Abstract
Objective
To examine the effect of concussion history and cumulative exposure to collision sports on baseline serum biomarker concentrations, as well as associations between biomarker concentrations and clinical assessments.
Methods
In this observational cohort study, β-amyloid peptide 42 (Aβ42), total tau, S100 calcium binding protein B (S100B), ubiquitin carboxy-terminal hydrolyzing enzyme L1 (UCH-L1), glial fibrillary acidic protein, microtubule associated protein 2, and 2′,3′-cyclic-nucleotide 3′-phosphodiesterase serum concentrations were measured in 415 (61% male, 40% white, aged 19.0 ± 1.2 years) nonconcussed collegiate athletes without recent exposure to head impacts. Regression analyses were used to evaluate the relationship between self-reported history of concussion(s), cumulative years playing collision sports, clinical assessments, and baseline biomarker concentrations. Football-specific analyses were performed using a modified Cumulative Head Impact Index. Clinical assessments included symptom, cognitive, balance, and oculomotor tests.
Results
Athletes with a greater number of concussions had a higher baseline Aβ42 concentration only (ρ = 0.140, p = 0.005, small effect size). No biomarker concentrations correlated with cumulative exposure to collision sports. Race status fully mediated the correlations of S100B, UCH-L1, and Aβ42 with cognitive scores. Football exposure, specifically, was not associated with serum biomarker concentrations or clinical assessment scores based on the modified Cumulative Head Impact Index.
Conclusion
Concussion-related serum biomarkers showed no consistent association with concussion history, cumulative exposure to collision sports, or clinical assessments in a sample of healthy collegiate athletes. Serum Aβ42 concentrations could increase following multiple previous concussions. Considering race status is essential when investigating links between biomarkers and cognition. The biomarkers studied may not detect residual effects of concussion or repetitive head impact exposure in otherwise asymptomatic collegiate athletes without recent exposure to head impacts. Much more research is needed for identifying reliable and valid blood biomarkers of brain trauma history.
Fluid biomarkers are increasingly studied as potentially objective measures of acute concussion, and interest extends to detecting residual effects of repetitive head impact exposure (RHIE), or “subconcussive” hits. For example, boxers following a bout without a knockout showed higher plasma tau after the fight compared to controls, but no effect on S100 calcium binding protein B (S100B), glial fibrillary acidic protein (GFAP), brain-derived neurotrophic factor, or β-amyloid peptide 42 (Aβ42) levels.1 Conversely, heading exposure in soccer was not associated with changes in neurofilament light, total tau, GFAP, or S100B.2
Plasma tau, specifically, has been proposed as an indicator of RHIE in football based on a study of retired National Football League (NFL) athletes. These retired athletes did not differ from age-matched controls in total tau concentration,3 but a plasma total tau concentration >3.56 pg/mL was 100% specific to former NFL athletes (12 of the 96 NFL athletes vs 0 of the 25 controls). Another study found plasma tau may also weakly correlate with estimated quantity of head impacts4 but not with number of years playing football or with neuropsychological test scores. There is a clear need for identifying a useful screening test for detection of neurobiological consequences of RHIE, and multiple biomarker candidates, in addition to total tau, remain to be explored.
A screening test should capture at-risk individuals before clinical symptom manifestation. Otherwise healthy collegiate athletes with RHIE are unlikely to exhibit overt symptoms of disorders such as chronic traumatic encephalopathy (CTE) but are potentially at risk of related pathology. Therefore, they are an important population for assessing the efficacy of screening tests like peripheral biomarkers of RHIE.
In Concussion BASICS: Part II, we examine the relationship of concussion history, total years playing collision sports, and estimated quantity of head impacts with concentrations of potential serum biomarkers of concussion and RHIE in current, healthy collegiate athletes. A secondary aim was to investigate associations between baseline serum biomarker concentrations and scores on baseline clinical measures.
Methods
Standard protocol approvals, registrations, and patient consents
The 415 male and female varsity athletes included in these analyses are described in our companion report, along with specifics of serum specimen collection, storage, and analysis.5 Briefly, ubiquitin carboxy-terminal hydrolyzing enzyme L1 (UCH-L1), GFAP, microtubule associated protein 2, and CNPase (2′,3′-cyclic-nucleotide 3′-phosphodiesterase) were analyzed using the Banyan ELISA (Banyan Biomarkers, Inc., Alachua, FL). S100B concentrations were determined using an electrochemiluminescence immunoassay (Cobas 6000; Roche Diagnostics, Indianapolis, IN). Aβ42 and total tau were analyzed with an ultrasensitive immunoassay using digital array technology (Quanterix Corp., Lexington, MA) via the Simoa Tau and Simoa Aβ42 kits. A trained phlebotomist performed all blood draws following individual athlete consent according to a protocol approved by an independent ethical and biomedical research review board (Western IRB) with approved language by the university's institutional review board (IRB-01).
