Abstract
Biologic sex influences many variables that are important to brain health in general, and to stroke or cerebral ischemia in particular, such as general health status, cerebrovascular anatomy and function, unique risk factors such as pregnancy and preeclampsia, symptomatology, and therapeutic response. A more complete understanding of the scale and depth of sexual dimorphism in the brain and the role of more general sex-based factors is crucial to reducing the burden of stroke in women and men. This focused review highlights recent findings in stroke, including sex differences in epidemiology, risk factor reduction, comparative use of stroke therapeutics in both sexes, the importance of frailty in women, and the biologic basis for sex differences in stroke. Such findings show tremendous promise for the future of personalized medicine in stroke prevention and treatment.
Keywords: Sex, sex differences, stroke, women
Introduction
Stroke is a massive public health concern. It is the fourth and fifth leading cause of death in women and men, respectively, and is the number 1 cause of disability in the US in both sexes. In its wake, victims often suffer from neurologic disability and mood disorders, as well as increased likelihood of re-hospitalization and complications such as infections, venous thromboembolism, falls, and fractures. Finally, the high cost of rehabilitation and long-term nursing care, and the loss in productivity for stroke survivors and their caregivers have created a financial burden that exceeds $75 billion a year in the US alone.
Biologic sex, the variable defined by characteristics encoded in DNA, and gender, the collection of social, cultural and psychological traits that are a function of being a human male or female, influences both health and disease. In stroke care and research, both sex and gender effects have been identified, either separately or as variables with intersectionality. A well-known example is the observation that coronary heart disease is more likely to be the first cardiovascular event for a man, whereas for a woman, the first such event is more likely to be stroke. This is important because risk factors for stroke vs. heart disease are somewhat different. Thus, in 2014, the American Heart Association/American Stroke Association published Guidelines for the Prevention of Stroke in Women.1 These guidelines not only highlight differences in risk factors associated with stroke vs. heart disease but also point to risk factors that are unique to, or more prevalent in, women. Preeclampsia/eclampsia, for example, increases the risk for stroke 14.5-fold during the first three years after delivery.2 Since the publication of these guidelines, ongoing research incorporates these unique risk factors, particularly preeclampsia, into risk scores to assess their contribution to stroke incidence over and above traditional stroke risk factors such as hypertension, hyperlipidemia, and diabetes.3 Another, often under-appreciated, sex difference in stroke is that women have worse outcomes, increased disability, and decreased quality of life. These quality of life challenges in women may be related to increased anxiety, depression, pain and discomfort, as well as decreased mobility compared to men.4
Unusual presentation of symptoms, timely recognition of symptoms, increased likelihood of a stroke mimic, delays in acute imaging, and a lower likelihood of receiving intravenous tissue plasminogen activator (IV tPA) are sex differences that can impact acute stroke treatment, particularly in women. Further, critically important differences in risk profiles, management of and adherence to hypertension and cholesterol medication regimens, and risks for both ischemic stroke and bleeding complications from atrial fibrillation (AF) are still poorly understood.5 In this selected overview, the authors have summarized some of the most important findings related to women and sex differences in stroke including treatment, prevention, and determinants of health across the entire lifespan, from child-bearing to advanced years. We believe that by raising awareness of the challenges in this arena, we will set the stage for urgently needed research that will lead to improved care for women after stroke.
Sex differences in critically important aspects of stroke
Epidemiology
It has been long recognized that stroke incidence is higher in men than in women globally, a sexually dimorphic epidemiology that persists well beyond the menopausal years until it is eclipsed by the effect of age. In children, sex differences in stroke risk and pathobiology are evident even before puberty. Understanding sex differences in stroke epidemiology is critical to the design of effective interventions for prevention, treatment, and recovery that are sex-tailored.
Due to sex differences in life expectancy as well as other contributing factors, women experience approximately 55,000 more strokes in the US each year.6,7 Stroke prevalence is also higher in women, with 4.1 million women currently living with stroke compared to 3.1 million men.6 Although stroke has declined to the fifth leading cause of death overall in the US, it remains the fourth leading cause of death among women, and the third leading cause of death among both African American and Hispanic women.8,9
Although the higher burden of stroke in women is largely a result of longer life expectancies, sex differences in stroke incidence rates may also play a role.7 Historically, data have shown that the age-adjusted incidence of stroke is higher in men vs. women10–12 except in the elderly.6,7 Recent analyses of temporal trends, however, suggest that stroke incidence may be declining to a greater extent in men than in women, and that the overall decrease in stroke incidence observed over time is being driven by a decrease in ischemic stroke among men.13 Thus, it is critical to continue to monitor these trends by sex, and to ensure strategies that maximize stroke care for both women and men.
Stroke incidence and outcomes
The higher age-adjusted incidence of stroke among men as compared to women is well documented.14 However, recent data suggest that reduced rates of stroke among men have not been equally experienced by women.13 Moreover, declines in stroke incidence and mortality over time among whites in the US have not been observed among other racial/ethnic groups.15,16 The intersectionality of these factors and multiple dimensions of marginalization, e.g. race/ethnicity, sex, age and socioeconomic factors, are important to stroke risk and outcome. However, these areas have not been adequately examined to date. For example, stroke risk is significantly higher among African Americans and Hispanics in the US as compared to their white counterparts.17,18 Yet racial differences may diminish with age such that the largest disparities in stroke risk are observed among younger adults. Results from the US National Vital Statistic System demonstrate that Latinos are the only racial/ethnic group to exhibit an increase in stroke mortality rates between 2013 to 2015.16 It is still unclear how sex may modify these age-related racial/ethnic differences in stroke.
The influence of socioeconomic variables and their potential to confound conclusions around changes in stroke risk may also vary depending on sex and/or race/ethnicity; however, there is a paucity of data exploring these interactions. A meta-analysis of 44 studies, representing 89 cohorts, examined associations between socioeconomic factors (education, area measures of deprivation, occupation, and income) and risk of cardiovascular disease (CVD) by sex. Of these studies only 11 examined stroke. Furthermore, the studies varied in their examination of specific socioeconomic factors. Eleven studies were identified which provided sex-specific data on risk of stroke and education, but only three studies were identified which provided data on occupation.19 Lower levels of education, lower income, and higher area deprivation (a measure of neighborhood socioeconomic disadvantage) were associated with increased risk of stroke among both men and women, equally. However, it is unclear whether these associations are consistent across age or race/ethnicity. For example, income and occupation may not adequately reflect measures of wealth and access to material resources among older adults due to retirement; therefore, estimates based on such measures among older adults may underestimate differences in stroke by sex.
Similar considerations are apparent regarding stroke outcome. Data from two population-based cohorts of middle-aged to older adults suggest that lower levels of education may be associated with poorer functional outcomes after stroke among men, but not women.20,21 However, other measures of socioeconomic status may be more salient predictors of functional limitations among women than education alone, especially when considering changes in educational attainment among women over time. Among participants in the Brain Attack Surveillance in Corpus Christi Project, activity of daily living (ADL)/instrumental ADL (IADL) scores were higher among women, suggesting worse functional outcomes, than in men 90 days after stroke. Notably, these scores did not change substantially after adjusting for education, medical insurance, and race/ethnicity.21 However, ADL/IADL scores did increase by 5% after adjusting for marital status. Women experienced poorer functional outcomes, suggesting that widowhood has a negative effect on outcome. Importantly, similar associations between sex-related socioeconomic factors and stroke outcomes have not been evaluated in younger adults or across racial/ethnic groups. Moreover, the observed role of widowhood suggests that investigators must consider the potential impact of other markers of socioeconomic position as well as socio-cultural factors. The latter may provide insight into dimensions of women’s access to material and social resources that may contribute to gender-related socioeconomic differences in stroke. Thus, it is imperative that research has a broader focus and considers the domains of social experience, e.g. sex, socioeconomic factors, and race/ethnicity, in shaping stroke disparities.
