et al. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc 2014; 3: e000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berwick J, Johnston D, Jones M, et al. Fine detail of neurovascular coupling revealed by spatiotemporal analysis of the hemodynamic response to single whisker stimulation in rat barrel cortex. J Neurophysiol 2008; 99: 787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilson DA, Traystman RJ, Rapela CE. Transient analysis of the canine cerebrovascular response to carbon dioxide. Circ Res 1985; 56: 596–605. [DOI] [PubMed] [Google Scholar]
- 64.Ainslie PN, Ashmead JC, Ide K, et al. Differential responses to CO2 and sympathetic stimulation in the cerebral and femoral circulations in humans. J Physiol 2005; 566: 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Willie CK, Macleod DB, Shaw AD, et al. Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol 2012; 590: 3261–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moore LE, Kirsch JR, Helfaer MA, et al. Hypercapnic blood flow reactivity not increased by alpha-blockade or cordotomy in piglets. Am J Physiol 1992; 262: H1884–H1890. [DOI] [PubMed] [Google Scholar]
- 67.McPherson RW, Kirsch JR, Ghaly RF, et al. Effect of nitric oxide synthase inhibition on the cerebral vascular response to hypercapnia in primates. Stroke 1995; 26: 682–687. [DOI] [PubMed] [Google Scholar]
- 68.Koehler RC, Traystman RJ. Bicarbonate ion modulation of cerebral blood flow during hypoxia and hypercapnia. Am J Physiol – Heart Circ Physiol 1982; 243: H33–H40. [DOI] [PubMed] [Google Scholar]
- 69.Kontos HA, Wei EP, Raper AJ, et al. Local mechanism of CO2 action of cat pial arterioles. Stroke 1977; 8: 226–229. [DOI] [PubMed] [Google Scholar]
- 70.Boedtkjer E. Acid-base regulation and sensing: accelerators and brakes in metabolic regulation of cerebrovascular tone. J Cereb Blood Flow Metab 2018; 38: 588–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Willie CK, Macleod DB, Shaw AD, et. al. Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol 2012; 590: 3261–3275. [DOI] [PMC free article] [PubMed]
- 72.Tameem A, Krovvidi H. Cerebral physiology. Contin Educ Anaesth Crit Care Pain 2013; 13: 113–118. [Google Scholar]
- 73.Meng L, Gelb AW. Regulation of cerebral autoregulation by carbon dioxide. Anesthesiology 2015; 122: 196–205. [DOI] [PubMed] [Google Scholar]
- 74.Bos MJ, Koudstaal PJ, Hofman A, et al. Transcranial Doppler hemodynamic parameters and risk of stroke: the Rotterdam study. Stroke 2007; 38: 2453–2458. [DOI] [PubMed] [Google Scholar]
- 75.Portegies ML, de Bruijn RF, Hofman A, et al. Cerebral vasomotor reactivity and risk of mortality: the Rotterdam Study. Stroke 2014; 45: 42–47. [DOI] [PubMed] [Google Scholar]
- 76.Ainslie PN, Shaw AD, Smith KJ, et al. Stability of cerebral metabolism and substrate availability in humans during hypoxia and hyperoxia. Clin Sci (Lond) 2014; 126: 661–670. [DOI] [PubMed] [Google Scholar]
- 77.Vestergaard MB, Lindberg U, Aachmann-Andersen NJ, et al. Acute hypoxia increases the cerebral metabolic rate – a magnetic resonance imaging study. J Cereb Blood Flow Metab 2016; 36: 1046–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ogoh S, Nakahara H, Ueda S, et al. Effects of acute hypoxia on cerebrovascular responses to carbon dioxide. Exp Physiol 2014; 99: 849–858. [DOI] [PubMed] [Google Scholar]
- 79.Kitano H, Kirsch JR, Hurn PD, et al. Inhalational anesthetics as neuroprotectants or chemical preconditioning agents in ischemic brain. J Cereb Blood Flow Metab 2007; 27: 1108–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ishida K, Berger M, Nadler J, et al. Anesthetic neuroprotection: antecedents and an appraisal of preclinical and clinical data quality. Curr Pharm Des 2014; 20: 5751–5765. [DOI] [PubMed] [Google Scholar]
- 81.Bilotta F, Stazi E, Zlotnik A, et al. Neuroprotective effects of intravenous anesthetics: a new critical perspective. Curr Pharm Des 2014; 20: 5469–5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zwerus R, Absalom A. Update on anesthetic neuroprotection. Curr Opin Anaesthesiol 2015; 28: 424–430. [DOI] [PubMed] [Google Scholar]
- 83.Oshima T, Karasawa F, Okazaki Y, et al. Effects of sevoflurane on cerebral blood flow and cerebral metabolic rate of oxygen in human beings: a comparison with isoflurane. Eur J Anaesthesiol 2003; 20: 543–547. [DOI] [PubMed] [Google Scholar]
- 84.Kadoi Y, Kawauchi CH, Ide M, et al. Differential increases in blood flow velocity in the middle cerebral artery after tourniquet deflation during sevoflurane, isoflurane or propofol anaesthesia. Anaesth Intensive Care 2009; 37: 598–603. [DOI] [PubMed] [Google Scholar]
- 85.Villa F, Iacca C, Molinari AF, et al. Inhalation versus endovenous sedation in subarachnoid hemorrhage patients: effects on regional cerebral blood flow. Crit Care Med 2012; 40: 2797–2804. [DOI] [PubMed] [Google Scholar]