Brain trauma exposure
Our primary predictor variables were (1) concussion history and (2) cumulative exposure to collision sports. Concussion history was determined via self-report and cross-validated with participant medical records wherever possible. Participants were provided a standardized definition of concussion reflecting the Concussion in Sport Group's consensus definition, which has also been directly reflected in the university's concussion management protocol for defining clinical diagnosis.6,7 Concussions diagnosed while enrolled at the university but before enrollment in the study were confirmed in the institution's medical record. Cumulative exposure to collision sports included the total combined years that a participant self-reported playing the following: football, soccer, diving, wrestling, and ice hockey. Both training/practices and competitive events for these sports involve a high risk of RHIE to varying degrees. We also performed analyses for current football players using a modified version of the Cumulative Head Impact Index (mCHII).4 This metric takes into account data from biomechanics studies characterizing the average number of head impacts sustained by different football positions separated by collegiate, high school, and youth football participation.
Standard Cumulative Head Impact Index calculations require retrospective self-report of the percentage of time spent playing various positions at different levels of play. We did not collect these data. Therefore, the position listed as the athlete's primary position at the time of enrollment in our study was used to calculate their Cumulative Head Impact Index with an assumed 100% of time spent in that position. Self-reported years playing football was assumed to represent consecutive playing years, so an individual's total years playing high school or pre–high school football was determined by counting backward from the academic year when they provided a blood sample.
Clinical measures
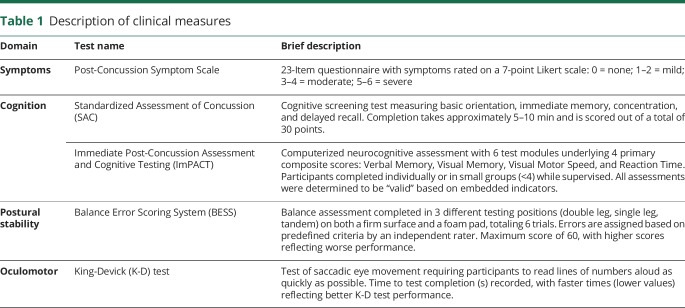
Participants completed the Post-Concussion Symptom Scale concurrently with the Immediate Postconcussion Assessment and Cognitive Testing (ImPACT).8 Cognitive testing included the Standardized Assessment of Concussion (SAC),9 and ImPACT (ImPACT Applications Inc., Pittsburgh, PA). Participants also completed the Balance Error Scoring System (BESS)10 and King-Devick (K-D) test.11 More detailed descriptions of these clinical measures are provided in table 1.
Table 1.
Description of clinical measures
Only participants who performed clinical tests within 30 days of their baseline blood draw were considered for analyses. All clinical measures were collected in the context of routine baseline concussion testing as mandated by the university's concussion management protocol. The available sample differed slightly between clinical tests: symptom scale (n = 228), ImPACT (n = 237), SAC (n = 135), BESS (n = 129), and K-D test (n = 135).
Statistical analyses
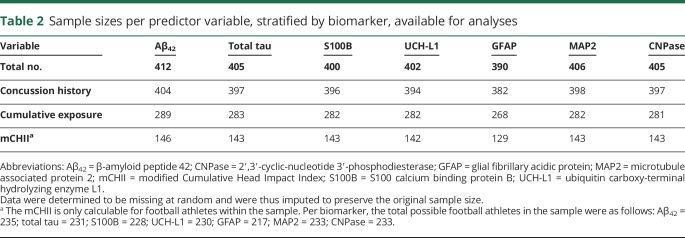
All analyses were performed using IBM-SPSS Statistical Software, version 22.0 (IBM Corp., Armonk, NY). We primarily utilized linear regression analyses for examining associations between our variables of interest. Analyses investigating brain trauma exposure included concussion history and cumulative years exposed to collision sports as independent variables and each biomarker as a dependent variable. Little's test was performed to assess for randomness of missing data (table 2). If missing at random, we imputed missing data to preserve the original sample size using a multiple imputations approach with 20 imputations performed.12
Table 2.
Sample sizes per predictor variable, stratified by biomarker, available for analyses
The relationships between concussion history, cumulative years playing collision sports, and each of the 7 biomarkers were first independently evaluated within the Spearman rank-order correlation matrix. If one or both predictors demonstrated a significant correlation with a given biomarker, they were included together in a follow-up linear regression model with demographic covariates using the “enter” method to include all variables. Subsequent inclusion of demographic covariates was biomarker-specific based on prior findings showing race and sex differences in expression of certain baseline biomarkers.5 Similar methods were used for evaluating the relationships between baseline serum biomarker concentration and clinical outcomes. We were particularly interested in potential mediating effects of race and sex based on the Part I findings combined with previous work showing the influence of race on clinical concussion tests.13
For football-specific analyses, we replaced the cumulative years exposed to collision sports factor with the mCHII score for each football player. Participants designating their primary position as placekicker or punter were excluded. We also examined potential interactions between self-reported concussion history and mCHII score.