Atrial fibrillation
AF has become increasingly common around the world, with an estimated prevalence of 596 cases per 100,000 men and 373 cases per 100,000 women.22 The actual figures, however, may be considerably higher because AF is under-diagnosed. Although the prevalence of AF is higher in men, women have a higher risk for stroke and death due to AF as well as worse outcomes after stroke secondary to AF.23 In addition, the prevalence of AF and AF-related healthcare costs are expected to increase exponentially as our senior populations grow—a statement that has special implications for women since the number of elderly women will continue to outpace elderly men in many countries.
Women with AF have a higher incidence of stroke and a higher mortality rate compared to men with AF.24,25 In the Framingham Heart Study, the adjusted risk for stroke was almost two-fold higher in women with AF than in men with AF (HR = 1.92; 95% CI, 1.2–3.07).26 Further, the adjusted odds ratio for death in women with AF vs. women without AF is 1.9 compared to 1.5 in men with AF vs. men without AF.27
Major risk factors for AF include age, body mass index, blood pressure and hypertension treatment, diabetes, valvular heart disease, heart failure, and myocardial infarction (MI).28 Advanced age carries the highest risk for AF. Indeed, the incidence of AF doubles with every 10-year increase in age29 which is particularly important in women, who have a higher life expectancy overall. In the Framingham Heart Study,28 74% of women with AF were 70 years or older, while only 58% of men with AF were over 70.
The American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS)30 recommends using the CHA2DS2-VASc score to predict stroke risk and need for anticoagulation therapy in patients with AF. The CHA2DS2-VASc scoring system assigns one point for heart failure, hypertension, age 65–74 years, diabetes mellitus, or vascular disease (prior MI, peripheral artery disease, or aortic plaque), and two points for female sex, prior stroke/TIA/thromboembolism, or age ≥75 years.
Oral anticoagulation therapy reduces the risk of stroke from AF. The AHA/ACC/HRS recommends anticoagulation therapy in patients with a CHA2DS2-VASc score ≥2. Warfarin, the oldest and best-known anticoagulant, reduces the risk for stroke in AF patients by 64% vs. placebo and by 39% vs. aspirin.31 Novel oral anticoagulants (NOACs) are now recommended over warfarin for stroke prevention in patients with non-valvular AF. NOACs are as effective as warfarin and have improved safety profiles in both men and women, although several studies indicate that the risk for bleeding is lower in women compared to men. Indeed, a meta-analysis of the ARISTOTLE, AVERROES, RELY, and ROCKET AF trials showed that women on NOACs have lower rates of major bleeding compared to men (OR = 0.84; 95% CI, 0.75–0.96).32
Finally, treatment of modifiable cardiovascular risk factors for stroke, including hypertension, obesity, and metabolic syndrome, can be highly effective in women, and can thus further optimize prevention of AF and stroke. However, understanding the effects of underlying biologic, socioeconomic, and cultural factors on sex disparities and treatment in AF is expected to have a significant impact on reducing the growing healthcare and financial burden of AF and stroke in women.
AF develops through a complex pathophysiological process associated with multiple risk factors that include genetic and vascular risk factors, and systemic and structural cardiomyopathy.33 Atrial cardiomyopathy with structural changes such as atrial dilation, fibrosis, loss of muscle mass, epicardial adipose tissue, extracellular matrix remodeling, inflammation, and disruption of gap junctions are important substrates for development of AF,34 in addition to abnormal electrical activity, resting membrane potential, and ionic influx to atrial myocytes.35 Although great advances have been made in understanding these structural and electrical AF substrates, the exact cellular and molecular mechanisms responsible for development of AF are largely unknown. Therefore, animal models are critical to study the mechanisms of development of AF and sex-specific AF predisposition. However, the complexity of AF development process has made studies of AF in animal models very challenging.
Several small and large animal AF models have been used.36 Vagal nerve stimulation or acetylcholine infusion and disease conditions such as pericarditis, heart failure, ischemia, or tachycardia were initially used to induce AF in animals. More recently, genetically modified animals have been used as more standard models to study AF. Transgenic mice models of AF such as cardiac-specific LKB1 knockout mice (with deletion of liver kinase B1, LKB1) have shown to spontaneously develop AF with electrical and anatomical changes in the atria that mimic the human AF process.37 Using these models that more accurately represents human AF, oxidative and metabolic stress, and inflammation leading to progressive atrial cardiomyopathy have been considered the cause of AF development and the source of electrical and structural remodeling characteristically observed in AF patients. Suppression of oxidative or metabolic stress and inflammation presents a promising therapeutic target for primary and secondary AF prevention. However, more work is needed to understand the mechanisms of development of AF. There is a current debate on the role of voltage-gated Ca(2+) in AF, the contribution of atrial fibrosis, epicardial fat, and the best techniques to identify structural and electrophysiological drivers of AF.38 Novel genomic data are emerging with candidate genes and short non-coding microRNAs that regulate gene expression and their involvement in the pathophysiology of AF. These are novel and potentially important biomarkers and novel therapeutic targets in AF. Clear elucidation is needed of an individual’s genetic predisposition and modifiable risk factors to facilitate personalized strategies to prevent and treat AF.
Pregnancy
During the peripartum period (two days before to one day after delivery), the risks for ischemic stroke, intracerebral hemorrhage (ICH), and subarachnoid hemorrhage (SAH) increase.39–42 Compared to non-pregnant periods or early pregnancy,41 the peripartum period is associated with a relative risk of 34 for ischemic stroke, 95 for ICH, and 47 for SAH. The postpartum period, which extends from two days to six weeks after delivery, is associated with a relative risk of 8 for ischemic stroke and 12 for ICH, but no excessive risk for SAH. Population-based studies have documented that pre-eclampsia and eclampsia account for 24%–48% of all pregnancy-related strokes,39,40 but associated factors influence these data.43 The reversible cerebral vasospasm syndrome (RCVS) and the posterior reversible vasospasm syndrome (PRES) are also common in post-partum stroke.44,45 These three conditions, pre-eclampsia, RCVS, and PRES, are often concurrent and share underlying features of endothelial dysfunction and failure of cerebrovascular autoregulation43,44 In a preclinical model, when the posterior cerebral arteries of rats were infused with plasma from women with pre-eclampsia, there was increased blood–brain barrier permeability and diminished vasodilation in response to calcium-activated potassium channels.46 There is evidence that endothelin-1 may play an important role in the pathogenesis of pre-eclampsia.47
Large studies have quantified the increased risk for stroke associated with pregnancy; however, only very limited data are available on the added risk for women with prior stroke who are considering a future pregnancy. Approximately 1 in every 200 women of childbearing age has had prior stroke,48 and thus quantitating this added risk is essential to making informed decisions on family planning. Limited data from case series49–51 suggest an absolute risk of 0.7% for recurrent arterial ischemic stroke associated with pregnancy, which is similar to the yearly risk of <1% for recurrent stroke among young adults who have no vascular risk factors.52 However, the 95% confidence interval is very wide (0.04%–4.4%), indicating the need for further study. At this time, therefore, counseling must be based on an uncertain estimate of average risk, interpreted in the context of the specific cause of the prior stroke, and current risk factor status.