- 86.Jung HS, Sung TY, Kang H, et al. Cerebral blood flow change during volatile induction in large-dose sevoflurane versus intravenous propofol induction: transcranial Doppler study. Korean J Anesthesiol 2014; 67: 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schlunzen L, Cold GE, Rasmussen M, et al. Effects of dose-dependent levels of isoflurane on cerebral blood flow in healthy subjects studied using positron emission tomography. Acta Anaesthesiol Scand 2006; 50: 306–312. [DOI] [PubMed] [Google Scholar]
- 88.Schlunzen L, Vafaee MS, Cold GE, et al. Effects of subanaesthetic and anaesthetic doses of sevoflurane on regional cerebral blood flow in healthy volunteers. A positron emission tomographic study. Acta Anaesthesiol Scand 2004; 48: 1268–1276. [DOI] [PubMed] [Google Scholar]
- 89.Li CX, Patel S, Wang DJ, et al. Effect of high dose isoflurane on cerebral blood flow in macaque monkeys. Magn Reson Imaging 2014; 32: 956–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fairgrieve R, Rowney DA, Karsli C, et al. The effect of sevoflurane on cerebral blood flow velocity in children. Acta Anaesthesiol Scand 2003; 47: 1226–1230. [DOI] [PubMed] [Google Scholar]
- 91.Lorenz IH, Kolbitsch C, Hormann C, et al. Subanesthetic concentration of sevoflurane increases regional cerebral blood flow more, but regional cerebral blood volume less, than subanesthetic concentration of isoflurane in human volunteers. J Neurosurg Anesthesiol 2001; 13: 288–295. [DOI] [PubMed] [Google Scholar]
- 92.Kolbitsch C, Lorenz IH, Hormann C, et al. A subanesthetic concentration of sevoflurane increases regional cerebral blood flow and regional cerebral blood volume and decreases regional mean transit time and regional cerebrovascular resistance in volunteers. Anesth Analg 2000; 91: 156–162. [DOI] [PubMed] [Google Scholar]
- 93.Mielck F, Stephan H, Weyland A, Sonntag H. Effects of one minimum alveolar anesthetic concentration sevoflurane on cerebral metabolism, blood flow, and CO2 reactivity in cardiac patients. Anesth Analg 1999; 89: 364–369. [DOI] [PubMed] [Google Scholar]
- 94.Kaisti KK, Metsahonkala L, Teras M, et al. Effects of surgical levels of propofol and sevoflurane anesthesia on cerebral blood flow in healthy subjects studied with positron emission tomography. Anesthesiology 2002; 96: 1358–1370. [DOI] [PubMed] [Google Scholar]
- 95.Rhondali O, Mahr A, Simonin-Lansiaux S, et al. Impact of sevoflurane anesthesia on cerebral blood flow in children younger than 2 years. Paediatr Anaesth 2013; 23: 946–951. [DOI] [PubMed] [Google Scholar]
- 96.Molnar C, Settakis G, Sarkany P, et al. Effect of sevoflurane on cerebral blood flow and cerebrovascular resistance at surgical level of anaesthesia: a transcranial Doppler study. Eur J Anaesthesiol 2007; 24: 179–184. [DOI] [PubMed] [Google Scholar]
- 97.Matta BF, Mayberg TS, Lam AM. Direct cerebrovasodilatory effects of halothane, isoflurane, and desflurane during propofol-induced isoelectric electroencephalogram in humans. Anesthesiology 1995; 83: 980–985. discussion 27A. [DOI] [PubMed] [Google Scholar]
- 98.Matta BF, Heath KJ, Tipping K, et al. Direct cerebral vasodilatory effects of sevoflurane and isoflurane. Anesthesiology 1999; 91: 677–680. [DOI] [PubMed] [Google Scholar]
- 99.Iida H, Ohata H, Iida M, et al. Isoflurane and sevoflurane induce vasodilation of cerebral vessels via ATP-sensitive K+channel activation. Anesthesiology 1998; 89: 954–960. [DOI] [PubMed] [Google Scholar]
- 100.McPherson RW, Kirsch JR, Moore LE, et al. N omega-nitro-L-arginine methyl ester prevents cerebral hyperemia by inhaled anesthetics in dogs. Anesth Analg 1993; 77: 891–897. [DOI] [PubMed] [Google Scholar]
- 101.McPherson RW, Traystman RJ. Effects of isoflurane on cerebral auto regulation in dogs. Anesthesiology 1988; 69: 493–499. [DOI] [PubMed] [Google Scholar]
- 102.Strebel S, Lam FA, Matta B, et al. Dynamic and static cerebral autoregulation during isoflurane, desflurane, and propofol anesthesia. Anesthesiology 1995; 83: 66–76. [DOI] [PubMed] [Google Scholar]
- 103.McCulloch TJ, Visco E, Lam AM. Graded hypercapnia and cerebral autoregulation during sevoflurane or propofol anesthesia. Anesthesiology 2000; 93: 1205–1209. [DOI] [PubMed] [Google Scholar]
- 104.McCulloch TJ, Boesel TW, Lam AM. The effect of hypocapnia on the autoregulation of cerebral blood flow during administration of isoflurane. Anesth Analg 2005; 100: 1463–1667. table of contents. [DOI] [PubMed] [Google Scholar]
- 105.Goettel N, Patet C, Rossi A, et al. Monitoring of cerebral blood flow autoregulation in adults undergoing sevoflurane anesthesia: a prospective cohort study of two age groups. J Clin Monit Comput 2016; 30: 255–264. [DOI] [PubMed] [Google Scholar]
- 106.McPherson RW, Derrer SA, Traystman RJ. Changes in cerebral CO2 responsivity over time during isoflurane anesthesia in the dog. J Neurosurg Anesthesiol 1991; 3: 12–19. [DOI] [PubMed] [Google Scholar]
- 107.Nishiyama T, Matsukawa T, Yokoyama T, et al. Cerebrovascular carbon dioxide reactivity during general anesthesia: a comparison between sevoflurane and isoflurane. Anesth Analg 1999; 89: 1437–1441. [DOI] [PubMed] [Google Scholar]