In the event of significantly skewed outcome variables, we performed analyses using both the original distribution as well as Blom normalized distributions and report both findings if they differed. For regression analyses, P-P plots and scatterplots of standardized residuals were examined to ensure assumptions of homoscedasticity were met for each outcome variable. Polynomial terms were also explored to assess for quadratic or cubic relationships. We adjusted for multiple comparisons using a Bonferroni correction reflecting the 7 dependent biomarker outcomes, resulting in an α criterion of p < 0.007 to control for type 1 error inflation (0.05/7 = 0.007).
Data availability
Any qualified investigator may contact the corresponding (B.M.A.) or senior (J.R.C.) author with a specific request that includes details of (1) the resources requested, (2) how the data and resources will be used in the proposed research, and (3) the qualifications of the investigator requesting the resources. Investigators may be asked to provide further details if necessary. All requests will be reviewed for availability of the requested resources, potential for duplication of ongoing studies, and scientific merit. The requesting investigator may be asked to partner with a University of Florida investigator to better understand the available data and will sometimes be encouraged to include a Florida representative as an academic consultant or collaborator. Requesting investigators will also be required to sign a Data Use Agreement ensuring data will not be shared with third parties.
Results
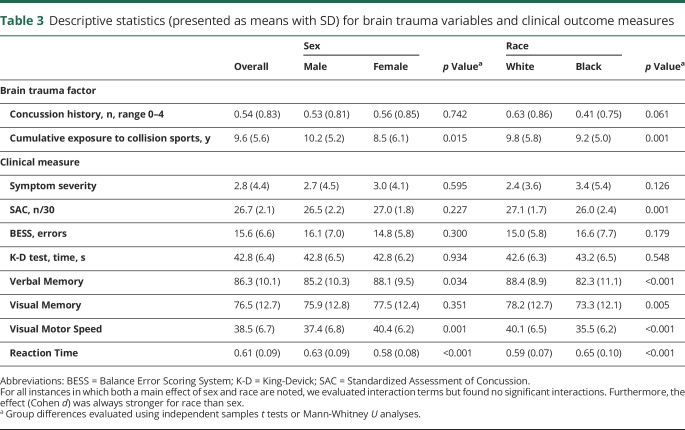
Descriptive data for concussion history, cumulative years playing collision sports, and clinical measures stratified by sex and race are provided in table 3. Corresponding normative descriptive data for baseline biomarker concentrations are shown elsewhere.5 Missing concussion history, cumulative exposure to collision sports, and mCHII data were determined to be missing at random (Little's missing completely at random test, p > 0.05). Therefore, multiple imputations were used for missing data, and pooled statistics are reported where applicable.
Table 3.
Descriptive statistics (presented as means with SD) for brain trauma variables and clinical outcome measures
Brain trauma history and baseline biomarker levels
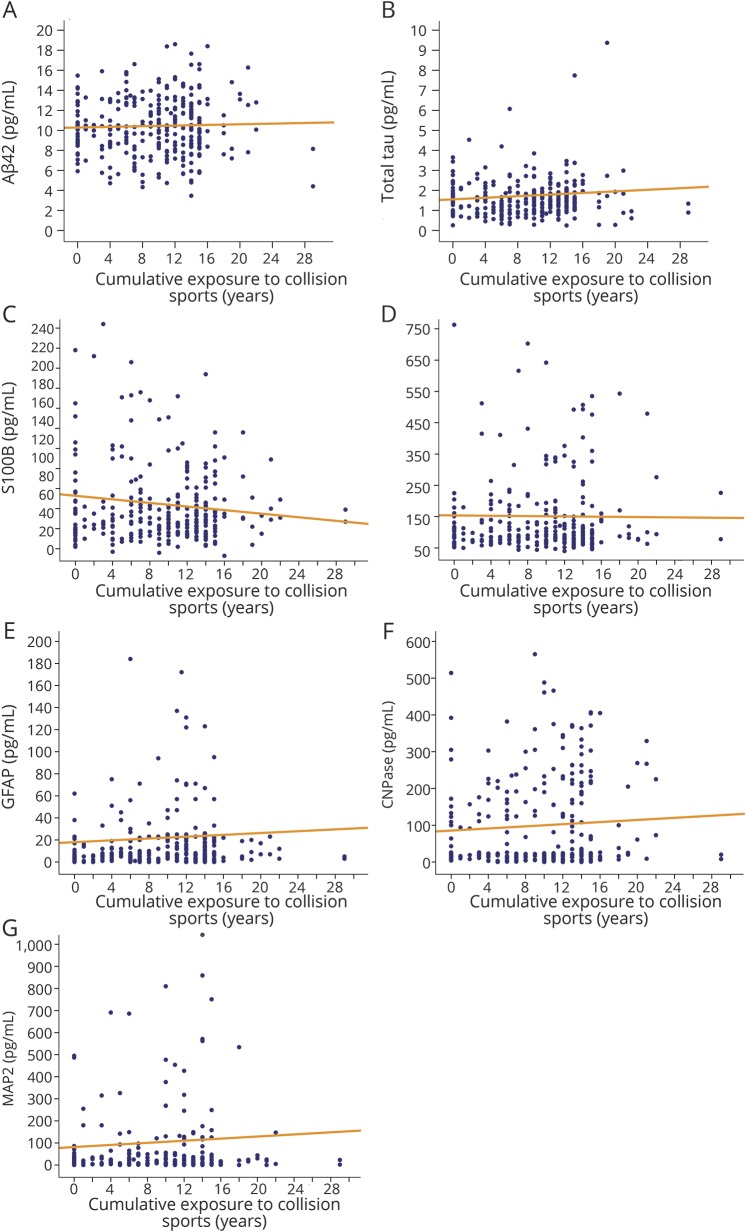
Athletes with a greater number of self-reported concussions had higher baseline Aβ42 levels ([pooled] ρ = 0.140, p = 0.005, small effect size). No other associations between concussion history and baseline biomarker levels were noted (figure 1). Similarly, there was no observed relationship between cumulative exposure to collision sports and baseline biomarker levels ([pooled] p > 0.007 for all analyses; figure 2).
