Abstract
Ischemic brain injury causes a local inflammatory response, involving the activation of resident brain cells such as microglia and the recruitment of infiltrating immune cells. Increasing evidence supports that plasticity of the myeloid cell lineage is determinant for the specific role of these cells on stroke outcome, from initiation and maintenance to resolution of post-ischemic inflammation. The aim of this review is to summarize some of the key characteristics of these cells and the mechanisms for their recruitment into the injured brain through interactions with platelets, endothelial cells and other leukocytes. Also, we discuss the existence of different leukocyte subsets in the ischemic tissue and, specifically, the impact of different myeloid phenotypes on stroke outcome, with special emphasis on neutrophils and their interplay with platelets. Knowledge of these cellular phenotypes and interactions may pave the way to new therapies able to promote protective immune responses and tissue repair after cerebral ischemia.
Keywords: Neuroinflammation, cerebral ischemia, leukocyte, microglia, neuroimmune, phenotypes
Introduction
Inflammation is recognized as an important contributor to the pathophysiology of stroke. The inflammatory response induces the release of reactive oxygen species (ROS) and promotes immune-derived mechanisms associated with cytotoxicity and brain damage.1,2 In particular, the innate immune system plays a pivotal role in the evolution of ischemic cerebral injury, as soluble mediators (i.e. cytokines and chemokines) and specialized cells, activated in the brain or recruited from the periphery, actively participate in the detrimental processes implicated in tissue damage.3,4 In this context, a vast number of experimental studies have explored the beneficial role of anti-inflammatory approaches to block/antagonize key pro-inflammatory pathways driven by cerebral ischemia.2 However, the dualistic role exerted by several mediators of the immune reaction may explain why most anti-inflammatory approaches, conceived disregarding the potential beneficial function of the target, have failed to reach the clinical setting.5 In addition, the fact that ischemic cerebral injury involves several mechanisms may explain why “single-target” therapies have resulted insufficient, a reason for which it has been proposed that therapeutic approaches should target multiple cell types to promote protection and recovery.6 An important factor to consider is that a part of the inflammatory response is its resolution, an active process that involves the participation of myeloid cells with distinct phenotypes, which could reflect either the local conversion of cells from a pro-inflammatory to an anti-inflammatory phenotype or the recruitment of cells with different phenotypes and functions. In this context, whereas polarization of microglia/macrophages has been extensively investigated,7 the roles of neutrophils are understudied. Increasing evidence shows the ability of neutrophils to switch towards a variety of phenotypes dependent on specific microenvironmental signals which are related to the spatio-temporal progression of ischemic brain damage.8–10 Another crucial issue which remains quite unknown is the important impact of the interaction of neutrophils with platelets in the onset of inflammation. Platelets are growingly considered a major actor of the immune response. Their binding to myeloid cells has been shown to enhance their function as well as recruitment to extravascular areas (rev. in Gomez-Moreno et al.11).
The aim of this review is to summarize the roles that have been identified so far for hematopoietic stem cell (HSC)-derived myeloid cell subsets (neutrophils, monocytes and platelets) and resident microglial cells, with a special emphasis on neutrophils and their interaction with platelets in the stroke setting. We focus on the mechanisms that regulate their activation and recruitment to inflamed tissues and on how they contribute to stroke pathogenesis. Also, we highlight the important role of immune cells after stroke and the therapeutic relevance of their polarization as a potential novel target in stroke neuroinflammation. We also propose that potential cell-based treatments using the pleiotropic mechanisms conferred by their polarization towards non-inflammatory phenotypes may be an effective therapeutic strategy to the acute, subacute and chronic phases of this pathology.
Monocytes/macrophages
Monocytes are a population of mononuclear leukocytes that originate in the bone marrow from HSCs and then are released into the circulation as non-dividing cells.12 These cells represent approximately 4 or 10% of total leukocytes in mice or human peripheral blood, respectively, with a considerable pool located in spleen and lungs, apart from their homing into inflammatory sites in response to specific chemokines.13 Monocytes are immune effector cells, equipped with chemokine and pathogen recognition receptors (PRRs) that mediate migration from blood to tissues during infection. Their functions include the killing of pathogens via phagocytosis, the expression of inflammatory cytokines and enzymes and the subsequent production of ROS and reactive nitrogen species (RNS).14 Under specific conditions, monocytes can stimulate or inhibit T-cell responses during cancer as well as infectious and autoimmune diseases. They are also involved in tissue repair and neovascularization.15 They can also differentiate into inflammatory dendritic cells (DC) or macrophages during inflammation and constitute the resident pool of macrophages in the lamina propria of the intestine and skin16,17 in steady state. Monocyte migration to tissues and differentiation to inflammatory DC and macrophages is likely determined by the inflammatory milieu and the activation of pathogen-associated PRRs.18
Two predominant subsets of circulating monocytes, “inflammatory” and “patrolling” monocytes, are found in the blood of rodents. Short-lived inflammatory monocytes are characterized by the expression of high levels of lymphocyte antigen 6 complex locus C1 (Ly6C), low to intermediate levels of CX(3)-C motif chemokine receptor 1 (CX3CR1) and the expression of the C–C chemokine receptor type 2 (CCR2): Ly6ChighCXC3CR1+CCR2+. This subset is actively recruited to inflamed tissues and requires CCR2 for mobilization and transmigration from the bone marrow to blood and subsequently for the transmigration into these sites of inflammation.19–22 In contrast, patrolling monocytes express Ly6C at low levels, high levels of CXC3CR1 and do not express CCR2 (Ly6ClowCXC3CR1brightCCR2). Their egress from bone marrow is Ccr2-independent but dependent on sphingosine-1 phosphate receptor 5 (S1pr5).23 They have a longer half-life, crawl on the vessel luminal wall24 and are recruited within the vasculature of inflamed and damaged tissues.25,26 To date, a specific precursor for patrolling monocytes has not been identified and Ly-6Chi monocytes are thought to give rise to Ly-6Clo monocytes either in the blood or in the bone marrow.27 C/EBPβ-dependent control of Nr4a1 expression in monocytes has been proposed as the molecular mechanism that mediates the maturation of Ly-6Chi monocytes into Ly-6Clo.28
Human monocytes have been classified into three subtypes based on the differential expression of CD14 and CD16: classical CD14++CD16− (also referred as CD14+ or CD14+CD16−), intermediate CD14++CD16+ (also referred as CD14+CD16+ or CD14+CD16int/low) and non-classical CD14+CD16++ (also referred as CD14lowCD16+ or CD14dimCD16+) monocytes.29 The marker of human monocytes, CD14, is a glycoprotein and myelomonocytic differentiation antigen that functions as an accessory protein to toll-like receptor (TLR4).30
Monocyte/macrophage lineages display a remarkable plasticity and are able to change their phenotype in response to the microenvironment, giving rise to different populations of cells with distinct functions.31 In damaged tissues, as in ischemic stroke, peripheral-blood monocytes could have the ability to differentiate into macrophages and further polarize into several subtypes with specific functions including the production of inflammatory molecules and phagocytic activity (Figure 1).32 To easily classify them and based on in vitro studies, two main activation states in macrophages, designated as M1 (classically activated) and M2 (alternatively activated) macrophages, have been proposed. M1 subset is mainly associated with cytotoxicity and is thought to play an important role in the killing phase of the inflammatory response, producing pro-inflammatory cytokines and ROS upon stimulation with interferon-γ (IFNγ) and lipopolysaccharide (LPS), whereas polarization of macrophages towards an M2 phenotype, induced by interleukin-4 (IL-4), IL-10 or transforming growth factor-β (TGF-β), promotes tissue repair and trophic functions.31,33–35 Moreover, associated markers to each polarization state have been used to distinguish between these two macrophage activation states: in mice, the inducible nitric oxide synthase (iNOS) for the M1 macrophages, and the mannose receptor (CD206), arginase 1 (Arg1), chitinase-like 3 (Chil3 or Ym1) and IL-10 for the M2 ones.36–38
Figure 1.