Sex differences in stroke treatment
Tissue plasminogen activator
The efficacy of intravenous tissue plasminogen activator (tPA) in treating acute ischemic stroke is well established.53,54 However, the rates of tPA use remain low both nationally and globally. Estimates of tPA use in the US are consistently <5%,55,56 and similar rates are reported in the UK.57 A 2009 meta-analysis of 16 administrative and clinical studies found that women with acute ischemic stroke are less likely than men to receive tPA (OR 0.70; 95% CI, 0.55–0.88), although significant heterogeneity among studies was observed.58 A 2009 analysis of 383,318 acute ischemic stroke admissions from the Get With The Guidelines Stroke registry found that women who presented within 2 h of stroke symptom onset were about 10% less likely than men to receive tPA within 3 h of symptom onset (OR = 0.91; 95% CI, 0.86–0.95), after adjusting for several potential confounders.59 Finally, data from the Nationwide Inpatient Sample from 2004 to 2010 showed that women are less likely than men to receive tPA at both primary stroke centers (OR = 0.87; 95% CI, 0.81–0.94) and non-primary stroke centers (OR = 0.88; 95% CI, 0.82–0.94), after adjusting for potential confounders.60
These sex differences in tPA use are particularly noteworthy when confronted with evidence suggesting that women actually benefit more from tPA than men,60–66 and with definitive evidence that women have worse outcomes after stroke than men.67–72 The reasons for these sex disparities in tPA administration are not clear.73 However, women may be more likely to live alone before stroke,69–70 potentially resulting in delayed symptom recognition and hospital arrival. A registry-based study of 10,048 Canadian patients with acute ischemic or hemorrhagic stroke found that subjects living alone are more likely to be women than men (61.5% vs. 38.5%),74 and almost 50% less likely to arrive at the hospital within 2.5 h of onset of symptoms (OR = 0.54; 95% CI, 0.48–0.60) and to receive tPA (OR = 0.52; 95% CI, 0.43–0.63), after adjusting for age and other potential confounders.
Women may also present with more atypical symptoms of acute stroke compared to men, which can result in delayed diagnosis, and thus lower tPA rates. Such atypical symptoms may include altered consciousness or mental status,75–79 fatigue, drowsiness, or lethargy,77 incontinence,78 pain,80 and generalized weakness.77 If emergency room providers do not recognize these symptoms as potentially representative of acute stroke, patients may be inappropriately triaged and become ineligible for thrombolysis.
Further, women may be less likely to consent to thrombolysis than men. In a survey-based study, 586 patients, recruited from ambulatory clinics at two Canadian hospitals, were presented with a hypothetical scenario in which they were in an emergency room with acute stroke. The scenario included death and disability rates from ischemic stroke with and without thrombolysis.81 Compared to men, women were less likely to accept thrombolysis (OR = 0.58; 95% CI, 0.37–0.92), after adjusting for potential confounders and were more likely to cite risk aversion and the need for more information before making a decision.
Finally, acute hypertension may be undertreated in women presenting with stroke, potentially decreasing their eligibility for thrombolysis. Indeed, a population-based study of ischemic stroke patients who presented to 1 of 16 emergency departments in the Greater Cincinnati/Northern Kentucky region in 2005, found that women and men received similar treatment with antihypertensive medication, even though more women had a systolic blood pressure >185 mm Hg or diastolic blood pressure >110 mm Hg (17.1% vs. 12.4% of men; p = 0.02).82
Carotid intervention
Sex differences in extracranial internal carotid artery disease are receiving increased attention. Pathologic evaluation of carotid endarterectomy (CEA) specimens shows that plaques from women have less macrophage infiltration and a more “stable phenotype,” compared to plaques from men.83 Imaging studies using high-resolution MRI have shown that, in men, plaques are more likely to have a thin fibrous cap with a lipid-rich/necrotic core.84 Areas of stenosis are more common in men.84
These observations may relate to clinical observations that women with carotid stenosis do better with drug therapy. In a pooled analysis of patients with symptomatic 50%–99% carotid stenosis, drug therapy was associated with a lower stroke rate in women but not in men.85 Further, one in nine carotid procedures prevented stroke in men, while only 1 procedure in 36 prevented stroke in women.85 In a combined analysis of two clinical trials related to asymptomatic carotid stenosis, CEA reduced stroke in men, but no definitive benefit was seen in women.86 Thus, some have advocated for a future trial of carotid intervention vs. medical management that includes only women with carotid stenosis.87
Sex and frailty, implications for stroke risk, and outcome
Frailty is a clinical syndrome that results from loss of physiologic reserve across multiple systems, and thus reduced capacity to compensate in response to common stressors.88,89 Frailty has consistently been linked to increased risk for stroke, CVD, and mortality.90 Like stroke, frailty disproportionately affects older women. Indeed, the prevalence of frailty in women is twice that in men.88,91 A recent systematic review of a community-based senior population found that the average prevalence of frailty in women is 9.6% compared to 5.2% in men.91 African American women are particularly vulnerable, with twice the prevalence observed among white women.92,93 Despite these findings, very little has been done to identify specific factors that contribute to such disparaties.94 Women constitute nearly 60% of the US population over 70,95 and as the number of seniors increases in the coming decades, the need to understand relationships between stroke and frailty in women is becoming urgent.
A standardized definition of frailty has not been established. This deficit continues to be an important challenge in the field. The Fried Index, developed by the Cardiovascular Health Study (CHS), is commonly used to assess frailty and is based on five criteria that measure physical attributes: slow walking speed, weakness assessed by handgrip, self-reported low physical activity level, exhaustion, and unintended weight loss.88 According to this construct, frailty is defined as the presence of at least three of these criteria, and pre-frailty is defined as the presence of one or two. However, the frailty index score, which measures the accumulated number of deficits,96 and gait or walking speed alone,97,98 are also used to define frailty.
Stroke and frailty have a bi-directional relationship, i.e. frailty is a risk factor for stroke and also impacts the ability to recover independent function after stroke, while stroke is a known predictor of onset and progression of frailty.99 The prevalency of frailty is in women and men with a history of stroke and CVD is reaching 50% according to some studies.100 Interestingly, in the AGES prospective cohort study from Iceland, frailty was associated with new onset (incident) CVD in women, but not in men, after adjusting for clinical factors including sub-clinical atherosclerosis.100 The Women’s Health Initiative (WHI), an observational study that involved more than 40,000 women 65 and older, found that stroke and coronary artery disease are the most important predictors of frailty.101 In the WHI study, baseline frailty, according to the Fried Index, was present in 16% of women, and the incidence of new onset frailty at three years was 15%.
In the CHS, slower walking speed (a measure of frailty) correlated with white matter hyperintensities and subclinical brain infarcts.102,103 Data from the WHI study showed that slow walking speed is an independent risk factor for ischemic stroke among postmenopausal women.104 Indeed, the risk for stroke was 69% higher in the slowest walking speed tertile compared to the fastest walking speed tertile (HR = 1.69; 95% CI, 1.21–2.36).