- 108.Michenfelder JD, Sundt TM, Fode N, et al. Isoflurane when compared to enflurane and halothane decreases the frequency of cerebral ischemia during carotid endarterectomy. Anesthesiology 1987; 67: 336–340. [DOI] [PubMed] [Google Scholar]
- 109.Hoffman WE, Charbel FT, Edelman G, et al. Comparison of the effect of etomidate and desflurane on brain tissue gases and ph during prolonged middle cerebral artery occlusion. Anesthesiology 1998; 88: 1188–1194. [DOI] [PubMed] [Google Scholar]
- 110.Jevtovic-Todorovic V. Exposure of developing brain to general anesthesiawhat is the animal evidence? Anesthesiology 2018; 128: 832–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Davidson AJ, Sun LS. Clinical evidence for any effect of anesthesia on the developing brain. Anesthesiology 2018; 128: 840–853. [DOI] [PubMed] [Google Scholar]
- 112.Russell GB, Snider MT, Richard RB, et al. Hyperbaric nitrous oxide as a sole anesthetic agent in humans. Anesth Analg 1990; 70: 289–295. [DOI] [PubMed] [Google Scholar]
- 113.Jevtovic-Todorovic V, Todorovic SM, Mennerick S, et al. Nitrous oxide (laughing gas) is an NMDA antagonist, neuroprotectant and neurotoxin. Nat Med 1998; 4: 460–463. [DOI] [PubMed] [Google Scholar]
- 114.Frost EAM. Nitrous oxide in neuroanesthesia: does it have a place? In: Scher CS, Clebone A, Miller SM, et al.(eds). You’re wrong, I’m right: dueling authors reexamine classic teachings in anesthesia, Cham: Springer International Publishing, 2017, pp. 207–208. [Google Scholar]
- 115.Wollman H, Alexander C, Cohen PJ, et al. Cerebral circulation during general anesthesia and hyperventilation in man. Anesthesiology 1965; 26: 329–334. [DOI] [PubMed] [Google Scholar]
- 116.Lorenz IH, Kolbitsch C, Hormann C, et al. Influence of equianaesthetic concentrations of nitrous oxide and isoflurane on regional cerebral blood flow, regional cerebral blood volume, and regional mean transit time in human volunteers. Br J Anaesth 2001; 87: 691–698. [DOI] [PubMed] [Google Scholar]
- 117.Leon JE, Bissonnette B. Transcranial Doppler sonography: nitrous oxide and cerebral blood flow velocity in children. Can J Anaesth 1991; 38: 974–979. [DOI] [PubMed] [Google Scholar]
- 118.Field LM, Dorrance DE, Krzeminska EK, et al. Effect of nitrous oxide on cerebral blood flow in normal humans. Br J Anaesth 1993; 70: 154–159. [DOI] [PubMed] [Google Scholar]
- 119.Reinstrup P, Ryding E, Ohlsson T, et al. Regional cerebral metabolic rate (positron emission tomography) during inhalation of nitrous oxide 50% in humans. Br J Anaesth 2008; 100: 66–71. [DOI] [PubMed] [Google Scholar]
- 120.Algotsson L, Messeter K, Rosen I, et al. Effects of nitrous oxide on cerebral haemodynamics and metabolism during isoflurane anaesthesia in man. Acta Anaesthesiol Scand 1992; 36: 46–52. [DOI] [PubMed] [Google Scholar]
- 121.Sakabe T, Kuramoto T, Kumagae S, et al. Cerebral responses to the addition of nitrous oxide to halothane in man. Br J Anaesth 1976; 48: 957–962. [DOI] [PubMed] [Google Scholar]
- 122.Kaisti KK, Langsjo JW, Aalto S, et al. Effects of sevoflurane, propofol, and adjunct nitrous oxide on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology 2003; 99: 603–613. [DOI] [PubMed] [Google Scholar]
- 123.Lam AM, Mayberg TS, Eng CC, et al. Nitrous oxide-isoflurane anesthesia causes more cerebral vasodilation than an equipotent dose of isoflurane in humans. Anesth Analg 1994; 78: 462–468. [DOI] [PubMed] [Google Scholar]
- 124.Eng C, Lam AM, Mayberg TS, et al. The influence of propofol with and without nitrous oxide on cerebral blood flow velocity and CO2 reactivity in humans. Anesthesiology 1992; 77: 872–879. [DOI] [PubMed] [Google Scholar]
- 125.Harrison JM, Girling KJ, Mahajan RP. Effects of propofol and nitrous oxide on middle cerebral artery flow velocity and cerebral autoregulation. Anaesthesia 2002; 57: 27–32. [DOI] [PubMed] [Google Scholar]
- 126.Karsli C, Luginbuehl IA, Bissonnette B. The effect of nitrous oxide on cerebral blood flow velocity in children anaesthetised with desflurane. Anaesthesia 2003; 58: 24–27. [DOI] [PubMed] [Google Scholar]
- 127.Rowney DA, Fairgrieve R, Bissonnette B. The effect of nitrous oxide on cerebral blood flow velocity in children anaesthetised with sevoflurane. Anaesthesia 2004; 59: 10–14. [DOI] [PubMed] [Google Scholar]
- 128.Inaba S, Sato J, Aono M, et al. Combined effects of nitrous oxide and propofol on the dynamic cerebrovascular response to step changes in end-tidal Pco2 in humans. Anesthesiology 2003; 98: 633–638. [DOI] [PubMed] [Google Scholar]
- 129.Pasternak JJ, Lanier WL. Is nitrous oxide use appropriate in neurosurgical and neurologically at-risk patients? Curr Opin Anaesthesiol 2010; 23: 544–550. [DOI] [PubMed] [Google Scholar]
- 130.Todd MM, Hindman BJ, Clarke WR, et al. Intraoperative Hypothermia for Aneurysm Surgery Trial I. Mild intraoperative hypothermia during surgery for intracranial aneurysm. N Engl J Med 2005; 352: 135–145. [DOI] [PubMed] [Google Scholar]