Circulating and infiltrated monocytes and resident microglia participate actively in an immune-inflammatory scenario after stroke. In this environment, these cells are able to respond to different signals, modulate their response and change their phenotype (M1/M2 polarization). Different mechanisms could be proposed as possible targets for modulating the neuroinflammation process.
In the context of cerebral ischemia, growing evidence suggests that monocytes/macrophages can contribute to both brain injury39,40 and repair (Figure 1).41,42 Monocytes massively infiltrate into brain infarct early after stroke.43,44 In ischemic stroke patients, the number of peripheral monocytes is significantly increased in blood,45–47 and a significantly positive correlation between monocyte count and NIHSS score on admission has been reported.48 Urra et al.45,46 also found that monocytes are major players in the prognosis and risk of infection after acute stroke. Ly6Chi monocytes, which are the predominant CCR2-expressing monocyte cell type, requiring CCR2 for extravasation from bone marrow into blood and subsequently for transmigration into sites of inflammation,22 appear to be a prominent cell subset recruited from the circulation into the ischemic brain in mice.49–51 As to the cytokines/chemokines involved, CCL2 (also known as monocyte chemoattractant protein 1, MCP-1) is the most potent activator of CCR2 signalling in mice, leading to monocyte transmigration after stroke. The implication of the CCL2/CCR2 axis in mediating monocyte infiltration into the inflamed tissue has also been shown in other neuroinflammatory conditions such as EAE or traumatic brain injury.49,52,53 CCR2 knock-out mice display an increased number of monocytes in the bone marrow, a decreased number of circulating monocytes and a subsequent decrease in ischemic tissue, thus explaining that the absence of this chemokine receptor is protective against cerebral ischemia/reperfusion injury.54 There is, however, also evidence showing that CCR2+ monocytes may play an important role in anti-inflammatory and recovery processes after cerebral ischemia in mice models. CCR2+ monocytes have been associated to the maintenance of the neurovascular unit integrity and have been implicated in mediating neuroblast migration from neurogenic regions to damaged areas of the brain after stroke.41,55 In agreement with a pro-repairing role of CCR2+ monocytes after stroke, monocyte depletion during the first week after cerebral ischemia leads to impaired recovery of the sensorimotor function and drastically decreases the tissue expression of anti-inflammatory genes, including TGFβ, CD163, and Ym1.32 Also, recently, the use of CX3CR1GFP/+CCR2RFP/+ bone marrow chimeric mice showed that CCR2+Ly6Chi monocytes are the primary monocytic cell infiltrating during the acute inflammatory response to brain ischemia. This is followed by CX3CR1+ cell accumulation at the border of the infarct core 14 days after MCAO. In addition, this study suggests the possibility that CCR2+ monocytes undergo a phenotypic transformation into CX3CR1+CCR2− cells following infiltration to the ischemic tissue because accumulation of CX3CR1+Ly6Clo monocytes was absent in the brains of CCR2-deficient mice, which exhibit deficiency in CCR2+Ly6Chi recruitment, but not in NR4A1−/− chimeric mice, which lack circulating CX3CR1+Ly6Clo monocytes.51 These findings strengthen the idea of the presence of different effector functions driven by monocytes/macrophages after CNS injury that play a pleiotropic role in mediating brain injury and repair. In addition, monocyte infiltration into the ischemic tissue follows a spatiotemporal pattern whose consequences are currently not well understood.
In this regard, immunoregulatory and anti-inflammatory cytokines released after stroke, such as IL-4, IL-10 and TGF-β, could influence monocyte/macrophage activation, fate and functionality.31 In the context of cell activation, only a few experimental studies have evaluated the therapeutic benefits of drugs acting by increasing the M2/M1 ratio in stroke. Among these, Amantea et al.56 have demonstrated that acute treatment with azithromycin attenuates blood–brain barrier (BBB) damage and cerebral ischemic damage in rodents subjected to MCAO, with a significant amelioration of neurological deficits up to seven days after the insult. Even more, chronic metformin treatment was reported to promote functional recovery and tissue repair via AMPK-dependent turning microglia/macrophages toward an M2 phenotype in mice after MCAO,57 and up-regulation of M2 markers has also been shown to underlie neuroprotection by exendin-4, used in clinic against type 2 diabetes, when administrated in young healthy and in aged diabetic/obese after MCAO.58 As it will be discussed below, the differential role of infiltrating monocytes/macrophages vs. resident microglia in these settings remains to be accurately established. Moreover, phenotypic characterization of myeloid subsets using the M1/M2 paradigm of activation could not reflect the real diversity and functionality of these cells in the context of brain inflammation as it relies on the assumption that a macrophage genetic program is recapitulated by the culture of monocyte-derived macrophages (MDMs). In this regard, identification of specific myeloid subsets with specific functions could be more useful to understand the diversity of the myeloid compartment in the context of stroke pathogenesis.
Microglia
Microglia derives from an extraembryonic erythromyeloid precursor and persist in adult mice independently of haematopoiesis.59,60 Therefore, they are not normally replaced by bone marrow-derived cells,61 strongly suggesting that these cells have an elaborate repertoire of brain-specific functions that may not be appropriately taken over by peripheral monocytes/macrophages. Microglial cells are the first immune responders in the CNS. Upon cerebral injury microglial processes quickly and autonomously converge on the site of the damage (Figure 1).
An increasing number of studies suggest that resident microglia are functionally distinct from HSC-derived macrophages and play unique roles within the CNS.22,62–64 Since the lack of reliable microglia-specific markers has made very difficult to discriminate between microglia and infiltrated MDMs in the inflamed brain, an essential step to allocate differential functions to these cell types requires being able to conclusively distinguish them from each other. One of the most common approaches is the observation that, in contrast to macrophages, microglia expresses low or intermediate levels of the leukocyte marker CD45. This allows the characterization of microglia by flow cytometry using the levels of expression of CD45 in CD11b+ cells, a myeloid marker similarly expressed in both resident and non-resident populations.65–67 Although, by flow cytometry, MDMs can be differentiated by the expression level of CD45 and Ly6C,68 however, these cells down-regulate the expression of Ly6C after entry into the CNS.51 Different in vivo strategies have been established to attempt this discrimination after stroke, such as adoptive transfer studies of infiltrating cells using bone marrow chimeric mice with labelled bone marrow cells (DsRed; red fluorescent reporter) replaced after irradiation, to characterize the phenotype of macrophages entering the ischemic cortex.69 The main limitation of this technique is that it might harm the CNS by affecting the integrity of the BBB,70 so it is important to protect the heads of recipient mice from irradiation by head shielding.49 Complementary data on the recruitment of peripheral myeloid cells into the CNS have been obtained by parabiosis. However, the rate of chimerism in the parabiosis model is generally low compared to irradiation, which might lead to an underestimation of myeloid cell engraftment.52,71 Also, in situ CFSE (carboxyfluorescein succinimidyl ester)-labelling of blood-derived cells has been extensively used in different models of cerebral ischemia,43,44,62,72 but this molecule labels all proliferating blood cells and may not be ideal for purifying cells for gene expression analysis. One of the best approaches in the recent years has been the use of genetically modified mouse models such as the LysM-EGFP knock-in transgenic mouse, in which EGFP is inserted into the lysozyme M locus labelling neutrophils and hematogenous macrophages but not microglia.73–76 This EGFP labelling helps to distinguish MDMs that have entered the damaged CNS from microglia that are LysMEGFP-negative and was confirmed using microglia-specific antibodies.75 Zarruk et al.77 used for the first time LysM-EGFP knock-in mice to separate MDMs from microglia by FACS and purified cell populations for expression analysis after ischemic stroke. They performed a PCR-array screen of 84 inflammatory genes revealing that pro-inflammatory chemokines and cytokines were predominantly upregulated in macrophages but down-regulated in microglia in the ischemic brain. These results show clear differences in the inflammatory expression profiles of microglia and macrophages 72 h post-ischemia which may shape repair and pro-regenerative mechanisms after stroke. A very good model to specifically label microglia is the Cx3cr1-ertCre.78
As previously indicated for macrophages, an M1- or M2-like activation has also been suggested to occur in microglia. In vitro studies in which primary microglial cultures were exposed to LPS or IFNγ showed the induction of an M1-like phenotype with reduced phagocytic activity and expression of IL-1β, TNFα, iNOS or IL-6, while IL-4 drives microglia towards a M2-like phenotype with increased Arg1, Ym1 and resistin-like α (FIZZ1) expression.79 Although M1/M2 activation can also take place in microglial cell cultures, it must be taken into account that, as previously indicated for monocytes, brain microglial gene expression signature could be lost in primary microglial cell cultures as well as in microglial cell lines such as BV2.53 Considering this limitation, in vivo M1/M2 activation of microglia has been proposed as a potential therapeutic option in different diseases.79–81
In stroke, modulation of the M1 and M2 phenotype in microglia/macrophages (Figure 1) has been linked to detrimental and neuroprotective actions, respectively (see, for instance, see Han et al.82). Further studies on different CNS pathologies such as traumatic brain injury or spinal cord injury also indicate that microglia shift from a transient M2 phenotype to become M1-like phagocytes.36,83 However, a major caveat of these studies relies on the characterization of the brain myeloid cells that express a limited array of M1/M2 markers that could not reflect the current activation state of the cell. In this regard, recent studies on AD using single-cell transcriptomics identified a novel microglial type associated with neurodegenerative diseases (disease-associated microglia or DAM) with a unique potential to restrict neurodegeneration84 that indicates a high degree of specialization in response to pathogenic stimuli of this myeloid subset that falls beyond the M1/M2 dichotomy.