Figure 1. Relationship between number of previous concussions and baseline serum biomarker concentrations.
A significant but weak association was found between more previous concussions and higher baseline Aβ42 levels (A). No other significant correlations were noted (B–G). Aβ42 = β-amyloid peptide 42; CNPase = 2′,3′-cyclic-nucleotide 3′-phosphodiesterase; GFAP = glial fibrillary acidic protein; MAP2 = microtubule associated protein 2; S100B = S100 calcium binding protein B; SRC = sport-related concussion; UCH-L1 = ubiquitin carboxy-terminal hydrolyzing enzyme L1.
Figure 2. Relationship between cumulative exposure to collision sports and baseline serum biomarker concentrations.
No significant correlations were noted for any biomarker (A–G). Aβ42 = β-amyloid peptide 42; CNPase = 2′,3′-cyclic-nucleotide 3′-phosphodiesterase; GFAP = glial fibrillary acidic protein; MAP2 = microtubule associated protein 2; S100B = S100 calcium binding protein B; UCH-L1 = ubiquitin carboxy-terminal hydrolyzing enzyme L1.
Association of baseline biomarker levels and clinical measures
We found no associations between baseline biomarker levels and self-reported symptom severity or performance on the SAC, BESS, or K-D test (p > 0.007 for all analyses).
Athletes with higher baseline levels of S100B performed more poorly on ImPACT Verbal Memory, Visual Motor Speed, and Reaction Time (p < 0.007 for all; figure 3A). Since previous analyses indicated that race and sex explain significant variance in baseline S100B levels,5 and are also important predictors of clinical test scores,13 we evaluated the relationship(s) between race and sex and these ImPACT composite scores in our sample. A main effect of race was found for Verbal Memory (F1,227 = 7.814, p = 0.006), Visual Motor Speed (F1,227 = 4.492, p = 0.035), and Reaction Time (F1,227 = 7.381, p = 0.007) when controlling for sex. There were no main effects of sex when controlling for race and no significant sex × race interactions. We therefore reevaluated the association between S100B and these ImPACT composite scores, covarying for the effect of race. Hierarchical regression indicated that the relationships between S100B and Verbal Memory, Visual Motor Speed, and Reaction Time were all fully mediated by race. Controlling for race, the associations between S100B and Verbal Memory, Visual Motor Speed, and Reaction Time all became nonsignificant (figure 3B). However, race remained an important predictor of all 3 composite scores such that participants self-reporting white race performed better on Verbal Memory, Visual Motor Speed, and Reaction Time (figure 3B).
Figure 3. Relationships between race, S100B, and cognitive measures.