The WHI also found that low weight, obesity, smoking, and depressive symptoms are strongly associated with the development of frailty, and potentially represent important targets for prevention.101 Thus, a better understanding of factors that predict frailty and its longitudinal course, and the extent to which such factors differ between women and men will provide crucial insight into the design of interventions to prevent or ameliorate the effects of frailty on stroke incidence and outcomes in women.105
Biology of sex differences in cerebral ischemia
The incidence of human stroke is sexually dimorphic until late in life, well beyond the years of reproductive senescence and menopause. From early through mid-adulthood years, stroke incidence is lower in women compared to men. However, with advancing age, the incidence of stroke and stroke-related mortality becomes higher in women.1 This overarching observation has led to much work and the notion that biologic mechanisms of cell death in the ischemic brain are influenced in part, by biologic sex and in part, by the availability of female and male sex steroids before or after injury. These hormones clearly contribute to, but do not fully account for, sex-specific responses to cerebral ischemia.106
In models of global cerebral ischemia after cardiac arrest, histologic damage is less in female mice compared to males. This relative protection from ischemic injury is linked to the presence or absence of circulating estradiol.107 In an innovative mouse model of pre-pubertal juvenile cardiac arrest, the relative protection afforded to female mice is lost in juvenile females compared to age-matched males.108 The mechanism of neuroprotection appears to be multi-factorial, as would be expected with a pleiotropic steroid such as estradiol. Molecular signaling likely involves estrogen receptor subtype B or G protein-coupled receptor 30.109
In models of focal cerebral ischemia, early studies showed that female stroke-prone hypertensive rats have a lower incidence of vascular events compared to males.110 Subsequent studies found that this protective phenotype is eliminated by ovariectomy and restored by supplemental estrogen.111 Interestingly, the neuroprotective effect of estrogen is not sex-dependent as exogenous estradiol protects both male and female brains.112 Indeed, acute estradiol therapy during reperfusion reduces tissue damage and enhances post-ischemic blood flow in adult male rats.113
However, since the vast majority of strokes occur in older adults, sex hormones have always been considered only a small piece of the puzzle. In female rodents, for example, the response to focal ischemia via vascular occlusion becomes altered during gonadal senescence.114,115 Middle-aged females have larger strokes and more inflammation compared to age-matched males or younger females.116 The underpinning mechanisms responsible for this shift from an “ischemia-protected” to an “ischemia-sensitive” phenotype in aging females are not clearly defined but involve loss of estrogen, increased systemic inflammation, and age-related changes in gene expression.117
Recent work has demonstrated changes in adipose immune cells that promote a pro-inflammatory milieu in middle-aged females, driven by a shift in the balance of pro-inflammatory and anti-inflammatory T cells.118 There is an age-related increase in CD8+ T cells in adipose tissue, but not in blood which is exacerbated in females. After ex vivo stimulation of T cells, this increase in CD8+ T cells results in higher levels of IFN-γ, TNF-α, and granzyme B. Middle-aged females also have lower levels of regulatory T cells (Tregs, an anti-inflammatory T-cell subtype) in adipose tissue compared to age-matched males. This shift in T-cell balance produces a pro-inflammatory milieu, which may contribute to the increased CVD burden observed in aging females.118
Sex differences in ischemic sensitivity and death pathways
The most convincing biologic evidence for intrinsic sex differences in stroke sensitivity arose when sex-specificity was modeled in sex-specific cell cultures grown in nourishing media that contained no sex steroids.119,120 The resulting data suggest that response to cerebral ischemia is mechanistically different in genetic female (XX) vs. male (XY) cells.120 If this is true, molecular signaling pathways activated by ischemia may be different in the male vs. female brain. One of the first pathways found to support this overarching hypothesis involved neuronal nitric oxide synthase (nNOS), poly-ADP ribose polymerase-1 (PARP-1), and apoptosis-inducing factor (AIF). This well-characterized “cell death” cascade begins with overproduction of nitric oxide (NO) via nNOS. The increased NO combines with available superoxide to initiate DNA damage, which in turn, activates the DNA repair enzyme PARP-1, leading to energy depletion, mitochondrial dysfunction, and release of pro-apoptotic molecules such as AIF.121
The evidence that originally defined this cell death mechanism was drawn exclusively from male animals and from cell-culture systems that were not sex specific.121 Thus, an inherent sex difference in the death pathway was missed initially. For example, female nNOS and PARP-1 knockout mice do not benefit from genetic deletion of these molecules, unlike their male counterparts.122 Similarly, pharmacologic inhibitors of these target molecules only protects the male brain from ischemic injury.123 Since the presence of sex hormones does not alter these outcomes, it was suggested that the nNOS-PARP pathway is quantitatively important only in males.123 However, later work showed that females are more vulnerable to cell death induced by activation of cytochrome-c and caspases.124 Caspases play key roles in inflammasome activation, and the first sex-specific microRNA regulators of cell death in experimental stroke involve caspase regulation.125 These initial studies have been replicated by others, and further work is continuing to identify cell death pathways that are influenced by sex (i.e. autophagy) in neurons and other cell types.126,127 Such findings have important translational implications for stroke patients as neuroprotective drugs may have different effects in males vs. females.128,129 To date, all clinical attempts to translate promising neuroprotective agents developed in the laboratory have failed. There are a multitude of potential reasons for these “translational failures” which have been recently highlighted.130 One potential contributing factor to these failures is the overwhelming use of young, male animals in pre-clinical studies.131 It is likely the mechanistic pathways activated after ischemic injury play a role. As an example, Li et al. have previously shown that minocycline, a drug that works in part as a PARP inhibitor, was robustly effective in protecting male animals after stroke, but showed no neuroprotective in females in a standard filament model of middle cerebral occlusion.132 In line with these pre-clinical results, a recent clinical study confirmed that minocycline only improved stroke outcomes in male patients.133 Similar sex differences have been noted in clinical trials when sex-specific outcomes were specifically examined. Uric acid treatment in combination with tPA doubled the effect of placebo for an excellent outcome in women but had no effect in men. Interactions with serum uric acid levels on infarct growth were only significant in women.134 These results demonstrate the importance of considering sex as a biological variable throughout therapeutic development of neuroprotective agents. Equally important is the recognition that many physiological differences are present in men and women and ensuring that clinical trials are designed and powered to perform sex-specific outcome analysis.
Sex-specific genetic and epigenetic influences
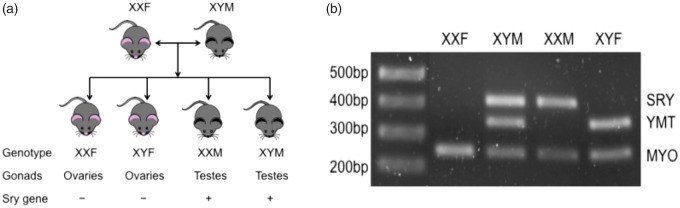
Only recently have relative contributions of X and Y chromosomes to stroke outcomes been explored. Investigators are using a four-core-genotype (FCG) mouse model to determine the role of sex chromosomes in ischemic sensitivity and outcome.135 In the FCG model, the testes-determining gene Sry is deleted from the Y chromosome and inserted on an autosome. If the mouse inherits the Sry gene, it is an XY Male (XYM), i.e. a gonadal and chromosomal male. Genetic crosses between XYM and wild-type females will produce FCG genotypes, as depicted in Figure 1. This model allows dissociation of chromosomal from gonadal sex in order to test the hypothesis that chromosomal sex is important to outcome from middle cerebral artery occlusion. In initial studies, gonadally intact FCG mice sustained significantly smaller strokes compared to phenotypic males. To evaluate the role of gonadal hormones in this result, a subsequent cohort of mice was tested after gonadectomy. All mice, regardless of their chromosome XX or XY compliment, experienced equivalent stroke damage suggesting that ischemic sensitivity is mediated exclusively by the effects of circulating gonadal hormones.135
Figure 1.