Neutrophils
Within the leukocyte populations, neutrophils play a key role in the innate immune response. They act as a fundamental defence in diseases associated with infections but also in sterile inflammation (as in stroke, cancer, autoimmune diseases, etc.). Their role in defence against pathogens has been extensively studied. Their ability to phagocytose and release molecules into their environment is well known.85 In addition, in response to different stimuli, neutrophils release neutrophil extracellular traps (NETs), which are large web-like structures composed of histones, DNA, and granule proteins, such as elastase and MPO.86 Although NETs primary function is to trap, restrain, and neutralize invading microbes,87 their role in diseases associated with sterile inflammation is increasingly demonstrated.
The bone marrow produces 1010 neutrophils per day. There, they will mature acquiring a high content in granules equipped with various proteins involved in the modulation of the immune response.88 Following maturation, neutrophils are released to the bloodstream where their half-life has been estimated to be 6 to 12 h, both in mice and humans.85 After that time, they are thought to undergo spontaneous apoptosis being then eliminated in different organs through phagocytosis.89 Besides circulating in the bloodstream, neutrophils can also be found in marginated pools in tissues such as the spleen and the lungs.90 Importantly, neutrophil biology can be altered by different situations. For example, their life span increases when an inflammatory process occurs. This process, called priming, expands neutrophils half-life when exposed to certain cytokines.91 It has been recently demonstrated that neutrophils are able to travel back to the bloodstream after they have infiltrated into an injured tissue. This process, so-called reverse transmigration, could explain such increased life span.92
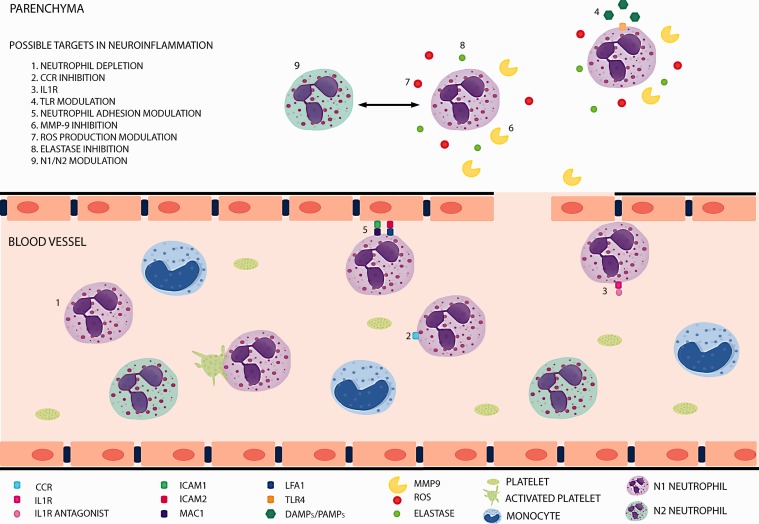
In response to inflammatory stimuli, neutrophils activate and contribute to the resolution of inflammation, to inflammation-associated immunopathology or to the modulation of the adaptive immune responses. Ischemic damage leads to neuroinflammation and cell death. Necrotic cells release pro-inflammatory cytokines that will induce subsequent neutrophil infiltration to the ischemic tissue. Neutrophils are the first leukocytes to be recruited into the parenchyma,93 reaching the peak of infiltration between 48 and 72 h after the insult.94 This process is facilitated by the characteristic disruption of the BBB that takes place after stroke (Figure 2).95 The presence of neutrophils in the ischemic tissue contributes to stroke-associated brain injury, oedema and also to BBB disruption. Many mechanisms underlie these effects, such as the synthesis/release of cytokines and chemokines and the production of ROS. In the bloodstream, neutrophils respond to cytokines such as TNFα, IL1β, IL6 and IL8. Also, they can be activated by DAMPS (damage-associated molecular patterns) like HMGB1 or HSP72 via the TLR family (Figure 2).96 Aside from the above mentioned, other molecules can modulate neutrophil migration, such as the leukotriene B4, that is required to recruit neutrophils to the lesion site.97
Figure 2.
Neutrophils, under inflammatory conditions, activate and contribute to the inflammatory process and its resolution. They are able to skew to different phenotypes (including N1/N2 polarization), produce and release different cytokines, form platelets–neutrophil complexes, adhere to the endothelium and infiltrate into damaged tissues. All these steps are potential targets to modulate neuroinflammation.
The specific role of neutrophils in brain ischemia is currently under debate (Figure 2). Classically, neutrophils have been shown to be mainly detrimental, by mechanisms that will affect outcome, severity and infarct volume. For instance, neutrophils account for the no-reflow phenomenon, increasing tissue damage.98 Even more, they can contribute to tissue injury through the release of elastase which damages the parenchyma and the production of ROS that contribute to BBB disruption.99 In this context, as we will discuss below, the formation and release of NETs within blood vessels may contribute to the formation of heterotypic aggregates, thus contributing to inflammation and thrombosis. However, in spite of their implication in tissue damage, there is not a clear correlation between the number of circulating neutrophils and the extent of the ischemic lesion.100 In this context, neutrophils are instrumental for the resolution of inflammation and their phagocytic activity contributes to the elimination of the necrotic tissue. In addition, other factors, such as the existence of neutrophil subsets with different roles in stroke pathogenesis, may account for possible beneficial actions of these cells. In this manner, the view of neutrophils as a homogeneous population is changing and nowadays it is believed that there are neutrophil subpopulations that exhibit different characteristics with anti-inflammatory, angiogenic or pro-resolutive properties. One of the first studies to describe the heterogeneity of neutrophil populations was published in the context of cancer and showed the existence of tumour-associated neutrophils (TANs), a subset of neutrophils that was named, by analogy to the macrophages M1/M2 paradigm, as N2 neutrophils or pro-tumorigenic.101 On the other side of this dichotomy, these authors found a population of neutrophils within the tumour that exhibited a pro-inflammatory profile and that was named as N1. The shift towards one or the other phenotype was determined by the environment. For example, the presence of IL-10, TGFβ, IL-13 and IL-4 would induce N2 polarization, whereas the presence of IFNβ, IFNγ and IL-12 would favour N1 polarization.102 A similar heterogeneity in the neutrophil compartment has been described by our group in the setting of stroke (Figure 2). Immunofluorescence characterization of the expression of prototypical M2 markers in ischemic tissue revealed that neutrophils located in the lesion differentially expressed some of these markers. Moreover, this phenotypic heterogeneity was modulated by PPARγ activation with the agonist rosiglitazone, that skewed neutrophils towards an M2-like or N2 phenotype which was associated with neuroprotection and resolution of inflammation after experimental stroke induced by pMCAO.103 Interestingly, this neutrophil subset was more efficiently cleared by microglial phagocytosis than the other subset. Through this mechanism, debris was promptly removed from the inflamed tissue by phagocytosis, contributing to the restoration of tissue homeostasis and improving the final outcome. In addition, one of the most accepted views proposes that there are different populations of neutrophils depending on their age: fresh neutrophils, recently released from the bone marrow, display a set of surface markers different to those present in aged neutrophils, close to be cleared away from the bloodstream.104,105 It has also been reported that neutrophil pro-inflammatory activity correlates positively with their ageing, while in circulation, a process driven by the microbiota via TLRs and MyD88-mediated signalling pathways.106 Consistently, our group has recently observed that a member of the TLR family, TLR4, one of the main actors for the initiation of inflammation in acute stroke,107 also plays a major role in the modulation of the neutrophil phenotype (García-Culebras et al., unpublished).