- 131.McGregor DG, Lanier WL, Pasternak JJ, et al. Effect of nitrous oxide on neurologic and neuropsychological function after intracranial aneurysm surgery. Anesthesiology 2008; 108: 568–579. [DOI] [PubMed] [Google Scholar]
- 132.Pasternak JJ, McGregor DG, Lanier WL, et al. Effect of nitrous oxide use on long-term neurologic and neuropsychological outcome in patients who received temporary proximal artery occlusion during cerebral aneurysm clipping surgery. Anesthesiology 2009; 110: 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Harris PD, Barnes R. The uses of helium and xenon in current clinical practice. Anaesthesia 2008; 63: 284–293. [DOI] [PubMed] [Google Scholar]
- 134.Cullen SC, Gross EG. The anesthetic properties of xenon in animals and human beings, with additional observations on krypton. Science 1951; 113: 580–582. [DOI] [PubMed] [Google Scholar]
- 135.Franks NP, Dickinson R, de Sousa SL, et al. How does xenon produce anaesthesia? Nature 1998; 396: 324. [DOI] [PubMed] [Google Scholar]
- 136.Dickinson R, Peterson BK, Banks P, et al. Competitive inhibition at the glycine site of the N-methyl-D-aspartate receptor by the anesthetics xenon and isoflurane: evidence from molecular modeling and electrophysiology. Anesthesiology 2007; 107: 756–767. [DOI] [PubMed] [Google Scholar]
- 137.Hecker K, Baumert JH, Horn N, et al. Xenon, a modern anaesthesia gas. Minerva Anestesiol 2004; 70: 255–260. [PubMed] [Google Scholar]
- 138.Derwall M, Coburn M, Rex S, et al. Xenon: recent developments and future perspectives. Minerva Anestesiol 2009; 75: 37–45. [PubMed] [Google Scholar]
- 139.Laitio RM, Kaisti KK, Laangsjo JW, et al. Effects of xenon anesthesia on cerebral blood flow in humans: a positron emission tomography study. Anesthesiology 2007; 106: 1128–1133. [DOI] [PubMed] [Google Scholar]
- 140.Laitio RM, Langsjo JW, Aalto S, et al. The effects of xenon anesthesia on the relationship between cerebral glucose metabolism and blood flow in healthy subjects: a positron emission tomography study. Anesth Analg 2009; 108: 593–600. [DOI] [PubMed] [Google Scholar]
- 141.Rex S, Schaefer W, Meyer PH, et al. Positron emission tomography study of regional cerebral metabolism during general anesthesia with xenon in humans. Anesthesiology 2006; 105: 936–943. [DOI] [PubMed] [Google Scholar]
- 142.Rex S, Meyer PT, Baumert JH, et al. Positron emission tomography study of regional cerebral blood flow and flow-metabolism coupling during general anaesthesia with xenon in humans. Br J Anaesth 2008; 100: 667–675. [DOI] [PubMed] [Google Scholar]
- 143.Yip GM, Chen ZW, Edge CJ, et al. A propofol binding site on mammalian GABAA receptors identified by photolabeling. Nat Chem Biol 2013; 9: 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jurd R, Arras M, Lambert S, et al. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J 2003; 17: 250–252. [DOI] [PubMed] [Google Scholar]
- 145.Schlunzen L, Juul N, Hansen KV, et al. Regional cerebral blood flow and glucose metabolism during propofol anaesthesia in healthy subjects studied with positron emission tomography. Acta Anaesthesiol Scand 2012; 56: 248–255. [DOI] [PubMed] [Google Scholar]
- 146.Park WK, Lynch C, 3rd, Johns RA. Effects of propofol and thiopental in isolated rat aorta and pulmonary artery. Anesthesiology 1992; 77: 956–963. [DOI] [PubMed] [Google Scholar]
- 147.Kadoi Y, Kawauchi C, Saito S, et al. The comparative effects of equipotent Bispectral Index dosages of propofol and sevoflurane on cerebrovascular carbon dioxide reactivity in elderly patients. J Clin Anesth 2009; 21: 173–177. [DOI] [PubMed] [Google Scholar]
- 148.Ishiyama T, Kotoda M, Asano N, et al. Effects of hyperventilation on cerebral oxygen saturation estimated using near-infrared spectroscopy: a randomised comparison between propofol and sevoflurane anaesthesia. Eur J Anaesthesiol 2016; 33: 929–935. [DOI] [PubMed] [Google Scholar]
- 149.Ružman T, Šimurina T, Gulam D, et al. Sevoflurane preserves regional cerebral oxygen saturation better than propofol: randomized controlled trial. J Clin Anesth 2017; 36: 110–117. [DOI] [PubMed] [Google Scholar]
- 150.Valencia L, Rodríguez-Pérez A, Kühlmorgen B, et al. Does sevoflurane preserve regional cerebral oxygen saturation measured by near-infrared spectroscopy better than propofol? Ann Franç d'Anesth de Réanimat 2014; 33: e59–e65. [DOI] [PubMed] [Google Scholar]
- 151.Schonenberger S, Uhlmann L, Hacke W, et al. Effect of conscious sedation vs general anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: a randomized clinical trial. JAMA 2016; 316: 1986–1996. [DOI] [PubMed] [Google Scholar]
- 152.Simonsen CZ, Yoo AJ, Sorensen LH, et al. Effect of general anesthesia and conscious sedation during endovascular therapy on infarct growth and clinical outcomes in acute ischemic stroke: a randomized clinical trial. JAMA Neurol 2018; 75(4): 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Roach GW, Newman MF, Murkin JM, et al. Ineffectiveness of burst suppression therapy in mitigating perioperative cerebrovascular dysfunction. Multicenter Study of Perioperative Ischemia (McSPI) Research Group. Anesthesiology 1999; 90: 1255–1264. [DOI] [PubMed] [Google Scholar]