The four-core-genotype (FCG) mouse model to determine the role of sex chromosomes in ischemic sensitivity and outcome. (a) Schematic of the generation of FCG mice in which the Sry gene (the testis-determining gene; sex-determining region, Y chromosome) is removed from the Y chromosome and inserted on an autosome (Chromosome 3).139 This produces XX and XY mice with testes, and XX and XY mice with ovaries, allowing for the independent assessment of the effects of sex chromosome complement. (b) PCR showing the presence of the Sry in XYM and XXM (gonadal males) and the absence of Sry in XXF and XYF (gonadal females). Ymt identifies the Y chromosome and Myo is used as a positive control.
When studies were repeated in aged FCG mice with low levels of endogenous gonadal hormones, the importance of XX chromosomes in stroke outcome, regardless of gonadal sex, was observed. Indeed, animals with an XX chromosome compliment had larger infarcts, higher neurologic deficit scores, and greater immune-cell infiltration and activation, compared to animals with an XY chromosome compliment.136 These results suggest a detrimental effect of the second X chromosome that is only evident after reproductive senescence, which implies a complex interaction between aging, ischemia, and sex chromosome genes. One mechanism that may account for the chromosomal dependence observed in the FCG model is related to function of microglia, the innate resident immune cells of the brain.137 In animals with 2 X chromosomes, microglial activation after focal stroke is enhanced, and thus inflammation is greatly enhanced.136 The search for gene candidates responsible for these effects is currently underway.
In summary, there is now ample evidence that the pathophysiology of stroke is sex specific. This implies that development of pharmacologic strategies for stroke prevention and treatment must consider sex as part of the design of any new clinical trial. However, despite the increasing awareness of the importance of sex differences in stroke and vascular disease, we continue to fall short in the laboratory and in the clinic. The over-reliance on data derived from young male animals has been recently highlighted,138 and the lack of sex-specific endpoints in clinical trials continues to retard stroke research. For example, the recently published SPRINT trial showed that lowering blood pressure significantly improves vascular outcomes in men, but not significantly in women, perhaps because female enrollment was low, and event rates are lower in females vs. males.124 This creates a major gap in our knowledge and leaves clinicians uncertain about how to provide the best care for their female patients. We urgently need adaptive trial designs that require enrollment of women until futility or endpoints are met. Acceptance that some therapies may work in only one sex should also not lead to the abandonment of the therapy. Recognizing that one drug will not “fit all” is the cornerstone of personalized medicine, and treating some stroke patients is better than treating none at all. Developing novel, efficacious, and safe neuroprotective agents with sex-specificity in mind is essential to reducing stroke-related morbidity and mortality.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and /or publication of this article.
References
- 1.Bushnell CD, McCullough LD, Awad IA, et al. Guidelines for the prevention of stroke in women. Stroke 2014; 45: 1545–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin YS, Tang CH, Yang CY, et al. Effect of pre-eclampsia-eclampsia on major cardiovascular events among peripartum women in Taiwan. Am J Cardiol 2011; 107: 325–530. [DOI] [PubMed] [Google Scholar]
- 3.Bushnell CD, Howard VJ, Lisabeth L, et al. Sex differences in the evaluation and treatment of acute ischemic stroke. Lancet Neurol 2018. in press. [DOI] [PubMed] [Google Scholar]
- 4.Bushnell CD, Reeves MJ, Pan W, et al. Sex differences in quality of life after ischemic stroke. Neurology 2014; 82: 922–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peacock WF, Tamayo S, Patel M, et al. CHA2DS2-VASc scores and major bleeding in patients with nonvalvular atrial fibrillation who are receiving rivaroxaban. Ann Emerg Med 2017; 69: 541–550. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics – 2017 update: a report from the American Heart Association. Circulation 2017; 135: e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reeves MJ, Bushnell CD, Howard G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol 2008; 7: 915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leading Causes of Death (LCOD) by Race/ethnicity, all females-United States, 2014, www.cdc.gov/women/lcod/2014/race-ethnicity/index.htm (accessed 1 February 2018).
- 9.Leading Causes of Death (LCOD) by race/ethnicity, all males-United States, www.cdc.gov/healthequity/lcod/men/2014/race-ethnicity/index.htm (2014, accessed 1 February 2018).
- 10.Carandang R, Seshadri S, Beiser A, et al. Trends in the incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA 2006; 296: 2939–2946. [DOI] [PubMed] [Google Scholar]
- 11.Petrea RE, Beiser AS, Seshadri S, et al. Gender differences in stroke incidence and post-stroke disability in the Framingham heart study. Stroke 2009; 40: 1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koton S, Schneider AL, Rosamond WD, et al. Stroke incidence and mortality trends in US communities, 1987–2011. JAMA 2014; 312: 259–268. [DOI] [PubMed] [Google Scholar]
- 13.Madsen TE, Khoury J, Alwell K, et al. Sex-specific stroke incidence over time in the Greater Cincinnati/Northern Kentucky Stroke Study. Neurology 2017; 89: 990–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benjamin EJ, Virani SS, Callaway CW, et al. American Heart Association Council on prevention, statistics, stroke statistics, heart disease and stroke statistics – 2018 update. Circulation 2018; 137: e67–e492. [DOI] [PubMed] [Google Scholar]
- 15.Kleindorfer DO, Khoury J, Moomaw CJ, et al. Stroke incidence is decreasing in whites but not in blacks: a population-based estimate of temporal trends in stroke incidence from the greater Cincinnati/Northern Kentucky Stroke Study. Stroke 2010; 41: 1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Q, Tong X, Schieb L, et al. Vital signs: recent trends in stroke death rates – United States 2000–2015. MMWR Morb Mortal Wkly Rep 2017; 66: 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgenstern LB, Smith MA, Sanchez BN, et al. Persistent ischemic stroke disparities despite declining incidence in mexican americans. Ann Neurol 2013; 74: 778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard G, Moy CS, Howard VJ, et al. Where to focus efforts to reduce the black-white disparity in stroke mortality: incidence versus case fatality? Stroke 2016; 47: 1893–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Backholer K, Peteres SAE, Bots SH, et al. Sex differences in the relatoinship between socioeconomic status and cardiovascular disease: a systematic review and meta-analysis. J Epi Comm Health 2017; 71: 550–557. [DOI] [PubMed] [Google Scholar]