Platelets
Platelets, or thrombocytes, are small non-nucleated cell fragments derived from a myeloid progenitor that circulate in the bloodstream. They are well-known for their role in thrombosis and haemostasis. Notably, an increasing number of studies indicate that platelets also play a critical part in inflammation (Figure 3).108,109
Figure 3.
Platelets switch between a quiescent state to an activated one under inflammatory conditions and release different inflammatory mediators from their storage compartments into circulation. They are capable of interacting with neutrophils through P-selectin. This neutrophil–platelet interaction is bidirectional. Activated platelets can induce NETs formation and, also, NET components can activate platelets. Theoretically, these steps could be manipulated in order to modulate the neuroinflammation process.
Under physiological conditions, platelets circulate in a quiescent state; however, when they become activated, platelets are able to express and release different inflammatory mediators, such as cytokines, chemokines and surface molecules110–113 to recruit leukocytes to the site of inflammation or injury (Figure 3). Many of these molecules are stored in platelet granules, of which three different types, dense-granules, α-granules and lysosomes, have been described, each of which is rich in certain chemicals that have an important role in platelet function.
Released from dense granules, ADP and ATP play a key role in inflammation and ischemia-reperfusion injury during platelet activation;114 serotonin has been implicated in neutrophil rolling and adhesion to inflamed endothelium.115 Moreover, inorganic polyphosphates (PolyP), also released upon platelet activation, have been suggested to cause neutrophil accumulation and increase vascular permeability,116,117 although their role has recently been questioned.118 The regulation of neuroinflammation by modulating the expression of platelet adhesion receptors interacting with leukocytes or by releasing cytokines that affect leukocyte function is mediated by proteins stored in α-granules.109,119,120 Platelet factor 4 (PF4), the most abundant protein secreted from α-granules, acts as a chemoattractant for monocytes and neutrophils.121 PF4 also promotes neutrophil granule release and adhesion to endothelial cells, mediated by L-selectin and leukocyte function antigen 1 (LFA-1).122 PF4 induces cytokine release from monocytes including CXCL8, CXCL3, IL-1α, IL-1β, IL-6, IL-19, TNFα, CCL2, CCL3 and CCL22.123 The chemokine RANTES (regulated on activation normal T cell expressed and secreted) is released either directly or in microparticles which can be immobilised on activated endothelium and promote monocyte recruitment by P-selectin.124,125 T cells can also induce platelet RANTES release promoting T cell recruitment to the endothelium.126 Among other platelet-derived chemokines, macrophage inflammatory protein- (MIP-) 1α is a chemoattractant for monocytes, macrophages, T-cells and neutrophils, and is involved in transendothelial migration at sites of inflammation.127 When platelets become activated, they also release IL-1 which has a major role in vascular inflammation.128 IL-1 is known to promote neutrophil adhesion to endothelial cells and mediate vascular NO production.129,130 Platelet-secreted IL-1α causes endothelial activation which leads to the expression of ICAM-1 and VCAM-1 adhesion molecules on endothelial cells, CXCL1 release, and neutrophil transendothelial migration.131 Another constituent of platelet α-granules is P-selectin,132 an important adhesion molecule which, in response to activating signals, is translocated to the surface of platelets and binds to P-selectin glycoprotein ligand-1 (PSGL-1) on immune cells.133 In vitro studies have suggested that leukocytes can roll and adhere to immobilized platelets adherent in the vessel wall causing a pro-inflammatory state.134 This result was corroborated in the cerebral ischemia setting by an in vivo study revealing that platelets modulate leukocyte recruitment via P-selectin in a mouse tMCAO model (Figure 3).135 In addition, our group has described in a mouse pMCAO model that, at early stages of inflammation, neutrophils recruited to the injured vessel use their PSGL-1 domain to scan for the presence of activated platelets, as a primary mechanism for the initiation of the inflammation process.136
Specifically, platelet–neutrophil crosstalk leading to the formation of NETS is being increasingly recognised as a driver of inflammation and thrombosis. In this context, cell activation appears to be bidirectional.137 Activated platelets can stimulate neutrophils to release NETs,86,138 for instance, through P-selectin and PSGL-1 binding.139 In addition, neutrophils-derived NETs can activate platelets, a process in which TLR-dependent mechanisms may be involved (Figure 3).140,141 NET formation, by leading to the formation of heterotypic aggregates within blood vessels, is likely to have a large impact on the outcome of inflammatory processes. Specifically, signs of NET formation have been described in perivascular spaces, in the brain parenchyma nearby blood vessels, and in the lumen of capillaries in the ischemic brain in the tMCAO model.142 Importantly, the administration of DNase I, which degrades NETs, has been shown to have a protective in vivo effect in murine models after ischemic stroke.143 To date, NETs have been detected in venous and arterial thrombosis in patients.140,144 Recent studies showed that thrombus NETs content is associated with poor outcome in cerebral ischemia and may be responsible for reperfusion resistance, including mechanical or pharmacological approaches with intravenous t-PA.145,146 Even more, Vallés et al.147 not only found that NETs were significantly elevated in the plasma of acute ischemic stroke patients when compared to healthy subjects, but also that NETs levels were increased in patients who were over 65 years of age or with a history of atrial fibrillation (AF), cardioembolic stroke, high glucose levels, and severe stroke scores at admission and discharge. However, data are still scarce and further studies are required to establish their role in stroke patients.
Modulation of neutrophil function as a novel therapeutic target in neuroinflammation
All the aforementioned data evidence the high degree of plasticity of the myeloid system. Monocytes, neutrophils and microglia have been described to play different functions and to be composed by heterogeneous cell populations with specialized functions that could be exploited to investigate novel therapeutic targets for the treatment of brain damage associated to neuroinflammation after stroke. The importance of monocytes/macrophages and microglial cells in this context has been thoroughly reviewed.148 Importantly, growing evidence supports the therapeutic interest of neutrophils and their interaction with platelets.
The inhibition of neutrophil recruitment to the ischemic tissue is a strategy that could be theoretically approached by targeting the signalling pathways involved (Figure 2). In this context, combined pharmacological inhibition of CXCR1 and CXCR2 reduced ischemic brain injury and improved outcome in a rat stroke model,149 although this improvement did not occur when only CXCR2 was blocked.150 CCR2 loss of function using CCR2−/− mice reduced the infarct volume and neutrophil infiltration but this target is not neutrophil-specific as it also acts on monocyte recruitment to the ischemic tissue.54 A promising target for the early phases of acute stroke at the level of neutrophil recruitment to the ischemic tissue is the inhibition of the P-selectin-PSGL-1 interaction that leads to platelet–neutrophil adhesion, a process that is neuroprotective in experimental models of stroke.136
A related potential approach is to impede neutrophil infiltration (Figure 2). This process is mediated by integrins and selectins. The blockade of ICAM-1 showed some promising results in animal models but failed to be effective in patients.151,152 The effect of blocking LFA1 (CD11a/CD18) and Mac1(CD11b/CD18), which mediate neutrophil adhesion through ICAM1 and ICAM2 in endothelial cells, has also been explored.153–155 Again, the results have been different depending on the timing and the model used. Inhibiting LFA1 and Mac1 have been tried out in patients with no success.156,157 It should be noted that the infiltration of neutrophils into the ischemic tissue is considered controversial. Some authors even claim that neutrophils do not infiltrate into the parenchyma but remain in the perivascular spaces,158 an effect that could depend on the ischemia model used, transient or permanent occlusion, as the presence of neutrophils within the brain parenchyma has also been shown.103 A crucial issue that needs to be emphasised is the relevance of reperfusion for post-stroke inflammatory response. An interesting possibility is that neutrophils have different fates and/or roles depending on their final location, either within the vasculature or into the parenchyma of the ischemic tissue. In relation with the intravascular location, an additional aspect to be considered is the interaction of neutrophils with platelets and the subsequent release of NETs which may lead to vascular “thromboinflammatory” injury.159 The understanding of these processes may provide new opportunities for the development of novel therapeutic strategies in ischemic stroke that remain to be studied.