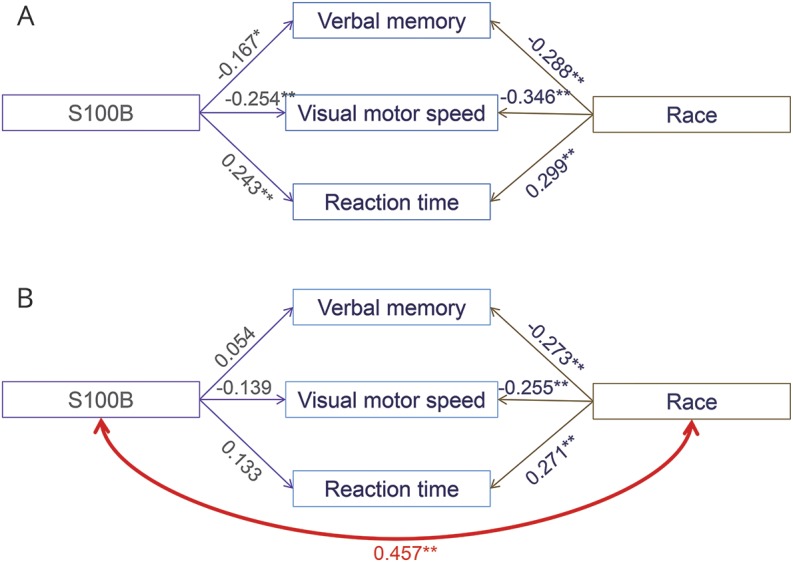
(A) S100B and race both significantly correlated with Verbal Memory, Visual Motor Speed, and Reaction Time. (B) Evidence of mediation: S100B and race significantly correlated. Controlling for race, the associations between S100B and Verbal Memory, Visual Motor Speed, and Reaction Time all became nonsignificant. Race remained significantly correlated with all composite scores while controlling for S100B effects. Numerical values represent regression weights (r or β). *p < 0.01, **p < 0.001. S100B = S100 calcium binding protein B.
Similar findings were noted elsewhere. Those with higher baseline UCH-L1 (ρ = −0.192, p = 0.003) and lower Aβ42 (ρ = 0.175, p = 0.007) levels performed worse on ImPACT Visual Motor Speed (small effect size). Controlling for race, the associations between UCH-L1 (β = −0.066, p = 0.309) and Aβ42 (β = 0.123, p = 0.053) and Visual Motor Speed became nonsignificant. In both cases, race remained a moderately strong predictor of Visual Motor Speed (β = −0.320, p < 0.001 and β = −0.305, p < 0.001, respectively) with white participants obtaining better scores.
Football-specific analyses
The mean mCHII (i.e., approximate number of head impacts sustained throughout their career) was 3,100 (SD = 915.1, median = 3,136) and ranged from 868 to 5,891 in our sample. We found no association between head impact exposure and baseline levels of any serum biomarkers ([pooled] p > 0.007 for all analyses). Furthermore, cumulative head impact exposure did not correlate with outcomes on any clinical measure ([pooled] p > 0.007 for all analyses).
Discussion
The pathophysiology of concussion and RHIE is complex and diverse within the CNS.14 Traumatic brain injury has classically been conceptualized as primarily affecting long white matter tracts thought to be most susceptible to shear-strain diffuse injury.15 The exact injury mechanism may actually be much broader and nonspecific, with many unrecognized person-level susceptibilities. The likely involvement of multiple components within the CNS, such as glia, cerebrovasculature, synapses, and dendrites, suggests that physiologic biomarker research must cast a wide net. Fluid biomarkers like those used within the present study offer the advantage of a panel-based approach to capturing injury effects. Such markers are likely differentially applicable based on timing (minutes, hours, or days after injury), severity (mild vs moderate/severe), and population of interest (acutely injured vs [a]symptomatic with repeated exposure history).14,16 Biomarker kinematics and the injury cascade process they purportedly reflect are both important considerations in this regard. The current lack of validated physiologic biomarkers for concussion management underscores both the novel and exploratory nature of this research to date.
Previous studies suggested that peripheral tau (measured in plasma) may be specific to extensive RHIE based on findings from a sample of retired NFL athletes.3,4 We were unable to replicate these findings with serum total tau or our 6 other biomarkers in a sample of collegiate athletes. Athletes in the present study presumably would have less overall RHIE than retired NFL athletes, but our data indicate that peripheral biomarkers are unlikely to detect any residual effects of multiple concussions or RHIE in college-aged athletes.
The literature on peripheral biomarker changes acutely and subacutely following exposure to subclinical head impacts, in the absence of a diagnosed concussion, remains conflicting.1,2,17,18 Plasma tau levels may elevate following a boxing match compared to age-matched “friends and relatives” controls, but then decrease after a rest period.1 A different study of boxers reported sustained elevations of neuron specific enolase (not measured in our study) following 2 months of rest, but no residual elevations in S100B or GFAP.18 Coupled with results showing no changes in tau, GFAP, S100B, or other fluid biomarkers acutely after controlled soccer heading,2 our data support the notion that serum biomarker evidence of CNS damage is not a requisite finding following RHIE, particularly after rest.