- 154.Fritz BA, Kalarickal PL, Maybrier HR, et al. Intraoperative electroencephalogram suppression predicts postoperative delirium. Anesth Analg 2016; 122: 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.List WF, Crumrine RS, Cascorbi HF, et al. Increased cerebrospinal fluid pressure after ketamine. Anesthesiology 1972; 36: 98–99. [DOI] [PubMed] [Google Scholar]
- 156.Wyte SR, Shapiro HM, Turner P, et al. Ketamine-induced intracranial hypertension. Anesthesiology 1972; 36: 174–176. [DOI] [PubMed] [Google Scholar]
- 157.Evans J, Rosen M, Weeks RD, et al. Ketamine in neurosurgical procedures. Lancet 1971; 1: 40–41. [DOI] [PubMed] [Google Scholar]
- 158.Nelson SR, Howard RB, Cross RS, et al. Ketamine-induced changes in regional glucose utilization in the rat brain. Anesthesiology 1980; 52: 330–334. [DOI] [PubMed] [Google Scholar]
- 159.Crosby G, Crane AM, Sokoloff L. Local changes in cerebral glucose utilization during ketamine anesthesia. Anesthesiology 1982; 56: 437–443. [DOI] [PubMed] [Google Scholar]
- 160.Wang X, Ding X, Tong Y, et al. Ketamine does not increase intracranial pressure compared with opioids: meta-analysis of randomized controlled trials. J Anesth 2014; 28: 821–827. [DOI] [PubMed] [Google Scholar]
- 161.Langsjo JW, Kaisti KK, Aalto S, et al. Effects of subanesthetic doses of ketamine on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology 2003; 99: 614–623. [DOI] [PubMed] [Google Scholar]
- 162.Langsjo JW, Salmi E, Kaisti KK, et al. Effects of subanesthetic ketamine on regional cerebral glucose metabolism in humans. Anesthesiology 2004; 100: 1065–1071. [DOI] [PubMed] [Google Scholar]
- 163.Zeiler FA, Sader N, Gillman LM, et al. The cerebrovascular response to ketamine: a systematic review of the animal and human literature. J Neurosurg Anesthesiol 2016; 28: 123–140. [DOI] [PubMed] [Google Scholar]
- 164.Oren RE, Rasool NA, Rubinstein EH. Effect of ketamine on cerebral cortical blood flow and metabolism in rabbits. Stroke 1987; 18: 441–444. [DOI] [PubMed] [Google Scholar]
- 165.Bell JD. In vogue: ketamine for neuroprotection in acute neurologic injury. Anesth Analg 2017; 124: 1237–1243. [DOI] [PubMed] [Google Scholar]
- 166.Forman SA. Clinical and molecular pharmacology of etomidate. Anesthesiology 2011; 114: 695–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Van Reempts J, Borgers M, Van Eyndhoven J, Hermans C. Protective effects of etomidate in hypoxic-ischemic brain damage in the rat. A morphologic assessment. Exp Neurol 1982; 76: 181–195. [DOI] [PubMed] [Google Scholar]
- 168.Frizzell RT, Meyer YJ, Borchers DJ, et al. The effects of etomidate on cerebral metabolism and blood flow in a canine model for hypoperfusion. J Neurosurg 1991; 74: 263–269. [DOI] [PubMed] [Google Scholar]
- 169.Van Aken J, Rolly G. Influence of etomidate, a new short acting anesthetic agent, on cerebral blood flow in man. Acta Anaesthesiol Belg 1976. 27 suppl: 175–180. [PubMed] [Google Scholar]
- 170.Renou AM, Vernhiet J, Macrez P, et al. Cerebral blood flow and metabolism during etomidate anaesthesia in man. Br J Anaesth 1978; 50: 1047–1051. [DOI] [PubMed] [Google Scholar]
- 171.Moss E, Powell D, Gibson RM, et al. Effect of etomidate on intracranial pressure and cerebral perfusion pressure. Br J Anaesth 1979; 51: 347–352. [DOI] [PubMed] [Google Scholar]
- 172.Cold GE, Eskesen V, Eriksen H, et al. CBF and CMRO2 during continuous etomidate infusion supplemented with N2O and fentanyl in patients with supratentorial cerebral tumour. A dose-response study. Acta Anaesthesiol Scand 1985; 29: 490–494. [DOI] [PubMed] [Google Scholar]
- 173.Milde LN, Milde JH, Michenfelder JD. Cerebral functional, metabolic, and hemodynamic effects of etomidate in dogs. Anesthesiology 1985; 63: 371–377. [DOI] [PubMed] [Google Scholar]
- 174.Drummond JC, McKay LD, Cole DJ, et al. The role of nitric oxide synthase inhibition in the adverse effects of etomidate in the setting of focal cerebral ischemia in rats. Anesth Analg 2005; 100: 841–846. table of contents. [DOI] [PubMed] [Google Scholar]
- 175.Batjer HH. Cerebral protective effects of etomidate: experimental and clinical aspects. Cerebrovasc Brain Metab Rev 1993; 5: 17–32. [PubMed] [Google Scholar]
- 176.Edelman GJ, Hoffman WE, Charbel FT. Cerebral hypoxia after etomidate administration and temporary cerebral artery occlusion. Anesth Analg 1997; 85: 821–825. [DOI] [PubMed] [Google Scholar]
- 177.Hindman BJ, Bayman EO, Pfisterer WK, et al. No association between intraoperative hypothermia or supplemental protective drug and neurologic outcomes in patients undergoing temporary clipping during cerebral aneurysm surgery: findings from the Intraoperative Hypothermia for Aneurysm Surgery Trial. Anesthesiology 2010; 112: 86–101. [DOI] [PubMed] [Google Scholar]