Neutrophil-mediated detrimental effects are also possible targets (Figure 2). Neutrophils contribute to the disruption of the BBB through the release of proteases (such as MMP-9 and elastase) and ROS. MMP-9 has been correlated with BBB disruption and haemorrhagic transformation both in animal models and patients,160 and elastase promotes the degradation of the basal lamina and the extracellular matrix.99 Pharmacological and genetic approaches to block these molecules have showed promising results in animal models.161,162 However, this potential therapy remains to be proved beneficial in patients, especially considering the potential reparative actions of MMP-9, also known to promote angiogenesis/neurogenesis in the chronic phase of stroke (see, for instance, Lee et al.,163 Barkho et al.164 and Hao et al.165).
Finally, the modulation of neutrophil phenotype towards phenotypes with pro-resolving properties in stroke is an interesting novel therapeutic possibility (Figure 2), aiming to specific targets such as, for instance, PPARγ and TLR receptors.103,106
In summary, current knowledge underlines the importance of the identification of the myeloid cell subsets directly associated to neuroprotection after stroke. In addition, it is also essential to characterize specific states of activation in myeloid cells associated to brain ischemia and to explore the signalling pathways by which these states determine stroke progression and outcome, as well as the mechanisms and consequences of platelet–leukocyte interactions. The comprehension of these processes may pave the way to novel therapeutic avenues to stop the cytotoxic phase of the inflammatory response and to promote neuroprotection and tissue repair after ischemic stroke. Furthermore, a better characterization of the myeloid cell subsets that orchestrate the response to brain ischemia and their dynamics may help us to design specific treatments within specific therapeutic windows with the aim to increase the benefits of the modulation of cell–cell interactions in the immune response after stroke.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from Spanish Ministry of Economy and Competitiveness SAF2015-68632-R (MAM) and SAF2016-81716-REDC (MAM), from Instituto de Salud Carlos III and cofinanced by Fondo Europeo de Desarrollo Regional (FEDER) «Una manera de hacer Europa» PI17/01601 (IL) and RD16/0019/0009 (IL), and from Regional Madrid Government B2017/BMD-3688 (IL).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Chamorro A, Meisel A, Planas AM, et al. The immunology of acute stroke. Nat Rev Neurol 2012; 8: 401–410. [DOI] [PubMed] [Google Scholar]
- 2.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med 2011; 17: 796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamel H, Iadecola C. Brain-immune interactions and ischemic stroke: clinical implications. Arch Neurol 2012; 69: 576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amantea D, Tassorelli C, Petrelli F, et al. Understanding the multifaceted role of inflammatory mediators in ischemic stroke. Curr Med Chem 2014; 21: 2098–2117. [DOI] [PubMed] [Google Scholar]
- 5.Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med 2008; 14: 497–500. [DOI] [PubMed] [Google Scholar]
- 6.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron 2010; 67: 181–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantovani A, Biswas SK, Galdiero MR, et al. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 2013; 229: 176–185. [DOI] [PubMed] [Google Scholar]
- 8.Jaillon S, Peri G, Delneste Y, et al. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med 2007; 204: 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amantea D, Micieli G, Tassorelli C, et al. Rational modulation of the innate immune system for neuroprotection in ischemic stroke. Front Neurosci 2015; 9: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaillon S, Galdiero MR, Del Prete D, et al. Neutrophils in innate and adaptive immunity. Semin Immunopathol 2013; 35: 377–394. [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Moreno D, Adrover JM, Hidalgo A. Neutrophils as effectors of vascular inflammation. Eur J Clin Invest. Epub ahead of print 12 April 2018. DOI: 10.1111/eci.12940. [DOI] [PubMed] [Google Scholar]
- 12.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol 2009; 27: 669–692. [DOI] [PubMed] [Google Scholar]
- 13.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 2014; 14: 392–404. [DOI] [PubMed] [Google Scholar]
- 14.Saha P, Geissmann F. Toward a functional characterization of blood monocytes. Immunol Cell Biol 2011; 89: 2–4. [DOI] [PubMed] [Google Scholar]
- 15.Nahrendorf M, Swirski FK, Aikawa E, et al. The healing myocardium sequentially mobilizes two monocyte sub sets with divergent and complementary functions. J Exp Med 2007; 204: 3037–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bain CC, Bravo-Blas A, Scott CL, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol 2014; 15: 929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoeffel G, Wang Y, Greter M, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med 2012; 209: 1167–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serbina NV, Jia T, Hohl TM, et al. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol 2008; 26: 421–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boring L, Gosling J, Chensue SW, et al. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest 1997; 100: 2552–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuziel WA, Morgan SJ, Dawson TC, et al. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci U S A 1997; 94: 12053–12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu B, Rutledge BJ, Gu L, et al. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med 1998; 187: 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu HX, Arumugam TV, Gelderblom M, et al. Role of CCR2 in inflammatory conditions of the central nervous system. J Cereb Blood Flow Metab 2014; 34: 1425–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Debien E, Mayol K, Biajoux V, et al. S1PR5 is pivotal for the homeostasis of patrolling monocytes. Eur J Immunol 2013; 43: 1667–1675. [DOI] [PubMed] [Google Scholar]
- 24.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003; 19: 71–82. [DOI] [PubMed] [Google Scholar]
- 25.Auffray C, Fogg D, Garfa M, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007; 317: 666–670. [DOI] [PubMed] [Google Scholar]
- 26.Carlin LM, Stamatiades EG, Auffray C, et al. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell 2013; 153: 362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yona S, Kim KW, Wolf Y, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013; 38: 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mildner A, Schönheit J, Giladi A, et al. Genomic characterization of murine monocytes reveals C/EBPβ transcription factor dependence of Ly6C. Immunity 2017; 46: 849–862.e847. [DOI] [PubMed] [Google Scholar]
- 29.Ziegler-Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010; 116: e74–80. [DOI] [PubMed] [Google Scholar]
- 30.Wacleche VS, Tremblay CL, Routy JP, et al. The biology of monocytes and dendritic cells: contribution to HIV pathogenesis. Viruses 2018. 10: pii: E65. DOI: 10.3390/v10020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 2010; 32: 593–604. [DOI] [PubMed] [Google Scholar]
- 32.Wattananit S, Tornero D, Graubardt N, et al. Monocyte-derived macrophages contribute to spontaneous long-term functional recovery after stroke in mice. J Neurosci 2016; 36: 4182–4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hao NB, Lu MH, Fan YH, et al. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol 2012; 2012: 948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benakis C, Garcia-Bonilla L, Iadecola C, et al. The role of microglia and myeloid immune cells in acute cerebral ischemia. Front Cell Neurosci 2014; 8: 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantovani A, Sica A, Sozzani S, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004; 25: 677–686. [DOI] [PubMed] [Google Scholar]
- 36.Hu X, Li P, Guo Y, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 2012; 43: 3063–3070. [DOI] [PubMed] [Google Scholar]