There are several possible interpretations for our findings: (1) there were no residual effects of brain trauma history to detect, (2) residual effects of brain trauma were insufficiently present to be detected, (3) the sample had insufficient exposure to RHIE, (4) residual effects, should they exist, progress and develop over time but are not detectable in college-aged individuals, or (5) this particular panel of biomarkers is not sensitive or specific enough to the pathophysiology of RHIE or multiple remote concussions.
The lack of correlation to clinical measures suggests that brain trauma history, as defined in the present study, does not influence performance on the cognitive, balance, and oculomotor assessments frequently utilized in athletic settings. In addition, we demonstrated that relationships between baseline serum biomarkers and cognitive scores were fully explained by race status. These data previously showed that both S100B and UCH-L1 vary significantly between white and black collegiate athletes,5 though the underlying biological mechanisms driving these differences is unclear. The links between brain trauma history and cognitive performance in collegiate athletes may be similarly influenced by demographic factors. Race/ethnicity (and correlated factors such as socioeconomic status and educational achievement) is a well-established modifier of cognitive test performance in the field of neuropsychology but is rarely incorporated into multivariate investigations of cognitive and functional outcomes following brain injury.
A recent study exhibited that race and socioeconomic status better predicted cognitive function than brain trauma history in collegiate athletes13; however, it must be acknowledged that approximately 35% of the sample from that study overlaps with the present sample. Other large studies of current collegiate athletes,19 including cadets from military service academies,20 have also found no difference in baseline cognitive performance between contact and noncontact sport athletes. The current study extends these findings by describing how serum biomarkers, cognition, and race might be related in a healthy collegiate athlete sample. More broadly, this further highlights the importance of appropriately considering relevant demographic factors (e.g., race, socioeconomic status, education quality) when utilizing neurocognitive endpoints in concussion and RHIE research.21,22
Concerns over long-term effects of concussion(s) and RHIE pervade sports at all participation levels because of the media attention and publicity surrounding conditions like CTE and its reported association with collision sport participation. The primary limitation to advancing CTE knowledge is arguably a lack of understanding of prevalence, incidence, and risk estimates. No validated in vivo diagnostic measures currently exist, though advanced neuroimaging and fluid biomarkers hold promise.23 This study did not hypothesize that CTE pathology would be prevalent in this sample; however, evaluating a large sample of subjectively healthy individuals with a range of exposure to RHIE helps advance the science of cumulative effects of concussion and RHIE. We believe these findings are important for demonstrating that blood biomarkers associated with CNS dysfunction are not disproportionately expressed with more exposure to head trauma in athletes during their college years.
Our analyses were restricted to college-aged participants from one institution and may not generalize to older or younger ages, or to other geographical regions with different sociodemographics. Reliance on self-report for determining concussion history and years playing collision sports is limited by inherent recall biases. Our assumption of football players playing the same primary position throughout their careers when calculating the mCHII may misrepresent certain individual's head impact exposure. In addition, there are several complexities with incorporating head impact biomechanics into predictive models. These include, but are not limited to, differential effects of helmeted vs nonhelmeted impacts, linear vs rotational acceleration, degree of anticipation, time between impacts, and other factors that are difficult to accurately quantify for research purposes.
While our data suggest that serum biomarker levels do not demonstrate residual effects of brain trauma history, we cannot directly imply similar findings in other fluid mediums such as plasma or CSF, and we did not have other modalities (e.g., advanced neuroimaging) against which to validate our serum biomarker data. It is also possible that our biomarker panel does not capture all potential pathophysiologic effects of concussion/RHIE history. Longitudinal data collection may better characterize individual changes in response to accumulated head impacts. We used total tau as a biomarker of interest, which may not characterize axonal damage as accurately as specific phosphorylated or cleaved tau products. Several factors beyond those measured in our study may influence peripheral biomarker expression, such as genetics (i.e., APOE genotype), glymphatic system functioning, sleep quality, and many others. These represent important avenues of future research that will continue to shed light on the ultimate utility of fluid biomarkers in the short- and long-term management of concussion and RHIE.
In conclusion, serum biomarkers associated with CNS dysfunction and collected at rest from healthy collegiate athletes in our sample demonstrated no association with concussion history or cumulative exposure to collision sports. Clinically, the relationship between serum biomarkers and cognitive performance seems mediated by race. The serum biomarkers studied may not detect residual effects of concussion or RHIE in otherwise asymptomatic collegiate athletes without recent exposure to head impacts. Much more research is needed for identifying reliable and valid blood biomarkers of brain trauma history.