- 20.Honjo K, Iso H, Ikeda A, et al. Education level and physical functional limitations among Japanese community residents – gender difference in prognosis from stroke. BMC Public Health 2009; 9: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lisabeth LD, Reeves MJ, Baek J, et al. Factors influencing sex differences in poststroke functional outcome. Stroke 2015; 46: 860–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014; 129: 837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emdin CA, Wong CX, Hsiao AJ, et al. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. BMJ 2016; 532: h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ball J, Carrington MJ, Wood KA, et al. Women versus men with chronic atrial fibrillation: insights from the Standard versus Atrial Fibrillation spEcific managemenT studY (SAFETY). PLoS One 2013; 8: e65795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heering AJ, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J 2006; 27: 949–953. [DOI] [PubMed] [Google Scholar]
- 26.Wang TJ, Massaro JM, Levy D, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA 2003; 290: 1049–1056. [DOI] [PubMed] [Google Scholar]
- 27.Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998; 98: 946–952. [DOI] [PubMed] [Google Scholar]
- 28.Schnabel RB, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet 2015; 386: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Study. JAMA 1994; 271: 840–844. [PubMed] [Google Scholar]
- 30.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014; 130: e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146: 857–867. [DOI] [PubMed] [Google Scholar]
- 32.Pancholy SB, Sharma PS, Pancholy DS, et al. Meta-analysis of gender differences in residual stroke risk and major bleeding in patients with nonvalvular atrial fibrillation treated with oral anticoagulants. Am J Cardiol 2014; 113: 485–490. [DOI] [PubMed] [Google Scholar]
- 33.Lubitz SA, Ozcan C, Magnani JW, et al. Advances in the genetics of atrial fibrillation: implications for future research directions and personalized medicine. Circ Arrhythm Electrophysiol 2010; 1: 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wakill R, Voigt N, Kaab S, et al. Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest 2011; 121: 2955–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nattel S, Burstein B, Dobrey D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol 2008; 1: 62–73. [DOI] [PubMed] [Google Scholar]
- 36.Ozcan C, Battaglia E, Young R, et al. LKB1 knockout mouse develops spontaneous atrial fibrillation and provides mechanistic insights into human disease process. J Am Heart Assoc 2015; 15: e001733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ikeda Y, Sato K, Pimentel DR, et al. Cardiac-specific deletion of LKB1 leads to hypertrophy and dysfunction. J Biol Chem 2009; 284: 35839–35849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schotten U, Dobrey D, Platonov PG, et al. Current controversies in determining the main mechanisms of atrial fibrillation. J Internal Med 2016; 279: 428–438. [DOI] [PubMed] [Google Scholar]
- 39.Sharshar T, Lamy C, Mas JL. Incidence and causes of strokes associated with pregnancy and puerperium. A study in public hospitals of Ile de France. Stroke in Pregnancy Study Group. Stroke 1995; 26: 930–936. [DOI] [PubMed] [Google Scholar]
- 40.Kittner SJ, Stern BJ, Feeser BR, et al. Pregnancy and the risk of stroke. N Engl J Med 1996; 335: 768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salonen Ros H, Lichtenstein P, Bellocco R, et al. Increased risks of circulatory diseases in late pregnancy and puerperium. Epidemiology 2001; 12: 456–460. [DOI] [PubMed] [Google Scholar]
- 42.Ban L, Sprigg N, Abdul Sultan A, et al. Incidence of first stroke in pregnant and non-pregnant women of childbearing age: a population-based cohort study from England. J Am Heart Assoc 2017; 6: pii e004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDermott M, Miller EC, Rundek T, et al. Preeclampsia: association with posterior reversible encephalopathy syndrome and stroke. Stroke 2018; 49: 524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller EC, Yaghi S, Boehme AD, et al. Mechanisms and outcomes of stroke during pregnancy and the postpartum period: a cross-sectional study. Neurol Clin Pract 2016; 6: 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshida K, Takahashi JC, Takenobu Y, et al. Strokes associated with pregnancy and puerperium: a nationwide study by the Japan Stroke Society. Stroke 2017; 48: 276–282. [DOI] [PubMed] [Google Scholar]
- 46.Wallace K, Tremble SM, Owens MY, et al. Plasma from patients with HELLP syndrome increases blood brain barrier permeability. Reprod Sci 2015; 22: 278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saleh L, Verdonk K, Visser W, et al. The emerging role of endothelin-1 in the pathogenesis of pre-eclampsia. Ther Adv Cardiovasc Dis 2016; 10: 282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singhal A B, Biller J, Elkind MS, et al. Recognition and management of stroke in young adults and adolescents. Neurology 2013; 81: 1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lamy C, Hamon JB, Coste J, et al. Ischemic stroke in young women: risk of recurrence during subsequent pregnancies. Neurology 2000; 55: 269–274. [DOI] [PubMed] [Google Scholar]
- 50.Coppage KH, Hinton AC, Moldenhauer J, et al. Maternal and perinatal outcome in women with a history of stroke. Am J Obstet Gynecol 2004; 190: 1331–1334. [DOI] [PubMed] [Google Scholar]
- 51.Crovetto F, Ossola MW, Spadaccini G, et al. Ischemic stroke recurrence during pregnancy: a case series and a review of the literature. Arch Gynecol Obstet 2012; 286: 599–604. [DOI] [PubMed] [Google Scholar]
- 52.Putaala J, Metso AJ, Metso TM, et al. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke the Helsinki young stroke registry. Stroke 2009; 40: 1195–1203. [DOI] [PubMed] [Google Scholar]
- 53.National Institute of Neurological Diseases and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. NEJM 1995; 333: 1581–1587. [DOI] [PubMed] [Google Scholar]
- 54.Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44: 870–947. [DOI] [PubMed] [Google Scholar]
- 55.Kleindorfer D, Lindsell CJ, Brass L, et al. National us estimates of recombinant tissue plasminogen activator use: ICD-9 codes substantially underestimate. Stroke 2008; 39: 924–928. [DOI] [PubMed] [Google Scholar]
- 56.Fang MC, Cutler DM, Rosen AB. Trends in thrombolytic use for ischemic stroke in the United States. J Hosp Med 2010; 5: 406–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lees KR, Ford GA, Muir KW, et al. Thrombolytic therapy for acute stroke in the United Kingdom: experience from the safe implementation of thrombolysis in stroke (sits) register. QJM 2008; 101: 863–869. [DOI] [PubMed] [Google Scholar]
- 58.Reeves M, Bhatt A, Jajou P, et al. Sex differences in the use of intravenous rt-PA thrombolysis treatment for acute ischemic stroke: a meta-analysis. Stroke 2009; 40: 1743–1749. [DOI] [PubMed] [Google Scholar]