- 37.Liu C, Li Y, Yu J, et al. Targeting the shift from M1 to M2 macrophages in experimental autoimmune encephalomyelitis mice treated with fasudil. PLoS One 2013; 8: e54841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014; 41: 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang Z, Gan Y, Liu Q, et al. CX3CR1 deficiency suppresses activation and neurotoxicity of microglia/macrophage in experimental ischemic stroke. J Neuroinflammation 2014; 11: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bao Y, Kim E, Bhosle S, et al. A role for spleen monocytes in post-ischemic brain inflammation and injury. J Neuroinflammation 2010; 7: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gliem M, Mausberg AK, Lee JI, et al. Macrophages prevent hemorrhagic infarct transformation in murine stroke models. Ann Neurol 2012; 71: 743–752. [DOI] [PubMed] [Google Scholar]
- 42.Gliem M, Krammes K, Liaw L, et al. Macrophage-derived osteopontin induces reactive astrocyte polarization and promotes re-establishment of the blood brain barrier after ischemic stroke. Glia 2015; 63: 2198–2207. [DOI] [PubMed] [Google Scholar]
- 43.Denes A, Vidyasagar R, Feng J, et al. Proliferating resident microglia after focal cerebral ischaemia in mice. J Cereb Blood Flow Metab 2007; 27: 1941–1953. [DOI] [PubMed] [Google Scholar]
- 44.Schilling M, Besselmann M, Muller M, et al. Predominant phagocytic activity of resident microglia over hematogenous macrophages following transient focal cerebral ischemia: an investigation using green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol 2005; 196: 290–297. [DOI] [PubMed] [Google Scholar]
- 45.Urra X, Villamor N, Amaro S, et al. Monocyte subtypes predict clinical course and prognosis in human stroke. J Cereb Blood Flow Metab 2009; 29: 994–1002. [DOI] [PubMed] [Google Scholar]
- 46.Urra X, Cervera A, Obach V, et al. Monocytes are major players in the prognosis and risk of infection after acute stroke. Stroke 2009; 40: 1262–1268. [DOI] [PubMed] [Google Scholar]
- 47.Pusch G, Debrabant B, Molnar T, et al. Early dynamics of P-selectin and interleukin 6 predicts outcomes in ischemic stroke. J Stroke Cerebrovasc Dis 2015; 24: 1938–1947. [DOI] [PubMed] [Google Scholar]
- 48.Kaito M, Araya S, Gondo Y, et al. Relevance of distinct monocyte subsets to clinical course of ischemic stroke patients. PLoS One 2013; 8: e69409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mildner A, Schmidt H, Nitsche M, et al. Microglia in the adult brain arise from Ly-6ChiCCR2 + monocytes only under defined host conditions. Nat Neurosci 2007; 10: 1544–1553. [DOI] [PubMed] [Google Scholar]
- 50.Chu HX, Broughton BR, Kim HA, et al. Evidence that Ly6C(hi) monocytes are protective in acute ischemic stroke by promoting M2 macrophage polarization. Stroke 2015; 46: 1929–1937. [DOI] [PubMed] [Google Scholar]
- 51.Garcia-Bonilla L, Faraco G, Moore J, et al. Spatio-temporal profile, phenotypic diversity, and fate of recruited monocytes into the post-ischemic brain. J Neuroinflammation 2016; 13: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ajami B, Bennett JL, Krieger C, et al. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci 2011; 14: 1142–1149. [DOI] [PubMed] [Google Scholar]
- 53.Butovsky O, Jedrychowski MP, Moore CS, et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat Neurosci 2014; 17: 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dimitrijevic OB, Stamatovic SM, Keep RF, et al. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke 2007; 38: 1345–1353. [DOI] [PubMed] [Google Scholar]
- 55.Yan YP, Sailor KA, Lang BT, et al. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J Cereb Blood Flow Metab 2007; 27: 1213–1224. [DOI] [PubMed] [Google Scholar]
- 56.Amantea D, Certo M, Petrelli F, et al. Azithromycin protects mice against ischemic stroke injury by promoting macrophage transition towards M2 phenotype. Exp Neurol 2016; 275(Pt 1): 116–125. [DOI] [PubMed] [Google Scholar]
- 57.Jin Q, Cheng J, Liu Y, et al. Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav Immun 2014; 40: 131–142. [DOI] [PubMed] [Google Scholar]
- 58.Darsalia V, Hua S, Larsson M, et al. Exendin-4 reduces ischemic brain injury in normal and aged type 2 diabetic mice and promotes microglial M2 polarization. PLoS One 2014; 9: e103114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kierdorf K, Katzmarski N, Haas CA, et al. Bone marrow cell recruitment to the brain in the absence of irradiation or parabiosis bias. PLoS One 2013; 8: e58544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schulz C, Gomez Perdiguero E, Chorro L, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 2012; 336: 86–90. [DOI] [PubMed] [Google Scholar]
- 61.Kierdorf K, Erny D, Goldmann T, et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci 2013; 16: 273–280. [DOI] [PubMed] [Google Scholar]
- 62.Denes A, McColl BW, Leow-Dyke SF, et al. Experimental stroke-induced changes in the bone marrow reveal complex regulation of leukocyte responses. J Cereb Blood Flow Metab 2011; 31: 1036–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Girard S, Brough D, Lopez-Castejon G, et al. Microglia and macrophages differentially modulate cell death after brain injury caused by oxygen-glucose deprivation in organotypic brain slices. Glia 2013; 61: 813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moraga A, Pradillo JM, Garcia-Culebras A, et al. Aging increases microglial proliferation, delays cell migration, and decreases cortical neurogenesis after focal cerebral ischemia. J Neuroinflammation 2015; 12: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Campanella M, Sciorati C, Tarozzo G, et al. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke 2002; 33: 586–592. [DOI] [PubMed] [Google Scholar]
- 66.Denker SP, Ji S, Dingman A, et al. Macrophages are comprised of resident brain microglia not infiltrating peripheral monocytes acutely after neonatal stroke. J Neurochem 2007; 100: 893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ballesteros I, Cuartero MI, Pradillo JM, et al. Rosiglitazone-induced CD36 up-regulation resolves inflammation by PPARγ and 5-LO-dependent pathways. J Leukoc Biol 2014; 95: 587–598. [DOI] [PubMed] [Google Scholar]
- 68.Ritzel RM, Patel AR, Grenier JM, et al. Functional differences between microglia and monocytes after ischemic stroke. J Neuroinflammation 2015; 12: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miro-Mur F, Urra X, Gallizioli M, et al. Antigen presentation after stroke. Neurotherapeutics 2016; 13: 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yuan H, Gaber MW, McColgan T, et al. Radiation-induced permeability and leukocyte adhesion in the rat blood-brain barrier: modulation with anti-ICAM-1 antibodies. Brain Res 2003; 969: 59–69. [DOI] [PubMed] [Google Scholar]
- 71.Ajami B, Bennett JL, Krieger C, et al. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci 2007; 10: 1538–1543. [DOI] [PubMed] [Google Scholar]
- 72.Kokovay E, Li L, Cunningham LA. Angiogenic recruitment of pericytes from bone marrow after stroke. J Cereb Blood Flow Metab 2006; 26: 545–555. [DOI] [PubMed] [Google Scholar]
- 73.Faust N, Varas F, Kelly LM, et al. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood 2000; 96: 719–726. [PubMed] [Google Scholar]
- 74.Greenhalgh AD, David S. Differences in the phagocytic response of microglia and peripheral macrophages after spinal cord injury and its effects on cell death. J Neurosci 2014; 34: 6316–6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Greenhalgh AD, Passos Dos Santos R, Zarruk JG, et al. Arginase-1 is expressed exclusively by infiltrating myeloid cells in CNS injury and disease. Brain Behav Immun 2016; 56: 61–67. [DOI] [PubMed] [Google Scholar]
- 76.Mawhinney LA, Thawer SG, Lu WY, et al. Differential detection and distribution of microglial and hematogenous macrophage populations in the injured spinal cord of lys-EGFP-ki transgenic mice. J Neuropathol Exp Neurol 2012; 71: 180–197. [DOI] [PubMed] [Google Scholar]
- 77.Zarruk JG, Greenhalgh AD, David S. Microglia and macrophages differ in their inflammatory profile after permanent brain ischemia. Exp Neurol 2018; 301: 120–132. [DOI] [PubMed] [Google Scholar]