Acknowledgment
The authors thank Thomas Glenn, PhD (Associate Professor, Department of Neurosurgery, Co-Director Cerebral Blood Flow Laboratory, University of California Los Angeles), for his technical review of the manuscript. The University of Florida has financial stake in Banyan Biomarkers, Inc. (see disclosures); an independent review of the manuscript was performed in accordance with predetermined requirements. The authors further thank Dr. Glenn for performing this review and for his determination that the manuscript is free of biased presentation.
Glossary
- Aβ42
β-amyloid peptide 42
- BASICS
Biomarkers Assessed in Collegiate Student-Athletes
- BESS
Balance Error Scoring System
- CTE
chronic traumatic encephalopathy
- GFAP
glial fibrillary acidic protein
- ImPACT
Immediate Postconcussion Assessment and Cognitive Testing
- K-D
King-Devick
- mCHII
modified Cumulative Head Impact Index
- NFL
National Football League
- RHIE
repetitive head impact exposure
- S100B
S100 calcium binding protein B
- SAC
Standardized Assessment of Concussion
- UCH-L1
ubiquitin carboxy-terminal hydrolyzing enzyme L1
Footnotes
Author contributions
Drafting/revising the manuscript for content, including medical writing for content: B.M.A., R.M.B., S.T.D., M.S.J., D.N.D., J.K.B., Z.M.H., C.C.M., A.G.W., J.R.C. Study concept or design: B.M.A., R.M.B., S.T.D., M.S.J., A.G.W., J.R.C. Analysis or interpretation of data: B.M.A., R.M.B., J.R.C. Acquisition of data: D.N.D., J.K.B., A.G.W., J.R.C. Statistical analysis: B.M.A. Study supervision or coordination: B.M.A., J.R.C. Obtaining funding: J.R.C. Other: Banyan Biomarkers, Inc. was responsible for organizing and conducting the biomarker analyses (blinded to all demographic and medical history information pertaining to each sample) and providing B.A./J.C. with these data. B.A. and J.C. had full access to all data throughout the study. Final approval of submitted manuscript: B.M.A., R.M.B., S.T.D., M.S.J., D.N.D., J.K.B., Z.M.H., C.C.M., A.G.W., J.R.C.
Study funding
This work was primarily supported by funds provided in a grant from the Head Health Initiative, a collaboration between GE and the NFL, Banyan Biomarkers, Inc., and the U.S. Army Medical Research and Material Command (USAMRMC) under contract W81XWH-06-1-0517. The University of Florida owns stock in Banyan Biomarkers, Inc., which is the sponsor of the study. In addition, the university has licensed technology to Banyan concerning blood biomarkers or proteins thereby allowing Banyan to use the technology. The company is interested in making a biomarker test for traumatic brain injury. If the biomarker test is sold commercially, then the University of Florida could benefit financially. Please feel free to ask any further questions you might have about this matter.
Disclosure
B. Asken received partial support from Banyan Biomarkers, Inc., for data collection and analysis. R. Bauer reports no disclosures relevant to the manuscript. S. DeKosky is supported by the 1Florida ADRC and AG047266. S.T.D. also reports serving on advisory boards for Amgen, Cognition Therapeutics, and Acumen, and chairs a drug monitoring committee for Biogen. Z. Houck and C. Moreno report no disclosures relevant to the manuscript. M. Jaffee is supported by McKnight Brain Institute and Florida Department of Elderly Affairs. D. Dubose received partial support from Banyan Biomarkers, Inc., for data collection and analysis. J. Boone received partial support from Banyan Biomarkers, Inc., for data collection and analysis. A. Weber holds stock in and is an employee of Banyan Biomarkers, Inc. J. Clugston is supported by Banyan Biomarkers, Inc., Florida High Tech Corridor Matching Funds Program, and NCAA-DoD CARE Consortium (award W81XWH-14-2-0151). Go to Neurology.org/N for full disclosures.
Publication history
Received by Neurology December 1, 2017. Accepted in final form August 23, 2018.