- 59.Reeves MJ, Fonarow GC, Zhao X, et al. Quality of care in women with ischemic stroke in the GWTG program. Stroke 2009; 40: 1127–1133. [DOI] [PubMed] [Google Scholar]
- 60.Boehme AK, Carr BG, Kasner SE, et al. Sex differences in rt-PA utilization at hospitals treating stroke: the national inpatient sample. Front Neurol 2017; 8: 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kent DM, Price LL, Ringleb P, et al. Sex-based differences in response to recombinant tissue plasminogen activator in acute ischemic stroke: a pooled analysis of randomized clinical trials. Stroke 2005; 36: 62–65. [DOI] [PubMed] [Google Scholar]
- 62.Shobha N, Sylaja PN, Kapral MK, et al. Differences in stroke outcome based on sex. Neurology 2010; 74: 767–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee SJ, Heo SH, Ambrosius WT, et al. Factors mediating outcome after stroke: gender, thrombolysis, and their interaction. Transl Stroke Res 2018; 9: 267–273. [DOI] [PubMed] [Google Scholar]
- 64.Nathanson D, Patrone C, Nystrom T, et al. Sex, diastolic blood pressure, and outcome after thrombolysis for ischemic stroke. Stroke 2014; 2014: 747458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lasek-Bal A, Puz P, Kazibutowska Z. Efficacy and safety assessment of alteplase in the treatment of stroke – gender differences. Neurol Res 2014; 36: 851–856. [DOI] [PubMed] [Google Scholar]
- 66.Lorenzano S, Ahmed N, Falcou A, et al. Does sex influence the response to intravenous thrombolysis in ischemic stroke?: answers from safe implementation of treatments in stroke – International Stroke Thrombolysis Register. Stroke 2013; 44: 3401–3406. [DOI] [PubMed] [Google Scholar]
- 67.Persky RW, Turtzo LC, McCullough LD. Stroke in women: disparities and outcomes. Curr Cardiol Rep 2010; 12: 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kapral MK, Fang J, Hill MD, et al. Sex differences in stroke care and outcomes: results from the registry of the Canadian stroke network. Stroke 2005; 36: 809–814. [DOI] [PubMed] [Google Scholar]
- 69.Glader EL, Stegmayr B, Norrving B, et al. Sex differences in management and outcome after stroke: a Swedish national perspective. Stroke 2003; 34: 1970–1975. [DOI] [PubMed] [Google Scholar]
- 70.Lai SM, Duncan PW, Dew P, et al. Sex differences in stroke recovery. Prev Chronic Dis 2005; 2: A13. [PMC free article] [PubMed] [Google Scholar]
- 71.Lisabeth LD, Reeves MJ, Baek J, et al. Factors influencing sex differences in poststroke functional outcome. Stroke 2015; 46: 860–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Di Carlo A, Lamassa M, Baldereschi M, et al. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in europe: data from a multicenter multinational hospital-based registry. Stroke 2003; 34: 1114–1119. [DOI] [PubMed] [Google Scholar]
- 73.Lisabeth LD, Brown DL, Morgenstern LB. Barriers to intravenous tissue plasminogen activator for acute stroke therapy in women. Gender Med 2006; 3: 270–278. [DOI] [PubMed] [Google Scholar]
- 74.Reeves MJ, Prager M, Fang J, et al. Impact of living alone on the care and outcomes of patients with acute stroke. Stroke 2014; 45: 3083–3085. [DOI] [PubMed] [Google Scholar]
- 75.Eriksson M, Glader EL, Norrving B, et al. Sex differences in stroke care and outcome in the Swedish National Quality Register for Stroke Care. Stroke 2009; 40: 909–914. [DOI] [PubMed] [Google Scholar]
- 76.Lisabeth LD, Brown DL, Hughes R, et al. Acute stroke symptoms: comparing women and men. Stroke 2009; 40: 2031–2036. [DOI] [PubMed] [Google Scholar]
- 77.Jerath NU, Reddy C, Freeman WD, et al. Gender differences in presenting signs and symptoms of acute ischemic stroke: a population-based study. Gender Med 2011; 8: 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gall SL, Donnan G, Dewey HM, et al. Sex differences in presentation, severity, and management of stroke in a population-based study. Neurology 2010; 74: 975–981. [DOI] [PubMed] [Google Scholar]
- 79.Niewada M, Kobayashi A, Sandercock PA, et al. Influence of gender on baseline features and clinical outcomes among 17,370 patients with confirmed ischaemic stroke in the international stroke trial. Neuroepidemiology 2005; 24: 123–128. [DOI] [PubMed] [Google Scholar]
- 80.Labiche LA, Chan W, Saldin KR, et al. Sex and acute stroke presentation. Ann Emerg Med 2002; 40: 453–460. [DOI] [PubMed] [Google Scholar]
- 81.Kapral MK, Devon J, Winter AL, et al. Gender differences in stroke care decision-making. Med Care 2006; 44: 70–80. [DOI] [PubMed] [Google Scholar]
- 82.Madsen TE, Khoury JC, Alwell KA, et al. Analysis of tissue plasminogen activator eligibility by sex in the greater Cincinnati/Northern Kentucky Stroke Study. Stroke 2015; 46: 717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hellings WE, Pasterkamp G, Verhoeven BA, et al. Gender-associated differences in plaque phenotype of patients undergoing carotid endarterectomy. J Vasc Surg 2007; 45: 289–296. [DOI] [PubMed] [Google Scholar]
- 84.Ota H, Reeves MJ, Zhu DC, et al. Sex differences in patients with asymptomatic carotid atherosclerotic plaque. Stroke 2010; 41: 1630–1635. [DOI] [PubMed] [Google Scholar]
- 85.Rothwell PM, Eliasziw M, Gutnikov SA, et al. Endarterectomy for symptomatic stenosis in relation to clinical subgroups and timing of surgery. Lancet 2004; 363: 915–924. [DOI] [PubMed] [Google Scholar]
- 86.Rothwell PM, Goldstein LB. Carotid endarterectomy for asymptomatic carotid stenosis: asymptomatic Carotid Surgery Trial. Stroke 2004; 35: 2425–2427. [DOI] [PubMed] [Google Scholar]
- 87.Marulanda-Londono E, Chaturvedi S. Carotid stenosis in women: time for a reappraisal. Stroke Vasc Neurol 2016; 1: 192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M156. [DOI] [PubMed] [Google Scholar]
- 89.Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm – issues and controversies. J Gerontol A Biol Sci Med Sci 2007; 62: 731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Singh M, Stewart R, White H. Importance of frailty in patients with cardiovascular disease. Eur Heart J 2014; 5: 1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Collard RM, Boter H, Schoevers RA, et al. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012; 60: 1487–1492. [DOI] [PubMed] [Google Scholar]
- 92.Hirsch C, Anderson ML, Newman A, et al. The association of race with frailty: the cardiovascular health study. Ann Epidemiol 2006; 16: 545–553. [DOI] [PubMed] [Google Scholar]
- 93.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci 2006; 61: 262–266. [DOI] [PubMed] [Google Scholar]
- 94.Hubbard RE. Sex differences in frailty. Interdiscip Topics Gerontol Geriatr 2015; 41: 41–53. [DOI] [PubMed] [Google Scholar]
- 95.US Census Bureau. Annual estimates of the resident population for selected age groups by sex for the United States, states, counties, and Puerto Rico commonwealth and municipios: April 1, 2010 to July 1, 2014. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=DEC_10_DP_DPDP1&src=pt.