- 78.Goldmann T, Wieghofer P, Muller PF, et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat Neurosci 2013; 16: 1618–1626. [DOI] [PubMed] [Google Scholar]
- 79.Michelucci A, Heurtaux T, Grandbarbe L, et al. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: effects of oligomeric and fibrillar amyloid-beta. J Neuroimmunol 2009; 210: 3–12. [DOI] [PubMed] [Google Scholar]
- 80.Cherry JD, Olschowka JA, O'Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 2014; 11: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 2011; 12: 388–399. [DOI] [PubMed] [Google Scholar]
- 82.Han L, Cai W, Mao L, et al. Rosiglitazone promotes white matter integrity and long-term functional recovery after focal cerebral ischemia. Stroke 2015; 46: 2628–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shechter R, London A, Varol C, et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med 2009; 6: e1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Keren-Shaul H, Spinrad A, Weiner A, et al. A unique microglia type associated with restricting development of Alzheimer's disease. Cell 2017; 169: 1276–1290.e1217. [DOI] [PubMed] [Google Scholar]
- 85.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013; 13: 159–175. [DOI] [PubMed] [Google Scholar]
- 86.Metzler KD, Goosmann C, Lubojemska A, et al. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep 2014; 8: 883–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science 2004; 303: 1532–1535. [DOI] [PubMed] [Google Scholar]
- 88.Borregaard N. Neutrophils, from marrow to microbes. Immunity 2010; 33: 657–670. [DOI] [PubMed] [Google Scholar]
- 89.Hong C, Kidani Y, A-Gonzalez N, et al. Coordinate regulation of neutrophil homeostasis by liver X receptors in mice. J Clin Invest 2012; 122: 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Summers C, Rankin SM, Condliffe AM, et al. Neutrophil kinetics in health and disease. Trends Immunol 2010; 31: 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Colotta F, Re F, Polentarutti N, et al. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood 1992; 80: 2012–2020. [PubMed] [Google Scholar]
- 92.Mathias JR, Perrin BJ, Liu TX, et al. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J Leukoc Biol 2006; 80: 1281–1288. [DOI] [PubMed] [Google Scholar]
- 93.Kim JY, Park J, Chang JY, et al. Inflammation after ischemic stroke: the role of leukocytes and glial cells. Exp Neurobiol 2016; 25: 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jickling GC, Liu D, Ander BP, et al. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab 2015; 35: 888–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 2011; 42: 3323–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Prince LR, Whyte MK, Sabroe I, et al. The role of TLRs in neutrophil activation. Curr Opin Pharmacol 2011; 11: 397–403. [DOI] [PubMed] [Google Scholar]
- 97.Lämmermann T, Afonso PV, Angermann BR, et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 2013; 498: 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ames A, Wright RL, Kowada M, et al. Cerebral ischemia. II. The no-reflow phenomenon. Am J Pathol 1968; 52: 437–453. [PMC free article] [PubMed] [Google Scholar]
- 99.Ikegame Y, Yamashita K, Hayashi S, et al. Neutrophil elastase inhibitor prevents ischemic brain damage via reduction of vasogenic edema. Hypertens Res 2010; 33: 703–707. [DOI] [PubMed] [Google Scholar]
- 100.Strecker JK, Sevimli S, Schilling M, et al. Effects of G-CSF treatment on neutrophil mobilization and neurological outcome after transient focal ischemia. Exp Neurol 2010; 222: 108–113. [DOI] [PubMed] [Google Scholar]
- 101.Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009; 16: 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sionov RV, Fridlender ZG, Granot Z. The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron 2015; 8: 125–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cuartero MI, Ballesteros I, Moraga A, et al. N2 neutrophils, novel players in brain inflammation after stroke: modulation by the PPARγ agonist rosiglitazone. Stroke 2013; 44: 3498–3508. [DOI] [PubMed] [Google Scholar]
- 104.Adrover JM, Nicolás-Ávila JA, Hidalgo A. Aging: a temporal dimension for neutrophils. Trends Immunol 2016; 37: 334–345. [DOI] [PubMed] [Google Scholar]
- 105.Casanova-Acebes M, Pitaval C, Weiss LA, et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 2013; 153: 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang D, Chen G, Manwani D, et al. Neutrophil ageing is regulated by the microbiome. Nature 2015; 525: 528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Caso JR, Pradillo JM, Hurtado O, et al. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation 2007; 115: 1599–1608. [DOI] [PubMed] [Google Scholar]
- 108.Ghoshal K, Bhattacharyya M. Overview of platelet physiology: its hemostatic and nonhemostatic role in disease pathogenesis. Sci World J 2014; 2014: 781857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thomas MR, Storey RF. The role of platelets in inflammation. Thromb Haemost 2015; 114: 449–458. [DOI] [PubMed] [Google Scholar]
- 110.Shi G, Morrell CN. Platelets as initiators and mediators of inflammation at the vessel wall. Thromb Res 2011; 127: 387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Boehlen F, Clemetson KJ. Platelet chemokines and their receptors: what is their relevance to platelet storage and transfusion practice?. Transfus Med 2001; 11: 403–417. [DOI] [PubMed] [Google Scholar]
- 112.Smyth SS, McEver RP, Weyrich AS, et al. Platelet functions beyond hemostasis. J Thromb Haemost 2009; 7: 1759–1766. [DOI] [PubMed] [Google Scholar]
- 113.Golebiewska EM, Poole AW. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev 2015; 29: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med 2013; 368: 1260. [DOI] [PubMed] [Google Scholar]
- 115.Duerschmied D, Suidan GL, Demers M, et al. Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood 2013; 121: 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Müller F, Mutch NJ, Schenk WA, et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell 2009; 139: 1143–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morrissey JH, Choi SH, Smith SA. Polyphosphate: an ancient molecule that links platelets, coagulation, and inflammation. Blood 2012; 119: 5972–5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Faxälv L, Boknäs N, Ström JO, et al. Putting polyphosphates to the test: evidence against platelet-induced activation of factor XII. Blood 2013; 122: 3818–3824. [DOI] [PubMed] [Google Scholar]
- 119.Herter JM, Rossaint J, Zarbock A. Platelets in inflammation and immunity. J Thromb Haemost 2014; 12: 1764–1775. [DOI] [PubMed] [Google Scholar]
- 120.Jenne CN, Urrutia R, Kubes P. Platelets: bridging hemostasis, inflammation, and immunity. Int J Lab Hematol 2013; 35: 254–261. [DOI] [PubMed] [Google Scholar]
- 121.Deuel TF, Senior RM, Chang D, et al. Platelet factor 4 is chemotactic for neutrophils and monocytes. Proc Natl Acad Sci U S A 1981; 78: 4584–4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Weber C, Springer TA. Neutrophil accumulation on activated, surface-adherent platelets in flow is mediated by interaction of Mac-1 with fibrinogen bound to alphaIIbbeta3 and stimulated by platelet-activating factor. J Clin Invest 1997; 100: 2085–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kasper B, Brandt E, Brandau S, et al. Platelet factor 4 (CXC chemokine ligand 4) differentially regulates respiratory burst, survival, and cytokine expression of human monocytes by using distinct signaling pathways. J Immunol 2007; 179: 2584–2591. [DOI] [PubMed] [Google Scholar]
- 124.Schober A, Manka D, von Hundelshausen P, et al. Deposition of platelet RANTES triggering monocyte recruitment requires P-selectin and is involved in neointima formation after arterial injury. Circulation 2002; 106: 1523–1529. [DOI] [PubMed] [Google Scholar]
- 125.von Hundelshausen P, Koenen RR, Sack M, et al. Heterophilic interactions of platelet factor 4 and RANTES promote monocyte arrest on endothelium. Blood 2005; 105: 924–930. [DOI] [PubMed] [Google Scholar]
- 126.Danese S, de la Motte C, Reyes BM, et al. Cutting edge: T cells trigger CD40-dependent platelet activation and granular RANTES release: a novel pathway for immune response amplification. J Immunol 2004; 172: 2011–2015. [DOI] [PubMed] [Google Scholar]