- 178.Lovell AT, Owen-Reece H, Elwell CE, et al. Continuous measurement of cerebral oxygenation by near infrared spectroscopy during induction of anesthesia. Anesth Analg 1999; 88: 554–548. [DOI] [PubMed] [Google Scholar]
- 179.Paris A, Tonner PH. Dexmedetomidine in anaesthesia. Curr Opin Anaesthesiol 2005; 18: 412–418. [DOI] [PubMed] [Google Scholar]
- 180.Ebert TJ, Hall JE, Barney JA, et al. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology 2000; 93: 382–394. [DOI] [PubMed] [Google Scholar]
- 181.Soliman RN, Hassan AR, Rashwan AM, et al. Prospective, randomized controlled study to assess the role of dexmedetomidine in patients with supratentorial tumors undergoing craniotomy under general anesthesia. Middle East J Anaesthesiol 2011; 21: 23–33. [PubMed] [Google Scholar]
- 182.Batra A, Verma R, Bhatia VK, et al. Dexmedetomidine as an anesthetic adjuvant in intracranial surgery. Anesth Essays Res 2017; 11: 309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Farag E, Argalious M, Abd-Elsayed A, et al. The use of dexmedetomidine in anesthesia and intensive care: a review. Curr Pharm Des 2012; 18: 6257–6265. [DOI] [PubMed] [Google Scholar]
- 184.Slupe AM, Minnier J, Merritt RH, et al. Dexmedetomidine sedation for paroxysmal supraventricular tachycardia ablation is not associated with alteration of arrhythmia inducibility. Anesth Analg. Epub ahead of print 11 April 2018. DOI: 10.1213/ANE.0000000000003359. [DOI] [PubMed] [Google Scholar]
- 185.McPherson RW, Koehler RC, Kirsch JR, et al. Intraventricular dexmedetomidine decreases cerebral blood flow during normoxia and hypoxia in dogs. Anesth Analg 1997; 84: 139–147. [DOI] [PubMed] [Google Scholar]
- 186.Prielipp RC, Wall MH, Tobin JR, et al. Dexmedetomidine-induced sedation in volunteers decreases regional and global cerebral blood flow. Anesth Analg 2002; 95: 1052–1059. table of contents. [DOI] [PubMed] [Google Scholar]
- 187.Zornow MH, Maze M, Dyck JB, et al. Dexmedetomidine decreases cerebral blood flow velocity in humans. J Cereb Blood Flow Metab 1993; 13: 350–353. [DOI] [PubMed] [Google Scholar]
- 188.Bonhomme V, Maquet P, Phillips C, et al. The effect of clonidine infusion on distribution of regional cerebral blood flow in volunteers. Anesth Analg 2008; 106: 899–909. [DOI] [PubMed] [Google Scholar]
- 189.Drummond JC, Dao AV, Roth DM, et al. Effect of dexmedetomidine on cerebral blood flow velocity, cerebral metabolic rate, and carbon dioxide response in normal humans. Anesthesiology 2008; 108: 225–232. [DOI] [PubMed] [Google Scholar]
- 190.Drummond JC, Sturaitis MK. Brain tissue oxygenation during dexmedetomidine administration in surgical patients with neurovascular injuries. J Neurosurg Anesthesiol 2010; 22: 336–341. [DOI] [PubMed] [Google Scholar]
- 191.Farag E, Kot M, Podolyak A, et al. The relative effects of dexmedetomidine and propofol on cerebral blood flow velocity and regional brain oxygenation: a randomised noninferiority trial. Eur J Anaesthesiol 2017; 34: 732–739. [DOI] [PubMed] [Google Scholar]
- 192.Mikkelsen MLG, Ambrus R, Rasmussen R, et al. The effect of dexmedetomidine on cerebral perfusion and oxygenation in healthy piglets with normal and lowered blood pressure anaesthetized with propofol-remifentanil total intravenous anaesthesia. Acta Vet Scand 2017; 59: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Firestone LL, Gyulai F, Mintun M, et al. Human brain activity response to fentanyl imaged by positron emission tomography. Anesth Analg 1996; 82: 1247–1251. [DOI] [PubMed] [Google Scholar]
- 194.Zelaya FO, Zois E, Muller-Pollard C, et al. The response to rapid infusion of fentanyl in the human brain measured using pulsed arterial spin labelling. MAGMA 2012; 25: 163–175. [DOI] [PubMed] [Google Scholar]
- 195.Schlaepfer TE, Strain EC, Greenberg BD, et al. Site of opioid action in the human brain: mu and kappa agonists' subjective and cerebral blood flow effects. Am J Psychiatry 1998; 155: 470–473. [DOI] [PubMed] [Google Scholar]
- 196.Jones AK, Friston KJ, Qi LY, et al. Sites of action of morphine in the brain. Lancet 1991; 338: 825. [DOI] [PubMed] [Google Scholar]
- 197.Hanel F, Werner C, von Knobelsdorff G, et al. The effects of fentanyl and sufentanil on cerebral hemodynamics. J Neurosurg Anesthesiol 1997; 9: 223–227. [DOI] [PubMed] [Google Scholar]
- 198.Weinstabl C, Mayer N, Spiss CK. Sufentanil decreases cerebral blood flow velocity in patients with elevated intracranial pressure. Eur J Anaesthesiol 1992; 9: 481–484. [PubMed] [Google Scholar]
- 199.Fodale V, Schifilliti D, Pratico C, et al. Remifentanil and the brain. Acta Anaesthesiol Scand 2008; 52: 319–326. [DOI] [PubMed] [Google Scholar]
- 200.Lorenz IH, Kolbitsch C, Schocke M, et al. Low-dose remifentanil increases regional cerebral blood flow and regional cerebral blood volume, but decreases regional mean transit time and regional cerebrovascular resistance in volunteers. Br J Anaesth 2000; 85: 199–204. [DOI] [PubMed] [Google Scholar]
- 201.Klimscha W, Ullrich R, Nasel C, et al. High-dose remifentanil does not impair cerebrovascular carbon dioxide reactivity in healthy male volunteers. Anesthesiology 2003; 99: 834–840. [DOI] [PubMed] [Google Scholar]