References
- 1.Neselius S, Zetterberg H, Blennow K, et al. Olympic boxing is associated with elevated levels of the neuronal protein tau in plasma. Brain Inj 2013;27:425–433. [DOI] [PubMed] [Google Scholar]
- 2.Zetterberg H, Jonsson M, Rasulzada A, et al. No neurochemical evidence for brain injury caused by heading in soccer. Br J Sports Med 2007;41:574–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alosco ML, Tripodis Y, Jarnagin J, et al. Repetitive head impact exposure and later-life plasma total tau in former National Football League players. Alzheimers Dement 2017;7:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montenigro PH, Alosco ML, Martin BM, et al. Cumulative head impact exposure predicts later-life depression, apathy, executive dysfunction, and cognitive impairment in former high school and college football players. J Neurotrauma 2017;34:328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asken BM, Bauer RM, DeKosky ST, et al. Concussion Biomarkers Assessed in Collegiate Student-Athletes (BASICS) I: normative study. Neurology 2018;91:e2109–e2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med 2013;47:250–258. [DOI] [PubMed] [Google Scholar]
- 7.McCrory P, Meeuwisse W, Dvorak J, et al. Consensus statement on concussion in sport—the 5th International Conference on Concussion in Sport held in Berlin, October 2016. Br J Sports Med 2017;51:838–847. [DOI] [PubMed] [Google Scholar]
- 8.Lovell MR, Iverson GL, Collins MW, et al. Measurement of symptoms following sports-related concussion: reliability and normative data for the post-concussion scale. Appl Neuropsychol 2006;13:166–174. [DOI] [PubMed] [Google Scholar]
- 9.McCrea M, Kelly JP, Randolph C, et al. Standardized Assessment of Concussion (SAC): on-site mental status evaluation of the athlete. J Head Trauma Rehabil 1998;13:27–35. [DOI] [PubMed] [Google Scholar]
- 10.Riemann BL, Guskiewicz KM. Effects of mild head injury on postural stability as measured through clinical balance testing. J Athl Train 2000;35:19–25. [PMC free article] [PubMed] [Google Scholar]
- 11.Oride MK, Marutani JK, Rouse MW, DeLand PN. Reliability study of the Pierce and King-Devick saccade tests. Am J Optom Visio Opt 1986;63:419–424. [DOI] [PubMed] [Google Scholar]
- 12.Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci 2007;8(3):206–213. [DOI] [PubMed] [Google Scholar]
- 13.Houck Z, Asken B, Clugston J, Perlstein W, Bauer R. Socioeconomic status and race outperform concussion history and sport participation in predicting collegiate athlete baseline neurocognitive scores. J Int Neuropsychol Soc 2017;24:1–10. [DOI] [PubMed] [Google Scholar]
- 14.Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery 2014;75:S24–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeKosky ST, Asken BM. Injury cascades in TBI-related neurodegeneration. Brain Inj 2017;31:1177–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papa L, Wang KK. Raising the bar for traumatic brain injury biomarker research: methods make a difference. J Neurotrauma 2017;34:2187–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olivera A, Lejbman N, Jeromin A, et al. Peripheral total tau in military personnel who sustain traumatic brain injuries during deployment. JAMA Neurol 2015;72:1109–1116. [DOI] [PubMed] [Google Scholar]
- 18.Zetterberg H, Tanriverdi F, Unluhizarci K, Selcuklu A, Kelestimur F, Blennow K. Sustained release of neuron-specific enolase to serum in amateur boxers. Brain Inj 2009;23:723–726. [DOI] [PubMed] [Google Scholar]
- 19.Katz BP, Kudela M, Harezlak J, et al. Baseline performance of NCAA athletes on a concussion assessment battery: a report from the CARE Consortium. Sports Med 2018;48:1971–1985. [DOI] [PubMed] [Google Scholar]
- 20.O’Connor KL, Dain Allred C, Cameron KL, et al. Descriptive analysis of a baseline concussion battery among US service academy members: results from the Concussion Assessment, Research, and Education (CARE) Consortium. Mil Med Epub 2018 Mar 28. [DOI] [PubMed]
- 21.Asken B, Sullan M, DeKosky S, Jaffee M, Bauer R. Research gaps and controversies in chronic traumatic encephalopathy. JAMA Neurol 2017;74:1255–1262. [DOI] [PubMed] [Google Scholar]
- 22.Asken BM, Sullan MJ, Snyder AR, et al. Factors influencing clinical correlates of chronic traumatic encephalopathy (CTE): a review. Neuropsychol Rev 2016;26:340–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCrea M, Meier T, Huber D, et al. Role of advanced neuroimaging, fluid biomarkers and genetic testing in the assessment of sport-related concussion: a systematic review. Br J Sports Med 2017;51:919–929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any qualified investigator may contact the corresponding (B.M.A.) or senior (J.R.C.) author with a specific request that includes details of (1) the resources requested, (2) how the data and resources will be used in the proposed research, and (3) the qualifications of the investigator requesting the resources. Investigators may be asked to provide further details if necessary. All requests will be reviewed for availability of the requested resources, potential for duplication of ongoing studies, and scientific merit. The requesting investigator may be asked to partner with a University of Florida investigator to better understand the available data and will sometimes be encouraged to include a Florida representative as an academic consultant or collaborator. Requesting investigators will also be required to sign a Data Use Agreement ensuring data will not be shared with third parties.