- 96.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 2011; 27: 17–26. [DOI] [PubMed] [Google Scholar]
- 97.Studenski S, Perera S, Patel K, et al. Gait speed and survival. JAMA 2011; 305: 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abellan van Kan G, Rolland Y, Bergman H, et al. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging 2008; 12: 29–37. [DOI] [PubMed] [Google Scholar]
- 99.Singh M, Stewart R and White H. Importance of frailty in patients with cardiovascular disease. Eur Heart J 2014; 35: 1726–1731. [DOI] [PMC free article] [PubMed]
- 100.Veronese N, Sigeirsdottir K, Eiriksdottir G, et al. Frailty and risk of cardiovascular diseases in older persons: the age, gene/environment Susceptibility-Reykjavik Study. Rejuvenation Res 2017; 20: 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fugate Woods N, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative observational study. J Am Geriatr Soc 2005; 53: 1321–1330. [DOI] [PubMed] [Google Scholar]
- 102.Newman AB, Gottdiener JS, Mcburnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci 2001; 56: M158–M166. [DOI] [PubMed] [Google Scholar]
- 103.Rosano C, Brach J, Longstreth WT, Jr, et al. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology 2006; 26: 52–60. [DOI] [PubMed] [Google Scholar]
- 104.McGinn AP, Kaplan RC, Verghese J, et al. Walking speed and risk of incident ischemic stroke among postmenopausal women. Stroke 2008; 39: 1233–1239. [DOI] [PubMed] [Google Scholar]
- 105.Ferrucci L, Guralnik JM, Studenski S, et al. Interventions on Frailty Working Group Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc 2004; 52: 625–634. [DOI] [PubMed] [Google Scholar]
- 106.McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab 2003; 14: 228–235. [DOI] [PubMed] [Google Scholar]
- 107.Noppens RR, Kofler J, Hurn PD, et al. Dose-dependent neuroprotection by 17beta-estradiol after cardiac arrest and cardiopulmonary resuscitation. Crit Care Med 2005; 33: 1595–1602. [DOI] [PubMed] [Google Scholar]
- 108.Dietz RM, Deng G, Orfila JE, et al. Therapeutic hypothermia protects against ischemia-induced impairment of synaptic plasticity following juvenile cardiac arrest in sex-dependent manner. Neuroscience 2016; 325: 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lu D, Qu Y, Shi F, et al. Activation of G protein-coupled estrogen receptor 1 (GPER1) ameliorates blood brain barrier permeability after global cerebral ischemia in ovariectomized rats. Biochem Biophys Res Comm 2016; 477: 209–214. [DOI] [PubMed] [Google Scholar]
- 110.Yamori Y, Horie R, Sato M, et al. Proceedings: prophylactic trials for stroke in stroke-prone SHR: effect of sex hormones. Japan Heart J 1976; 17: 404–406. [DOI] [PubMed] [Google Scholar]
- 111.Alkayed NJ, Harukuni I, Kimes AS, et al. Gender-linked brain injury in experimental stroke. Stroke 1998; 29: 159–165. [DOI] [PubMed] [Google Scholar]
- 112.Toung TJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke 1998; 29: 1666–1670. [DOI] [PubMed] [Google Scholar]
- 113.McCullough LD, Alkayed NJ, Traystman RJ, et al. Postischemic estrogen reduces hypoperfusion and secondary ischemia after experimental stroke. Stroke 2001; 32: 796–802. [DOI] [PubMed] [Google Scholar]
- 114.Manwani B, Liu F, Scranton V, et al. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp Neurol 2013; 249: 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu F, Benashski SE, Xu Y, et al. Effects of chronic and acute oestrogen replacement therapy in aged animals after experimental stroke. J Neuroendocrinol 2012; 24: 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Selvamani A, Sohrabji F. Reproductive age modulates the impact of focal ischemia on the forebrain as well as the effects of estrogen treatment in female rats. Neurobiol Aging 2010; 31: 1618–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sohrabji F, Park MJ, Mahnke AH. Sex differences in stroke therapies. J Neurosci Res 2017; 95: 681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ahnstedt H, Roy-O’Reilly M, Spychala M, et al. Sex differences in adipose tissue CD8+ T cells and regulatory t cells in middle-aged mice. Front Immunol 2018; 9: 659 https://doi.org/10.3389/fimmu.2018.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Du L, Bayir H, Lai Y, et al. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem 2004; 279: 38563–38570. [DOI] [PubMed] [Google Scholar]
- 120.Li H, Pin S, Zeng Z, et al. Sex differences in cell death. Ann Neurol 2005; 58: 317–321. [DOI] [PubMed] [Google Scholar]
- 121.Eliasson MJ, Sampei K, Mandir AS, et al. Poly (ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med 1997; 3: 1089–1095. [DOI] [PubMed] [Google Scholar]
- 122.McCullough LD, Zeng Z, Blizzard KK, et al. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab 2005; 25: 502–512. [DOI] [PubMed] [Google Scholar]
- 123.Yuan M, Siegel C, Zeng Z, et al. Sex differences in the response to activation of the poly (ADP-ribose) polymerase pathway after experimental stroke. Exp Neurol 2009; 217: 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu F, Li Z, Li J, et al. Sex differences in caspase activation after stroke. Stroke 2009; 40: 1842–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Siegel C, Li J, Liu F, et al. miR-23a regulation of X-linked inhibitor of apoptosis (XIAP) contributes to sex differences in the response to cerebral ischemia. Proc Natl Acad Sci U S A 2011; 108: 11662–11667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Du L, Hickey RW, Bayir H, et al. Starving neurons show sex difference in autophagy. J Biol Chem 2009; 284: 2383–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Boddu R, Fan C, Rangarajan S, et al. Unique sex- and age-dependent effects in protective pathways in acute kidney injury. Am J Physiol 2017; 313: F740–F755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li J, McCullough LD. Sex differences in minocycline-induced neuroprotection after experimental stroke. J Cereb Blood Flow Metab 2009; 29: 670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Amiri-Nikpour MR, Nazarbaghi S, Hamdi-Holasou M, et al. An open-label evaluator-blinded clinical study of minocycline neuroprotection in ischemic stroke: gender-dependent effect. Acta Neurol Scand 2015; 131: 45–50. [DOI] [PubMed] [Google Scholar]
- 130.Bosetti F, Koenig JI, Avata C, et al. Translational stroke research: vision and opportunities. Stroke 2017; 48: 2632–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van der Worp HB, de Haan P, Morrema E, et al. Methodological quality of animal studies on neuroprotection in focal cerebral ischaemia. J Neurol 2005; 252: 1108–1114. [DOI] [PubMed] [Google Scholar]
- 132.Li J, McCullough LD. Sex differences in minocycline-induced neuroprotection after experimental stroke. J Cereb Blood Flow Metab 2009; 29: 670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Amiri-Nikpour MR, Nazarbaghi S, Hamdi-Holason M, et al. An open-label evaluation-blinded clinical study of minocycline neuroprotection in ischemic stroke: gender-dependent affect. Acta Neurol Scand 2015; 131: 45–50. [DOI] [PubMed] [Google Scholar]
- 134.Llull L, Laredo C, Renu A, et al. Uric acid improves clinical outcome in women with acute ischemic stroke. Stroke 2015; 47: 2874–2876. [DOI] [PubMed] [Google Scholar]
- 135.Manwani B, Bentivegna K, Benashski SE, et al. Sex differences in ischemic stroke sensitivity are influenced by gonadal hormones, not by sex chromosome complement. J Cereb Blood Flow Metab 2015; 35: 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McCullough LD, Mirza MA, Xu Y, et al. Stroke sensitivity in the aged: sex chromosome complement vs. gonadal hormones. Aging 2016; 8: 1432–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rahimijan R, Cordeau P, Kriz J. Brain response to injuries: when microglia go sexist. Neuroscience 2018. pii: S0306-4522(18)30173-8 epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 138.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature 2014; 509: 282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Itoh Y, Mackie R, Kampf K, et al. Four core genotypes mouse model: localization of the Sry transgene and bioassay for testicular hormone levels. BMC Res Notes 2015; 8: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]