- 202.Baker KZ, Ostapkovich N, Sisti MB, et al. Intact cerebral blood flow reactivity during remifentanil/nitrous oxide anesthesia. J Neurosurg Anesthesiol 1997; 9: 134–140. [DOI] [PubMed] [Google Scholar]
- 203.Adler LJ, Gyulai FE, Diehl DJ, et al. Regional brain activity changes associated with fentanyl analgesia elucidated by positron emission tomography. Anesth Analg 1997; 84: 120–126. [DOI] [PubMed] [Google Scholar]
- 204.Gerlach K, Uhlig T, Huppe M, et al. Remifentanil-propofol versus sufentanil-propofol anaesthesia for supratentorial craniotomy: a randomized trial. Eur J Anaesthesiol 2003; 20: 813–820. [PubMed] [Google Scholar]
- 205.Balakrishnan G, Raudzens P, Samra SK, et al. A comparison of remifentanil and fentanyl in patients undergoing surgery for intracranial mass lesions. Anesth Analg 2000; 91: 163–169. [DOI] [PubMed] [Google Scholar]
- 206.Gemma M, Tommasino C, Cozzi S, et al. Remifentanil provides hemodynamic stability and faster awakening time in transsphenoidal surgery. Anesth Analg 2002; 94: 163–168. table of contents. [DOI] [PubMed] [Google Scholar]
- 207.Chunhua C, Chunhua X, Megumi S, et al. Kappa opioid receptor agonist and brain ischemia. Transl Perioper Pain Med 2014; 1: 27–34. [PMC free article] [PubMed] [Google Scholar]
- 208.Zhang J, Gibney GT, Zhao P, et al. Neuroprotective role of delta-opioid receptors in cortical neurons. Am J Physiol Cell Physiol 2002; 282: C1225–C1234. [DOI] [PubMed] [Google Scholar]
- 209.Dong HP, Zhou W, Ma XX, et al. Salvinorin A preserves cerebral pial artery autoregulation after forebrain ischemia via the PI3K/AKT/cGMP pathway. Braz J Med Biol Res 2018; 51: e6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Su D, Riley J, Armstead WM, et al. Salvinorin A pretreatment preserves cerebrovascular autoregulation after brain hypoxic/ischemic injury via extracellular signal-regulated kinase/mitogen-activated protein kinase in piglets. Anesth Analg 2012; 114: 200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hayes RL, Lyeth BG, Jenkins LW, et al. Possible protective effect of endogenous opioids in traumatic brain injury. J Neurosurg 1990; 72: 252–261. [DOI] [PubMed] [Google Scholar]
- 212.Theodore WH, Leiderman D, Gaillard W, et al. The effect of naloxone on cerebral blood flow and glucose metabolism in patients with complex partial seizures. Epilepsy Res 1993; 16: 51–54. [DOI] [PubMed] [Google Scholar]
- 213.Adams HP, Olinger CP, Barsan WG, et al. A dose-escalation study of large doses of naloxone for treatment of patients with acute cerebral ischemia. Stroke 1986; 17: 404–409. [DOI] [PubMed] [Google Scholar]
- 214.Olinger CP, Adams HP, Jr, Brott TG, et al. High-dose intravenous naloxone for the treatment of acute ischemic stroke. Stroke 1990; 21: 721–725. [DOI] [PubMed] [Google Scholar]
- 215.Clark WM, Raps EC, Tong DC, et al. Cervene (Nalmefene) in acute ischemic stroke. Stroke 2000; 31: 1234. [DOI] [PubMed] [Google Scholar]
- 216.Bracken MB, Holford TR. Effects of timing of methylprednisolone or naloxone administration on recovery of segmental and long-tract neurological function in NASCIS 2. J Neurosurg 1993; 79: 500–507. [DOI] [PubMed] [Google Scholar]
- 217.Flamm ES, Young W, Demopoulos HB, et al. Experimental spinal cord injury: treatment with naloxone. Neurosurgery 1982; 10: 227–231. [PubMed] [Google Scholar]
- 218.Erman T, Iskender Göçer A, Sule Yldz M, et al. Effect of naloxone after spinal cord injury in the rat: inducible nitric oxide synthase expression, superoxide dismutase activity, and ultrastructural changes. Neurosurg Q 2004; 14: 249–256.
- 219.Cotev S, Shalit MN. Effects on diazepam on cerebral blood flow and oxygen uptake after head injury. Anesthesiology 1975; 43: 117–122. [DOI] [PubMed] [Google Scholar]
- 220.Nugent M, Artru AA, Michenfelder JD. Cerebral effects of midazolam and diazepam. Anesthesiology 1980; 53: S8. [DOI] [PubMed] [Google Scholar]
- 221.Mathew RJ, Wilson WH, Daniel DG. The effect of nonsedating doses of diazepam on regional cerebral blood flow. Biol Psychiatry 1985; 20: 1109–1116. [DOI] [PubMed] [Google Scholar]
- 222.Matthew E, Andreason P, Pettigrew K, et al. Benzodiazepine receptors mediate regional blood flow changes in the living human brain. Proc Natl Acad Sci USA 1995; 92: 2775–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Finelli LA, Landolt HP, Buck A, et al. Functional neuroanatomy of human sleep states after zolpidem and placebo: a H215O-PET study. J Sleep Res 2000; 9: 161–173. [DOI] [PubMed] [Google Scholar]
- 224.Roy-Byrne P, Fleishaker J, Arnett C, et al. Effects of acute and chronic alprazolam treatment on cerebral blood flow, memory, sedation, and plasma catecholamines. Neuropsychopharmacology 1993; 8: 161–169. [DOI] [PubMed] [Google Scholar]
- 225.Veselis R, Reinsel R, Feshchenko V, et al. Dose related decreases in rCBF in R and L prefrontal cortices (PFC) during memory impairment with midazolam and propofol. NeuroImage 2001; 13: 757.