- 127.Lentzsch S, Gries M, Janz M, et al. Macrophage inflammatory protein 1-alpha (MIP-1 alpha) triggers migration and signaling cascades mediating survival and proliferation in multiple myeloma (MM) cells. Blood 2003; 101: 3568–3573. [DOI] [PubMed] [Google Scholar]
- 128.Loppnow H, Libby P. Proliferating or interleukin 1-activated human vascular smooth muscle cells secrete copious interleukin 6. J Clin Invest 1990; 85: 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bevilacqua MP, Pober JS, Wheeler ME, et al. Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest 1985; 76: 2003–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ignarro LJ, Buga GM, Wei LH, et al. Role of the arginine-nitric oxide pathway in the regulation of vascular smooth muscle cell proliferation. Proc Natl Acad Sci U S A 2001; 98: 4202–4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Thornton P, McColl BW, Greenhalgh A, et al. Platelet interleukin-1alpha drives cerebrovascular inflammation. Blood 2010; 115: 3632–3639. [DOI] [PubMed] [Google Scholar]
- 132.Romo GM, Dong JF, Schade AJ, et al. The glycoprotein Ib-IX-V complex is a platelet counterreceptor for P-selectin. J Exp Med 1999; 190: 803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Frenette PS, Denis CV, Weiss L, et al. P-Selectin glycoprotein ligand 1 (PSGL-1) is expressed on platelets and can mediate platelet-endothelial interactions in vivo. J Exp Med 2000; 191: 1413–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hammer DA, Apte SM. Simulation of cell rolling and adhesion on surfaces in shear flow: general results and analysis of selectin-mediated neutrophil adhesion. Biophys J 1992; 63: 35–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ishikawa M, Cooper D, Arumugam TV, et al. Platelet-leukocyte-endothelial cell interactions after middle cerebral artery occlusion and reperfusion. J Cereb Blood Flow Metab 2004; 24: 907–915. DOI: 10.1097/01.WCB.0000132690.96836.7F. [DOI] [PubMed] [Google Scholar]
- 136.Sreeramkumar V, Adrover JM, Ballesteros I, et al. Neutrophils scan for activated platelets to initiate inflammation. Science 2014; 346: 1234–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ghasemzadeh M, Hosseini E. Platelet-leukocyte crosstalk: linking proinflammatory responses to procoagulant state. Thromb Res 2013; 131: 191–197. [DOI] [PubMed] [Google Scholar]
- 138.Massberg S, Grahl L, von Bruehl ML, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med 2010; 16: 887–896. [DOI] [PubMed] [Google Scholar]
- 139.Etulain J, Martinod K, Wong SL, et al. P-selectin promotes neutrophil extracellular trap formation in mice. Blood 2015; 126: 242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A 2010; 107: 15880–15885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Semeraro F, Ammollo CT, Morrissey JH, et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood 2011; 118: 1952–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Perez-de-Puig I, Miró-Mur F, Ferrer-Ferrer M, et al. Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol 2015; 129: 239–257. [DOI] [PubMed] [Google Scholar]
- 143.De Meyer SF, Suidan GL, Fuchs TA, et al. Extracellular chromatin is an important mediator of ischemic stroke in mice. Arterioscler Thromb Vasc Biol 2012; 32: 1884–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mangold A, Alias S, Scherz T, et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ Res 2015; 116: 1182–1192. [DOI] [PubMed] [Google Scholar]
- 145.Laridan E, Denorme F, Desender L, et al. Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol 2017; 82: 223–232. [DOI] [PubMed] [Google Scholar]
- 146.Ducroux C, Di Meglio L, Loyau S, et al. Thrombus neutrophil extracellular traps content impair tPA-induced thrombolysis in acute ischemic stroke. Stroke 2018; 49: 754–757. [DOI] [PubMed] [Google Scholar]
- 147.Vallés J, Lago A, Santos MT, et al. Neutrophil extracellular traps are increased in patients with acute ischemic stroke: prognostic significance. Thromb Haemost 2017; 117: 1919–1929. [DOI] [PubMed] [Google Scholar]
- 148.Kanazawa M, Ninomiya I, Hatakeyama M, et al. Microglia and monocytes/macrophages polarization reveal novel therapeutic mechanism against stroke. Int J Mol Sci 2017. 18: pii: E2135. DOI: 10.3390/ijms18102135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Villa P, Triulzi S, Cavalieri B, et al. The interleukin-8 (IL-8/CXCL8) receptor inhibitor reparixin improves neurological deficits and reduces long-term inflammation in permanent and transient cerebral ischemia in rats. Mol Med 2007; 13: 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Brait VH, Rivera J, Broughton BR, et al. Chemokine-related gene expression in the brain following ischemic stroke: no role for CXCR2 in outcome. Brain Res 2011; 1372: 169–179. [DOI] [PubMed] [Google Scholar]
- 151.Zhang RL, Chopp M, Jiang N, et al. Anti-intercellular adhesion molecule-1 antibody reduces ischemic cell damage after transient but not permanent middle cerebral artery occlusion in the Wistar rat. Stroke 1995; 26: 1438–1442, discussion 1443. [DOI] [PubMed] [Google Scholar]
- 152.Investigators EAST. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology 2001; 57: 1428–1434. [DOI] [PubMed] [Google Scholar]
- 153.Arumugam TV, Salter JW, Chidlow JH, et al. Contributions of LFA-1 and Mac-1 to brain injury and microvascular dysfunction induced by transient middle cerebral artery occlusion. Am J Physiol Heart Circ Physiol 2004; 287: H2555–2560. [DOI] [PubMed] [Google Scholar]
- 154.Soriano SG, Coxon A, Wang YF, et al. Mice deficient in Mac-1 (CD11b/CD18) are less susceptible to cerebral ischemia/reperfusion injury. Stroke 1999; 30: 134–139. [DOI] [PubMed] [Google Scholar]
- 155.Zhang L, Zhang ZG, Zhang RL, et al. Effects of a selective CD11b/CD18 antagonist and recombinant human tissue plasminogen activator treatment alone and in combination in a rat embolic model of stroke. Stroke 2003; 34: 1790–1795. [DOI] [PubMed] [Google Scholar]
- 156.Becker KJ. Anti-leukocyte antibodies: LeukArrest (Hu23F2G) and enlimomab (R6.5) in acute stroke. Curr Med Res Opin 2002; 18(Suppl 2): s18–s22. [DOI] [PubMed] [Google Scholar]
- 157.Krams M, Lees KR, Hacke W, et al. Acute stroke therapy by inhibition of neutrophils (ASTIN): an adaptive dose-response study of UK-279,276 in acute ischemic stroke. Stroke 2003; 34: 2543–2548. [DOI] [PubMed] [Google Scholar]
- 158.Enzmann G, Mysiorek C, Gorina R, et al. The neurovascular unit as a selective barrier to polymorphonuclear granulocyte (PMN) infiltration into the brain after ischemic injury. Acta Neuropathol 2013; 125: 395–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.De Meyer SF, Denorme F, Langhauser F, et al. Thromboinflammation in stroke brain damage. Stroke 2016; 47: 1165–1172. [DOI] [PubMed] [Google Scholar]
- 160.Castellanos M, Sobrino T, Millán M, et al. Serum cellular fibronectin and matrix metalloproteinase-9 as screening biomarkers for the prediction of parenchymal hematoma after thrombolytic therapy in acute ischemic stroke: a multicenter confirmatory study. Stroke 2007; 38: 1855–1859. [DOI] [PubMed] [Google Scholar]
- 161.Gidday JM, Gasche YG, Copin JC, et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol 2005; 289: H558–568. [DOI] [PubMed] [Google Scholar]
- 162.Stowe AM, Adair-Kirk TL, Gonzales ER, et al. Neutrophil elastase and neurovascular injury following focal stroke and reperfusion. Neurobiol Dis 2009; 35: 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee SR, Kim HY, Rogowska J, et al. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci 2006; 26: 3491–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Barkho BZ, Munoz AE, Li X, et al. Endogenous matrix metalloproteinase (MMP)-3 and MMP-9 promote the differentiation and migration of adult neural progenitor cells in response to chemokines. Stem Cells 2008; 26: 3139–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hao Q, Su H, Palmer D, et al. Bone marrow-derived cells contribute to vascular endothelial growth factor-induced angiogenesis in the adult mouse brain by supplying matrix metalloproteinase-9. Stroke 2011; 42: 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]