- 226.Liang P, Manelis A, Liu X, et al. Using arterial spin labeling perfusion MRI to explore how midazolam produces anterograde amnesia. Neurosci Lett 2012; 522: 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Knudsen L, Cold GE, Holdgard HO, et al. Effects of flumazenil on cerebral blood flow and oxygen consumption after midazolam anaesthesia for craniotomy. Br J Anaesth 1991; 67: 277–280. [DOI] [PubMed] [Google Scholar]
- 228.Ogawa Y, Iwasaki K, Aoki K, et al. The effects of flumazenil after midazolam sedation on cerebral blood flow and dynamic cerebral autoregulation in healthy young males. J Neurosurg Anesthesiol 2015; 27: 275–281. [DOI] [PubMed] [Google Scholar]
- 229.Forster A, Juge O, Louis M, et al. Effects of a specific benzodiazepine antagonist (RO 15-1788) on cerebral blood flow. Anesth Analg 1987; 66: 309–313. [PubMed] [Google Scholar]
- 230.Steinbach JH, Akk G. Modulation of GABA(A) receptor channel gating by pentobarbital. J Physiol 2001; 537: 715–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wechsler RL, Dripps RD, Kety SS. Blood flow and oxygen consumption of the human brain during anesthesia produced by thiopental. Anesthesiology 1951; 12: 308–314. [DOI] [PubMed] [Google Scholar]
- 232.Pierce EC, Jr, Lambertsen CJ, Deutsch S, et al. Cerebral circulation and metabolism during thiopental anesthesia and hyper-ventilation in man. J Clin Invest 1962; 41: 1664–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Donegan JH, Traystman RJ, Koehler RC, et al. Cerebrovascular hypoxic and autoregulatory responses during reduced brain metabolism. Am J Physiol 1985; 249: H421–H429. [DOI] [PubMed] [Google Scholar]
- 234.Levasseur JE, Kontos HA. Effects of anesthesia on cerebral arteriolar responses to hypercapnia. Am J Physiol 1989; 257: H85–H88. [DOI] [PubMed] [Google Scholar]
- 235.Traystman RJ. Anesthetic mediated neuroprotection: established fact or passing fancy? J Neurosurg Anesthesiol 2004; 16: 308–312. [DOI] [PubMed] [Google Scholar]
- 236.Al-Hashimi S, Zaman M, Waterworth P, et al. Does the use of thiopental provide added cerebral protection during deep hypothermic circulatory arrest? Interact Cardiovasc Thorac Surg 2013; 17: 392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Euasobhon P, Dej-Arkom S, Siriussawakul A, et al. Lidocaine for reducing propofol-induced pain on induction of anaesthesia in adults. Cochrane Database Syst Rev 2016; 2: CD007874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Qi DY, Wang K, Zhang H, et al. Efficacy of intravenous lidocaine versus placebo on attenuating cardiovascular response to laryngoscopy and tracheal intubation: a systematic review of randomized controlled trials. Minerva Anestesiol 2013; 79: 1423–1435. [PubMed] [Google Scholar]
- 239.Adinoff B, Devous MD, Sr, Cooper DC, et al. Neural response to lidocaine in healthy subjects. Psychiatry Res 2009; 173: 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kastrup J, Petersen P, Dejgard A. Intravenous lidocaine and cerebral blood flow: impaired microvascular reactivity in diabetic patients. J Clin Pharmacol 1990; 30: 318–323. [DOI] [PubMed] [Google Scholar]
- 241.Lam AM, Donlon E, Eng CC, et al. The effect of lidocaine on cerebral blood flow and metabolism during normocapnia and hypocapnia in humans. J Neurosurg Anesthesiol 1993; 5: 307. [Google Scholar]
- 242.Bedford RF, Persing JA, Pobereskin L, et al. Lidocaine or thiopental for rapid control of intracranial hypertension? Anesth Analg 1980; 59: 435–437. [PubMed] [Google Scholar]
- 243.Sutherland G, Ong BY, Louw D, et al. Effect of lidocaine on forebrain ischemia in rats. Stroke 1989; 20: 119–122. [DOI] [PubMed] [Google Scholar]
- 244.Shokunbi MT, Gelb AW, Wu XM, et al. Continuous lidocaine infusion and focal feline cerebral ischemia. Stroke 1990; 21: 107–111. [DOI] [PubMed] [Google Scholar]
- 245.Rasool N, Faroqui M, Rubinstein EH. Lidocaine accelerates neuroelectrical recovery after incomplete global ischemia in rabbits. Stroke 1990; 21: 929–935. [DOI] [PubMed] [Google Scholar]
- 246.Wang D, Wu X, Li J, et al. The effect of lidocaine on early postoperative cognitive dysfunction after coronary artery bypass surgery. Anesth Analg 2002; 95: 1134–1141. table of contents. [DOI] [PubMed] [Google Scholar]
- 247.Mitchell SJ, Merry AF, Frampton C, et al. Cerebral protection by lidocaine during cardiac operations: a follow-up study. Ann Thorac Surg 2009; 87: 820–825. [DOI] [PubMed] [Google Scholar]
- 248.Mathew JP, Mackensen GB, Phillips-Bute B, et al. Randomized, double-blinded, placebo controlled study of neuroprotection with lidocaine in cardiac surgery. Stroke 2009; 40: 880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Peng Y, Zhang W, Zhou X, et al. Lidocaine did not reduce neuropsychological-cognitive decline in patients 6 months after supratentorial tumor surgery: a randomized, controlled trial. J Neurosurg Anesthesiol 2016; 28: 6–13. [DOI] [PubMed] [Google Scholar]
- 250.Soeding PF, Currigan DA, Mamo Y, et al. Effect of interscalene anaesthesia on cerebral oxygen saturation. Anaesth Intensive Care 2016; 44: 359–363.27246935 [Google Scholar]
- 251.Friedman DB, Friberg L, Mitchell JH, et al. Effect of axillary blockade on regional cerebral blood flow during static handgrip. J Appl Physiol (1985) 1991; 71: 651–656. [DOI] [PubMed] [Google Scholar]
- 252.Jorgensen LG, Perko G, Payne G, et al. Effect of limb anesthesia on middle cerebral response to handgrip. Am J Physiol 1993; 264: H553–H559. [DOI] [PubMed] [Google Scholar]
- 253.Vianna LC, Deo SH, Jensen AK, et al. Impaired dynamic cerebral autoregulation at rest and during isometric exercise in type 2 diabetes patients. Am J Physiol – Heart Circ Physiol 2015; 308: H681–H687. [DOI] [PMC free article] [PubMed] [Google Scholar]